Abstract
We report here on a feasibility study of initiating buprenorphine/naloxone prior to release from incarceration and linking participants to community treatment providers upon release. Study consisted of a small number of Rhode Island (RI) prisoners (N=44) diagnosed with opioid dependence. The study design is a single arm, open-label pilot study with a 6-month follow up interview conducted in the community. However, a natural experiment arose during the study comparing pre-release initiation of buprenorphone/naloxone to initiation post-release. Time to post-release prescriber appointment (mean days) for initiation of treatment Outside Rhode Island Department of Corrections (RIDOC) vs. Inside RIDOC was 8.8 and 3.9, respectively (p=.1). Median post release treatment duration (weeks) for Outside RIDOC vs. Inside RIDOC was 9 and 24, respectively (p=.007). We conclude that initiating buprenorphine/naloxone prior to release from incarceration may increase engagement and retention in community-based treatment.
Introduction
Among the 2.3 million individuals currently incarcerated in the United States (Sabol, West, & Cooper, 2010), an estimated 200,000 have current opiate dependence (Rich et al., 2005). Those who might benefit from opiate replacement therapy (ORT) most often do not receive appropriate care (Nunn et al., 2009). As a result, many return to opioid use after their release (Baker, Kochan, Dixon, Wodak, & Heather, 1995; Binswanger et al., 2007) and are at increased risk for infectious disease(Hammett, 2006; Inciardi & Needle, 1998), overdose (Binswanger et al., 2007) and reincarceration (Binswanger et al., 2007; Gore, Bird, & Ross, 1995; Lipton, Falkin, & Wexler, 1992). Provision of ORT during incarceration or linkage to ORT immediately post release is rare in correctional facilities currently (Nunn et al., 2009; Rich et al., 2005), despite evidence that ORT is effective in reducing morbidity and mortality associated with opiate dependence (Fiellin, Rosenheck, & Kosten, 2001). Buprenorphine made available as ORT has been shown to decrease drug use, criminal activity, recidivism and HIV risk behavior in a variety of settings (Auriacombe, Franques, & Tignol, 2001; Carrieri et al., 2006; Fhima, Henrion, Lowenstein, & Charpak, 2001).
Although methadone maintenance therapy (MMT) remains the most prevalent form of ORT in the U.S. Criminal Justice System (Nunn et al., 2009), buprenorphine has shown similar effectiveness as methadone in suppressing opioid use (Johnson, Strain, & Amass, 2003; Mattick, et al 2008). A recent study in a New York correctional facility reported that while methadone and buprenorphine completion rates were equivalent during treatment, buprenorphine patients were more likely to continue treatment after release than methadone patients (Magura et al., 2009). Another New York study found that patients released from jail had comparable retention and opioid abstinence rates as patients without recent incarceration in a primary care buprenorphine treatment setting (Lee et al., 2012). Reported patient ambivalence to MMT, especially in correctional settings (Zaller, Bazazi, Velazquez, & Rich, 2009), is consistent with demonstrated preference for buprenorphine over MMT because of its few side effects, alleviation of cravings, and overall patient preference (Dasgupta et al., 2010). Additionally, buprenorphine has less associated stigma and lower risk of overdose than methadone (Dasgupta et al, 2010; Awgu, Magura, & Rosenblum, 2010). Diversion and illicit use of buprenorphine is of growing concern (Alho, Sinclair, Vuori, & Holopainen, 2007; Auriacombe, Fatseas, Dubernet, Daulouede, & Tignol, 2004; Schuman-Olivier et al., 2010; Yokell, Zaller, Green, & Rich, 2011), but initial reports indicate that much of diverted use is for self-management of withdrawal (Bazazi, Yokell, Fu, Rich, & Zaller, 2011; Johanson, Arfken, di Menza, & Schuster, 2012).
While some research has reported favorably on the use of buprenorphine/naloxone in correctional facilities, especially in its comparison to methadone (Magura et al., 2009), the link between incarceration intervention and post-release care, including effects on recidivism, overdose, relapse and crime rate, is not well understood. We report here on a feasibility study of initiating buprenorphine/naloxone prior to release from incarceration and linking participants to community treatment providers upon release. The study was supported through a supplement to a NIDA funded randomized controlled trial parent study assessing MMT initiation during incarceration and linkage to MMT upon release (R01 DA018641).
Methods
The study population was composed of 44 male and female prisoners with a DSM-IV diagnosis of opioid dependence. The planned study design was a single arm, open-label pilot study with a 6-month follow up interview conducted in the community. Buprenorphine-naloxone (Suboxone) was donated by the manufacturer (Reckitt-Benkiser Pharmaceuticals Inc.) to all study participants who received medication; both during incarceration and up to six months in the community post release. The Institutional Review Board of the Miriam Hospital, the federal OHRP and the Medical Research Advisory Group of the RIDOC approved all study procedures and revisions to protocol.
The setting for recruitment was the Rhode Island Department of Corrections (RIDOC). RIDOC manages a unified, centralized and comprehensive state correctional system that encompasses a jail/prison and rehabilitative services, including community corrections (probation/parole). Rhode Island has no county jails and just two intake service centers, one for men and one for women. The prison facilities of the RIDOC and all correctional divisions are centralized on a single campus in Cranston, Rhode Island. Approximately 20% of commitments at the RIDOC are for drug offenses (RIDOC, 2011).
Recruitment occurred primarily through referral from discharge planning or counseling RIDOC staff. In addition, study staff held weekly informational meetings in the different facilities that house sentenced inmates. These group meetings provided information about buprenorphine treatment in general and about the study in specific and allowed self-referral among the inmates. Potential participants were screened for eligibility based on DSM-IV criteria for opioid dependence by study staff. Diagnosis was confirmed independently through clinical assessment by the prescribing physician.
During the first several months of recruitment we were still negotiating details with the RIDOC for initiating buprenorphine/naloxone treatment during incarceration. Therefore, a natural experiment arose in which participants recruited during the first four months (Outside RIDOC Group) were screened, consented and administered a baseline assessment approximately 30 days prior to release from incarceration, but were referred to initiate buprenorphine/naloxone treatment after release. The clinic appointment was arranged to occur within approximately three days of release. Transportation to the appointment was provided to participants in order to minimize barriers to treatment initiation.
The last two months of recruitment, participants were able to initiate treatment before release from incarceration (Inside RIDOC Group). Dosing began up to two weeks prior to release. The study physician supervised the initial dose and any adjustments; a research nurse administered subsequent doses on a daily basis. Symptom review checklists administered by the research nurse and the study physician’s assessment informed dosage adjustments. On the day of release from incarceration, patients who initiated buprenorphine prior to release were provided with enough medication to make it to their scheduled follow-up appointment in the community.
Outcomes
The primary outcomes was successful initiation of buprenorphine/naloxone prior to release from incarceration defined as receiving one or more doses of buprenorphine/naloxone and weeks of retention in buprenorphine/naloxone treatment in the community.
The secondary aims were to explore the effect of pre-release buprenorphine/naloxone on: a) time to first doctor visit upon release from incarceration and retention in treatment; b) HIV risk behaviors; c) overdose; d) reincarceration; and e) comparison to methadone treatment participants on similar measures from the parent study of this supplement.
Trained interviewers collected outcome data by self-report during a face-to-face interviews at baseline, 2 weeks, 6 and 12 months post release from incarceration. Only data from baseline and 6 months follow up interviews are included in the analyses presented here. Buprenorphine/naloxone data were also abstracted from clinical charts. The interview instrument included the Addiction Severity Index(McLellan, Kushner, et al., 1992), variables from the Risk Behavior Assessment(Branch), 1993; Risk Behavior Assessment, 1993), and Treatment Services Review(McLellan, Alterman, Cacciola, Metzger, & O'Brien, 1992).
Analysis
Descriptive statistics were calculated to characterize the overall study population. Comparisons between the outcomes of those who initiated buprenorphine within and outside the RIDOC as well as outcomes for the full sample at follow up were conducted using Chi-square or Fisher’s exact tests for categorical data and t-tests or Mann Whitney U tests for continuous data.
Results
Of the 44 participants, 32 (73%) were referred to buprenorphine/naloxone treatment post-release (Outside RIDOC); 12 (27%) started buprenorphine/naloxone while incarcerated (Inside RIDOC). The two groups were similar in drug use and other socio-demographics at baseline (Table 1). Of study participants, 82 percent (36/44) completed the 6 month follow-up interview (Table 2).
Table 1.
Characteristics of the buprenorphine/naloxone pilot sample*
| Variable | Site of buprenorphine/naloxone initiation |
Total | ||
|---|---|---|---|---|
| Outside RIDOC N (%) 32 (73) |
Inside RIDOC N (%) 12 (27) |
N (%) 44 total |
||
| Mean age (SD) | 36.7 (7.1) | 38.7 (7.7) | 37.3 (7.3) | |
| Male | 26 (78.8) | 11 (91.6) | 37 (84.1) | |
| Female | 6 (18.7) | 1 (8.3) | 7 (15.9) | |
| Race/Ethnicity | ||||
| Hispanic/Latino | 10 (31.2) | 3 (25) | 13 (29.5) | |
| Black/African American | 0 | 1 (8.3) | 1 (2.2) | |
| White | 22 (68.8) | 8 (66.7) | 30 (68.1) | |
| Other | 0 | 0 | 0 | |
| Marital status | ||||
| Married | 6 (18.8) | 1 (8.3) | 7 (15.9) | |
| Separated | 1 (3.1) | 1 (8.3) | 2 (4.6) | |
| Divorced | 3 (9.3) | 2 (16.7) | 5 (11.4) | |
| Never Married | 22 (68.8) | 8 (66.7) | 30 (68.2) | |
| Mean (SD) years use | ||||
| Heroin | 8.3 (5.7) | 11.5 (10.3) | 9.1 (7.1) | |
| Other opiates | 5.7 (6.0) | 7.6 (10.0) | 6.2 (7.2) | |
| Ever overdosed | 7 (21.9) | 5 (41.7) | 12 (27.3) | |
| Attended previous methadone maintenance program |
13 (40.6) | 6 (50) | 19 (43.2) | |
| Mean (SD) months ever incarcerated |
50.7 (37.5) | 45.8 (35.6) | 49.3 (36.7) | |
No statistically significant differences between groups
RIDOC=Rhode Department of Corrections, Cranston, Rhode Island
SD=standard deviation
Table 2.
Chart Review - Post Release Clinic Attendance
| Variable | Site of buprenorphine/naloxone initiation |
p-value | |
|---|---|---|---|
| Outside RIDOC N (%) 32(73) |
Inside RIDOC N (%) 12 (27) |
44 total |
|
| Linked to community provider post release |
25 (78) | 11 (92) | 0.30 |
| Time to post release prescriber appointment (days) |
8.8 | 3.9 | 0.10 |
| Remained in tx at 6 months | 11 (34) | 10 (91) | 0.005 |
| Post release average tx duration (weeks) |
13 | 20 | 0.05 |
| Post release median tx duration (weeks) |
9 | 24 | 0.007* |
Wilcoxon-Mann-Whitney rank sum test to compare median values between the two groups.
Outside RIDOC
Of the 32 (73%) participants who were referred to buprenorphine/naloxone post-release, 25 (78%) linked to care in the community, and 7 (22%) attended only the initial appointment. The average number of days to first treatment appointment post release in this group was 9.2. Eleven of the 32 (34%) remained in care for the entire six months for which buprenorphine/naloxone was available through the study. For those participants who initiated and remained in treatment post-release, the mean and median number of weeks of treatment was 13.2 and 9, respectively.
Inside RIDOC
The average length of buprenorphine/naloxone treatment pre-release was approximately one week (6.33 days, with a range of 2–14 days). Eleven of 12 (92%) participants were linked to care (defined as attending at least one appointment with a community based provider) in the community for continued buprenorphine/naloxone treatment. One participant who started buprenorphine/naloxone treatment inside the RIDOC could not tolerate the medication and decided to enter a drug free residential treatment program instead (Rich et al., 2011). The average number of days to first treatment appointment post release was 3.9. Ten participants (83%) remained in care for the entire six months for which buprenorphine/naloxone was available through the study. For those participants who engaged in treatment post release, the mean and median number of weeks of treatment was 20.3 and 24, respectively (Table 2).
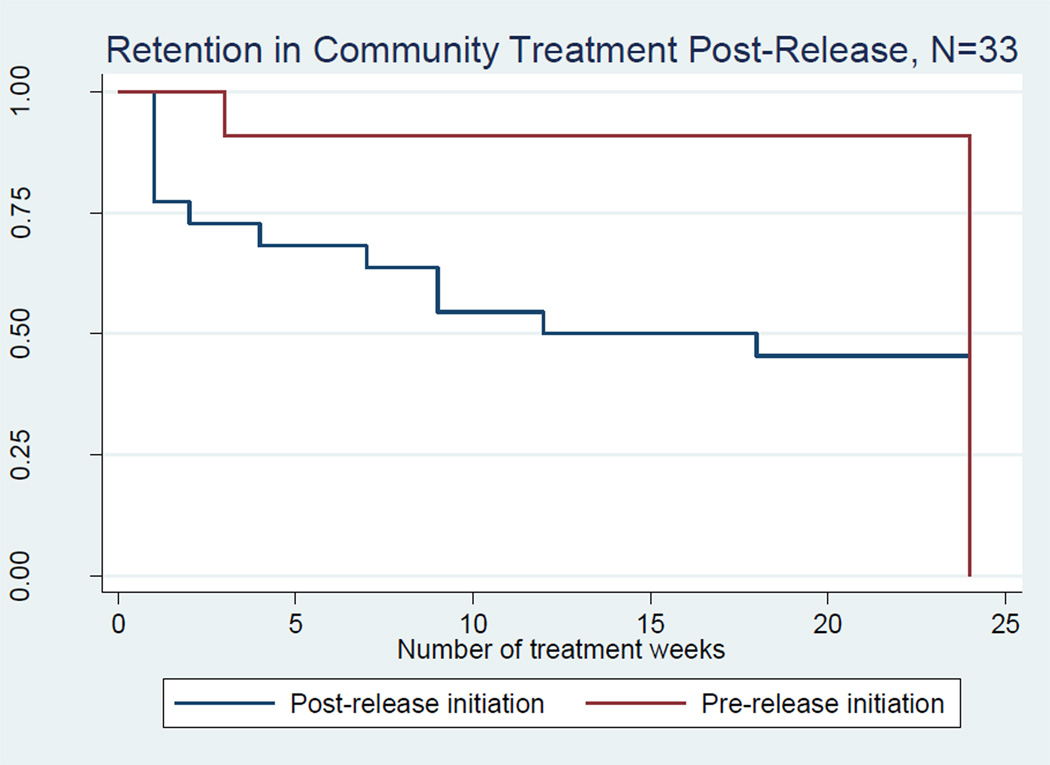
Figure 1 provides a survival curve depicting the number of weeks participants remained in community based treatment post-release. At 24 weeks, 91% of participants who initiated treatment prior to release (n= 10) remained in treatment compared with 34% of participants who initiated treatment post-release (n=11, p= 0.005). The mean time to this first appointment is provided in Table 2 (3.9 days for participants initiated prior to release and 9.2 days for participants initiated post-release).
Figure 1.
*Three participants did not attend any treatment in the community and therefore could not contribute any observation time. These participants were subsequently dropped from the analysis.
Non-fatal overdose and drug use to opiate use
Table 3 reports the overdose and drug use outcomes at the 6-month follow up for participants by treatment start location. Overall, the number of non-fatal overdoses experienced was small (3 of 36, 8.3%) and drug use and injection drug use were low (17% and 19%, respectively, Table 2). Of the 11 individuals who started buprenorphine/naloxone while incarcerated, none reported an overdose during the follow up period compared to 3 (11.5%) of the 25 post-release initiators who reported a non-fatal overdose during the follow-up period. None of the participants who initiated while incarcerated reported use of any opiates or drug injection during the 6 month follow up; 6 (23.1%) of the post-release initiators reported heroin use (P=.08) and 7 (26.9%) reported drug injection (P=.05) during the follow up period.
Table 3.
Post release and six month outcomes
| Variable | Site of buprenorphine/naloxone initiation |
p-value 36 total |
||
|---|---|---|---|---|
| Outside RIDOC N (%) 25(69) |
Inside RIDOC N (%) 11 (30.1) |
|||
| Drug use, past 30 days | ||||
| Heroin | 6 (24) | 0 | 0.08 | |
| Other opioid | 1 (4) | 0 | 0.50 | |
| Crack/Cocaine | 5 (20) | 3 (27) | 0.63 | |
| Other substance use, past 30 days | ||||
| Alcohol | 8 (47) | 1 (10) | 0.14 | |
| Sedatives | 5 (20) | 1 (9) | 0.42 | |
| Prescribed methadone, past 30 days |
1 (4) | 0 | 0.50 | |
| Any drug injecting, past 30 days | 7 (39) | 0 | 0.05 | |
| Arrests, past 30 days | 6 | 0 | 0.08 | |
| Overdose, past 6 months | 3 (12) | 0 | 0.23 | |
Criminal involvement
Overall, self-reported re-arrest during the follow-up period was uncommon (6 of 36, 17%). However, all re-arrests (both past 6 months and past 30 days) occurred in the group that initiated buprenorphine/naloxone post-release (Table 3). Upon further analysis, individuals who were not continuously in treatment during the follow-up period were more likely to report being re-arrested at 6 months (37.5% vs. 0%, p= 0.003, data not shown in tables). Participants who were re-arrested spent a mean of 4 nights in jail.
Discussion
This study found that initiating buprenorphine/naloxone prior to release from incarceration and continuing treatment in the community is feasible. These findings are consistent with previous research (Cropsey et al., 2011; Garcia et al., 2007; Gordon, Kinlock, & Miller, 2011; Magura et al., 2009) reporting on the efficacy and challenges of pre-release buprenorphine/naloxone initiation. Furthermore, initiating buprenorphine/naloxone replacement therapy prior to release increased linkages to treatment post release. Research investigating methadone maintenance treatment (Kinlock, Gordon, Schwartz, & Fitzgerald, 2010; McKenzie et al., 2012) has consistently found that pre-release initiation of ORT increases post release treatment attendance. Trends identified at six months (decreased opiate use, injection drug use and non-fatal overdose among participants who initiated buprenorphine/naloxone prelease) are also consistent with this literature.
Significant findings in the parent study which assessed initiation of methadone maintenance treatment (MMT) to referral to post release MMT were similar to the findings here. Participants who initiated MMT pre-release were significantly more likely to attend clinic post release and in fewer days. At the six month assessment, they were significantly less likely to have used heroin or to have injected in the previous 30 days. In the parent study, we did not find a difference in the number of self reported nonfatal overdoses or in re-arrest rates at the 6 month assessment (re-arrest was higher in the parent study with an average of 29%).
Gordon et al. (2011 report the challenges of initiating opiate replacement therapy in an incarcerated setting. Beginning ORT in a population that has been withdrawn from opiates for long enough to lose their tolerance can be challenging. This challenge is especially true for methadone; Kinlock et al took approximately 3–6 months and in our parent study we took approximately 1 month to initiate MMT during incarceration with few reaching a blocking dose at the time of release. The initiation of methadone prior to release is also challenged by the fact that often release dates change, sometimes at the last minute. Buprenorphine/naloxone induction is easily done in a short time frame (days) and is often more easily performed within correction settings as prisoners generally do not have opiates in their system and therefore are not likely to experience buprenorphine-induced withdrawal symptoms.
Participants who started inside typically started 2 or 4 mg daily and over about a week were titrated up to 8 mg at time of release; titration was based upon toxicity, not withdrawal symptoms. The mean dose of buprenorphine upon discharge was 8 mg. A “stabilization dose” was not achieved prior to release. Most participants had a clinical appointment within a week after release, and the dosage was typically titrated up to alleviate craving or other symptoms, as reported by participants. There was variability in stabilization dose by patient and provider.
We found that community resources were virtually non-existent to maintain uninsured individuals (56% of the entire sample at 6 month follow up) on buprenorphine/naloxone treatment after release from incarceration. Further, few buprenorphine/naloxone treatment providers accepted Medicaid, so even the 14% of participants with Medicaid had limited options. A challenge for Rhode Island and other states is to increase the number of prescribing physicians who accept Medicaid. One method to do this is to engage the community health and community mental health center networks to become certified to prescribe buprenorphine/naloxone and to partner with behavioral health specialists to provide addiction recovery support. Academic medical centers that provide collaborative primary care can also play a role Alford DP, et al, 2011). Community buprenorphine/naloxone providers who accept Medicaid will be particularly important when the Medicaid expansion authorized by the Affordable Care Act is fully implemented since many individuals involved in the criminal justice system will become eligible for Medicaid under this expansion.
Limitations
This study was subject to several limitations. First, the small sample size reduced the power to detect some differences between the groups who initiated treatment inside the DOC compared to outside of the DOC. Also, data on the number of participants who refused to participate were not collected. The small and single-site sample may limit the generalizability of findings, but the consistency of our findings with other pilot studies of buprenorphine/naloxone treatment supports its external validity. In addition, with respect to criminal data, we did not ascertain dates of criminal behaviors so were not able to correlate these with treatment data. Ahe reliance on self-reported data is another limitation, particularly with respect to drug use and criminal history. While self-reports are useful when collecting sensitive information, they are subject to important biases such as recall and/or social desirability bias. Finally, only participant data at six months are reported here since twelve month follow up rates were too low for comparative analyses.
Conclusion
Initiating buprenorphine/naloxone prior to release from incarceration with continued treatment in the community is feasible and may increase treatment retention post-release. Providing buprenorphine/naloxone treatment for prisoners prior to release is a promising strategy to decrease post incarceration HIV risk behaviors, as well as improve linkage to care and reduce drug use, criminal behavior, and recidivism. However, future studies, which include larger numbers of participants who initiate buprenorphine prior to release from correctional settings and longer duration of follow up are needed to better elucidate optimal medication dosing, both inside and outside of correctional settings, as well as factors associated with longer term engagement and retention in treatment.
Acknowledgements
This project was supported by grant numbers: R01DA18641, K24DA022112 and 2U01DA016191 from the National Institute of Drug Abuse (NIDA) as well as P30-AI-42853 from the National Institutes of Health, Center for AIDS Research (NIH/CFAR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alford DP, LaBelle CT, Kretsch N, Bergeron A, Winter M, Botticelli M, Samet JH. Collaborative care of opioid-addicted patients in primary care using buprenorphine. Arch Intern Med. 2011;171(5):425–431. doi: 10.1001/archinternmed.2010.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alho H, Sinclair D, Vuori E, Holopainen A. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88(1):75–78. doi: 10.1016/j.drugalcdep.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Auriacombe M, Fatseas M, Dubernet J, Daulouede JP, Tignol J. French field experience with buprenorphine. Am J Addict. 2004;13(Suppl 1):S17–S28. doi: 10.1080/10550490490440780. [DOI] [PubMed] [Google Scholar]
- Auriacombe M, Franques P, Tignol J. Deaths attributable to methadone vs buprenorphine in France. JAMA. 2001;Vol. 285:45. doi: 10.1001/jama.285.1.45. United States. [DOI] [PubMed] [Google Scholar]
- Awgu E, Magura S, Rosenblum A. Heroin-dependent inmates' experiences with buprenorphine or methadone maintenance. J Psychoactive Drugs. 2010;42(3):339–346. doi: 10.1080/02791072.2010.10400696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A, Kochan N, Dixon J, Wodak A, Heather N. HIV risk-taking behaviour among injecting drug users currently, previously and never enrolled in methadone treatment. Addiction. 1995;90(4):545–554. doi: 10.1046/j.1360-0443.1995.9045458.x. [DOI] [PubMed] [Google Scholar]
- Bazazi AR, Yokell M, Fu JJ, Rich JD, Zaller ND. Illicit use of buprenorphine/naloxone among injecting and noninjecting opioid users. J Addict Med. 2011;5(3):175–180. doi: 10.1097/ADM.0b013e3182034e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenko S, Peugh J. Estimating drug treatment needs among state prison inmates. Drug Alcohol Depend. 2005;77(3):269–281. doi: 10.1016/j.drugalcdep.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, Koepsell TD. Release from prison--a high risk of death for former inmates. N Engl J Med. 2007;356(2):157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrieri MP, Amass L, Lucas GM, Vlahov D, Wodak A, Woody GE. Buprenorphine use: the international experience. Clin Infect Dis. 2006;43(Suppl 4):S197–S215. doi: 10.1086/508184. [DOI] [PubMed] [Google Scholar]
- Cropsey KL, Lane PS, Hale GJ, Jackson DO, Clark CB, Ingersoll KS, Stitzer ML. Results of a pilot randomized controlled trial of buprenorphine for opioid dependent women in the criminal justice system. Drug Alcohol Depend. 2011;119(3):172–178. doi: 10.1016/j.drugalcdep.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta N, Bailey EJ, Cicero T, Inciardi J, Parrino M, Rosenblum A, Dart RC. Post-marketing surveillance of methadone and buprenorphine in the United States. Pain Med. 2010;11(7):1078–1091. doi: 10.1111/j.1526-4637.2010.00877.x. [DOI] [PubMed] [Google Scholar]
- Elkader A, Sproule B. Buprenorphine: clinical pharmacokinetics in the treatment of opioid dependence. Clin Pharmacokinet. 2005;44(7):661–680. doi: 10.2165/00003088-200544070-00001. [DOI] [PubMed] [Google Scholar]
- Fhima A, Henrion R, Lowenstein W, Charpak Y. [Two-year follow-up of an opioid-user cohort treated with high-dose buprenorphine (Subutex)] Ann Med Interne (Paris) 2001;152(Suppl 3):IS26–I36. [PubMed] [Google Scholar]
- Fiellin DA, Rosenheck RA, Kosten TR. Office-based treatment for opioid dependence: reaching new patient populations. Am J Psychiatry. 2001;158(8):1200–1204. doi: 10.1176/appi.ajp.158.8.1200. [DOI] [PubMed] [Google Scholar]
- Garcia CA, Correa GC, Viver AD, Kinlock TW, Gordon MS, Avila CA, Schwartz RP. Buprenorphine-naloxone Treatment for Pre-release Opioid-dependent Inmates in Puerto Rico. J Addict Med. 2007;1(3):126–132. doi: 10.1097/ADM.0b013e31814b8880. [DOI] [PubMed] [Google Scholar]
- Gordon MS, Kinlock TW, Miller PM. Medication-assisted treatment research with criminal justice populations: challenges of implementation. Behav Sci Law. 2011;29(6):829–845. doi: 10.1002/bsl.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore SM, Bird AG, Ross AJ. Prison rites: starting to inject inside. BMJ. 1995;311(7013):1135–1136. doi: 10.1136/bmj.311.7013.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammett TM. HIV/AIDS and other infectious diseases among correctional inmates: transmission, burden, and an appropriate response. Am J Public Health. 2006;Vol. 96:974–978. doi: 10.2105/AJPH.2005.066993. United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DS, Jones RT, Welm S, Upton RA, Lin E, Mendelson J. Buprenorphine and naloxone co-administration in opiate-dependent patients stabilized on sublingual buprenorphine. Drug Alcohol Depend. 2000;61(1):85–94. doi: 10.1016/s0376-8716(00)00126-5. [DOI] [PubMed] [Google Scholar]
- Inciardi JA, Needle RH. Editors' introduction: HIV/AIDS interventions for out-of treatment drug users. J Psychoactive Drugs. 1998;30(3):225–229. doi: 10.1080/02791072.1998.10399696. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Arfken CL, di Menza S, Schuster CR. Diversion and abuse of buprenorphine: Findings from national surveys of treatment patients and physicians. Drug Alcohol Depend. 2012;120(1–3):190–195. doi: 10.1016/j.drugalcdep.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(2 Suppl):S59–S77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT. Developing and Implementing a New Prison-Based Buprenorphine Treatment Program. J Offender Rehabil. 2010;49(2):91–109. doi: 10.1080/10509670903534951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Grossman E, Truncali A, Rotrosen J, Rosenblum A, Magura S, Gourevitch MN. Buprenorphine-naloxone maintenance following release from jail. Subst Abus. 2012;33(1):40–47. doi: 10.1080/08897077.2011.620475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton DS, Falkin GP, Wexler HK. Correctional drug abuse treatment in the United States: an overview. NIDA Res Monogr. 1992;118:8–30. [PubMed] [Google Scholar]
- Magura S, Lee JD, Hershberger J, Joseph H, Marsch L, Shropshire C, Rosenblum A. Buprenorphine and methadone maintenance in jail and post-release: a randomized clinical trial. Drug Alcohol Depend. 2009;99(1–3):222–230. doi: 10.1016/j.drugalcdep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews. 2008;(2):CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- McKenzie M, Zaller N, Dickman SL, Green TC, Parihk A, Friedmann PD, Rich JD. A randomized trial of methadone initiation prior to release from incarceration. Subst Abus. 2012;33(1):19–29. doi: 10.1080/08897077.2011.609446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Alterman AI, Cacciola J, Metzger D, O'Brien CP. A new measure of substance abuse treatment. Initial studies of the treatment services review. J Nerv Ment Dis. 1992;180(2):101–110. doi: 10.1097/00005053-199202000-00007. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Jones RT. Clinical and pharmacological evaluation of buprenorphine and naloxone combinations: why the 4:1 ratio for treatment? Drug Alcohol Depend. 2003;70(2 Suppl):S29–37. doi: 10.1016/s0376-8716(03)00057-7. [DOI] [PubMed] [Google Scholar]
- Nunn A, Zaller N, Dickman S, Trimbur C, Nijhawan A, Rich JD. Methadone and buprenorphine prescribing and referral practices in US prison systems: results from a nationwide survey. Drug Alcohol Depend. 2009;105(1–2):83–88. doi: 10.1016/j.drugalcdep.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode Island Department of Corrections. Population Report FY2011: State of Rhode Island. 2011. [Google Scholar]
- Rich JD, Boutwell AE, Shield DC, Key RG, McKenzie M, Clarke JG, Friedmann PD. Attitudes and practices regarding the use of methadone in US state and federal prisons. J Urban Health. 2005;82(3):411–419. doi: 10.1093/jurban/jti072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich JD, McKenzie M, Dickman S, Bratberg J, Lee JD, Schwartz RP. An Adverse Reaction to Buprenorphine/Naloxone Induction in Prison: A Case Report. Addict Disord Their Treat. 2011;10(4):199–200. doi: 10.1097/ADT.0b013e3182133949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risk Behavior Assessment. Rockville, MD: National Institute on Drug Abuse (Community Research Branch); 1993. [Google Scholar]
- Sabol, William, West, Heather, Cooper, Matthew . Prisoners in 2008. Bureau of Justice Statistics: U.S. Department of Justice; 2010. [Google Scholar]
- Schuman-Olivier Z, Albanese M, Nelson SE, Roland L, Puopolo F, Klinker L, Shaffer HJ. Self-treatment: illicit buprenorphine use by opioid-dependent treatment seekers. J Subst Abuse Treat. 2010;39(1):41–50. doi: 10.1016/j.jsat.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Taxman FS, Perdoni ML, Harrison LD. Drug treatment services for adult offenders: the state of the state. J Subst Abuse Treat. 2007;32(3):239–254. doi: 10.1016/j.jsat.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55(5):569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- Yokell MA, Zaller ND, Green TC, Rich JD. Buprenorphine and buprenorphine/naloxone diversion, misuse, and illicit use: an international review. Curr Drug Abuse Rev. 2011;4(1):28–41. doi: 10.2174/1874473711104010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaller ND, Bazazi AR, Velazquez L, Rich JD. Attitudes toward methadone among out-of-treatment minority injection drug users: implications for health disparities. Int J Environ Res Public Health. 2009;6(2):787–797. doi: 10.3390/ijerph6020787. [DOI] [PMC free article] [PubMed] [Google Scholar]