Abstract
Background
Post-traumatic stress disorder (PTSD) develops in a minority of traumatized individuals. Attention biases to threat and abnormalities in fear learning and extinction are processes likely to play a critical role in the creation and/or maintenance of PTSD symptomatology. However, the relationship between these processes has not been established, particularly in highly traumatized populations; understanding their interaction can help inform neural network models and treatments for PTSD.
Method
Attention biases were measured using a dot probe task modified for use with our population; task stimuli included photographs of angry facial expressions, which are emotionally salient threat signals. A fear-potentiated startle paradigm was employed to measure atypical physiological response during acquisition and extinction phases of fear learning. These measures were administered to a sample of 64 minority (largely African American), highly traumatized individuals with and without PTSD.
Results
Participants with PTSD demonstrated attention biases toward threat; this attentional style was associated with exaggerated startle response during fear learning and early and middle phases of extinction, even after accounting for the effects of trauma exposure.
Conclusions
Our findings indicate that an attentional bias toward threat is associated with abnormalities in ‘ fear load ’ in PTSD, providing seminal evidence for an interaction between these two processes. Future research combining these behavioral and psychophysiological techniques with neuroimaging will be useful toward addressing how one process may modulate the other and understanding whether these phenomena are manifestations of dysfunction within a shared neural network. Ultimately, this may serve to inform PTSD treatments specifically designed to correct these atypical processes.
Keywords: Attention bias, extinction, fear, PTSD, startle, threat
Introduction
Post-traumatic stress disorder (PTSD) is a debilitating condition that can develop in the aftermath of psychological trauma. Given that a minority of individuals develop PTSD after a traumatic experience (Kessler et al. 1995; Liebschutz et al. 2007), researchers have examined symptom manifestations, including intrusive trauma re-experiencing and hyperarousal, to guide investigation of specific underlying cognitive and physiological processes. Two associated processes have been highlighted in integrative theories of anxious psychopathology (Bishop, 2007): attention biases to threat-related cues; atypical fear conditioning/extinction processes.
With regard to the former, behavioral findings suggest that individuals with PTSD preferentially allocate attentional resources toward threat-related cues (Buckley et al. 2000). Attention biases to trauma stimuli, whether in the form of facilitated orientation to or delayed disengagement from such cues, are highly maladaptive in the absence of actual threat. Such biases preclude adequate processing of corrective information and lead to an inefficient cognitive processing style, disrupting downstream processes such as explicit memory retrieval.
Evidence for these biases has largely emerged from modified Stroop tasks, although other tasks, including lexical decision-making tasks, have also been used (Pineles et al. 2007). Stroop studies have highlighted clear processing disruptions during attention to trauma-related words in PTSD (McNally et al. 1990, 1993; Foa et al. 1991; Cassiday et al. 1992; Kaspi et al. 1995; Vrana et al. 1995; Dalgleish et al. 2003). However, the Stroop is limited in its ability to measure attention biases, i.e. facilitated orientation toward, or avoidance of, these cues. In this regard, another task, the dot probe, has inherent strengths; it is equipped to measure direction of bias and, given that it can accommodate words or images, can be a more precise and adaptable measure for examining attention biases. Further, it does not require semantic processing, unlike the Stroop or other lexically based tasks. However, only a minority of PTSD attention bias studies have employed this measure (Bryant & Harvey, 1995; Dalgleish et al. 2003; Elsesser et al. 2004, 2005; Pine et al. 2005; Fani et al. 2010). Findings from these studies have been mixed with regard to direction or type of bias and a select few used stimuli that were directly relevant to participants’ trauma(s).
In sum, more precise and adaptable attention bias measures are needed in PTSD research. To address these concerns, our research group adapted a standard dot probe task (Bradley et al. 1997) to include photographs of African American (AA) and Caucasian (C) models displaying emotional facial expressions, including anger. Considering the high rates of interpersonal trauma experienced by participants in our study population (Schwartz et al. 2005; Gillespie et al. 2009), most of whom are AA, these stimuli are particularly salient.
Trauma-related stimuli not only capture attentional resources in individuals with PTSD, but also provoke exaggerated physiological reactions (Pitman et al. 1999; McTeague et al. 2010), including startle response, that persist in the absence of these stimuli. Fear conditioning and extinction paradigms have been valuable for exploring the basis of this heightened physiological arousal. Specifically, individuals with PTSD have shown exaggerated physiological response compared with PTSD-free controls during conditioned fear learning (Lissek et al. 2005) and extinction processes (Norrholm et al. 2011).
During fear conditioning, a previously neutral stimulus comes to elicit a defensive physiological response (e.g. increased arousal) after repeated pairings with a threat-related or aversive stimulus (Pavlov, 1927, see review by Phelps & LeDoux, 2005). Some theorists have proposed that individuals with PTSD demonstrate exaggerated physiological and behavioral responses in response to trauma-related or neutral stimuli as a result of abnormal fear conditioning processes (Davis, 1992). Fear conditioning paradigms have been useful for identifying atypical fear processes in PTSD (e.g. see Morgan et al. 1997; Grillon & Morgan, 1999; Bremner et al. 2005; Jovanovic et al. 2009b, 2009c; Milad et al. 2009).
Extinction is a type of learning that occurs when a neutral cue, previously paired with an aversive stimulus during conditioning, appears repeatedly in the absence of the aversive stimulus. This process promotes attenuation of the heightened physiological response observed during conditioning (Phelps et al. 2004). Findings from recent studies indicate that PTSD is also characterized by an inability to inhibit fear responses during fear extinction (Norrholm et al. 2011).
In sum, attention biases to trauma-related cues, conditioned fear learning and extinction are processes that have been implicated in the pathophysiology of PTSD, but surprisingly few studies have directly investigated the relationship between these processes. In one study, (Elsesser et al. 2004) a dot probe task (including trauma-relevant, generally aversive, neutral and positive pictures) and a startle paradigm were administered to healthy controls (HCs) and trauma survivors, a minority of whom met criteria for acute stress disorder (1%) or PTSD (21%). Trauma survivors and PTSD patients viewed trauma-related pictures longer than HCs, but no statistically significant between-group differences were found for attention bias scores. However, PTSD patients demonstrated a higher startle amplitude than HCs. Correlations between attention bias scores and startle response were not reported, but viewing time for trauma-related images was negatively correlated with startle response in traumatized participants, possibly indicating that exaggerated startle responses were related to attentional avoidance of trauma cues in traumatized individuals. Altogether, their findings suggest that, in trauma survivors, greater physiological arousal may be related to attentional disruption to trauma cues, but provide no information about whether attention biases are associated with atypical physiological responses during fear acquisition or extinction in PTSD.
In sum, previous studies have demonstrated relationships between attentional disruption and response to fear-potentiated startle probes (Elsesser et al. 2005). However, few studies have investigated the relationship between attention biases to threat and fear acquisition and extinction processes in individuals with PTSD. Establishing a link between these associative and attentional processes would provide a richer understanding of pathophysiological processes that maintain PTSD symptomatology. Thus, the objective of this study was to examine associations among PTSD, attention bias for threat (conveyed in angry facial expressions), fear acquisition and extinction. We hypothesized that: (1) participants with current PTSD would demonstrate significant attention biases for threat, relative to traumatized controls, given the mixed findings in the current literature, these biases may manifest either toward or away from the cue; (2) PTSD participants would demonstrate increased fear expression during fear acquisition and impaired fear inhibition during fear extinction, relative to controls; (3) attention biases for threat would be significantly associated with increased startle during fear learning and impaired fear inhibition during extinction in individuals with PTSD, but not traumatized controls, after controlling for trauma exposure.
Method
Participants
Participants were recruited through an ongoing study of risk factors for PTSD in a highly traumatized urban population. Study procedures were approved by the Institutional Review Boards of Emory University and Georgia State University, Atlanta, Georgia. Patients were deemed eligible for participation if they were able to give informed consent. A total of 69 adult males and females aged 18–59 years participated in this study. Data from five of these participants, however, were excluded from analyses due to poor dot probe task performance (more than 20% skipped trials or trial errors), yielding a final sample of 64 participants.
Participants in the final sample were primarily female (61%) and AA (95%) and were, on average, 38 years of age (S.D.=12.5), demographically similar to our earlier study (Fani et al. 2010). Most participants had obtained ≤12 years of education (77%) and reported household monthly incomes of ≤$1000 (68%). Participants were administered the PTSD Symptom Scale (PSS), described below, to assess for the presence of PTSD symptoms and as proxy for assessment of DSM-IV criteria for current PTSD. Based on these criteria, 39 participants were classified as trauma controls (Control) and 25 were classified as PTSD+. Most participants experienced different trauma types, predominantly interpersonal in nature, as evidenced by previous studies sampling this population (e.g. Schwartz et al. 2005; Bradley et al. 2008). All clinical measures were administered orally by trained interviewers to decrease potential confounds introduced by literacy problems common to this population.
No significant differences were found in demographic characteristics between PTSD and Control groups, including age, household monthly income and educational level (p>0.05). Mean total PSS score for controls was 8.4 (S.D.=6.6); mean total PSS score for PTSD participants was 27.4 (S.D.=10.8). We did not exclude participants due to use of psychotropic medication, given that psychotropic medication use was not a common occurrence in this study. None of the 39 controls was taking psychotropic medication. Of the 25 PTSD participants, one was taking selective serotonin re-uptake inhibitors and benzodiazepines and another participant was taking only benzodiazepines.
Measures
PTSD Symptom Scale
The PSS (Falsetti et al. 1993) was used to evaluate for presence and severity of PTSD symptomatology; it includes 17 items assessing for the presence of PTSD symptoms in the past 2 weeks based on DSM-IV criteria and a final item measuring symptom duration. For the purposes of this study, participants were classified as PTSD+ if they endorsed one or more symptoms in the re-experiencing cluster, three or more symptoms in the avoidance/numbing cluster, two or more symptoms in the hyperarousal cluster and three or more months symptom duration, in keeping with DSM-IV PTSD criteria.
Traumatic Events Interview (TEI)
The TEI is a clinician-administered questionnaire that assesses number and type of traumatic incidents experienced throughout the lifetime. Consistent with prior research (Gillespie et al. 2009), total level of trauma exposure was measured by number of types of traumatic experiences reported by study participants.
Childhood Trauma Questionnaire (CTQ) (Bernstein et al. 2003)
The CTQ is a 28-item self-report measure of child maltreatment and neglect that yields a total childhood trauma index used for the purposes of the present study.
Dot Probe Task
Details regarding task structure have been published previously (Fani et al. 2010) and are described briefly in Fig. 1. Emotion bias scores were calculated by subtracting response time to emotion-congruent stimuli (probes that replace neutral pictures) from response time to emotion-incongruent stimuli (probes that replace happy or threatening pictures). Emotion bias scores were further decomposed into threat and happy bias scores (for both AA and C faces); racespecific bias scores were also generated. A positive score indicates attention bias toward the selected emotion; negative scores indicate bias away from the emotion. This task was administered to participants following the fear acquisition paradigm and immediately prior to the fear extinction paradigm.
Fig. 1.
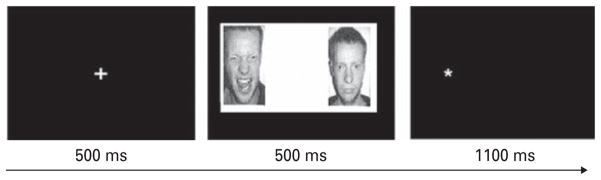
Dot Probe Task. Each dot probe trial began with the presentation of a central fixation cross for 500 ms, immediately followed by a pair of face photographs (both of the same actor) for 500 ms; in each pair, a threatening, happy or neutral face was paired with a neutral face. After the offset of the face pair, an asterisk was presented in place of one of the faces. Participants indicated as quickly as possible with a forced-choice button press response whether the asterisk appeared on the left- or right-hand side of the screen. This task consisted of 80 randomly ordered trials (32 positive-neutral, 32 threat-neutral and 16 neutral-neutral face pairs, all posed by female actors). The faces used in this task were selected from three separate sets of stimuli; African American (AA) faces were selected from the Center for Productive Aging (Minear & Park, 2004) and NimStim (Tottenham et al. 2009) databases and Caucasian (C) faces were selected from a commonly used version of the dot probe (Bradley et al. 1997). A total of 50% AA and 50% C face pairs were used in this version of the dot probe.
Startle response measurement
Startle response data were acquired using the electromyography (EMG) module of the Biopac MP150 for Windows (Biopac Systems, Inc., USA). The acquired data were sampled at 1000 Hz, filtered, rectified and smoothed using MindWare software (MindWare Technologies Ltd, USA) and exported for statistical analyses. The EMG signal was filtered with low- and high-frequency cut-offs at 28 and 500 Hz, respectively. The maximum amplitude of the eyeblink muscle contraction 20–200 ms after presentation of the startle probe was used as a measure of acoustic startle response. As previously described (Jovanovic et al. 2005, 2006), the eyeblink component of acoustic startle response was measured by EMG recordings of the right orbicularis oculi muscle with two 5-mm Ag/AgCl electrodes filled with electrolyte gel. One electrode was positioned 1 cm below the pupil of the right eye and the other was 1 cm below the lateral canthus. Impedance levels were <6 kΩ for each participant. The startle probe was a 108-dB(A) sound pressure level (SPL), 40 ms burst of broadband noise with near instantaneous rise time, delivered binaurally through headphones.
Fear-potentiated startle
The fear-potentiated startle task included two phases: fear acquisition; extinction. The fear acquisition phase began with six startle probes presented alone [noisealone (NA) trials] in order to reduce initial startle reactivity. This phase was followed by a conditioned stimulus (CS) habituation phase, in which stimuli were presented without the aversive unconditioned stimulus (UCS). Immediately following habituation, participants underwent the conditioning phase, which consisted of three blocks, each comprising four trials of each CS type and four NA trials, for a total of 12 trials per block. All CS+ trials were reinforced with the UCS; CS− trials were not reinforced. The UCS was a 250-ms airblast with an intensity of 140 psi directed to the larynx. This UCS has been used in our previous studies (Jovanovic et al. 2005; Norrholm et al. 2006) and produces robust fear-potentiated startle. The conditioned stimuli were different colored shapes presented on a computer monitor, 6 s in duration. The extinction phase began 10 min after fear acquisition, consisting of six blocks, each with four trials of each CS type and four NA trials. During this phase, neither CS was reinforced with the UCS. In all phases of the experiment, inter-trial intervals were of randomized duration ranging from 9 to 22 s.
Statistical analyses
Fear-potentiated startle was calculated using a difference score obtained by subtracting startle magnitude to the NA trials from the startle magnitude on CS+ trials and CS− trials for each conditioning block. To examine differences in fear conditioning between groups, a repeated-measure analysis of variance (ANOVA) was conducted with diagnostic group as the between-groups factor (PTSD, Control) and trial type as the repeated measure (CS+, CS−) during late acquisition (i.e. blocks 2 and 3, when discrimination is maximal). Significant interaction effects were followed by univariate ANOVA comparing diagnostic groups within each trial type.
Extinction to the previously reinforced CS+ was divided into early (blocks 1 and 2), mid (blocks 3 and 4) and late (blocks 5 and 6) phases. Fear-potentiated startle during extinction was entered as a withingroups variable with three levels (three phases) in a repeated-measures ANOVA, with diagnosis as the between-subjects variable. Significant interaction effects were decomposed by comparing diagnostic groups within each phase using univariate ANOVA. Extinction data from nine subjects (three PTSD, six Controls) were missing due to computer or experimenter error. Thus, extinction analyses included 55 subjects; dot probe and fear acquisition analyses included 64 subjects.
Mean attention bias scores for AA and C threatening faces were compared using a univariate ANOVA. Where significant (p<0.05) differences were found between PTSD and control groups for mean attention bias to threat, these scores were entered into correlational analyses with startle variables (late acquisition for CS+, late acquisition for CS−, early extinction, middle extinction, late extinction). Finally, regression analyses including trauma incidence, PTSD status and attention bias score as predictors were included to examine their unique associations with fear learning and extinction. Significance threshold was set at α<0.05. Repeated-measures analyses used the Sphericity-Assumed statistic.
Results
Significant between-group differences were found for mean PSS (PTSD=27.4, S.D.=10.8; Control=8.3, S.D.=6.6, F1,63=76.3), CTQ (PTSD=51.9, S.D.=22.6; Control=38.6, S.D.=14.1, F1,62=8.3, p<0.05) and TEI (means: PTSD=3.9, S.D.=2; Control=2.7, S.D.=1.7, F1,63=5.9, p<0.05) scores. A three-way ANOVA of attention bias for emotion (threatening, happy), race (C and AA), by diagnosis demonstrated a significant interaction of race and PTSD (F1,62=4.39, p<0.05) and a trend for emotion (F1,62=3.17, p=0.08). Follow-up ANOVA indicated that participants with PTSD demonstrated significant attentional biases toward threat, compared with Controls, expressed in C (F1,62=5.17, p<0.05), but not AA (F1,62=1.11, p>0.05) faces (see Table 1). However, no significant between-group differences were found (PTSD versus Controls) for mean threat bias score (AA and C faces, combined; F1,62=0.91, p>0.05) or mean happy bias score (AA and C faces, combined; F1,62=0.38, p>0.05). No significant effects of gender or gender/PTSD interaction were found on threat bias.
Table 1.
Mean (S.D.) attention bias scores for PTSD and control groups (n=64)
| Group | n | Threat bias | Happy bias | AA face threat bias | C face threat bias | AA face happy bias | C face happy bias |
|---|---|---|---|---|---|---|---|
| Control | 39 | 3.43 (50.6) | −6.66 (38.6) | 10.22 (57.15) | −3.79 (65.73) | −9.32 (69) | −3.06 (49) |
| PTSD | 25 | 15.44 (46.8) | 0.15 (49.89) | −6.33 (67.26) | 35.26 (69.02)* | −8.63 (62.27) | 6.9 (59.66) |
PTSD, Post-traumatic stress disorder; AA, African American; C, Caucasian.
Indicates significant between-group difference.
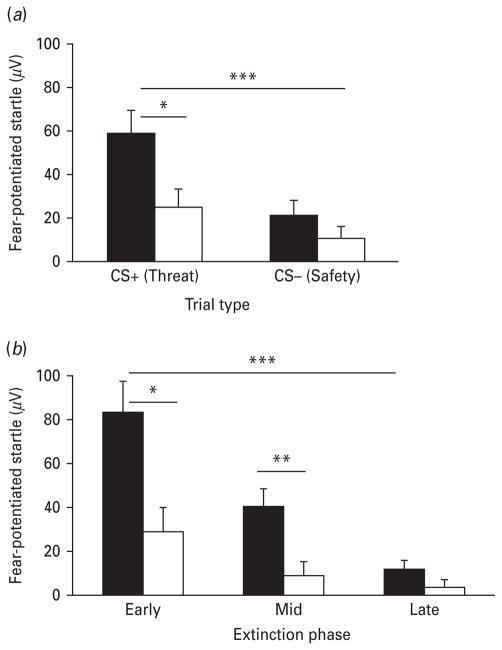
Fig. 2a shows fear conditioning results between PTSD and Controls. A repeated-measures ANOVA of fear-potentiated startle during the late acquisition phase with trial type (CS+, CS−) as a within-groups variable and diagnostic group (PTSD, Control) as a between-subjects factor revealed a significant main effect of trial type (F1,62=19.40, p<0.001), a significant main effect of group (F1,62=4.89, p<0.05) and an interaction effect (F1,62=3.95, p=0.05). Follow-up ANOVA of diagnostic groups within each trial type showed that PTSD subjects had higher fear-potentiated startle to the CS+ (threat cue) than Controls (F1,62=6.21, p<0.05); however, there was no group difference in fear-potentiated startle to the CS−.
Fig. 2.
Mean+S.E. (a) Fear-potentiated startle during the late acquisition phase across conditioned stimulus (CS) type and group. Startle was higher during the reinforced conditioned stimulus (CS+) than the non-reinforced conditioned stimulus (CS−) in both groups. The traumatized group with post-traumatic stress disorder (■, PTSD) had increased startle to the CS+ compared with the traumatized Control group (□). Fear-potentiated startle was defined as the difference score between the startle magnitude to the CS minus startle magnitude to noise alone. (b) Fear-potentiated startle to the CS+ during extinction across phase and group. Startle decreased over the three phases of extinction in both groups. Startle was higher during the early and middle extinction phases in the PTSD+ group compared with the traumatized Control group. Fear-potentiated startle was defined as the difference score between the startle magnitude to the CS minus startle magnitude to noise alone. * p<0.05; ** p<0.01; *** p<0.001.
Fig. 2b shows fear extinction results between PTSD and Controls. A repeated-measures ANOVA of fearpotentiated startle to the CS+ with extinction phase (early, mid, late) as a within-groups variable and diagnostic group (PTSD, Control) as a between-subjects factor revealed a significant main effect of phase (F2,106=31.59, p<0.001), a significant main effect of group (F1,53=8.94, p<0.005), and a significant interaction effect (F2,106=6.88, p<0.005). Follow-up ANOVA of diagnostic groups within each phase of extinction indicated that PTSD subjects had higher fearpotentiated startle than Controls during early extinction (F1,54=8.99, p<0.005) and mid-extinction (F1,54=9.15, p<0.005), but not during late extinction. To examine the effect of the degree of fear acquisition on extinction, we compared fear-potentiated startle during extinction divided by each individual’s level of fear-potentiated startle to the CS+ during late acquisition. After correcting for fear acquisition, PTSD subjects still displayed higher levels of fear-potentiated startle during early extinction (F1,54=6.58, p<0.05); however, there were no longer group differences in mid-extinction.
Threat bias for C faces significantly and positively correlated with startle response during late acquisition to danger signals (r=0.41, p<0.05) as well as startle response during early extinction (r=0.52, p<0.05) in PTSD subjects (see Table 2). Although threat bias for C faces demonstrated a weak negative correlation with acquisition of safety signals (r=−0.31, p=0.055) in Controls, no significant correlations were found for threat bias for C faces and late acquisition of danger signals or early or mid-extinction.
Table 2.
Intercorrelations among attention bias for threatening Caucasian faces and fear-potentiated startle
| Participants with PTSD (n=25) | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|
| 1. FPS late acquisition (CS+) | 0.474* | 0.633** | 0.391 | −0.131 | 0.413* |
| 2. FPS late acquisition (CS−) | 0.380 | 0.377 | 0.132 | 0.154 | |
| 3. FPS early extinction (CS+) | 0.826** | 0.509* | 0.516* | ||
| 4. FPS middle extinction (CS+) | 0.709* | 0.215 | |||
| 5. FPS late extinction (CS+) | 0.081 | ||||
| 6. Attention bias for threat | – |
| Controls (n=39) | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|
| 1. FPS late acquisition (CS+) | 0.653** | 0.582** | 0.34 | 0.142 | 0.130 |
| 2. FPS late acquisition (CS−) | 0.58** | 0.467** | 0.105 | −0.310 | |
| 3. FPS early extinction (CS+) | 0.711** | 0.538** | −0.044 | ||
| 4. FPS middle extinction (CS+) | 0.498** | −0.134 | |||
| 5. FPS late extinction (CS+) | 0.083 | ||||
| 6. Attention bias for threat | – |
PTSD, Post-traumatic stress disorder; FPS, fear-potentiated startle; CS, conditioned stimulus.
p<0.05,
p<0.01.
Hierarchical regressions including trauma history, PTSD diagnosis, threat bias, and the interaction of PTSD/threat bias were conducted to examine independent contributions of PTSD and threat bias to fear expression after controlling for trauma incidence. In the first model (see Table 3), total incidence of child and adult trauma exposure did not contribute a significant amount of variance to fear acquisition (R2=0.01, p>0.05). However, when added to this model, a PTSD diagnosis contributed significantly (R2 change=0.13, p<0.05), making the overall model significant (R2=0.14, p=0.03). Added to this model, attention bias for threatening C faces (R2 change=0.09, p<0.05) also contributed significantly to the variance in fear acquisition (R2 of overall model=0.23). Finally, an interaction term of threat bias for C faces and PTSD added significantly to the overall model (R2 change= 0.08, p<0.05); the overall model was significant (F5,62=4.88, p<0.01 and accounted for 30% of the variance in fear acquisition.
Table 3.
Contributions of PTSD, attention bias for threat, and interaction of PTSD/threat bias on late fear acquisition after controlling for incidence of childhood and adult trauma (n=64)
| R2 | β | p | ||
|---|---|---|---|---|
| Step 1 | CTQ total | 0.01 | −0.37 | 0.41 |
| TEI total | 2.54 | 0.57 | ||
| Step 2 | CTQ total | 0.14 | −0.68 | 0.18 |
| TEI total | 1.07 | 0.80 | ||
| PTSD diagnosis | 43.29 | 0.005* | ||
| Step 3 | CTQ total | 0.23 | −0.79 | 0.06 |
| TEI total | 0.92 | 0.82 | ||
| PTSD diagnosis | 35.42 | 0.017* | ||
| Threat bias† | 0.25 | 0.013* | ||
| Step 4 | CTQ total | 0.3 | −1.05 | 0.013* |
| TEI total | 4.87 | 0.24 | ||
| PTSD diagnosis | 25.19 | 0.09 | ||
| Threat bias† | −0.46 | 0.13 | ||
| PTSD/Threat bias Interaction | 0.5 | 0.017* |
PTSD, Post-traumatic stress disorder; CTQ, Childhood Trauma Questionnaire; TEI, Traumatic Events Interview.
Dependent variable=startle response, late acquisition (p<0.05).
Represents threat bias for Caucasian faces only.
In the second model (see Table 4), total incidence of child and adult trauma exposure did not contribute a significant amount of variance to fear inhibition (R2=0.01, p>0.05. However, when added to this model, a PTSD diagnosis contributed significantly (R2 change=0.16, p<0.05) to the overall model (R2=0.18, p<0.05). Attention bias for threatening C faces produced a significant R2 change (0.10, p<0.05) and addition of the interaction of threat bias for C faces and PTSD (R2 change=0.17, p<0.05) improved the overall model (R2=0.44, p<0.001).
Table 4.
Contributions of PTSD, attention bias for threat, and interaction of PTSD/threat bias on early extinction after controlling for incidence of childhood and adult trauma (n=55)
| R2 | β | p | ||
|---|---|---|---|---|
| Step 1 | CTQ total | 0.01 | −0.19 | 0.74 |
| TEI total | 5.1 | 0.39 | ||
| Step 2 | CTQ total | 0.18 | −0.73 | 0.2 |
| TEI total | 3.53 | 0.52 | ||
| PTSD diagnosis | 64.18 | 0.003* | ||
| Step 3 | CTQ total | 0.28 | −0.87 | 0.11 |
| TEI total | 3.35 | 0.52 | ||
| PTSD diagnosis | 52.06 | 0.011* | ||
| Threat bias† | 0.35 | 0.012* | ||
| Step 4 | CTQ total | 0.44 | −1.3 | 0.01* |
| TEI total | 11.04 | 0.03* | ||
| PTSD diagnosis | 29.21 | 0.12 | ||
| Threat bias† | −1.12 | 0.008* | ||
| PTSD/Threat bias Interaction | 1.0 | <0.001* |
PTSD, Post-traumatic stress disorder; CTQ, Childhood Trauma Questionnaire; TEI, Traumatic Events Interview.
Dependent variable=startle response, early extinction (p<0.05).
Represents threat bias for Caucasian faces only.
Discussion
We used a direct, emotionally salient visual attention task and a fear-potentiated startle paradigm to examine associations among attention biases and learning to acquire and extinguish fear. The findings from this study were largely consistent with our primary hypotheses. Relative to traumatized controls, participants with PTSD demonstrated significantly greater attention bias toward threat, indicating that PTSD in this population is characterized by a threat-orienting attention style. Although this may be adaptive in the presence of actual threat, in the absence of danger this bias can prevent adequate processing of other relevant environmental information. Neglecting other important environmental cues (including signals indicating safety) may lead to an inappropriate, exaggerated fear response, which is a characteristic of PTSD.
Additionally, this bias was specific to threatening C, but not AA, faces in participants with PTSD, relative to controls. This may suggest that threatening facial expressions in racial ‘out-group’ members may be particularly arousing, and possibly more threatening, than those of ‘in-group’ members for this sample of minority individuals with PTSD. Given that other social cognitive studies have observed tendencies to view familiar faces as more positively valenced than novel faces (Claypool et al. 2007), our findings may indicate that expressions of threat in less familiar, other-race faces capture greater attentional resources in this minority sample. Due to the likelihood that individuals in this population lived in racially homogenous neighborhoods and interacted with other-race individuals less frequently, C faces (particularly those conveying anger) may be more arousing, and potentially more threatening, to individuals with PTSD, as compared to threatening facial expressions of same-race individuals.
Relative to traumatized controls, this group of individuals with PTSD also demonstrated enhanced fear-potentiated startle to conditioned stimuli that signaled threat (CS+) and an impaired ability to inhibit this response during early and middle extinction phases. These data indicate that PTSD subjects have exaggerated fear expression to threat cues during conditioning and that it takes longer for this exaggerated fear response to extinguish. These findings support our primary hypotheses and are consistent with other recent findings in PTSD (Jovanovic et al. 2009b, 2010; Norrholm et al. 2011). Interestingly, individuals with PTSD demonstrated startle responses comparable with those of controls during the latest phase of extinction. This suggests that PTSD is best characterized by fear inhibition deficits during earlier extinction learning stages and that these deficits may not be apparent after repeated, prolonged exposure trials. Data from our recent extinction study (Norrholm et al. 2011) suggest that startle in the early phase of extinction is related to fear expression, as it is correlated with the level startle to the CS+ (i.e. danger signal) at the end of acquisition. We have termed this heightened fear expression in PTSD ‘ fear load’, evidenced by exaggerated fear during late acquisition and early extinction (Norrholm et al. 2011). It is important to note that even after accounting for the degree of fear in late acquisition, PTSD subjects still demonstrated higher fear-potentiated startle (i.e. less fear inhibition) during early extinction relative to controls.
We found that this increased fear load is associated with attentional orienting to threat, even after controlling for trauma exposure, and that these associations were relevant to a diagnosis of PTSD (demonstrated by a significant threat bias/PTSD diagnosis interaction). This implies that enhanced physiological response during fear learning is closely linked to a threat-orienting attentional style in individuals with PTSD, but not controls. It remains unclear whether attention biases or exaggerated responses during fear conditioning are first to emerge. Some studies of healthy individuals suggest that attention biases develop due to atypical fear learning processes (Kelly & Forsyth, 2007), while others suggest that these biases modulate magnitude of fear responses during startle conditioning (Filion et al. 1998). Nonetheless, there may be common physiological mechanisms associated with these phenomena; one possibility is hypersensitization of excitatory neural networks that facilitate rapid detection and response to threat (Bishop, 2007).
Regression analyses indicated that, in isolation, a PTSD diagnosis was not significant in the overall models. However, the interaction of a PTSD diagnosis and attention bias to threat was significant, indicating that the combined effects of PTSD and threat bias contribute significantly to fear-potentiated startle response during acquisition and inhibition. Successful fear extinction is likely to be modulated by the attentional resources available to the individual and an inefficient, over-vigilant attentional style may impair adequate inhibition of the fear response. Additionally, childhood maltreatment, but not adult trauma, was also found to be a significant predictor of this fear load, which is consistent with earlier findings (Pole et al. 2007; Jovanovic et al. 2009a), suggesting that exaggerated startle may be a physiological marker of childhood maltreatment.
These findings provide evidence for associations between two processes that are likely to play critical roles in the creation or maintenance of PTSD. Although their neural correlates have not been extensively investigated in PTSD, some evidence suggests that a common neural circuit may mediate the expression of these phenomena. Components of this circuit include the ventrolateral prefrontal cortex (vlPFC), medial prefrontal cortex [(mPFC); including the anterior cingulate cortex (ACC)] and the amygdala. The individual components of this circuit are differentially responsible for facilitated attention to threat and inhibition/control of fear response and have functioned atypically in anxious individuals during attention and response inhibition tasks. For example, one study indicated that, anxious adolescents, relative to healthy adolescents, demonstrated increased vlPFC activation to threatening facial expressions presented in the context of a dot probe task (Monk et al. 2006). Increased activity in the amygdala, a brain region that mediates rapid attention to threat (Phelps & LeDoux, 2005), has also been implicated in fear conditioning (LaBar et al. 1998; Bremner et al. 2005; Knight et al. 2005) and the mPFC appears relevant to both fear learning and extinction in adults. In a study of healthy individuals, Milad and colleagues found increased mPFC [specifically, dorsal aspects of the ACC (dACC)] activation during presentation of reinforced conditioned stimuli (danger cues) relative to presentation of non-reinforced conditioned stimuli (safety cues), indicating that this ACC region is related to fear expression, whereas ventromedial aspects of the prefrontal cortex (vmPFC) were related to fear inhibition (Milad et al. 2007). In a study of PTSD, Milad and colleagues found less activation in vmPFC and more activation in the dACC during extinction learning in individuals with PTSD compared with traumatized controls (Milad et al. 2009).
Taken together, these findings suggest that alterations in activation in distinct components of these neural circuits, including the amygdala, vlPFC and dorsal and ventral aspects of the mPFC, are relevant to attention biases to threat as well as fear learning and inhibition. Given the findings of the present study and the indications of previous studies, attentional biases and learned fear responses may be associated with imbalances in the neural network that mediates these functions. Future studies combining these behavioral and psychophysiological techniques with neuroimaging will be useful toward elucidating patterns of dysfunction in this network.
Several study limitations must be noted. As stated earlier, the design of this study precluded determination of the primacy of these processes (i.e. which process was first to emerge and which modulates the other). It is unclear whether attentional manipulation during fear acquisition or extinction would lead to attenuated fear expression during either phase; this is a worthy target for future investigation. Also, participants were not excluded for psychotropic medication usage, a potential confounding variable. Other limitations include our measures of PTSD and trauma exposure; like most self-report measures, there is the possibility for biases in reporting (over- or underreporting). Another limitation is related to our attention bias paradigm; given that the dot probe stimuli exclusively comprised female photographs, we were unable to examine possible gender-specific effects on biases. We were likewise unable to determine whether there were between-group differences in valence or arousal for these stimuli. Additionally, although we chose to study a population that is largely neglected in the PTSD literature, the selection of such a racially homogenous sample may limit the generalizability of these results to other traumatized populations.
In summary, we found that preferential allocation of attention toward threat was associated with heightened fear expression in this sample of individuals with PTSD. Given that attention bias toward threat is linked with psychopathology, it is likely that this bias represents a maladaptive attention style, which may create or maintain symptoms. We found that this bias toward threat in other-race faces was associated with enhanced fear-potentiated startle responses during fear acquisition and extinction in individuals with PTSD. In contrast, traumatized controls demonstrated little to no attention bias to threat and had lower physiological reactions during fear learning and inhibition. By elucidating the nature and direction of the associations between these processes in PTSD, future treatments can be manipulated to better target and correct these processes. We speculate that atypical patterns of activation in medial prefrontal circuits may be responsible for these phenomena; thus, neuroimaging research combining these paradigms is valuable toward obtaining a richer understanding of neural circuits engaged in these processes.
Acknowledgments
This work was primarily supported by National Institutes Health Grants MH071537 (K.J.R.) and the Georgia State University Brains and Behavior Fellowship (N.F.). Support was also received from Emory and Grady Memorial Hospital General Clinical Research Center, NIH National Centers for Research Resources (M01RR00039 and P20RR16435), the American Foundation for Suicide Prevention (B.B.) and the Burroughs Wellcome Fund (K.J.R.). We thank Karin Mogg and Brendan Bradley for their assistance with the development of this dot probe measure and the staff of the Grady Trauma Project, especially Allen Graham, Angelo Brown, India Karapanou, Daniel Crain, Lamya Khouri and Nineequa Blanding for their excellent technical support.
Footnotes
Declaration of Interest
None.
References
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse and Neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive Sciences. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Lee SC. Attentional biases for negative information in induced and naturally occurring dysphoria. Behaviour Research and Therapy. 1997;35:911–927. doi: 10.1016/s0005-7967(97)00053-3. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropinreleasing hormone receptor gene. Archives of General Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related posttraumatic stress disorder. Psychological Medicine. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Harvey AG. Processing of threatening information in posttraumatic stress disorder. Journal of Abnormal Psychology. 1995;104:537–541. doi: 10.1037//0021-843x.104.3.537. [DOI] [PubMed] [Google Scholar]
- Buckley TC, Blanchard EB, Neill WT. Information processing and PTSD: a review of the empirical literature. Clinical Psychology Review. 2000;28:1041–1065. doi: 10.1016/s0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Cassiday KL, McNally RJ, Zeitlin SB. Cognitive processing of trauma cues in rape victims with post-traumatic stress disorder. Cognitive Therapy and Research. 1992;16:283–295. [Google Scholar]
- Claypool HM, Hugenberg K, Housley MK, Mackie DM. Familiar eyes are smiling: on the role of familiarity in the perception of facial affect. European Journal of Social Psychology. 2007;37:856–866. [Google Scholar]
- Dalgleish T, Taghavi R, Hamid N, Moradi A, Canterbury R, Yule W. Patterns of processing bias for emotional information across clinical disorders: a comparison of attention, memory, and prospective cognition in children and adolescents with depression, generalized anxiety, and Posttraumatic Stress Disorder. Journal of Clinical Child and Adolescent Psychology. 2003;32:10–21. doi: 10.1207/S15374424JCCP3201_02. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends in Pharmacological Sciences. 1992;13:35–41. doi: 10.1016/0165-6147(92)90014-w. [DOI] [PubMed] [Google Scholar]
- Elsesser K, Sartory G, Tackenberg A. Attention, heart rate, and startle response during exposure to trauma-relevant pictures: a comparison of recent trauma victims and patients with posttraumatic stress disorder. Journal of Abnormal Psychology. 2004;113:289–301. doi: 10.1037/0021-843X.113.2.289. [DOI] [PubMed] [Google Scholar]
- Elsesser K, Sartory G, Tackenberg A. Initial symptoms and reactions to trauma-related stimuli and the development of posttraumatic stress disorder. Depression and Anxiety. 2005;21:61–70. doi: 10.1002/da.20047. [DOI] [PubMed] [Google Scholar]
- Falsetti SA, Resnick HS, Resick PA, Kilpatrick DG. The modified PTSD symptom scale: a brief self-report measure of posttraumatic stress disorder. The Behaviour Therapist. 1993;16:161–162. [Google Scholar]
- Fani N, Bradley RG, Ressler KJ, McClure-Tone EB. Attention bias in adult survivors of childhood maltreatment with and without posttraumatic stress disorder. Cognitive Therapy and Research. 2010;35:57–67. [Google Scholar]
- Filion DL, Dawson ME, Schell AM. The psychological significance of human startle eyeblink modification: a review. Biological Psychology. 1998;47:1–43. doi: 10.1016/s0301-0511(97)00020-3. [DOI] [PubMed] [Google Scholar]
- Foa EB, Feske U, Murdock TB, Kozac MJ, McCarthy PR. Processing of threat-related material in rape victims. Journal of Abnormal Psychology. 1991;100:156–162. doi: 10.1037//0021-843x.100.2.156. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, Ressler KJ. Trauma exposure and stress-related disorders in inner city primary care patients. General Hospital Psychiatry. 2009;31:505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Morgan CA., 3rd Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Blanding NQ, Norrholm SD, Duncan E, Bradley B, Ressler KJ. Childhood abuse is associated with increased startle reactivity in adulthood. Depression and Anxiety. 2009a;26:1018–1026. doi: 10.1002/da.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, Duncan EJ. Fear potentiation and fear inhibition in a human fear-potentiated startle paradigm. Biological Psychiatry. 2005;57:1559–1564. doi: 10.1016/j.biopsych.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ. Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and Anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Davis M, Duncan EJ. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Research. 2009b;167:151–160. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Keyes M, Fiallos A, Jovanovic S, Myers KM, Davis M, Duncan EJ. Contingency awareness and fear inhibition in a human fear-potentiated startle paradigm. Behavioral Neuroscience. 2006;120:995–1004. doi: 10.1037/0735-7044.120.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Sakoman AJ, Esterajher S, Kozaric-Kovacic D. Altered resting psychophysiology and startle response in Croatian combat veterans with PTSD. International Journal of Psychophysiology. 2009c;71:264–268. doi: 10.1016/j.ijpsycho.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspi SP, McNally RJ, Amir N. Cognitive processing of emotional information in posttraumatic stress disorder. Cognitive Therapy and Research. 1995;19:433–444. [Google Scholar]
- Kelly MM, Forsyth JP. Observational fear conditioning in the acquisition and extinction of attentional bias for threat: an experimental evaluation. Emotion. 2007;7:324–335. doi: 10.1037/1528-3542.7.2.324. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. Neuroimage. 2005;26:1193–1200. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Liebschutz J, Saltz R, Brower V, Keane TM, Lloyd-Travaglini C, Averbuch T, Samet JH. PTSD in urban primary care: high prevalence and low physician recognition. Journal of General Internal Medicine. 2007;22:719–726. doi: 10.1007/s11606-007-0161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behaviour Research & Therapy. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- McNally RJ, English GE, Lipke HJ. Assessment of intrusive cognition in PTSD: use of the modified Stroop paradigm. Journal of Traumatic Stress. 1993;6:33–41. [Google Scholar]
- McNally RJ, Kaspi SP, Reimann BC, Zeitlin SB. Selective processing of threat cues in post-traumatic stress disorder. Journal of Abnormal Psychology. 1990;99:398–402. doi: 10.1037//0021-843x.99.4.398. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Shumen JR, Bradley MM. Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity. Biological Psychiatry. 2010;67:346–356. doi: 10.1016/j.biopsych.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biological Psychiatry. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Minear M, Park DC. A lifespan database of adult facial stimuli. Behavior Research Methods, Instruments, & Computers. 2004;36:630–633. doi: 10.3758/bf03206543. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, Blair RJ, Chen G, Charney DS, Ernst M, Pine DS. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Morgan CA, 3rd, Grillon C, Lubin H, Southwick SM. Startle reflex abnormalities in women with sexual assault-related posttraumatic stress disorder. American Journal of Psychiatry. 1997;154:1076–1080. doi: 10.1176/ajp.154.8.1076. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biological Psychiatry. 2011;69:553–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, Duncan EJ. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learning & Memory. 2006;13:681–685. doi: 10.1101/lm.393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditional Reflexes. Dover Publications; New York: 1927. [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pine DS, Mogg K, Bradley BP, Montgomery L, Monk CS, McClure EB, Guyer AE, Ernst M, Charney DS, Kaufman J. Attention bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. American Journal of Psychiatry. 2005;162:291–296. doi: 10.1176/appi.ajp.162.2.291. [DOI] [PubMed] [Google Scholar]
- Pineles SL, Shipherd JC, Welch LP, Yovel I. The role of attentional biases in PTSD: is it interference or facilitation ? Behaviour Research and Therapy. 2007;45:1903–1913. doi: 10.1016/j.brat.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Shalev AY, Metzger LJ, Mellman TA. Psychophysiological alterations in post-traumatic stress disorder. Seminars in Clinical Neuropsychiatry. 1999;4:234–241. doi: 10.153/SCNP00400234. [DOI] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Metzler TJ, Best SR, Henn-Haase C, Marmar CR. Associations between childhood trauma and emotion-modulated psychophysiological responses to startling sounds: a study of police cadets. Journal of Abnormal Psychology. 2007;116:352–361. doi: 10.1037/0021-843X.116.2.352. [DOI] [PubMed] [Google Scholar]
- Schwartz AC, Bradley RL, Sexton M, Sherry A, Ressler KJ. Posttraumatic stress disorder among African Americans in an inner city mental health clinic. Psychiatric Services. 2005;56:212–215. doi: 10.1176/appi.ps.56.2.212. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168:241–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana SR, Roodman A, Beckham JC. Selective processing of trauma-relevant words in posttraumatic stress disorder. Journal of Anxiety Disorders. 1995;9:515–530. [Google Scholar]