Abstract
Purpose
We determined the role of opioid and metabotropic glutamate 5 receptors in the pudendal inhibition of bladder overactivity.
Materials and Methods
Cystometrograms were performed in 11 cats under α-chloralose anesthesia by slowly infusing the bladder with saline or 0.25% acetic acid. Pudendal nerve stimulation at intensities of multiple times the threshold to induce observable anal twitching was applied during cystometrogram to inhibit the bladder overactivity induced by acetic acid irritation. Naloxone (0.1, 0.3 and 1 mg/kg intravenously) was administered to block opioid receptors, followed by MTEP (3 and 10 mg/kg intravenously) to block metabotropic glutamate 5 receptors. After each drug dose, pudendal inhibition of bladder overactivity was examined during cystometrogram.
Results
Acetic acid irritated the bladder, induced bladder overactivity and significantly decreased mean ± SE bladder capacity to 23.6% ± 2.7% of saline control capacity. Pudendal nerve stimulation at 1 to 1.5 and 4 × threshold suppressed bladder overactivity and significantly increased mean capacity to 57.5% ± 8.1% (p = 0.0005) and 106% ± 15% (p = 0.0002), respectively, of saline control capacity. Naloxone had no effect on pudendal inhibition but MTEP eliminated the inhibition induced by low intensity stimulation and significantly decreased the inhibition induced by high intensity stimulation (p <0.05). Neither naloxone nor MTEP altered baseline bladder overactivity.
Conclusions
Opioid receptors are not involved in pudendal inhibition of bladder overactivity but metabotropic glutamate 5 receptors are partially involved. Understanding neurotransmitter mechanisms could improve the efficacy of neuromodulation therapy for overactive bladder and identify molecular targets for developing new drugs for overactive bladder.
Keywords: urinary bladder, overactive, receptors, opioid, metabotropic glutamate receptor 5, muscle contraction, cats
Overactive bladder is a symptom complex of urgency with or without urge incontinence, usually with increased daytime frequency and nocturia in the absence of a known pathological cause.1 The OAB prevalence is estimated to be 16% to 17% in the United States and Western Europe.2,3 Currently, OAB pathophysiology is not fully understood, causing significant difficulty in effectively treating OAB symptoms.4 Sacral, pudendal or tibial neuromodulation5–7 has been used clinically to treat OAB after pharmacotherapy fails. Therefore, it is important to understand the mechanisms of neuromodulation at the molecular level because it may provide information that could be used to develop new pharmacological treatments for OAB.
Our recent studies in cats revealed that opioid receptors are involved in TNS induced suppression of the bladder overactivity elicited by AA irritation.8 The opioid receptor antagonist naloxone eliminated the increase in bladder capacity elicited by TNS in AA irritated bladders but did not alter the inhibitory effects of TNS in nonirritated bladders. In addition, a low dose of the opioid receptor agonist tramadol significantly enhanced the TNS inhibition of bladder overactivity, suggesting that a combination of drug and neuromodulation therapies might yield more efficacious treatment for OAB with fewer adverse effects.9 These results raise the possibility that opioid mechanisms might also be involved in other neuromodulation (sacral or pudendal) therapies.
Our previous study showed that opioid receptors are partially involved in pudendal afferent nerve inhibition of the reflex bladder activity induced by saline distention of the bladder.10 However, to our knowledge the role of these receptors in pudendal afferent inhibition of the bladder overactivity induced by AA irritation has not been examined. Glutamatergic mechanisms involving mGluR5 receptors underlie part of the pudendal inhibition of bladder overactivity in cats,11 leaving the possibility for opioid receptors to also have a role in pudendal inhibition.
Therefore, we determined 1) whether opioid receptors are involved in pudendal inhibition of AA induced bladder overactivity and 2) whether interactions between opioid and mGluR5 receptor mechanisms are important for pudendal inhibition of bladder overactivity. Intravesical infusion of 0.25% AA was used to irritate the bladder, activate nociceptive bladder afferent C fibers and induce bladder overactivity in anesthetized cats. Unilateral pudendal nerve stimulation was used to inhibit bladder overactivity. The opioid receptor antagonist naloxone and the mGluR5 receptor antagonist MTEP (3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine) were administered intravenously to determine the role of different receptors in pudendal inhibition. Understanding the neurotransmitter mechanisms involved in pudendal inhibition of bladder overactivity may identify new molecular targets for developing pharmacological therapies for OAB.
MATERIALS AND METHODS
The University of Pittsburgh animal care and use committee approved all protocols involving animal use in this study. Experiments were performed in 6 female and 5 male cats weighing 3.0 to 4.4 kg while under α-chloralose anesthesia (65 mg/kg intravenously, supplemented as necessary) after induction with isoflurane (2% to 3% in O2). Systemic blood pressure was monitored throughout the experiment via a catheter inserted in the right carotid artery. Tracheotomy was performed and a tube was inserted to keep the airway patent. A catheter for intravenous infusion was introduced in the right cephalic vein. The ureters were cut and drained externally. A double lumen catheter was inserted through the urethra into the bladder and secured by a ligature around the urethra. One catheter lumen was connected to a pump to infuse the bladder with saline or 0.25% AA at the rate of 1 to 2 ml per minute. The other lumen was connected to a pressure transducer to measure bladder pressure. The pudendal nerve was dissected from the right side via a 3 to 4 cm incision in the sciatic notch lateral to the tail. An NC223pt tripolar cuff electrode (MicroProbe, Gaithersburg, Maryland) was applied around the nerve and connected to an S88 stimulator (Grass Instruments, Quincy, Massachusetts) for electrical stimulation (5 Hz and 0.2-millisecond pulse width). The intensity T to induce observable anal twitching was determined by gradually increasing stimulus intensity. The 5 Hz frequency was based on our previous studies of pudendal inhibition of bladder activity.10,11
Initially, multiple CMGs were performed with saline infusion to determine bladder capacity, defined as the bladder volume T inducing bladder contraction of large amplitude (greater than 30 cm H2O) and long duration (greater than 20 seconds). After bladder capacity was determined by saline infusion, another 3 to 5 CMGs were performed during 30 to 50 minutes with infusion of 0.25% AA to irritate the bladder, activate nociceptive C-fiber bladder afferents and induce bladder overactivity. After the capacity of the irritated bladder was stabilized, 4 CMGs were done during AA infusion, including 1) control CMG without stimulation, 2) CMG during 1 to 1.5T stimulation, 3) CMG during 4T stimulation and 4) control CMG without stimulation to determine any post-stimulation effect. Increasing cumulative doses (0.1, 0.3 and 1 mg/kg, intravenously) of naloxone (Sigma-Aldrich®) were then administered. Five minutes after administering each naloxone dose the series of 4 CMGs (control, 1T to 1.5T, 4T and control) was repeated during AA infusion. In 8 cats after naloxone tests increasing cumulative doses of MTEP (3 and 10 mg/kg intravenously) were administered. Ten minutes after administering each MTEP dose the 4 CMGs (control, 1T to 1.5T, 4T and control) were repeated during AA infusion. A 5-minute rest period was inserted between repeat CMGs to allow the distended bladder to recover.
Bladder capacity was measured during repeat CMGs. Capacity measured during the first saline control CMG in each cat served as the baseline (ie 100%) to normalize other capacity measurements in the same cat. Repeat measurements in the same cat under the same conditions were averaged. Normalized data on different cats are shown as the mean ± SE. ANOVA followed by the Dunnett or Bonferroni multiple comparison and Student t test were used to determine statistical significance (p <0.05).
RESULTS
Pudendal Inhibition of Bladder Overactivity Induced by AA Irritation
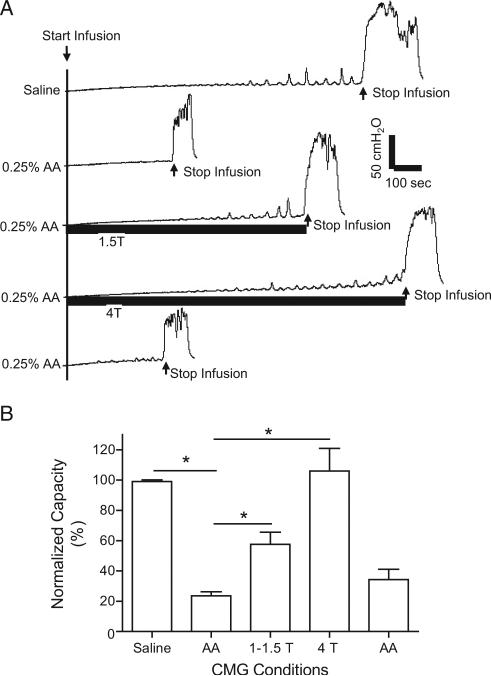
Bladder infusion with 0.25% AA irritated the bladder, induced bladder overactivity and significantly decreased mean bladder capacity to 23.6% ± 2.7% of control capacity measured during saline CMGs (2.5 ± 1.3 vs 10.7 ± 4.6 ml, p <0.0001, fig. 1). Pudendal nerve stimulation applied during AA CMGs at the intensity of 1T to 1.5T or 4T suppressed bladder overactivity and significantly increased bladder capacity to 57.5% ± 8.1% and 106% ± 15% of saline control capacity (p = 0.0005 and 0.0002, respectively, fig. 1). After pudendal nerve stimulation, bladder capacity measured during AA CMG did not differ from that measured before stimulation, ie there was no post-stimulation effect.
Figure 1.
Inhibition of bladder overactivity by pudendal nerve stimulation. A, repeat CMGs during saline or 0.25% AA infusion with/without pudendal nerve stimulation. Infusion rate was 1 ml per minute. AA infusion markedly decreased bladder capacity and pudendal nerve stimulation reversibly increased bladder capacity. Horizontal black bars indicate duration of stimulation at 5 Hz, 0.2 milliseconds and T of 0.6 V. B, summarized results in 11 cats. Bladder capacity was normalized to capacity measured during saline CMG. Infusion rate was 1 to 2 ml per minute. Asterisk indicates statistically significantly different.
Naloxone and MTEP Effect on Pudendal Inhibition of Bladder Overactivity
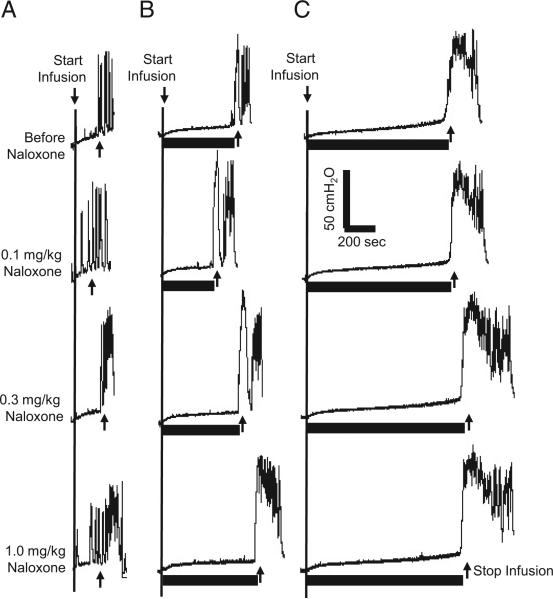
Cumulative doses of naloxone (0.1, 0.3 and 1 mg/kg intravenously) did not significantly change AA control bladder capacity or significantly alter pudendal inhibition of bladder overactivity (figs. 2 and 3). After various naloxone doses, bladder capacity during 1T to 1.5T and 4T stimulation was significantly increased over that measured during AA control CMGs and it increased by the same percent as in experiments without naloxone treatment (p <0.01 and <0.001, respectively, fig. 3).
Figure 2.
Naloxone did not alter pudendal inhibition of bladder overactivity during CMGs with infusion of 0.25% AA at 2 ml per minute. CMGs at increasing cumulative doses of naloxone were performed in sequence from left to right and top to bottom. A, without stimulation. B, during 1T stimulation. C, during 4T stimulation. Horizontal black bars indicate duration of stimulation at 5 Hz, 0.2 milliseconds and T of 1.6 V. Arrow indicates bladder infusion start and stop.
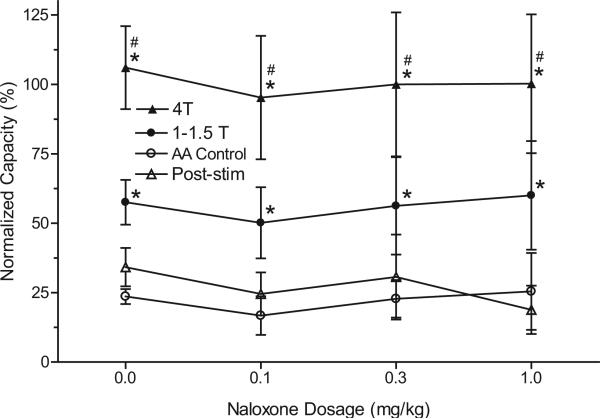
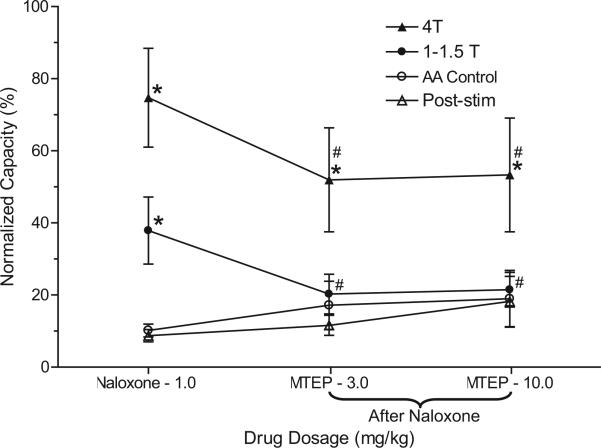
Figure 3.
Summarized results of naloxone effect on pudendal nerve inhibition of bladder overactivity induced by 0.25% AA irritation. Stimulation was done at 5 Hz, 0.2 milliseconds and T of 0.6 to 6.5 V in 11 cats. Post-stim, after stimulation. Asterisk indicates statistically significantly different vs AA control. Pound sign indicates 1T to 1.5T vs 4T statistically significantly different.
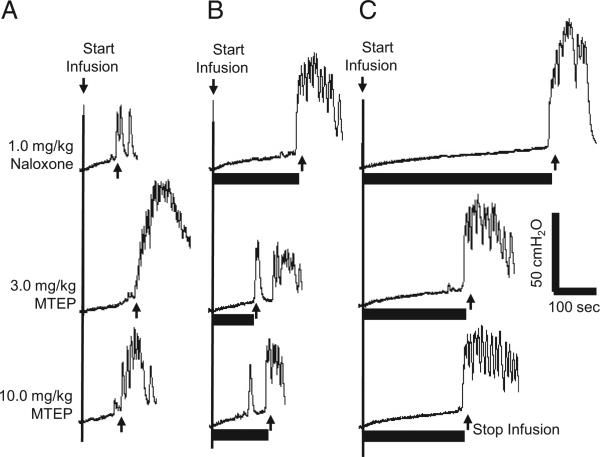
After the maximal dose of naloxone (1 mg/kg), cumulative MTEP doses (3 and 10 mg/kg intravenously) did not significantly change AA control bladder capacity but it significantly decreased pudendal inhibition of bladder overactivity (p <0.05, figs. 4 and 5). The increase in bladder capacity induced by pudendal nerve stimulation at low stimulation intensity (1T to 1.5T) was completely eliminated by MTEP but only partially decreased at the high 4T stimulation intensity (fig. 5). These MTEP effects in naloxone treated cats were similar to the effects of MTEP alone on pudendal inhibition in our previous experiments.11 In each series MTEP (3 and 10 mg/kg) completely blocked the pudendal inhibition evoked by 1T to 1.5T stimulation (fig. 5). It also decreased the inhibition evoked by 4T stimulation from a more than fourfold to a threefold increase in bladder capacity. Repeat stimulation of the pudendal nerve did not induce a post-stimulation effect after naloxone or MTEP treatment (Fig. 3 and Fig. 5).
Figure 4.
MTEP effect on pudendal inhibition of AA induced bladder overactivity after 1 mg/kg naloxone. Infusion rate was 1 cc per minute. CMGs at different doses were performed in sequence from left to right and top to bottom. A, without stimulation. B, during 1T stimulation. C, during 4T stimulation. Horizontal black bars indicate duration of stimulation at 5 Hz, 0.2 milliseconds and T of 0.6 V. Arrow indicates bladder infusion start and stop.
Figure 5.
Summarized results of MTEP effect on pudendal inhibition of AA induced bladder overactivity after 1 mg/kg naloxone in 8 cats. Post-stim, after stimulation. Asterisk indicates significantly different vs AA control data. Pound sign indicates significantly different vs 1 mg/kg naloxone.
DISCUSSION
This study in cats shows that naloxone treatment did not change the pudendal inhibition of bladder overactivity induced by 0.25% AA irritation (figs. 2 and 3). However, after the maximal dose of naloxone (1 mg/kg), MTEP (3 to 10 mg/kg) completely blocked the pudendal inhibition elicited by 1T to 1.5T stimulation and decreased the inhibition elicited by 4T stimulation (figs. 4 and 5). The effects of MTEP were similar to its effects on pudendal inhibition of bladder overactivity in the absence of naloxone.11 These results indicate that opioid receptors are not involved in pudendal inhibition of bladder overactivity and opioid mechanisms are not essential for MTEP induced suppression of the pudendal inhibition. The residual inhibition of bladder overactivity induced by high intensity pudendal nerve stimulation that persists after naloxone-MTEP treatment might be mediated by another transmitter, such as γ-aminobutyric acid or glycine.
The failure of naloxone to decrease pudendal inhibition of bladder overactivity was unexpected because our previous study showed that this drug partially reduced pudendal inhibition of normal bladder activity during saline infusion CMGs.10 This finding suggests that different neurotransmitters are involved in pudendal inhibition of normal and overactive bladder activity.
Bladder activity in irritated and nonirritated bladders depends on different reflex pathways. Bladder distention by saline infusion primarily activates nonnociceptive Aδ bladder afferents, which induces a supraspinal micturition reflex, while AA irritation of the bladder also activates nociceptive bladder C-fiber afferents, which trigger a spinal micturition reflex.12 Therefore, opioid receptors may be partially involved in pudendal inhibition of the supraspinal reflex but not of the spinal reflex.
However, our recent studies in cats indicated that opioid receptors are significantly involved in tibial/foot inhibition of bladder overactivity induced by AA irritation8,13 and a low dose of the opioid receptor agonist tramadol significantly enhances tibial/foot inhibition.9,14 These data coupled with differences between tibial/foot and pudendal inhibition in regard to frequency-response characteristics and the existence of post-stimulation inhibition indicate that tibial/foot inhibition and pudendal inhibition of reflex bladder activity in the cat are mediated by different mechanisms. It was suggested that the combination of tramadol and tibial/foot neuromodulation might be developed as a new OAB treatment.9,14 However, the failure of naloxone to affect pudendal inhibition of bladder overactivity in our study suggests that combining tramadol with pudendal neuromodulation would not have the same synergistic interaction.
Recent studies indicated that mGluR receptors may interact with opioid receptors in the modulation of somatic nociception.15–18 Therefore, we investigated the possibility of opioid-mGluR interactions in the current experiments, in which we examined the neuromodulation of bladder overactivity triggered by the response of bladder nociceptive afferents to intravesical AA infusion. However, failure of the maximal dose of naloxone (1 mg/kg) to alter the effect of MTEP on pudendal inhibition, as described in our previous experiments,11 indicates that opioid receptors do not interact with mGluR5 receptors during pudendal inhibition of nociceptive bladder activity. More studies are warranted to further investigate the neurotransmitter mechanisms underlying pudendal inhibition of nociceptive bladder activity.
While to our knowledge the site where MTEP acts to affect pudendal inhibition is unknown, group I mGluRs (mGluR1 and mGluR5), which are targeted by MTEP, are expressed on spinal inhibitory interneurons.19 Therefore, it is reasonable that pudendal inhibition of bladder overactivity occurs via direct glutamatergic (mGluR5 mediated) excitatory synaptic connections with spinal inhibitory interneurons, which in turn suppress excitatory transmission in spinal or supraspinal components of micturition reflexes. However, MTEP did not change the baseline bladder overactivity induced by AA irritation. This suggests that mGluR5 receptors expressed on interneurons in the spinal cord are not involved in the excitatory spinal micturition reflex activated by C-fiber bladder afferents or in a tonic inhibition of this reflex.
CONCLUSIONS
This study in cats shows that opioid receptors, which are involved in pudendal inhibition of normal reflex bladder activity, are not involved in pudendal inhibition of bladder overactivity induced by AA irritation of the bladder. Although this is a negative result, it provides important information on neurotransmitter mechanisms underlying neuromodulation therapy, especially because opioid receptors have a major role in tibial neuromodulation.8,9 Understanding the neurotransmitter mechanisms underlying different types of neuromodulation and the different effects of neuromodulation on normal and overactive bladder reflexes may improve our understanding of OAB pathophysiology and lead to the development of new therapies, such as combinations of drugs and electrical stimulation, to treat this disorder.
Acknowledgments
Supported by National Institutes of Health Grants DK-068566, DK-090006 and DK-091253.
Abbreviations and Acronyms
- AA
acetic acid
- CMG
cystometrogram
- mGluR
metabotropic glutamate receptor
- OAB
overactive bladder
- T
threshold
- TNS
tibial nerve stimulation
REFERENCES
- 1.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Subcommittee of the International Continence Society. Neurourol Urodyn. 2002;21:167. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 2.Milsom I, Abrams P, Cardozo L, et al. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87:760. doi: 10.1046/j.1464-410x.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- 3.Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 4.Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56:581. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- 5.MacDiarmid SA, Peters KM, Shobeiri SA, et al. Long-term durability of percutaneous tibial nerve stimulation for the treatment of overactive bladder. J Urol. 2010;183:234. doi: 10.1016/j.juro.2009.08.160. [DOI] [PubMed] [Google Scholar]
- 6.Peters KM, Killinger KA, Boguslawski BM, et al. Chronic pudendal neuromodulation: expending available treatment options for refractory uro-logic symptoms. Neurourol Urodyn. 2010;29:1267. doi: 10.1002/nau.20823. [DOI] [PubMed] [Google Scholar]
- 7.van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol. 2007;178:2029. doi: 10.1016/j.juro.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 8.Tai C, Larson JA, Ogagan PD, et al. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am J Physiol Renal Physiol. 2012;302:F1090. doi: 10.1152/ajprenal.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang F, Mally AD, Ogagan PD, et al. Inhibition of bladder overactivity by a combination of tibial neuromodulation and tramadol treatment in cats. Am J Physiol Renal Physiol. 2012;302:F1576. doi: 10.1152/ajprenal.00107.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen ML, Shen B, Wang J, et al. Influence of naloxone on inhibitory pudendal-to-bladder reflex in cats. Exp Neurol. 2010;224:282. doi: 10.1016/j.expneurol.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson JA, Ogagan PD, Chen G, et al. Involvement of metabotropic glutamate receptor 5 in pudendal inhibition of nociceptive bladder activity in cats. J Physiol. 2011;589:5833. doi: 10.1113/jphysiol.2011.215657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tai C, Ogagan PD, Chen G, et al. Involvement of opioid receptors in inhibition of bladder overactivity induced by foot stimulation in cats. J Urol. 2012;188:1012. doi: 10.1016/j.juro.2012.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mally AD, Zhang F, Matsuta Y, et al. Combination of foot stimulation and tramadol treatment reverses irritation induced bladder overactivity in cats. J Urol. 2012;188:2426. doi: 10.1016/j.juro.2012.07.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osikowicz N, Mika J, Makuch W, et al. Glutamate receptor ligands attenuate allodynia and hyperal gesia and potentiate morphine effects in a mouse model of neuropathic pain. Pain. 2008;139:117. doi: 10.1016/j.pain.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Fischer BD, Zimmerman EI, Picker MJ, et al. Morphine in combination with metabotropic glutamate receptor antagonists on schedule-controlled responding and thermal nociception. J Pharmacol Exp Ther. 2008;324:732. doi: 10.1124/jpet.107.131417. [DOI] [PubMed] [Google Scholar]
- 17.Yoon MH, Choi J, Bae HB, et al. Antinociceptive effects and synergistic interaction with morphine of intrathecal metabotropic glutamate receptor 2/3 antagonist in the formalin test of rats. Neurosci Lett. 2006;394:222. doi: 10.1016/j.neulet.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 18.Fischer BD, Miller LL, Henry FE, et al. Increased efficacy of micro-opioid agonist-induced antinociception by metabotropic glutamate receptor antagonists in C57BL/6 mice: comparison with (–)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboxylic acid (LY235959). Psychopharmacology (Berl) 2008;198:271. doi: 10.1007/s00213-008-1130-y. [DOI] [PubMed] [Google Scholar]
- 19.Walker K, Reeve A, Bowes M, et al. mGlu5 receptors and nociceptive function II. mGlu5 receptors functionally expressed on peripheral sensory neurons mediate inflammatory hyperalgesia. Neuropharmacol. 2001;40:10. doi: 10.1016/s0028-3908(00)00114-3. [DOI] [PubMed] [Google Scholar]