Abstract
Objectives
Estimating the prevalence of undiagnosed HIV in emergency departments (EDs) is not straightforward. Regional epidemiologic data are unlikely to translate directly to a single ED setting, and the prevalence of undiagnosed HIV likely differs between EDs within a region. We propose a simple method for estimating the prevalence of undiagnosed HIV in individual EDs.
Methods
First, incident cases are grouped by zip codes and combined with census data to calculate zip code–specific case rates. Second, the proportion of ED patients living in each zip code is determined. Third, the prevalence of undiagnosed disease is estimated as the mean zip code case rate, weighted by the proportion of ED patients living in each zip code, multiplied by the estimated time from infection to diagnosis. We applied this method to 3 EDs in a metropolitan region with an annual HIV/AIDS case rate of 6.2 per 100,000.
Results
From 1999 through 2003, the annual HIV case rate was estimated to range from 6.4 to 12.7 at an urban academic ED, 5.9 to 10.2 at an urban community ED, and 2.1 to 4.9 at a suburban community ED. The estimated prevalence of undiagnosed disease was 0.05% (urban academic), 0.04% (urban community), and 0.02% (suburban community).
Conclusion
Publicly reported regional AIDS or HIV statistics do not reflect ED-specific HIV epidemiology, but ED-specific case rates can be crudely estimated from readily available data. This method promises to be a valuable aid for translating HIV screening to ED settings.
INTRODUCTION
Deciding where and how to apply resources to screening for a disease depends in part on the prevalence of undiagnosed disease. At sites with high prevalence, applying resources to universal screening might be cost-effective. Where prevalence is lower, more targeted approaches might be beneficial. The Centers for Disease Control and Prevention (CDC) recommend routine HIV testing unless the prevalence of undiagnosed HIV is less than 0.1% or, in the absence of prevalence data, when the testing yield decreases below 1 HIV-positive patient per 1,000 patients tested.1 Individual emergency departments (EDs) would therefore benefit from establishing the prevalence of undiagnosed disease. A simple approach is to offer testing routinely and monitor the rate of positivity. However, this approach requires extensive resources that would not be justified if prevalence is less than 0.1%. The testing of remnant blood specimens can be used to garner some understanding of the extent of undiagnosed disease,2 but this also requires significant resources and can be biased because specimens are not available for all patients.
Given these barriers, individual EDs might be tempted to rely on publicly reported incidence and prevalence data. However, the prevalence of undiagnosed disease is not reported, and the estimate that nationwide 21% of HIV-infected persons are unaware of their disease status3 is unlikely to reflect the prevalence of undiagnosed HIV in an individual ED. Even regional data are unlikely to be representative of individual EDs whose catchment area is not classified in terms of state, county, or Metropolitan Statistical Area. To overcome these limitations, we propose a method for using available data to crudely estimate the prevalence of undiagnosed HIV in individual EDs.
CALCULATION FOR ESTIMATING PREVALENCE OF UNDIAGNOSED HIV FOR AN INDIVIDUAL ED
We propose a 3-step method. First, zip code–specific case rates are estimated. Second, the proportion of ED patients residing in each zip code is estimated. Finally, the setting-specific prevalence of undiagnosed disease is computed as the weighted mean of zip code–specific case rates (with weighting based on the geographic distribution of ED patients), multiplied by the time from infection to diagnosis.
- Step 1: Estimate zip code–specific case rates:
- Obtain zip code of residence for of all new cases. Sources could include health departments, screening programs, or HIV treatment centers.
- Estimate the population residing in each zip code by using census data.
- The zip code–specific case rate is the ratio of new cases within the zip code to population in the zip code.
- Step 2: Determine the proportion of ED patients residing in each zip code:
- Obtain zip code data for all ED patients.
- Compute the proportion of patients residing in each zip code.
- Step 3: Estimate the setting-specific prevalence of undiagnosed disease:
-
Compute the estimated case rate for the ED:ED case rate = Σ(zip code case rate×proportion of ED patients residing in zip code)
- Multiply the estimated ED case rate by the average time from infection to diagnosis. Divide by 1,000 to estimate the prevalence of undiagnosed disease.
-
METHODS
This demonstration project used a combination of publicly reported prevalence and incidence data, census data, zip code data for newly diagnosed patients, and zip code data for ED patients to estimate the prevalence of undiagnosed HIV for 3 EDs in a Midwestern metropolitan area. Use of identifiable patient data was approved by the local institutional review board.
Setting
The 3 EDs are located in a single county with a population of 845,303 that is 23% black. The EDs are a 665-bed urban teaching hospital, a 550-bed urban community hospital located a mile away, and a 200-bed suburban community hospital located 10 miles away. The academic ED is the region’s primary indigent care provider: about 45% of patients are uninsured or self-pay, 20% receive Medicaid, and 15% receive Medicare. There are approximately 80,000 visits annually, and patients are predominantly (57%) black. The urban community ED has approximately 45,000 visits annually; the patient population comprises black and white patients, most of whom are insured. The suburban community ED has approximately 35,000 visits annually; the patient population is largely white and insured.
Sources of Data
Annual HIV/AIDS incident case rates were obtained from CDC, the state health department, and an HIV advocacy group.4–6 Census data were extracted from the US Census Web site (http://www.census.gov) to estimate the size of the population aged 15 to 64 years and residing in each zip code tabulation area from 1999 through 2003. We also obtained estimates for specific groups in this age group: black men, black women, nonblack men, and nonblack women (nonblack includes all races except black).
Data for patients who received a new diagnosis were obtained from a regional provider of specialized HIV care located on the same campus as the academic ED. This center provides care for 90% of the HIV-positive population in care in the county. Patients who received a new diagnosis were those who had no previous awareness of their condition and no previous treatment of HIV.7 We extracted demographics, CD4 count, International Classification of Diseases, Ninth Revision (ICD-9) codes, and zip code of residence at diagnosis. Complete data were available for 1999 through 2003.
For ED patients, we extracted data from hospital administrative databases: visit data, demographics, ICD-9 codes, and zip code of residence. We included each patient’s first visit and removed cases for which ICD-9 codes denoted HIV infection (042 and V08).
Primary Data Analysis
To compute zip code–specific case rates, we divided the number of new cases in each zip code (residence data from treatment center) by the population of that zip code (from census data). Case rates were computed for all persons aged 15 to 64 years and separately for black men, nonblack men, black women, and nonblack women.
To compute the geographic distribution of ED patients, we divided the number of ED patients living in each zip code by the total number of ED patients for all patients aged 15 to 64 years and for black men, nonblack men, black women, and nonblack women.
To estimate the setting-specific prevalence of undiagnosed disease, we computed the ED case rates as the mean of the zip code case rate weighted by the number of ED patients residing in each zip code. The duration of infection before diagnosis was estimated from the difference between an assumed normal CD4 count of 600 cells/µL and the region’s median CD4 count and then divided by an estimated rate of decrease after infection of 60 to 100 cells/µL per year.8–10
RESULTS
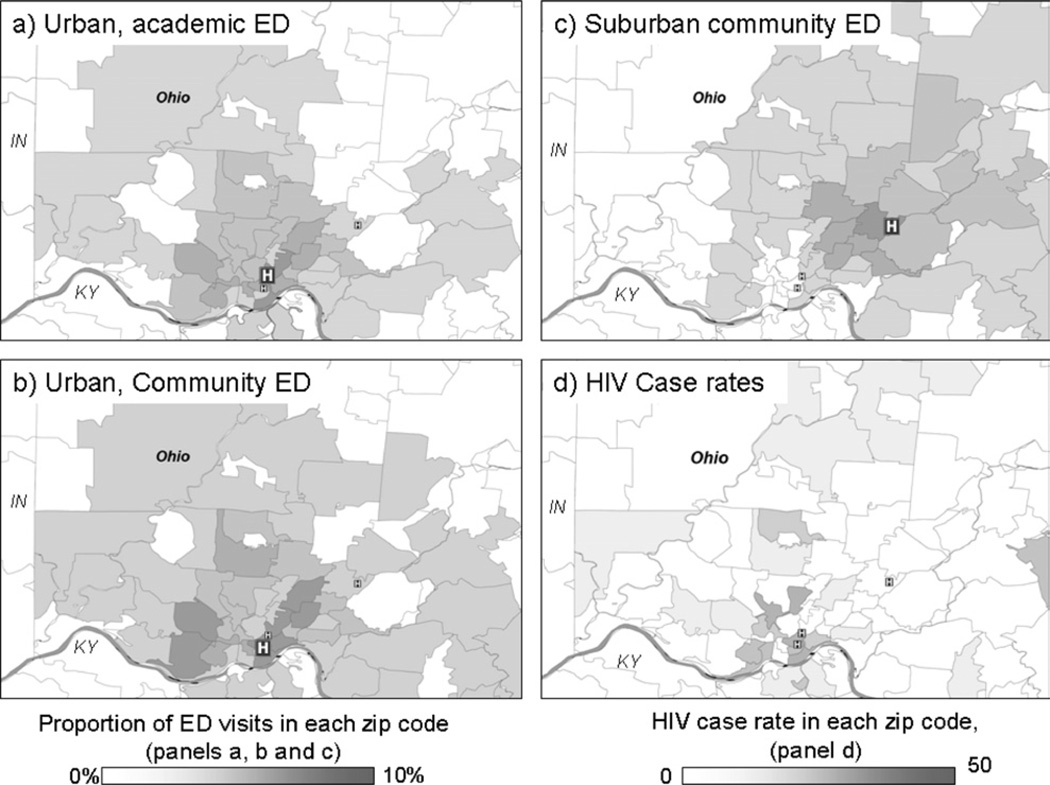
In 2003, a total of 43,718 patients aged 15 to 64 years presented to the urban academic ED, 16,689 to the urban community ED, and 15,145 to the suburban community ED. The zip code–specific case rates and geographic distribution of ED patients are shown in the Figure. The median CD4 count at diagnosis in our region is 324 cells/µL; it is 276/µL for patients identified in the academic ED.7 The CD4 count is about 300/µL lower than normal, suggesting that the time to diagnosis is between 3 and 5 years. Because the CD4 count in the ED was lower than that for the region, we assumed a 5-year duration of infection before diagnosis and summed the case rates for 5 years. The Table shows the estimated ED case rates for 1999 through 2003 and the estimated prevalence of undiagnosed disease. In the absence of 5 years of data, we would have multiplied the 1-year case rate by 5. For context, in 2003 the HIV/AIDS case rate for the county was estimated at 15 per 100,000 (0.015%); for the Metropolitan Statistical Area, the HIV/AIDS case rate was 6.2 (0.0062%), and the AIDS case rate was 3.8 (0.0038%).
Figure.
A–C, Geographic distribution of ED visits. D, HIV case rates. Zip code tabulation areas are plotted.
Table.
Estimated HIV case rates and prevalence of undiagnosed disease, by type of ED.*
| Total | Black Men | Black Women | Men of Other Races |
Women of Other Races |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Facility and Year | No. | Rate | No. | Rate | No. | Rate | No. | Rate | No. | Rate |
| Urban academic | ||||||||||
| 1999 | 31,563† | 10.73 | 7,823 | 31.14 | 8,678 | 7.25 | 8,174 | 12.96 | 6,888 | 1.51 |
| 2000 | 42,200 | 12.66 | 10,555 | 38.84 | 12,015 | 27.22 | 10,520 | 11.31 | 9,110 | 1.08 |
| 2001 | 43,837 | 8.22 | 11,097 | 14.04 | 12,243 | 16.09 | 11,151 | 10.37 | 9,345 | 0.62 |
| 2002 | 44,394 | 9.06 | 11,092 | 28.96 | 12,034 | 9.00 | 11,569 | 8.21 | 9,699 | 0.36 |
| 2003 | 43,718 | 6.41 | 10,864 | 22.75 | 11,516 | 9.69 | 11,661 | 5.77 | 9,677 | 0.80 |
| Estimated prevalence, % | 0.05 | 0.14 | 0.07 | 0.05 | 0.004 | |||||
| Urban community | ||||||||||
| 1999 | 15,243† | 7.92 | 2,109 | 28.39 | 3,482 | 7.12 | 4,252 | 10.19 | 5,400 | 1.57 |
| 2000 | 17,168 | 10.20 | 2,615 | 36.61 | 4,594 | 24.74 | 4,297 | 9.32 | 5,662 | 0.84 |
| 2001 | 17,269 | 7.02 | 2,764 | 14.16 | 4,835 | 15.87 | 4,066 | 9.21 | 5,604 | 0.65 |
| 2002 | 16,920 | 7.83 | 2,747 | 28.75 | 4,945 | 8.26 | 3,892 | 6.90 | 5,335 | 0.26 |
| 2003 | 16,689 | 5.91 | 2,707 | 26.08 | 4,938 | 9.70 | 3,780 | 6.03 | 5,264 | 0.56 |
| Estimated prevalence, % | 0.04 | 0.13 | 0.07 | 0.04 | 0.004 | |||||
| Suburban community | ||||||||||
| 2001 | 4,049 | 4.47 | 429 | 19.48 | 636 | 9.18 | 1,348 | 3.65 | 1,636 | 0.71 |
| 2002 | 15,382 | 4.90 | 1,567 | 27.74 | 2,434 | 5.31 | 5,211 | 5.07 | 6,170 | 0.49 |
| 2003 | 15,145 | 2.13 | 1,550 | 8.20 | 2,398 | 5.19 | 5,050 | 2.08 | 6,146 | 0.13 |
| Estimated prevalence, %† | 0.02 | 0.09 | 0.03 | 0.02 | 0.002 | |||||
Rates are per 100,000 population. Prevalence was estimated from the sum of the case rates for 5 years.
Electronic records did not cover the full year.
Only 3 years of data were available; estimated prevalence was computed as the mean of the case rates for the 3 available years multiplied by 5.
LIMITATIONS
The proposed method for crudely estimating the prevalence of undiagnosed disease for a single health care setting involves several assumptions. Most important, estimates are limited by the accuracy of the data used. In our demonstration, we used data from an HIV treatment center to estimate zip code–specific case rates and time from infection to diagnosis. Our estimates represent only the lower bound of undiagnosed disease prevalence because the center treats only 90% of HIV patients in care regionally and does not collect data on persons not linked to care. Further, although the center provides care regionally, it may have been less likely to capture data on cases in persons who lived farther from the center. Variability in annual incidence rates within and between zip codes might also have resulted in some instability in the estimated prevalence of undiagnosed disease. In choosing a data source, one should consider the desired accuracy and stability of the prevalence estimate: regional incidence data from public health authorities, including those reported to the CDC, would probably result in more accurate zip code case rates than regional center case data such as we have used, and the period during which cases are counted should be sufficient to ensure stability of the estimates.
Our demonstration shows that the proposed method has face validity. The estimated prevalence of undiagnosed disease was higher among blacks than among nonblacks, among men than among women, in urban than in suburban settings, and in academic than in community settings. A more rigorous test of validity would compare the estimates from our method against the proportion of positive test results from universal screening, from the testing of remnant specimens, or other methods for estimating undiagnosed disease prevalence. We are currently using universal testing and the testing of remnant specimens to obtain prevalence estimates for our setting. Comparison of the estimates reported here with those data will more fully test the validity of our proposed method.
DISCUSSION
Our data show clear differences between EDs—differences that are probably due to differing risk factors among the populations served. In our demonstration, the academic ED, which serves a primarily indigent, majority black patient population, has an estimated prevalence of undiagnosed HIV that is higher than that in the suburban community ED, which serves a more affluent, majority white patient population. Similarly, the estimated prevalence of undiagnosed disease at the urban community ED, located less than a mile from the academic center, is lower than that of the urban academic ED; the urban community ED serves an older population with a higher proportion of insured patients. Overall, these data support our assertion that the prevalence of undiagnosed disease in an ED cannot be inferred from its location or from the population served. The method we propose makes it possible to identify the EDs in which the greatest testing yield could be achieved, and thus the EDs that most need the resources to conduct HIV screening.
Where and how to apply the resources required to conduct screening for undiagnosed disease determines the cost-effectiveness of the approach. For HIV, the CDC recommends universal screening when the prevalence of undiagnosed HIV infection in a health care setting has been documented to be less than 0.1%.1 Previously proposed methods for estimating setting-specific prevalence are highly resource intensive. Our method was developed to overcome this limitation: the only required data that are not publicly available are counts of persons receiving a new diagnosis and ED visits for each zip code. If only crude estimates of prevalence are required, one can use data from public health authorities and from administrative databases (typically available for individual health care settings). The more comprehensive the data obtained, the more accurate the prevalence estimate will be.
CONCLUSIONS
The prevalence of undiagnosed HIV in individual EDs cannot be estimated from publicly reported HIV/AIDS case rates, but it can be crudely estimated by using a combination of data on ED visits and other publicly available data. Although further validation is required, we believe that the use of this proposed method will help determine whether individual EDs should provide universal screening for HIV.
Acknowledgments
Funding and support: This study was supported in part by National Institute of Allergy and Infectious Diseases K23 AI068453.
Publication of this article was supported by Centers for Disease Control and Prevention, Atlanta, GA.
Footnotes
By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org).
REFERENCES
- 1.Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. [PubMed] [Google Scholar]
- 2.Kelen GD, Fritz S, Qaqish B, et al. Unrecognized human immunodeficiency virus infection in emergency department patients. N Engl J Med. 1988;318:1645–1650. doi: 10.1056/NEJM198806233182503. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. HIV prevalence estimates—United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:1073–1076. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. AIDS cases, by geographic area of residence and metropolitan statistical area of residence, 2004. [Accessed September 24, 2010];HIV/AIDS Surveill Suppl Rep. 2006 12:1–65. Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports. [Google Scholar]
- 5.Avert. United States statistics by state and city. [Accessed September 27, 2010];Avert Web site. Available at: http://www.avert.org/usa-states-cities.htm. [Google Scholar]
- 6.Ohio Department of Health. State of Ohio HIV infections annual surveillance statistics [Ohio and county tables] [Accessed September 24, 2010];Ohio Department of Health Web site. Available at: http://www.odh.ohio.gov/healthStats/disease/hivann/hcty1.aspx. Updated April 22, 2010.
- 7.Lyons MS, Lindsell CJ, Hawkins DA, et al. Contributions to early HIV diagnosis among patients linked to care vary by testing venue. BMC Public Health. 2008;8:220. doi: 10.1186/1471-2458-8-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang W, Perkins H, Anderson RE, et al. Patterns of T lymphocyte changes with human immunodeficiency virus infection: from seroconversion to the development of AIDS. J Acquir Immune Defic Syndr. 1989;2:63–69. [PubMed] [Google Scholar]
- 9.Samet JH, Freedberg KA, Savetsky JB, et al. Understanding delay to medical care for HIV infection: the long-term non-presenter. AIDS. 2001;15:77–85. doi: 10.1097/00002030-200101050-00012. [DOI] [PubMed] [Google Scholar]
- 10.Kirschner D, Webb GF, Cloyd M. Model of HIV-1 disease progression based on virus-induced lymph node homing and homing-induced apoptosis of CD4+ lymphocytes. J Acquir Immune Defic Syndr. 2000;24:352–362. doi: 10.1097/00126334-200008010-00010. [DOI] [PubMed] [Google Scholar]