Abstract
Purpose
Malignant middle cerebral artery (MCA) infarctions are thought to be rare in children. In a recent hospital-based study, only 1.3 % of pediatric ischemic strokes were malignant MCA infarctions. However, population-based rates have not been published. We performed subgroup analysis of a population-based study to determine the rate of malignant MCA infarctions in children.
Methods
In 2005 and 2010, all ischemic stroke-related emergency visits and hospital admissions among the 1.3 million residents of the five-county Greater Cincinnati/Northern Kentucky area were ascertained. Cases that occurred in patients 18 years and younger were reviewed in detail, and corresponding clinical and neuroimaging findings were recorded. Infarctions were considered malignant if they involved 50 % or more of the MCA territory and resulted in cerebral edema and mass effect.
Results
In 2005, eight pediatric ischemic strokes occurred in the study population, none of which were malignant infarctions. In 2010, there were also eight ischemic strokes. Of these, two malignant MCA infarctions were identified: (1) a 7-year-old boy who underwent hemicraniectomy and survived with moderate disability at 30 days and (2) a 17-year-old girl with significant prestroke disability who was not offered hemicraniectomy and died following withdrawal of care. Thus, among 16 children over 2 years, there were two malignant MCA infarctions (12.5 %, 95 % CI 0–29).
Conclusions
Malignant MCA infarctions in children may not be as rare as previously thought. Given the significant survival and functional outcome benefit conferred by hemicraniectomy in adults, future studies focusing on its potential role in pediatric patients are warranted.
Keywords: Decompressive craniectomy, Hemicraniectomy, Ischemic stroke, Malignant infarction, Middle cerebral artery, Pediatric
Introduction
In adults, large infarctions that involve 50 % or more of the middle cerebral artery (MCA) territory often have a malignant course, characterized by massive cytotoxic edema and mass effect, ultimately leading to transtentorial herniation and brain death within 2–4 days [8, 22]. Under conservative medical management, malignant MCA infarction is associated with an 80 % mortality rate [3, 8, 22]. In a recent pooled analysis of three European randomized controlled trials, decompressive hemicraniectomy was unequivocally shown to reduce mortality and improve functional outcome in adults with this condition [21]. In fact, 78 % of patients in the surgical arm survived and 95 % of these had only mild or moderate disability (modified Rankin scale (mRS) 2–4) [21]. Furthermore, we recently demonstrated that, despite their physical disability, the vast majority of adult survivors were satisfied with life and did not regret having undergone surgery [15, 22]. However, despite the efficacy of hemicraniectomy and its increasing rate in the USA [1], its applicability to the adult stroke population as a whole remains very limited. In a recent population-based study, we found that only 1.8 % (39 out of 2,227) of adults who presented with a severe ischemic stroke, i.e., an NIH stroke scale (NIHSS) of 10 or more, and had no preexistent disability (mRS <2), had a large infarction involving 50 % or more of the MCA territory [14]. Moreover, using the inclusion criteria of the European pooled analysis [21], only 0.3 % of patients (6 out of 2,227) would have been eligible for hemicraniectomy [14].
Young age remains the strongest predictor of favorable outcome in patients with malignant MCA infarction who undergo hemicraniectomy [7, 20]. Children, in particular, may have a higher potential for neurological recovery after stroke, considering their remarkable brain plasticity [2]. Unfortunately, the literature on malignant MCA infarction and hemicraniectomy in children remains scarce [6, 9, 12, 16, 18, 19]. This is likely due to a very low incidence of ischemic stroke in the pediatric population, approximately 1.2–3.6/100,000 per year [5, 10, 13], and perhaps a concomitantly low rate of malignant MCA infarction in this age group. In fact, in a recent multicenter hospital-based study, malignant MCA infarctions were identified in only 1.3 % of more than 700 children treated for ischemic stroke [18]. However, given their inherent referral and selection biases, the results of hospital-based studies may not be generalizable to the population. In fact, over three 1-year periods in our population, 17.2 % (5 out of 29) of children with ischemic strokes presented to adult and/or community hospitals, rather than the region’s pediatric referral center [13]. To gain better understanding of the incidence of malignant MCA infarction and the rate of hemicraniectomy in children, we performed a subgroup analysis of pediatric patients in the population-based Greater Cincinnati/Northern Kentucky Stroke Study (GCNKSS).
Materials and methods
The GCNKSS is a population-based epidemiological study designed to measure incidence rates and temporal trends of stroke within a large biracial population whose demographic characteristics reflect the black and white populations of the USA as a whole [4, 11]. The study population is defined as the 1.3 million residents of the Greater Cincinnati/Northern Kentucky region, which includes two counties in southwestern Ohio and three contiguous counties in northern Kentucky, separated by the Ohio River. Seventeen hospitals are located within this area. It has been previously documented that residents of these five counties seek care exclusively at these hospitals rather than at more distant hospitals in the outlying region [17]. Although residents of nearby counties also seek care at these hospitals, only residents of the five study counties were included in the analysis.
The methods for data collection and case ascertainment have been previously described in detail [4, 11, 17]. In summary, study nurses visit all 17 hospitals in the study region and retrospectively review and abstract the medical records of all-age emergency visitors and inpatients that are residents of the five study counties and have primary or secondary stroke-related International Classification of Diseases 9 (ICD-9) discharge diagnoses (430–436). For pediatric patients, the following ICD-9 codes are also included: 437 (other and ill-defined cerebrovascular disease), 438 (late effects of cerebrovascular disease), 747.81–747.89 (anomalies of the cerebrovascular system), 325 (phlebitis and thrombophlebitis of the intracranial vessels), 997.02 (iatrogenic cerebrovascular infarction or hemorrhage), 671.5 [other phlebitis and thrombosis (cerebral) in children], and 674.00–674.04 (cerebrovascular disorders in the puerperium). Medical records are abstracted using standardized case report forms, including demographic information, stroke symptoms and physical examination findings, past medical and surgical history, NIHSS score, premorbid mRS score, laboratory and imaging tests performed, treatments received, and short-term outcomes. Study physicians review every abstract, determine whether a stroke has occurred, and assign a stroke subtype and mechanism based on all available information, using previously reported definitions [4]. To be ascertained as an actual stroke case, symptoms must have been abrupt in onset, localized to a focal area of the brain, and unresolved without treatment 24 h after onset. The study was approved by the institutional review boards at all participating hospitals.
For the present analysis, we pooled data from two calendar years (i.e., January 1–December 31): 2005 and 2010. Two authors (R.R., L.J.) independently reviewed all clinical and neuroimaging data for patients 18 years of age or younger. Given the lack of a standardized definition in children, malignant MCA infarction was defined based on adult criteria, as an ischemic stroke involving 50 % or more of the MCA territory, with associated cerebral edema and mass effect-related neurological deterioration during the course of hospitalization [21]. Whenever disagreement occurred between the two reviewers, it was resolved by consensus. Statistical analysis was performed using SAS® version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
During calendar year 2005, eight ischemic strokes occurred in the study population among children 18 years of age or younger. All strokes were small, none fulfilling the criteria of malignant MCA infarction. In 2010, eight ischemic strokes occurred in the pediatric study population. Of these, two malignant MCA infarctions were identified. The first patient, a previously healthy 7-year-old boy, presented with a large left MCA territory stroke of undetermined etiology and an initial NIHSS score of 4. A few days later, he exhibited worsening mental status secondary to stroke-related mass effect and underwent decompressive hemicraniectomy. The patient survived with moderate disability (mRS 3) at 30 days (Fig. 1). The second patient, a 17-year-old girl with moyamoya syndrome and significant pre-stroke disability (mRS 3), presented with a large left MCA stroke and an NIHSS score of 15. She exhibited mass effect-related neurological deterioration, but was not offered hemicraniectomy at the parents’ request, and ultimately died following withdrawal of care (Fig. 2).
Fig. 1.
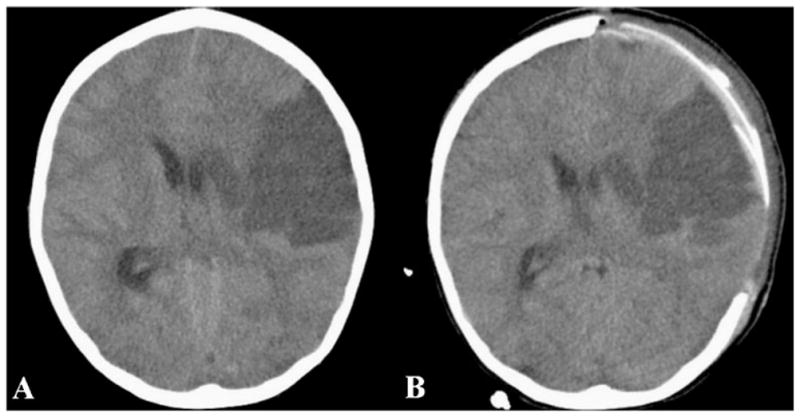
A 7-year-old boy with left MCA infarction. a Head CT reveals a large hypodensity involving 50 % of the MCA territory, with significant mass effect and midline shift. Following neurological deterioration, decompressive hemicraniectomy was performed. b Postoperative head CT
Fig. 2.
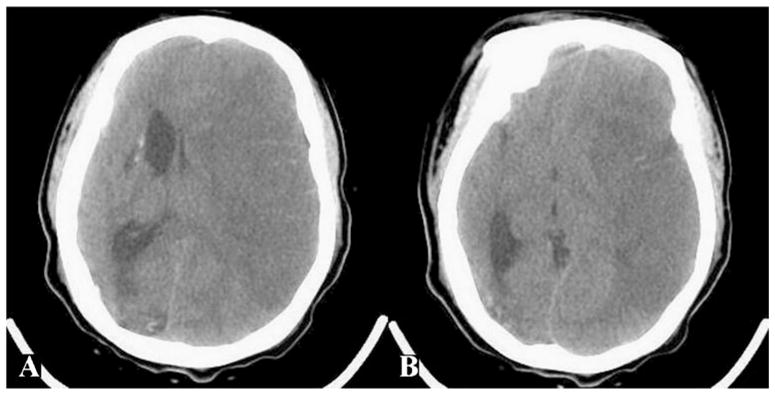
A 17-year-old girl with moyamoya syndrome and left MCA ischemic stroke. a, b Head CT demonstrates infarction of the entire MCA territory, with substantial mass effect and midline shift. Despite neurological deterioration, hemicraniectomy was not performed and the patient died following withdrawal of care
Overall, during the two 1-year study periods, 16 children suffered an ischemic stroke. These were 7 boys and 9 girls with a median age of 4.5 years (range 0–18), 12 white and 4 black. Stroke etiologies were: cerebral arteriopathy (including moyamoya and vasculitis) in four cases, sickle cell disease in two, cardioembolism in one, posterior reversible encephalopathy syndrome in one, venous sinus thrombosis in one, cerebral arteriovenous malformation in one, mitochondrial disorder in one, maternal chorioamniotitis in one, and unknown in four. Of the 16 stroke cases, 2 were malignant MCA infarctions, thus yielding an overall rate of 12.5 % (95 % CI 0–29). Interestingly, of the 16 patients, 2 (12.5 %) were managed outside the region’s pediatric referral center: an 18 year-old was admitted to the adult university hospital and a 2-day-old neonate was treated in a community hospital. None of these two patients had a malignant infarction.
Discussion
In this population-based study, 12.5 % of all pediatric ischemic strokes over two 1-year periods were malignant MCA infarctions. However, given the small number of patients, the 95 % confidence interval was wide, ranging from 0 to 29 %. Nevertheless, our 12.5 % point estimate appears to be higher than that recently reported in a hospital-based study [18]. In their retrospective review of more than 700 ischemic stroke patients admitted to five pediatric tertiary care centers over several years, Smith et al. [18] reported that only 1.3 % (10 cases) were malignant MCA infarctions. However, the results of any hospital-based study should be interpreted with caution, given the inevitable referral and selection biases that are inherent to such studies. Because of their grave condition, children with malignant MCA infarction are typically transferred to large pediatric referral centers earlier and more often than those with milder strokes. Thus, it is expected that a hospital-based study would overestimate rather than underestimate the true incidence of this condition. Surprisingly however, our population-based estimate was considerably higher than the hospital-based rate reported by these authors [18]. While random variation, given the small patient numbers and wide confidence intervals, may underlie this discrepancy, other factors could also be at play. For instance, it could be that, in the study of Smith et al. [18], children with extensive strokes, worsening mental statuses, and unstable clinical conditions were not frequently transferred to referral centers. Instead, many such patients could have either undergone hemicraniectomy at the admitting hospital or received conservative management and died prior to referral.
To date, malignant MCA infarction in children has not been sufficiently studied and, except for two small case series (10 and 4 patients, respectively) [16, 18], has been mostly dealt with in anecdotal case reports [6, 9, 12, 19]. The results of our study suggest that, in the pediatric population, malignant MCA infarctions may not be as rare as previously thought. Compared with adults, children have a lower cerebral compliance and smaller subarachnoid and cisternal compartments, which may limit their capacity to tolerate stroke-related cerebral edema and mass effect. However, despite the clear benefit of hemicraniectomy in adults [15, 21, 22], outcome data in children undergoing decompressive surgery remain scarce [6, 9, 12, 16, 18, 19]. Likewise, eligibility criteria for hemicraniectomy in the pediatric population have not been clearly defined and, for the most part, surgery is offered on a case-by-case basis, typically to children with large infarctions and mass effect-related neurological deterioration [6, 9, 12, 16, 18, 19]. Nevertheless, the bulk of available evidence seems to suggest that, compared with adults, pediatric survivors of malignant MCA infarction and hemicraniectomy tend to enjoy an even more favorable functional outcome, with the majority being able to walk independently and speak fluently at follow-up, likely as a result of higher central nervous system plasticity in this age group [6, 16, 18].
Our study has significant limitations, most notably the very small number of pediatric stroke cases within our population and the subsequently very wide 95 % confidence interval (0 to 29 %), making it virtually impossible to draw firm conclusions. Moreover, given the retrospective chart review design, some cases may have been missed due to misclassification of ICD-9 codes, incomplete documentation, underdiagnosis by clinicians, or presentation to outpatient settings. Nevertheless, this is the first study to assess the rate of malignant MCA infarction in children at a population level, thereby virtually eliminating referral and selection biases commonly encountered in hospital-based series. Furthermore, our population-based study ascertained cases from all hospitals in the region, both adult and pediatric. Thus, we were able to capture 2 pediatric stroke cases (out of 16) that were treated in adult and/or community hospitals. These cases would have been easily missed by a study conducted exclusively at the pediatric referral center.
Conclusions
Malignant MCA infarctions in the pediatric population may not be as rare as previously thought. Given the significant survival and functional outcome benefit conferred by hemicraniectomy in adults and the encouraging preliminary results in children, future multicenter collaborative efforts are warranted to better elucidate the role of decompressive surgery in pediatric stroke.
Footnotes
This paper was presented as poster at the 2012 American Association of Neurological Surgeons Annual Meeting, Miami, FL, USA, April 2012.
Contributor Information
Ralph Rahme, Department of Neurosurgery, University of Cincinnati, Cincinnati, OH, USA.
Lincoln Jimenez, Department of Neurosurgery, University of Cincinnati, Cincinnati, OH, USA.
Umair Bashir, Department of Neurosurgery, University of Cincinnati, Cincinnati, OH, USA.
Opeolu M. Adeoye, Department of Neurosurgery, University of Cincinnati, Cincinnati, OH, USA
Todd A. Abruzzo, Department of Neurosurgery, University of Cincinnati, Cincinnati, OH, USA
Andrew J. Ringer, Department of Neurosurgery, University of Cincinnati, Cincinnati, OH, USA
Brett M. Kissela, Department of Neurology, University of Cincinnati, 260 Stetson Street, Suite 2300, Cincinnati, OH 45219, USA
Jane Khoury, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA.
Charles J. Moomaw, Department of Neurology, University of Cincinnati, 260 Stetson Street, Suite 2300, Cincinnati, OH 45219, USA
Heidi Sucharew, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA.
Simona Ferioli, Department of Neurology, University of Cincinnati, 260 Stetson Street, Suite 2300, Cincinnati, OH 45219, USA.
Matthew L. Flaherty, Department of Neurology, University of Cincinnati, 260 Stetson Street, Suite 2300, Cincinnati, OH 45219, USA
Daniel Woo, Department of Neurology, University of Cincinnati, 260 Stetson Street, Suite 2300, Cincinnati, OH 45219, USA.
Pooja Khatri, Department of Neurology, University of Cincinnati, 260 Stetson Street, Suite 2300, Cincinnati, OH 45219, USA.
Kathleen Alwell, Department of Neurology, University of Cincinnati, 260 Stetson Street, Suite 2300, Cincinnati, OH 45219, USA.
Dawn Kleindorfer, Email: dawn.kleindorfer@uc.edu, Department of Neurology, University of Cincinnati, 260 Stetson Street, Suite 2300, Cincinnati, OH 45219, USA.
References
- 1.Adeoye O, Hornung R, Khatri P, Ringer A, Kleindorfer D. The rate of hemicraniectomy for acute ischemic stroke is increasing in the United States. J Stroke Cerebrovasc Dis. 2011;20:251–254. doi: 10.1016/j.jstrokecerebrovasdis.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Bernard TJ, Goldenberg NA, Armstrong-Wells J, Amlie-Lefond C, Fullerton HJ. Treatment of childhood arterial ischemic stroke. Ann Neurol. 2008;63:679–696. doi: 10.1002/ana.21406. [DOI] [PubMed] [Google Scholar]
- 3.Berrouschot J, Sterker M, Bettin S, Köster J, Schneider D. Mortality of space-occupying (‘malignant’) middle cerebral artery infarction under conservative intensive care. Intensive Care Med. 1998;24:620–623. doi: 10.1007/s001340050625. [DOI] [PubMed] [Google Scholar]
- 4.Broderick J, Brott T, Kothari R, Miller R, Khoury J, Pancioli A, Gebel J, Mills D, Minneci L, Shukla R. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke. 1998;29:415–421. doi: 10.1161/01.str.29.2.415. [DOI] [PubMed] [Google Scholar]
- 5.Broderick J, Talbot GT, Prenger E, Leach A, Brott T. Stroke in children within a major metropolitan area: the surprising importance of intracerebral hemorrhage. J Child Neurol. 1993;8:250–255. doi: 10.1177/088307389300800308. [DOI] [PubMed] [Google Scholar]
- 6.Farooq MU, Abbed KM, Fletcher JJ. Decompressive hemicraniectomy in a 19-month-old female after malignant cerebral infarction. Pediatr Neurosurg. 2009;45:146–150. doi: 10.1159/000209654. [DOI] [PubMed] [Google Scholar]
- 7.Gupta R, Connolly ES, Mayer S, Elkind MS. Hemicraniectomy for massive middle cerebral artery territory infarction: a systematic review. Stroke. 2004;35:539–543. doi: 10.1161/01.STR.0000109772.64650.18. [DOI] [PubMed] [Google Scholar]
- 8.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53:309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 9.Kirton A, DeVeber G. Ischemic stroke complicating pediatric cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2007;4:163–166. doi: 10.1038/ncpcardio0825. [DOI] [PubMed] [Google Scholar]
- 10.Kleindorfer D, Khoury J, Kissela B, Alwell K, Woo D, Miller R, Schneider A, Moomaw C, Broderick JP. Temporal trends in the incidence and case fatality of stroke in children and adolescents. J Child Neurol. 2006;21:415–418. doi: 10.1177/08830738060210050301. [DOI] [PubMed] [Google Scholar]
- 11.Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, Khatri P, Adeoye O, Ferioli S, Broderick JP, Kissela BM. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke. 2010;41:1326–1331. doi: 10.1161/STROKEAHA.109.575043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee MC, Frank JI, Kahana M, Tonsgard JH, Frim DM. Decompressive hemicraniectomy in a 6-year-old male after unilateral hemispheric stroke. Case report and review. Pediatr Neurosurg. 2003;38:181–185. doi: 10.1159/000069096. [DOI] [PubMed] [Google Scholar]
- 13.Lehman LL, Kleindorfer DO, Khoury JC, Alwell K, Moomaw CJ, Kissela BM, Khatri P. Potential eligibility for recombinant tissue plasminogen activator therapy in children: a population-based study. J Child Neurol. 2011;26:1121–1125. doi: 10.1177/0883073811408091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahme R, Curry R, Kleindorfer D, Khoury JC, Ringer AJ, Kissela BM, Alwell K, Moomaw CJ, Flaherty ML, Khatri P, Woo D, Ferioli S, Broderick J, Adeoye O. How often are patients with ischemic stroke eligible for decompressive hemicraniectomy? Stroke. 2012;43:550–552. doi: 10.1161/STROKEAHA.111.635185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahme R, Zuccarello M, Kleindorfer D, Adeoye OM, Ringer AJ. Decompressive hemicraniectomy for malignant MCA territory infarction: is life worth living? J Neurosurg. 2012 doi: 10.3171/2012.6.JNS111140. (in press) [DOI] [PubMed] [Google Scholar]
- 16.Ramaswamy V, Mehta V, Bauman M, Richer L, Massicotte P, Yager JY. Decompressive hemicraniectomy in children with severe ischemic stroke and life-threatening cerebral edema. J Child Neurol. 2008;23:889–894. doi: 10.1177/0883073808314960. [DOI] [PubMed] [Google Scholar]
- 17.Schneider AT, Kissela B, Woo D, Kleindorfer D, Alwell K, Miller R, Szaflarski J, Gebel J, Khoury J, Shukla R, Moomaw C, Pancioli A, Jauch E, Broderick J. Ischemic stroke subtypes: a population-based study of incidence rates among blacks and whites. Stroke. 2004;35:1552–1556. doi: 10.1161/01.STR.0000129335.28301.f5. [DOI] [PubMed] [Google Scholar]
- 18.Smith SE, Kirkham FJ, Deveber G, Millman G, Dirks PB, Wirrell E, Telfeian AE, Sykes K, Barlow K, Ichord R. Outcome following decompressive craniectomy for malignant middle cerebral artery infarction in children. Dev Med Child Neurol. 2011;53:29–33. doi: 10.1111/j.1469-8749.2010.03775.x. [DOI] [PubMed] [Google Scholar]
- 19.Tan MA, Salonga AM, Jamora RD. Decompressive hemicraniectomy in a 2-year-old girl with a left middle cerebral artery infarct. Childs Nerv Syst. 2006;22:523–525. doi: 10.1007/s00381-005-0001-7. [DOI] [PubMed] [Google Scholar]
- 20.Uhl E, Kreth FW, Elias B, Goldammer A, Hempelmann RG, Liefner M, Nowak G, Oertel M, Schmieder K, Schneider GH. Outcome and prognostic factors of hemicraniectomy for space occupying cerebral infarction. J Neurol Neurosurg Psychiatry. 2004;75:270–274. [PMC free article] [PubMed] [Google Scholar]
- 21.Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, Amelink GJ, Schmiedeck P, Schwab S, Rothwell PM, Bousser MG, van der Worp HB, Hacke W DECIMAL DESTINY, and HAMLET investigators. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–222. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- 22.Weil AG, Rahme R, Moumdjian R, Bouthillier A, Bojanowski MW. Quality of life following hemicraniectomy for malignant MCA territory infarction. Can J Neurol Sci. 2011;38:434–438. doi: 10.1017/s0317167100011835. [DOI] [PubMed] [Google Scholar]


