Abstract
GluN2D-containing NMDA receptors are characterized by an unusually low open probability (0.023), even in the presence of saturating glutamate and glycine. Here, we show that recombinant GluN1/GluN2D NMDA receptors can enter brief periods with exceptionally high open probability (0.65) in excised outside-out and cell-attached single channel recordings. GluN1/GluN2D channels during the enhanced gating mode have similar open durations as occurs outside of the high open probability burst of activity. However, the periods in the high gating mode only exhibit 4 brief closed duration exponential components similar to the briefest observed for openings outside the burst. GluN1/GluN2D receptors also open to a more prominent subconductance level compared to activity outside the high open probability burst. Evaluation of a five-state NMDA receptor gating model suggests that both the opening and closing rate constants differ for the periods of higher open probability compared to the high open probability arm of a gating model previously published for GluN1/GluN2D fit to a representative full length single channel recording. These data demonstrate that GluN2D-containing NMDA receptors can enter a conformation or mode that allows the pore to gate with high probability.
Keywords: Ionotropic glutamate receptors, Electrophysiology, Single channel recordings, NR2D
1. Introduction
N-methyl-d-aspartate (NMDA) receptors are ionotropic glutamate receptors typically composed of two glycine-binding GluN1 subunits and two glutamate-binding GluN2 subunits. Four GluN2 subunits (GluN2A-D) have been identified and are thought to control many of the kinetic properties of the NMDA receptor, including open probability, conductance levels, and deactivation time course (Monyer et al., 1994; Kuner and Schoepfer, 1996; Vicini et al., 1998; Qian et al., 2005; Dravid et al., 2008; Yuan et al., 2009; Traynelis et al., 2010; Vance et al., 2012). In addition, eight GluN1 splice variants, which are formed by mRNA splicing of a single gene, have been identified (Hollmann et al., 1993). The single channel and macroscopic current properties as well as the neuronal expression of GluN2D-containing NMDA receptors differ substantially from other NMDA receptor subunits. Individual recombinant GluN1-1a/GluN2D NMDA receptors have a particularly low single channel open probability of approximately 0.02, which is 25-fold lower than GluN1/GluN2A receptors (open probability around 0.5) (Popescu and Auerbach, 2003; Erreger et al., 2005; Yuan et al., 2009; Vance et al., 2012). GluN2D-containing receptors also exhibit a prominent single channel subconductance level, reduced sensitivity to inhibition by Mg2+, and an exceptionally slow deactivation time course following the removal of glutamate (Wyllie et al., 1996; Vicini et al., 1998; Wyllie et al., 1998; Clarke and Johnson, 2006; Yuan et al., 2009; Vance et al., 2011). The expression of the GluN2D subunit peaks early in development and later can only be found in particular regions of the adult brain (Monyer et al., 1994; Standaert et al., 1994; Dunah et al., 1996; Wenzel et al., 1996), including the subthalamic nucleus, substantia nigra, spinal cord, cerebellar Golgi and Purkinje cells, and interneurons (Laurie and Seeburg, 1994; Monyer et al., 1994; Standaert et al., 1994, 1996; Dunah et al., 1996; Wenzel et al., 1996; Goebel and Poosch, 1999; Standaert et al., 1999).
NMDA receptors have long been known to exhibit periods of high open probability. Jahr and Stevens (1987) and Gibb and Colquhoun (1991) reported brief periods of extremely high open probability in single channel recordings of hippocampal NMDA receptors activated by the selective agonist NMDA (Jahr and Stevens, 1987; Gibb and Colquhoun, 1991), while Howe et al. (1988, 1991) identified high open probability bursts in native NMDA receptors in cerebellar granule cells when activated by several agonists, including NMDA, glutamate, and aspartate (Howe et al., 1988, 1991). These high open probability bursts were correlated to an increase in mean open time (Jahr and Stevens, 1987) and were independent of agonist concentration (Gibb and Colquhoun, 1991). More recently, recombinant AMPA and NMDA receptors have been shown to exhibit multiple modes in gating when expressed in HEK 293 cells. Both GluN1/GluN2A (Popescu and Auerbach, 2003; Popescu et al., 2004; Zhang et al., 2008; Kussius and Popescu, 2009) and GluN1/GluN2B (Amico-Ruvio and Popescu, 2010) NMDA receptors exhibit modal gating, although on a much longer time scale than observed in the hippocampal or cerebellar NMDA receptor recordings. Moreover, unlike hippocampal NMDA channels, recombinant GluN2A-containing receptors are capable of entering high-, medium-, and low-gating modes. Modal gating described for GluN1/GluN2A and GluN1/GluN2B receptors also is correlated to mean open time, as open time is longest in high mode and shortest in low mode (Popescu and Auerbach, 2003; Popescu et al., 2004; Zhang et al., 2008; Kussius and Popescu, 2009; Amico-Ruvio and Popescu, 2010; Popescu, 2012). Additionally, single channel recordings of GluA1-4 AMPA receptors also reveal distinct gating modes when recorded in the presence of cyclothiazide to reduce desensitization (Poon et al., 2010; Prieto and Wollmuth, 2010; Poon et al., 2011). While both NMDA and AMPA receptors appear able to enter multiple gating modes, the mechanisms that control modal gating remain elusive.
The goal of this study was to determine whether GluN1/GluN2D NMDA receptors also can undergo modal gating. We show here that GluN1-1a/GluN2D NMDA receptors are capable of entering brief periods of exceptionally high open probability when activated by saturating concentrations of glutamate and glycine in excised, outside-out patch recordings with a single active channel. We further demonstrate that GluN2D-containing receptors can enter a high gating mode regardless of recording condition or the GluN1 splice variant assembled within the receptor, as cell-attached single channel recordings of GluN1-1b/GluN2D receptors also exhibit a high gating mode. Finally, we show that the rate constants of a five-state NMDA receptor gating model are altered when fit to the high open probability bursts from GluN1-1a/GluN2D receptors in outside-out patches that contain a single active channel when compared to the channel in lower open probability gating mode.
2. Methods
2.1. Cell culture
Human embryonic kidney 293 cells (CRL 1573; ATCC, Rockville, MD; hereafter HEK 293 cells) were plated onto 5 mm diameter glass coverslips (Warner Instruments, Hamden, CT) that were coated in 100 μg/mL poly-d-lysine. Cells were maintained in 5% humidified CO2 at 37 °C in Dulbecco's Modified Eagle Medium (Invitrogen, Carlsbad, CA) that was supplemented with 10% fetal bovine serum, 10 units/ml penicillin, and 10 μg/ml streptomycin. HEK 293 cells were transfected transiently using Fugene 6 (Roche Diagnostics, Basel, Switzerland) with cDNA encoding rat GluN1-1a (GenBank U11418, U08261) or GluN1-1b (GenBank U08263), GluN2D (GenBank L31611), and green fluorescent protein (GFP) at a ratio of 1:1:1 (0.5 μg total cDNA/well), as previously described (Vance et al., 2012). Following transfection, the HEK 293 cells were incubated in media supplemented with d,l-2-amino-5-phosphonovalerate (200 μM) and 7-chlorokynurenic acid (200 μM).
2.2. Electrophysiology
Voltage-clamp recordings were made from excised, outside-out patches (VHOLD −80 mV) or cell-attached patches (VHOLD +100 mV) from transiently transfected HEK 293 cells. Single channel recordings were obtained using an Axopatch 200B amplifier (Molecular Devices, Union City, CA), and were digitized at 40 kHz using Axon pClamp10 software and filtered at 8 kHz using an eight-pole Bessel filter (−3 dB; Frequency Devices, Haverhill, MD). Thick-walled borosilicate glass (Warner Instruments, Hamden, CT) was used to form recording electrodes, which were then coated with Sylgard 184 (Dow Corning, Midland, MI). Electrodes were filled with a pipette solution containing (in mM) 110 d-gluconate, 110 CsOH, 30 CsCl, 5 HEPES, 4 NaCl, 0.5 CaCl2, 2 MgCl2, 5 BAPTA, 2 NaATP, and 0.3 NaGTP (pH 7.35) for excised, outside-out patches. Cells were bathed at 23 °C in an extracellular solution composed of (in mM) 150 NaCl, 10 HEPES, 30 d-mannitol, 3 KCl, 0.5 CaCl2, and 0.01 EDTA at pH 8.0. The agonist solution was composed of extracellular solution supplemented with 0.05 mM glycine and 1 mM l-glutamate. Recording electrodes for the cell-attached recordings were filled with a pipette solution composed of the external solution with 1 mM l-glutamate and 0.05 mM glycine at pH 8.0. The holding potential was corrected for a junction potential of +5.4 mV when determining chord conductance (Vance et al., 2012).
2.3. Single channel analysis
Recordings were determined to have a single functional NMDA receptor when no double openings were observed throughout the duration (>3 min) of the recording (Colquhoun and Hawkes, 1990). The single channel records were idealized using the time course fitting method (SCAN; Colquhoun and Sigworth, 1995). The high open probability bursts were identified either through visual evaluation of the data records or by plotting the open probability as a function of time in 50–100 ms increments for each idealized data record. Only high gating modes that endured for a minimum of 50 ms with open probabilities of at least 0.25 were analyzed. Maximum likelihood fitting was used to analyze dwell time and amplitude histograms (EKDIST; www.ucl.ac.uk/Pharmacology/dcpr95.html). Adjacent open periods with different amplitudes were combined, and a 53 μs open resolution and 31 μs shut resolution were imposed on the data record (Colquhoun and Sigworth, 1995). The sequence of individual open and closed transitions within periods of the high gating mode were analyzed to evaluate unitary current amplitude, open duration properties, and shut duration characteristics. Fitted single channel unitary current amplitudes ranged from 0.0 to 7.0 pA. Open-point amplitude histograms were constructed, which could be fitted best by the sum of two Gaussian components.
When open periods within defined amplitude ranges (openings within 1.5 standard deviations of the mean amplitude) were evaluated, only openings longer than 246 μs, which is 2.5 times the filter rise time of 98.6 μs and long enough to reach 99.8% of the maximal amplitude, were included in the fit (Wyllie et al., 1996). In this analysis, adjacent openings to different conductance levels were treated as independent openings, each with a unique duration. The open duration histograms of openings within defined amplitude ranges were best fit by a single exponential equation.
The idealized data were converted to a QUB-compatible format (www.qub.buffalo.edu) to allow NMDA receptor gating models to be fitted to the data using maximum interval likelihood fitting (MIL) (Qin et al., 1997). A 50 μs resolution was imposed on the data record during maximum likelihood fitting on the data records. Consecutive opening to different amplitudes were joined and treated as a single open duration. We evaluated various gating schemes by comparing their log likelihood values, and a change of more than 10 log likelihood units was our threshold for an improvement in the fit.
2.4. Statistical analysis
Data are reported as mean ± s.e.m. to two significant figures and were evaluated statistically using an unpaired t-test or a Mann–Whitney test. Significance for all tests was set at p < 0.05.
3. Results
3.1. GluN1/GluN2D exhibits brief periods of high open probability
A feature of NMDA receptor function is modal gating, in which the characteristics of channel behavior change over a time scale of seconds (Popescu and Auerbach, 2003; Popescu et al., 2004; Zhang et al., 2008; Kussius and Popescu, 2009; Amico-Ruvio and Popescu, 2010). To evaluate whether GluN1/GluN2D NMDA receptors are capable of undergoing modal gating, we recorded GluN1-1a/GluN2D single channel currents in excised, outside-out patches pulled from transiently transfected HEK 293 cells for prolonged periods of time. The single channels were activated by steady application of 1 mM l-glutamate at 0.05 mM glycine at pH 8.0 and 0.5 mM Ca2+. In a subset of our GluN1-1a/GluN2D recordings (six out of a total of 23 recordings) that contained one active channel, we observed brief periods of extraordinarily high open probability, which endured for 50–600 ms (Fig. 1). These periods of high open probability, while relatively short in duration, were similar to the prominent high gating mode observed in GluN1/GluN2A (Popescu and Auerbach, 2003; Popescu et al., 2004; Kussius and Popescu, 2009). The mean percentage of time during which the receptor exhibited a high gating mode across all six patches in which they were evident was 0.11%.
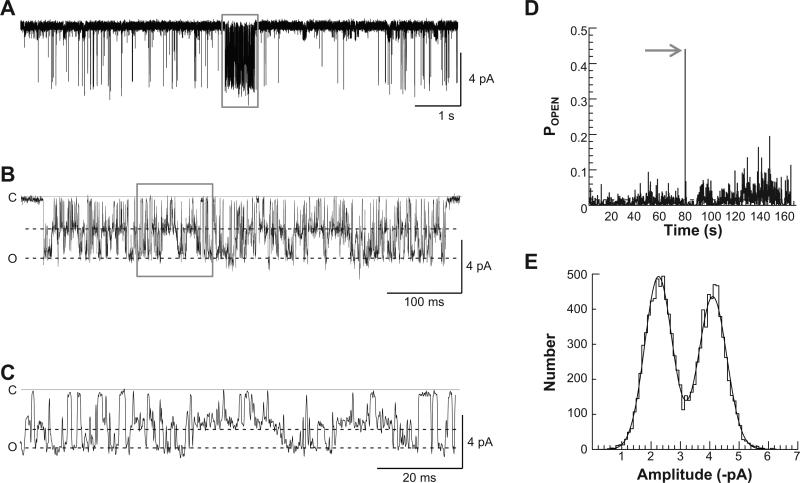
Fig. 1.
GluN1/GluN2D receptors exhibit high gating modes in excised outside-out patches. A, A representative trace of an outside-out GluN1-1a/GluN2D single channel recording exhibiting a mode of high open probability (box) than is sustained for 470 ms. The full burst is shown in (B) and expanded further in (C). An open probability stability plot is given in (D), where the POPEN of the recording was analyzed in 100 ms increments. Note the sharp increase in POPEN at about 90 s into the recording when the channel displays a high gating mode (arrow). E, A representative open-point histogram of fitted channel amplitudes for a high gating mode from the GluN1-1a/GluN2D single channel recording shown in A; this histogram was fitted with two Gaussian components, which we interpret to reflect two levels with chord conductances of 32 pS and 53 pS.
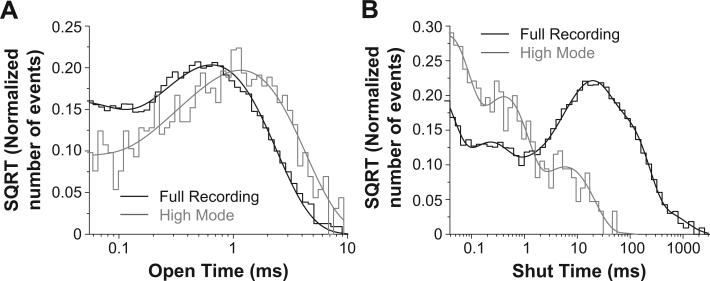
GluN1-1a/GluN2D open probability increased nearly 30-fold from 0.023 in the more common low mode to 0.65 in the rarer high gating mode (Fig. 1A–D; Table 1). Interestingly, while the amplitude of the subconductance level does not change significantly while in the high gating mode, the subconductance level becomes significantly more prominent than in the typical, low mode recordings (41% vs. 28%; Fig. 1E, Table 1; p < 0.05; t-test), differing from NMDA receptors in hippocampal and cerebellar neurons (Jahr and Stevens, 1987; Gibb and Colquhoun, 1991; Howe et al., 1988, 1991). The mean open duration of openings during high mode gating increased significantly to 1.2 ± 0.069 ms from 0.42 ± 0.037 ms (p < 0.05; Mann–Whitney test; Table 1). The open duration histograms for the periods in high mode were best fit by the sum of two exponential components (0.12 ± 0.046 ms and 1.3 ± 0.061 ms), which were significantly longer than the open duration histogram components for the more typical periods during recordings of GluN1-1a/GluN2D receptors (p < 0.05; t-test; Table 1; Fig. 2A). In addition, the mean shut time decreased significantly in the high mode to 0.95 ± 0.36 ms from 24 ± 5.6 ms (p < 0.05; Mann–Whitney; Table 1). While the average GluN1-1a/GluN2D single channel recording shut time distribution histograms were best fit by a sum of 7 exponential functions, the three longest shut time components appeared to be absent in the high gating mode (Fig. 2B).
Table 1.
Single channel and macroscopic properties of GluN1-1a/GluN2D full recordings and the high open probability bursts.
| Parameters | Full recording | High PO burst |
|---|---|---|
| N | 6 | 8 |
| POPEN | 0.023 ± 0.0068 | 0.65 ± 0.084* |
| Mean open duration (ms) | 0.42 ± 0.037 | 1.2 ± 0.069* |
| τ1 open, ms (%) | 0.032 ± 0.0070 (38%) | 0.12 ± 0.046* (6.0%*) |
| τ2 open, ms (%) | 0.66 ± 0.040 (62%) | 1.3 ± 0.061* (94%*) |
| Number of open periods | 11,202 | 1110 |
| Mean shut duration (ms) | 24 ± 5.6 | 0.95 ± 0.36* |
| τ0 shut, ms (%) | 0.019 ± 0.00040 (30%) | 0.038 ± 0.012 (66%*) |
| τ1 shut, ms (%) | 0.22 ± 0.012 (14%) | 0.44 ± 0.070* (22%) |
| τ2 shut, ms (%) | 6.1 ± 0.76 (10%) | 7.7 ± 3.7 (11%) |
| τ3 shut, ms (%) | 20 ± 2.5 (24%) | 24 ± 5 (1%*) |
| τ4 shut, ms (%) | 61 ± 7.1 (18%) | – |
| τ5 shut, ms (%) | 280 ± 110 (3%) | – |
| τ6 shut, ms (%) | 3200 ± 1100 (<1%) | – |
| Amplitude 1 (pA) | 2.7 ± 0.12 | 2.7 ± 0.12 |
| Amplitude 2 (pA) | 4.6 ± 0.13 | 4.6 ± 0.12 |
| γ1, pS (%) | 32 ± 1.4 (28%) | 32 ± 1.4 (41%*) |
| τ2, pS (%) | 54 ± 1.5 (72%) | 53 ± 1.4 (59%*) |
Excised, outside-out single channel recordings of GluN1-1a/GluN2D were conducted at 1 mM l-glutamate and 0.05 mM glycine at pH 8.0. Data are reported as mean ± s.e.m. and are given to two significant figures. Contiguous open periods of differing unitary currents were combined into a single opening. Data were analyzed for statistical significance by Mann–Whitney test (open time) or unpaired two-tailed t-test, as appropriate. The analysis for the full recordings excluded the high gating mode. N is the total number of full recordings or number of high gating mode bursts analyzed; data are from 6 outside-out patches.
p < 0.05 when compared to the corresponding value for the full GluN1-1a/GluN2D single channel recording.
Fig. 2.
The high open probability bursts have similar open time components but fewer shut time components than the GluN1/GluN2D single channel recordings for low open probability periods. A, Composite open time histograms from 8 bursts in 6 patches for the high open probability bursts (gray) and the low gating mode in 6 GluN1/GluN2D single channel recordings were best fit by the sum of two exponential components. The high open probability bursts had significantly longer open time components than the openings that occurred outside of the high open probability bursts. B, The composite shut time histogram for all high open probability bursts was best fit by four exponential components, while the histogram for the low open probability periods from the 6 single channel recordings was best fit with 7 exponential recordings. The longer shut time components are absent in the high open probability bursts. There were a total of 1110 open and 1118 closed durations fitted for the high open probability bursts.
Because the subconductance level is more prominent in the high gating mode, we evaluated the extent to which the increase in open probability in the high gating mode was due to an increase in the mean open time of the subconductance state by evaluating conditional open duration histograms for channel openings within each amplitude level (Wyllie et al., 1996). We found that the only the mean open time of the higher conductance level (0.72 ± 0.055 ms) was longer in the high gating mode compared to the more typical low gating mode (0.47 ± 0.035 ms; p < 0.05; Mann–Whitney test). These values are lower than open periods reported above (Table 1), which included duration of adjacent openings that reflected a direct transition between two different conductance levels. The mean open time of the subconductance level in high gating mode was not significantly longer than when in the low gating mode (0.54 ± 0.053 ms and 0.41 ± 0.035 ms, respectively; p = 0.1; Mann–Whitney test). These data show that the GluN1/GluN2D receptor enters the subconductance level more frequently when in the high gating mode, but does not remain at the subconductance level for longer durations than when gating in the low mode.
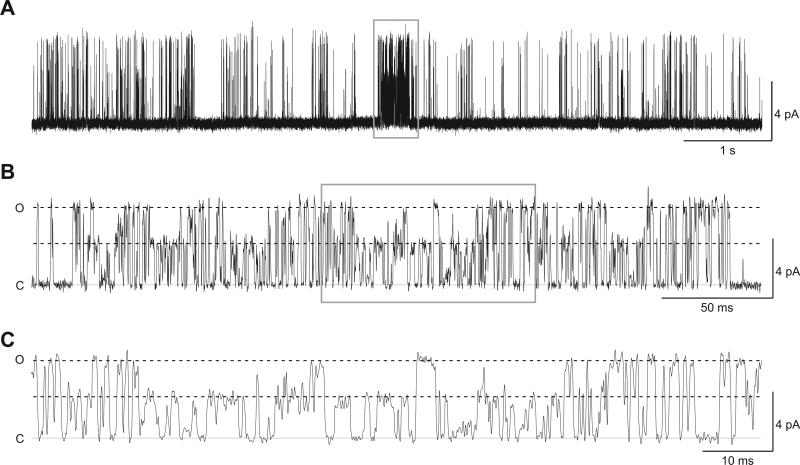
We also observed the presence of high gating modes in cell-attached single channel recordings of GluN1-1a/GluN2D and GluN1-1b/GluN2D, suggesting that the high mode occurs regardless of recording condition or GluN1 splice variant. That is, the high gating mode is not a consequence of removal of the intracellular compartment, disruption of the cytoskeleton, or extensive dialysis of the intracellular compartment. In one out of five cell-attached GluN1-1b/GluN2D single channel recordings, we observed several periods of high gating mode, with one period enduring for 260 ms (Fig. 3). The open probability of this high gating mode increased to 0.52 compared to the open probability of 0.036 in the remainder of the recording. The open duration histogram for the high mode in this cell-attached patch was best fit with one exponential component of 0.47 ms (Fig. 3B), while the shut duration histogram was best fit by two exponential components of 0.051 ms (41%) and 0.69 ms (59%) (Fig. 3C); this patch had a mean shut time of 0.43 ms during the high open probability period. These data show that GluN2D-containing NMDA receptors are capable of adopting (albeit rarely) a conformation in which pore opening occurs with high probability for agonist-bound receptors.
Fig. 3.
GluN1-1b/GluN2D receptors enter high gating modes in cell-attached patches. A, A representative trace of a cell-attached GluN1-1b/GluN2D receptor single channel recording shows a high gating mode that endures for 260 ms. The entire period of high gating mode is given in (B) and is expanded in (C).
3.2. Increases in open probability reflect changes in GluN1/GluN2D gating
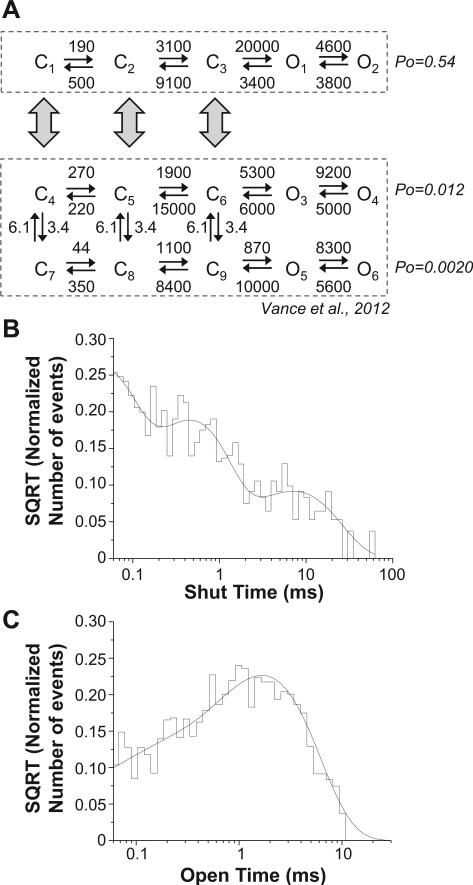
We next evaluated whether the high gating mode exhibited in our excised, outside-out patch recordings with a single active channel had differing gating rate constants compared to typical periods of GluN1-1a/GluN2D single channel activity (Wyllie et al., 1998; Yuan et al., 2009; Vance et al., 2012). A gating model capable of describing GluN1-1a/GluN2D single channel and macroscopic current data has previously been described (Vance et al., 2012). However, this model was designed to describe data with at least six shut time components. By contrast, periods of high gating mode in the GluN1-1a/GluN2D recordings had fewer identifiable shut time components (Fig. 2B; Table 1). This model of GluN2D function involved a high open probability arm and a low open probability arm that could interconvert. We reasoned that a third arm of this model might similarly be represented as a linear scheme (Fig. 4A). Because this arm is rarely visited, we assumed rate constants into and out of it were low and could not be ascertained from the limited number of high open probability bursts in our data set. We therefore evaluated the ability of a linear model with 3 or 4 closed states and two open states to fit the isolated high open probability data. This linear model was similar to those that previously have been used to describe modal gating in GluN1/GluN2A and GluN1/GluN2B single channel recordings (Fig. 4) (Popescu and Auerbach, 2003; Popescu et al., 2004; Zhang et al., 2008; Kussius and Popescu, 2009; Amico-Ruvio and Popescu, 2010; Popescu, 2012). Evaluation of the log likelihood for the high open probability data suggested the best linear model included 3 closed and two open states.
Fig. 4.
Gating rates differ during high probability bursts. A, A model with three closed and two open states that previously has been used to describe GluN1/GluN2A and GluN1/GluN2B single channel recordings (Popescu and Auerbach, 2003; Popescu et al., 2004; Erreger et al., 2005; Zhang et al., 2008; Amico-Ruvio and Popescu, 2010) as well as describe each arm of a GluN1/GluN2D model (Vance et al., 2012) was fitted to the composite sequence of single channel openings during the high gating for all recordings during high gating mode. The open probability was considerably higher than that predicted by the upper, fast-gating arm of a model developed for GluN1/GluN2D single channel recordings (Vance et al., 2012) fit to the normal, low mode openings within the representative recording in Fig. 1 (open probability of 0.012). B, The shut time histogram for the composite of all 8 high gating mode bursts is given, overlaid with the probability density function predicted by fitting the gating model to the data. C, The open time histogram for the composite of all 8 high gating mode bursts is given, overlaid with the probability density function predicted by fitting the gating model to the data.
When the NMDA receptor gating model was fit simultaneously to data from all high open probability bursts, the opening rate constant was accelerated when compared to the corresponding rate constant in either arm of a gating model previously published for GluN1/GluN2D that was fit to a representative full length GluN1/GluN2D single channel recording (Fig. 4A). This finding is consistent with the changes in gating rate constants between the modes previously observed for GluN1/GluN2A and GluN1/GluN2B single channel recordings (Popescu and Auerbach, 2003; Zhang et al., 2008; Kussius and Popescu, 2009; Amico-Ruvio and Popescu, 2010; Popescu, 2012). The model adequately predicted the composite open and shut duration histograms for all 8 high gating mode bursts of GluN1-1a/GluN2D (Fig. 4B,C), and the model predicted the GluN1-1a/GluN2D high gating mode had an open probability of 0.54, similar to the open probability of 0.65 determined from our experimental data. These data suggest that GluN1/GluN2D NMDA receptors are capable of entering periods of high open probability with similar channel properties and gating rate constants as has been observed in the modal gating of other NMDA receptors.
4. Discussion
There are several key findings in our study of the high open probability bursts in GluN1/GluN2D single channel recordings. First, although GluN1/GluN2D typically has a very low single channel open probability around 0.02, the receptor is capable of adopting protein conformations that allow it to enter periods of extremely high open probability. This increase in single channel open probability is accompanied by a significant increase in the mean open duration time and a significant decrease in the mean shut time. Second, while in high gating mode, GluN1-1a/GluN2D openings in the subconductance level occur more frequently than while in normal, low gating mode. Finally, the opening rate constant was more rapid in the high gating mode than the in the upper, elevated probability arm of a GluN1/GluN2D gating model fit to the full length, low open probability recording period of a representative recording. These data raise the possibility that GluN1/GluN2D receptors can support brief periods of high activity, which may influence neuronal function by increasing charge transfer by many fold. Although the mechanisms underlying these periods of high open probability are unknown, this property holds important implications for structural features of gating as well as the properties of triheteromeric receptors that contain GluN2D and a different GluN2 subunit.
Modal gating, in which a channel slowly shuttles between two modes with distinct properties, has been observed in a number of ion channels and appears to be a common feature that regulates channel function. In l-type and P/Q-type calcium channels, modal gating has been attributed to both phosphorylation and voltage dependence (Hess and Tsien, 1984; Ochi and Kawashima, 1990; Yue et al., 1990; Dzhura et al., 2000; Alt et al., 2004; Fellin et al., 2004; Luvisetto et al., 2004; Hashambhoy et al., 2009). In addition, phosphorylation has been implicated in modal gating in K+ channels (Marrion, 1996; Smith and Ashford, 1998; Singer-Lahat et al., 1999; Mullner et al., 2003). Voltage, auxiliary subunits, G-protein subunits, and other intracellular signaling molecules lead to models of modal gating in a number of other channels, including voltage-activated Na+ and Ca2+ channels as well as acetylcholine activated channels (Howe and Ritchie, 1992; Delcour et al., 1993; Naranjo and Brehm, 1993; Imredy and Yue, 1994; Chang et al., 1996; Peterson et al., 1999; Singer-Lahat et al., 1999; Wakamori et al., 1999; Meir et al., 2000).
Our data suggest that GluN1/GluN2D NMDA receptors may undergo a form of modal gating, as we observed brief periods of exceptionally high open probability in our single channel recordings similar to that observed in other channels, notably GluN2A-containing NMDA channels. However, unlike modal gating in GluN2A- and GluN2B-containing NMDA receptors and AMPA receptors, which exhibit several gating states that endure over a period of many seconds, we only observed brief periods of high open probability and no other distinguishable gating modes other than the low open probability behavior that previously has been described (Wyllie et al., 1998; Yuan et al., 2009; Vance et al., 2012). Therefore, it remains to be determined whether the high gating mode we observed in GluN1/GluN2D is a mechanistically similar form of modal gating that has been reported for other NMDA and AMPA receptors or is unique to GluN2D-containing receptors.
The structural basis for the high gating mode is unknown. One possibility is that high gating mode could signal a rearrangement of the amino-terminal domains within the NMDA receptor family, as this domain is particularly important in controlling single channel open probability (Gielen et al., 2009; Yuan et al., 2009). Alternatively, modal gating might reflect a change in the coupling of the receptor to an intracellular scaffold or binding of an intracellular protein (Rosenmund and Westbrook, 1993; Ehlers et al., 1996, 1998; Zhang et al., 1998; Krupp et al., 1999; Chavis and Westbrook, 2001; Rycroft and Gibb, 2002, 2004). Whereas we recorded from recombinant GluN1/GluN2D expressed in HEK 293 cells, it is possible that GluN2D-containing NMDA receptors expressed in native tissues could undergo a more prominent form of modal gating, or transition to high open probability states more often for longer durations than we observed in our recordings. We anticipate such an effect could occur as a result of the presence of potential NMDA receptor auxiliary subunits, neuron-specific posttranslational modifications, and/or physiological temperatures.
A model for GluN2D gating recently has been described that allows the GluN1/GluN2D receptor to shift in any closed state from gating at a higher open probability to gating at a lower open probability (Vance et al., 2012). This could be interpreted as a model describing the modal gating of GluN1/GluN2D NMDA receptors. However, our observation here of very brief periods in a high gating mode are not a manifestation of that model, as the open probability we observe here (0.65) is much higher than predicted for the enhanced open probability arm of the model (0.012). Because the high gating mode described here occurs rarely, it suggests a limited number of access points to this state, in contrast to the model presented in Vance et al. (2012). One way to model this behavior is as a separate phenomenon that is connected to the model previously described, but with slow rate constants that limit the visitations to the high gating mode.
While the periods of high gating mode for GluN1/GluN2D are rare, it would take nearly 30 s for the receptor in normal, low gating mode to pass as much current as the receptor would in one second in high gating mode. If the GluN2D subunit is expressed at synapses, a few GluN2D-containing receptors gating in high mode could dramatically impact the shape of the synaptic currents, increasing peak amplitude and enhancing charge transfer. Indeed, such a dramatic effect in GluN2D gating could also lead to pronounced variation in the peak amplitudes or charge transfer in excitatory postsynaptic currents, and thus would be expected to make important contributions to neuronal signaling.
5. Conclusion
In conclusion, our data show for the first time that the GluN1/GluN2D NMDA receptor is capable of entering brief periods of high open probability. It seems likely that gating in a high open probability mode could dramatically influence the synaptic currents in a synapse in which the GluN2D subunit is expressed, making this property a potentially important determinant of channel function.
Acknowledgments
This work was supported by NIH-NINDS (NS036654, NS065371, S.F.T.), Training Grants 5T32-NS007480-07, T32-DA01504006, and T32-ES012870 (K.M.V.), the Villum Kann Rasmussen Foundation (K.B.H.), and the Lundbeck Foundation (K.B.H.).
References
- Alt A, Weiss B, Ogden AM, Knauss JL, Oler J, Ho K, Large TH, Bleakman D. Pharmacological characterization of glutamatergic agonists and antagonists at recombinant human homomeric and heteromeric kainate receptors in vitro. Neuropharmacology. 2004;46(6):793–806. doi: 10.1016/j.neuropharm.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Amico-Ruvio SA, Popescu GK. Stationary gating of GluN1/GluN2B receptors in intact membrane patches. Biophys. J. 2010;98(7):1160–1169. doi: 10.1016/j.bpj.2009.12.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SY, Satin J, Fozzard HA. Modal behavior of the mu 1 Na+ channel and effects of coexpression of the beta(1)-subunit. Biophys. J. 1996;70(6):2581–2592. doi: 10.1016/S0006-3495(96)79829-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis P, Westbrook G. Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature. 2001;411(6835):317–321. doi: 10.1038/35077101. [DOI] [PubMed] [Google Scholar]
- Clarke RJ, Johnson JW. NMDA receptor NR2 subunit dependence of the slow component of magnesium unblock. J. Neurosci. 2006;26(21):5825–5834. doi: 10.1523/JNEUROSCI.0577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Hawkes A. Stochastic properties of ion channel openings and bursts in a membrane patch that contains two channels: evidence concerning the number of channels present when a record containing only single openings is observed. Proc. R Soc. Lond., B Biol. Sci. 1990;240(1299):453–457. doi: 10.1098/rspb.1990.0048. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Sigworth F. Fitting and statistical analysis of single-channel records. In: Sakmann B, Neher E, editors. Single-channel Recording. Plenum Press; New York, NY: 1995. pp. 483–587. [Google Scholar]
- Delcour AH, Lipscombe D, Tsien RW. Multiple modes of N-type calcium channel activity distinguished by differences in gating kinetics. J. Neurosci. 1993;13(1):181–194. doi: 10.1523/JNEUROSCI.13-01-00181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravid SM, Prakash A, Traynelis SF. Activation of recombinant NR1/NR2C NMDA receptors. J. Physiol. 2008;586(18):4425–4439. doi: 10.1113/jphysiol.2008.158634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Yasuda RP, Wang Y-H, Luo J, Dávila-García MI, Gbadegesin M, Vicini S, Wolfe BB. Regional and ontogenic expression of the NMDA receptor subunit NR2D protein in rat brain using a subunit-specific antibody. J. Neurochem. 1996;67(6):2335–2345. doi: 10.1046/j.1471-4159.1996.67062335.x. [DOI] [PubMed] [Google Scholar]
- Dzhura I, Wu YJ, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat. Cell Biol. 2000;2(3):173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Zhang S, Bernhardt JP, Huganir RL. Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell. 1996;84(5):745–755. doi: 10.1016/s0092-8674(00)81052-1. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Fung ET, O'Brien RJ, Huganir RL. Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. J. Neurosci. 1998;18(2):720–730. doi: 10.1523/JNEUROSCI.18-02-00720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJA, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J. Physiol. 2005;563(2):345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Luvisetto S, Spagnolo M, Pietrobon D. Modal gating of human Ca(v)2.1 (P/Q-type) calcium channels: II. The b mode and reversible uncoupling of inactivation. J. Gen. Physiol. 2004;124(5):463–474. doi: 10.1085/jgp.200409035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb AJ, Colquhoun D. Glutamate activation of a single NMDA receptor-channel produces a cluster of channel openings. Proc. Biol. Sci. 1991;243(1306):39–45. doi: 10.1098/rspb.1991.0007. [DOI] [PubMed] [Google Scholar]
- Gielen M, Retchless BS, Mony L, Johnson JW, Paoletti P. Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature. 2009;459(7247):703–707. doi: 10.1038/nature07993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel DJ, Poosch MS. NMDA receptor subunit gene expression in the rat brain: a quantitative analysis of endogenous mRNA levels of NR1Com, NR2A, NR2B, NR2C, NR2D and NR3A. Mol. Brain Res. 1999;69(2):164–170. doi: 10.1016/s0169-328x(99)00100-x. [DOI] [PubMed] [Google Scholar]
- Hashambhoy YL, Winslow RL, Greenstein JL. CaMKII-induced shift in modal gating explains L-type Ca2+ current facilitation: a modeling study. Biophys. J. 2009;96(5):1770–1785. doi: 10.1016/j.bpj.2008.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P, Tsien RW. Mechanism of ion permeation through calcium channels. Nature. 1984;309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Boulter J, Maron C, Beasley L, Sullivan J, Pecht G, Heinemann S. Zinc potentiates agonist-induced currents at certain splice variants of the NMDA receptor. Neuron. 1993;10(5):943–954. doi: 10.1016/0896-6273(93)90209-a. [DOI] [PubMed] [Google Scholar]
- Howe JR, Colquhoun D, Cullcandy SG. On the kinetics of large-conductance glutamate receptor ion channels in rat cerebellar granule neurons. Proc. Royal Soc. B Biol. Sci. 1988;233(1273):407–422. doi: 10.1098/rspb.1988.0030. [DOI] [PubMed] [Google Scholar]
- Howe JR, Cull-Candy SG, Colquhoun D. Currents through single glutamate receptor channels in outside-out patches from rat cerebellar granule cells. J. Physiol. 1991;432(1):143–202. doi: 10.1113/jphysiol.1991.sp018381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe JR, Ritchie JM. Multiple kinetic components of sodium channel inactivation in rabbit Schwann cells. J. Physiol. 1992;455(1):529–566. doi: 10.1113/jphysiol.1992.sp019315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imredy JP, Yue DT. Mechanisms of Ca2+-sensitive inactivation of L-type Ca2+ channels. Neuron. 1994;12(6):1301–1318. doi: 10.1016/0896-6273(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. Interactions of calmodulin and alpha-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors. J. Neurosci. 1999;19(4):1165–1178. doi: 10.1523/JNEUROSCI.19-04-01165.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner T, Schoepfer R. Multiple structural elements determine subunit specificity of Mg2+ block in NMDA receptor channels. J. Neurosci. 1996;16(11):3549–3558. doi: 10.1523/JNEUROSCI.16-11-03549.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kussius CL, Popescu GK. Kinetic basis of partial agonism at NMDA receptors. Nat. Neurosci. 2009;12(9):1114–1120. doi: 10.1038/nn.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J. Neurosci. 1994;14(5):3180–3194. doi: 10.1523/JNEUROSCI.14-05-03180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luvisetto S, Fellin T, Spagnolo M, Hivert B, Brust PF, Harpold MM, Stauderman KA, Williams ME, Pietrobon D. Modal gating of human Ca(v)2.1 (P/Q-type) calcium channels: I. The slow and the fast gating modes and their modulation by beta subunits. J. Gen. Physiol. 2004;124(5):445–461. doi: 10.1085/jgp.200409034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion NV. Calcineurin regulates M channel modal gating in sympathetic neurons. Neuron. 1996;16(1):163–173. doi: 10.1016/s0896-6273(00)80033-1. [DOI] [PubMed] [Google Scholar]
- Meir A, Bell DC, Stephens GJ, Page KM, Dolphin AC. Calcium channel beta subunit promotes voltage-dependent modulation of alpha 1B by G beta gamma. Biophys. J. 2000;79(2):731–746. doi: 10.1016/S0006-3495(00)76331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Mullner C, Yakubovich D, Dessauer CW, Platzer D, Schreibmayer W. Single channel analysis of the regulation of GIRK1/GIRK4 channels by protein phosphorylation. Biophys. J. 2003;84(2):1399–1409. doi: 10.1016/S0006-3495(03)74954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo D, Brehm P. Modal shifts in acetylecholine receptor channel gating confer subunit-dependent desensitization. Science. 1993;260(5115):1811–1814. doi: 10.1126/science.8511590. [DOI] [PubMed] [Google Scholar]
- Ochi R, Kawashima Y. Modulation of slow gating process of calcium channels by isoprenaline in guinea-pig ventricular cells. J. Physiol.-London. 1990;424:187–204. doi: 10.1113/jphysiol.1990.sp018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BZ, DeMaria CD, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of 1-type calcium channels. Neuron. 1999;22(3):549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- Poon K, Nowak LM, Oswald RE. Characterizing single-channel behavior of GluA3 receptors. Biophys. J. 2010;99(5):1437–1446. doi: 10.1016/j.bpj.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon K, Ahmed AH, Nowak LM, Oswald RE. Mechanisms of modal activation of GluA3 receptors. Mol. Pharmacol. 2011;80(1):49–59. doi: 10.1124/mol.111.071688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu G, Auerbach A. Modal gating of NMDA receptors and the shape of their synaptic response. Nat. Neurosci. 2003;6(5):476–483. doi: 10.1038/nn1044. [DOI] [PubMed] [Google Scholar]
- Popescu G, Robert A, Howe JR, Auerbach A. Reaction mechanism determines NMDA receptor response to repetitive stimulation. Nature. 2004;430(7001):790–793. doi: 10.1038/nature02775. [DOI] [PubMed] [Google Scholar]
- Popescu GK. Modes of glutamate receptor gating. J. Physiol.-London. 2012;590(1):73–91. doi: 10.1113/jphysiol.2011.223750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto ML, Wollmuth LP. Gating modes in AMPA receptors. J. Neurosci. 2010;30(12):4449–4459. doi: 10.1523/JNEUROSCI.5613-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian A, Buller AL, Johnson JW. NR2 subunit-dependence of NMDA receptor channel block by external Mg2+. J. Physiol.-London. 2005;562(2):319–331. doi: 10.1113/jphysiol.2004.076737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Auerbach A, Sachs F. Maximum likelihood estimation of aggregated Markov processes. Proc. Royal Soc. B Biol. Sci. 1997;264(1380):375–383. doi: 10.1098/rspb.1997.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Westbrook GL. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993;10(5):805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Rycroft BK, Gibb AJ. Direct effects of calmodulin on NMDA receptor single-channel gating in rat hippocampal granule cells. J. Neurosci. 2002;22(20):8860–8868. doi: 10.1523/JNEUROSCI.22-20-08860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rycroft BK, Gibb AJ. Regulation of single NMDA receptor channel activity by alpha-actinin and calmodulin in rat hippocampal granule cells. J. Physiol.-London. 2004;557(3):795–808. doi: 10.1113/jphysiol.2003.059212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Lahat D, Dascal N, Lotan I. Modal behavior of the Kv1.1 channel conferred by the Kv beta 1.1 subunit and its regulation by dephosphorylation of Kv1.1. Pflugers Archiv.-European J. Physiol. 1999;439(1–2):18–26. doi: 10.1007/s004249900139. [DOI] [PubMed] [Google Scholar]
- Smith MA, Ashford MLJ. Mode switching characterizes the activity of large conductance potassium channels recorded from rat cortical fused nerve terminals. J. Physiol.-London. 1998;513(3):733–747. doi: 10.1111/j.1469-7793.1998.733ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert DG, Testa CM, Young A, Penney JB., Jr. Organization of N-methyl-D-aspartate glutamate receptor gene expression in the basal ganglia of the rat. J. Comp. Neurol. 1994;343:1–16. doi: 10.1002/cne.903430102. [DOI] [PubMed] [Google Scholar]
- Standaert DG, Bernhard Landwehrmeyer G, Kerner JA, Penney JB, Young AB. Expression of NMDAR2D glutamate receptor subunit mRNA in neurochemically identified interneurons in the rat neostriatum, neocortex and hippocampus. Mol. Brain Res. 1996;42(1):89–102. doi: 10.1016/s0169-328x(96)00117-9. [DOI] [PubMed] [Google Scholar]
- Standaert DG, Friberg IK, Landwehrmeyer GB, Young AB, Penney JB. Expression of NMDA glutamate receptor subunit mRNAs in neurochemically identified projection and interneurons in the striatum of the rat. Mol. Brain Res. 1999;64(1):11–23. doi: 10.1016/s0169-328x(98)00293-9. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance KM, Simorowski N, Traynelis SF, Furukawa H. Ligand-specific deactivation time course of GluN1/GluN2D NMDA receptors. Nat. Commun. 2011;2:11. doi: 10.1038/ncomms1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance KM, Hansen KB, Traynelis SF. GluN1 splice variant control of GluN1/GluN2D NMDA receptors. J. Physiol.-London. 2012;590(16):3857–3875. doi: 10.1113/jphysiol.2012.234062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J. Neurophysiol. 1998;79(2):555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Wakamori M, Mikala G, Mori Y. Auxiliary subunits operate as a molecular switch in determining gating behaviour of the unitary N-type Ca2+ channel current in Xenopus oocytes. J. Physiol.-London. 1999;517(3):659–672. doi: 10.1111/j.1469-7793.1999.0659s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, Villa M, Mohler H. Developmental and regional expression of NMDA receptor subtypes containing the NR2D subunit in rat brain. J. Neurochem. 1996;66(3):1240–1248. doi: 10.1046/j.1471-4159.1996.66031240.x. [DOI] [PubMed] [Google Scholar]
- Wyllie DJA, Behe P, Nassar M, Schoepfer R, Colquhoun D. Single-channel currents from recombinant NMDA NR1a/NR2D receptors expressed in Xenopus oocytes. Proc. Royal Soc. B Biol. Sci. 1996;263(1373):1079–1086. doi: 10.1098/rspb.1996.0159. [DOI] [PubMed] [Google Scholar]
- Wyllie DJA, Behe P, Colquhoun D. Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. J. Physiol. 1998;510(1):1–18. doi: 10.1111/j.1469-7793.1998.001bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Hansen KB, Vance KM, Ogden KK, Traynelis SF. Control of NMDA receptor function by the NR2 subunit amino-terminal domain. J. Neurosci. 2009;29(39):12045–12058. doi: 10.1523/JNEUROSCI.1365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue DT, Backx PH, Imredy JP. Calcium-sensitive inactivation in the gating of single calcium channels. Science. 1990;250(4988):1735–1738. doi: 10.1126/science.2176745. [DOI] [PubMed] [Google Scholar]
- Zhang S, Ehlers MD, Bernhardt JP, Su CT, Huganir RL. Calmodulin mediates calcium-dependent inactivation of N-methyl-D-aspartate receptors. Neuron. 1998;21(2):443–453. doi: 10.1016/s0896-6273(00)80553-x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Howe JR, Popescu GK. Distinct gating modes determine the biphasic relaxation of NMDA receptor currents. Nat. Neurosci. 2008;11(12):1373–1375. doi: 10.1038/nn.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]