Abstract
Background
Chronic selective serotonin reuptake inhibitor (SSRI) administration to rodents desensitizes or downregulates raphe 5-HT1A autoreceptors. We previously found elevated 5-HT1A binding in antidepressant-naïve and not recently-medicated major depressive disorder, and now report the effect of SSRI treatment on 5-HT1A autoreceptors in depressed patients.
Methods
5-HT1A binding (BPF) was quantified in medication-free subjects using PET with [11C]-WAY-100635 before and after treatment of major depressive disorder (MDD) with an SSRI for 5 to 9 weeks (mean 47±8 days). 19 subjects without recent history of antidepressant pharmacotherapy completed both [11C]WAY-100635 PET scans with a metabolite-corrected arterial input function and depression severity was rated before and after the treatment course.
Results
5-HT1A autoreceptor BPF in the raphe was reduced 18% on SSRI treatment (df=1,18; F=5.12; p=0.036). However, the degree of reduction in 5-HT1A autoreceptor BPF was unrelated to improvement in depression (df=1,16; F=1.27; p=0.276).
Conclusion
Downregulation of 5-HT1A autoreceptor binding by SSRI treatment of major depression is consistent with animal studies. This may be a necessary but insufficient requirement for clinical response to SSRIs. A PET agonist ligand that binds selectively to the high affinity conformation of this receptor can determine whether SSRIs also cause desensitization of the autoreceptor as reported by some rodent studies, and whether that effect may be related to clinical response.
Keywords: depression, major depressive disorder, major depressive episode, kinetic modeling, compartmental models, bootstrap
INTRODUCTION
Serotonin plays an important role in the pathophysiology and treatment of major depression (1, 2). The three major classes of antidepressants, monoamine oxidase inhibitors, tricyclic antidepressants, and selective serotonin reuptake inhibitors (MAOI, TCA, and SSRI, respectively), all inhibit synaptic clearance or metabolism of serotonin (5-hydroxytryptamine, 5-HT). However, while maximal inhibition of serotonin uptake (or monoamine oxidase activity) occurs in hours, therapeutic response takes several weeks. This therapeutic lag indicates that there are slower, adaptive processes that result in the improvement of mood. In contrast, tryptophan depletion studies have demonstrated that acutely reducing serotonin availability can induce the reemergence of depressive symptoms in hours in patients remitted on SSRI treatment (3). These data suggest a model in which adaptive changes induced by chronic treatment and maintenance of adequate serotonin availability are both required for ongoing antidepressant effect.
The 5-HT1A receptor is both an autoreceptor located somatodendritically on serotonin neurons in the raphé nuclei, and located on target neurons throughout much of the brain. There is regional variation in its signaling mechanisms, but it is generally coupled to Gi and Go proteins associated with inhibition of adenylate cyclase, opening of potassium channels, and inhibition of voltage-dependent calcium channels (4). Somatodendritic 5-HT1A autoreceptors respond to locally-released serotonin by inhibiting the firing of serotonergic neurons, thereby regulating the release of serotonin throughout the brain. It has been observed in animal studies that the firing rate of serotonergic neurons is reduced when SSRI treatment is first initiated, and then slowly recovers over weeks, corresponding with the time-course of autoreceptor desensitization (5, 6). Since postsynaptic 5-HT1A sites in the terminal fields do not desensitize or downregulate (6, 7), the net effect enhances serotonergic transmission. SSRIs work by inhibiting serotonin reuptake and this mechanism of signal amplification is dependent on serotonin release after serotonin neuron firing. Attenuated firing will undermine the signal-enhancing effect of SSRIs, since less serotonin is being released in the first place. The 5-HT1A autoreceptor desensitization model of antidepressant action postulates that chronic antidepressant treatment reduces this autoinhibitory feedback within the raphé nucleus, by desensitization/downregulation of autoreceptor, more firing and more serotonin release and increase in postsynaptic serotonergic signaling over time (5).
Indirect evidence indicates downregulation/desensitization of the 5-HT1A receptor in human subjects receiving SSRI treatment. Acute 5-HT1A receptor agonist challenge in man produces transient hypothermia, and an increase in plasma levels of adrenocorticotropic hormone (ACTH), cortisol, and prolactin. Following chronic antidepressant use, these responses are blunted in control subjects (8), depressed patients (9, 10), and in obsessive-compulsive patients (11). In depressed subjects, treatment response correlates with blunting of the hypothermic response to the 5-HT1A agonist ipsapirone (12) and to a blunted effect of buspirone on ACTH and cortisol levels (10).
Relatedly, we have shown that subjects who have never been exposed to antidepressants, or not exposed for at least 4 years, have higher 5-HT1A BPF compared to currently antidepressant-free subjects who have recently been treated with antidepressants, suggesting that these receptors are downregulated and may take months or years off medication to revert to higher levels again (13, 14). Because the PET radioligand, WAY-100635, used in these studies binds equally to low- and high-agonist affinity receptor conformations, these findings suggest that downregulation of 5-HT1A receptors may be a therapeutically relevant effect of antidepressant treatment, independent but perhaps complementary to autoreceptor desensitization.
We have found higher autoreceptor (and terminal field) 5-HT1A receptor binding in two different cohorts comprising antidepressant naïve and not medicated within 4-years MDD subjects, and in a third group of medication-free MDD between episodes of major depression (13–15). Based on animal models and antidepressant exposure data in our previously scanned patients, we hypothesized that SSRI treatment would reduce raphe 5-HT1A autoreceptor binding in MDD. 5-HT1A binding was quantified using positron emission tomography and the radioligand [11C]-WAY-100635 before and after an average 7 weeks treatment of DSM-IV diagnosed MDD with an SSRI.
METHODS AND MATERIALS
Subjects
Twenty-three depressed subjects enrolled in our previously reported studies (13, 15) were recruited to have a second scan following SSRI treatment. Criteria for inclusion were: a diagnosis of MDD based on the Structured Clinical Interview for DSM-IV (SCID I), no current psychiatric medication, and age >18 yrs. One subject was subsequently found to have Bipolar Disorder II. To eliminate potential long-lasting effects of SSRI use on binding of WAY-100635 (14), subjects (N=4) with a history of treatment in the prior four years were excluded from further analysis. We found previously that antidepressant treatment might have relatively long-term effects on 5-HT1A binding (13, 24); hence, any effect of antidepressant treatment in the current study might be obscured by a ‘floor’ effect, in that resuming SSRI treatment would not be expected to further suppress 5-HT1A binding. The excluded four subjects with a history of antidepressant use in the prior four years had baseline raphé 5-HT1A binding a trend-level lower relative to the not recently medicated subjects (BPF 23.4 vs 29.9; df=1,21; F=3.43; p=0.078). Exclusion criteria included current or past diagnosis of schizophrenia, schizoaffective disorder, anorexia nervosa, bulimia nervosa, or drug or alcohol dependence; substance abuse within the past 2 months; any lifetime history of IV drug use, or ecstasy use more than twice; first-degree relative with schizophrenia (for subjects under 33 yrs); significant medical comorbidity; lack of capacity to provide informed consent; suicidal ideation; ECT within the past 6 months; other radiation exposure (e.g., occupational exposure, multiple diagnostic X-rays in previous year, prior nuclear medicine studies); pacemaker or metallic implant/foreign object; head injury resulting in prolonged loss of consciousness; current lactation or recent abortion, miscarriage or pregnancy (<2 months); and current or planned pregnancy. Anxiety disorders were not an exclusion criteria; lifetime co-morbidities in our sample included PTSD (n=3), panic disorder (n=4), social phobia (n=2), specific phobia (n=1), dysthymia (n=3), and ADHD (n=1).
All subjects were free of psychotropic medication for a minimum of two months and serotonergic antidepressants for 4 years prior to the first PET scan, with the exception of one subject who had been on bupropion until two weeks prior to pretreatment scanning. Bupropion has minimal affinity for the 5-HT1A receptor; and excluding this subject did not significantly alter findings. Following completion of the baseline Hamilton Depression Rating Scale (HDRS) and [11C]WAY-100635 scan, subjects were placed on paroxetine (n = 12, 20 – 50 mg daily), citalopram (n = 6, 20 – 40 mg daily), or escitalopram (n = 1, 10 mg daily), under the care of a research psychiatrist. Although the protocol permitted small doses of short-acting benzodiazepines up to 72 hours before PET scans, none of the subjects required benzodiazepines. No other psychotropic medication was permitted during the course of the study. Subjects completed a second [11C]WAY-100635 PET scan and HDRS following a mean of 47 days treatment (range: 34 – 63), and were given the option to continue outpatient treatment for six months.
All subjects provided written, informed consent following a description of the study protocol and associated risks. The protocol was approved by the Institutional Review Board of the New York State Psychiatric Institute.
Radiochemistry and Input Function Measurement
[11C]WAY-100635 (N-(2-4-2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinal) cyclohexane carboxamide) was prepared as previously described (17). Specific activity, injected mass, and injected dose did not differ between pre-treatment and post-treatment scans (table 1). Arterial blood samples were collected from a radial artery catheter and corrected for metabolites as previously described (17). Plasma free fraction (fp) was determined in triplicate as previously described (15).
Table 1.
Demographics and radiochemistry.
| Subjects (n = 19) | |||
|---|---|---|---|
| Male N (%) | 8 (42%) | ||
| Age (±SD) | 41.8 (±13.7) | ||
| Paroxetine N (%) | 12 (63%) | ||
| Citalopram* N (%) | 7 (37%) | ||
| Age of Onset, Years (±SD) | 27.1 (±13.3) | ||
| Number of Episodes (±SD) | 20.4 (±37.1) | ||
| Length of Current Episode, Weeks (±SD) | 44.2 (±53.3) | ||
| Genotype C-1019G | CC=1, CG=11, GG=7 | ||
| Pre-Treatment | Post-Treatment | P = | |
| HDRS-24 (±SD, n=18**) | 23.5(±7.8) | 11.2 (±7.8) | <0.001 |
| Injected Dose, mCi (±SD) | 7.07 (±3.15) | 7.37 (±4.05) | 0.69 |
| Injected Mass, μ g (±SD) | 1.78 (±1.25) | 2.57 (±2.24) | 0.11 |
| Specific Activity, mCi/nmol | 2.28 (±1.27) | 1.77 (±0.98) | 0.08 |
| Free Fraction (±SD) | 0.0632 (±0.0197) | 0.0667 (±0.0159) | 0.54 |
Means ± standard deviation.
One subject received escitalopram.
One subject did not receive a second Hamilton rating.
Image Acquisition and Analysis
Structural T1-weighted MRI images were acquired using either a 1.5T Signa Advantage or a 3T Signa HDx system (General Electric Medical Systems, Milwaukee, WI), at a resolution of 1.5×0.9×0.9mm or 1.0mm isotropic, respectively. PET data were acquired using an ECAT EXACT HR+ scanner (Siemens/CTI, Knoxville, TN) as previously described (17). Briefly, a ten minute transmission scan was acquired, followed by the bolus injection over 45 seconds of [11C]WAY-100635 and a 110 minute emission scan consisting of twenty frames of increasing duration (3×20s, 3×1min, 3×2min, 2×5 min, 9×10 min).
Image analysis was conducted using custom routines created for MATLAB 2006b (The Mathworks, Natick, MA) and functions from the FMRIB Software Library (FSL; FMRIB, Oxford, UK) and Statistical Parametric Mapping (SPM5; University College London, UK). Parameters for motion correction of PET scans were determined by applying FMRIB’s Linear Image Registration Tool (FLIRT) to denoised data. Briefly, each frame was successively registered to the mean of already-registered frames, beginning with the eighth frame. Movies of sagittal, axial, and coronal slices were then manually inspected for residual motion. Coregistration to MRI, also using FLIRT, involved performing multiple registrations using various cost functions and target images (e.g., T1, probabilistic gray matter map from SPM, etc), and the best fit was selected (18). Finally, the concatenated motion correction and coregistration transforms were applied to each frame of the scan, moving the PET data into MRI space.
All regions of interest (ROIs) except the raphé were hand drawn by a single technician on each subject’s MRI by a trained technician relying on standard human brain atlases (19, 20). The ROIs used were orbital prefrontal cortex (OPFC), medial PFC (MPFC), dorsolateral PFC (DLPFC), anterior cingulate (ACC), cingulate cortex (CIN), amygdala (AMY), hippocampus (HIP), parahippocampal gyrus (PIP), insular cortex (INS), temporal cortex (TEM), parietal cortex (PAR), and occipital cortex (OCC). Cortical ROIs were further masked using the probabilistic gray matter map produced by SPM’s MRI segmentation procedure. Where applicable, data for each ROI represent the average of the left and right sides. A cylindrical region of reference, approximately 2.5 cm3 in volume, was drawn in the cerebellar white matter, as this region of cerebellum is virtually devoid of 5-HT1A (21).
Because the boundaries of the median and dorsal raphe nuclei (RN) are not identifiable on MRI, the RN ROI was drawn directly on the PET image. Briefly, an ellipsoid measuring 12mm×12mm×26mm was manually placed on the mean PET image for each scan. This volume completely encompassed the high [11C]WAY100635 binding regions, and corresponded on the MRI to the posterior midbrain and the midbrain/pons junction at the midline, just anterior to the cerebral aqueduct.
Time-activity curves for each ROI were constructed from the mean voxel intensity in each frame. The outcome measure BPF was used for all statistical analyses, as it is the closest readily achievable surrogate of Bmax : BPF is equal to Bmax/KD , and the KD of the 5-HT1A receptor for an antagonist is assumed to be unaffected by SSRI treatment. Time activity curves were fit using the arterial input function and a two tissue constrained compartment model, in which the ratio K1/k2 is assumed to be comparable throughout the brain, including the reference region. Linear mixed-effects modeling was performed in R (R Project for Statistical Computing; www.R-project.org) with subject and scan nested within subject as random effects and all other factors as fixed effects. Observations were weighted according to standard errors computed using a bootstrap algorithm that accounts for error in metabolite, plasma, and brain data (22). To allow for proportional changes in binding across regions, to stabilize the variance across regions, and to correct for some slight skewness, analysis was performed on the log transformed BPF values.
Genotyping
Genotyping for the serotonin 1A receptor C-1019G promoter polymorphism was conducted as previously described (15).
RESULTS
Patient demographics, Hamilton Depression Scale scores, and radiotracer dose parameters of the 19 subjects are presented in Table 1. There was a 52% mean reduction in the 24-item HDRS score (pre-treatment HDRS = 24.3 ± 7.2; post-treatment HDRS = 11.7 ± 7.5; p < 0.001; paired-samples t-test). Baseline binding was not significantly different between those who were antidepressant-naïve (n=7) and those who had been medicated more than 4 years earlier (mean 6.8 ± 3.6 years) (BPF 28.6 vs 30.7; df=1,17; F=0.041; p = 0.84), and so the two groups were combined for further analyses.
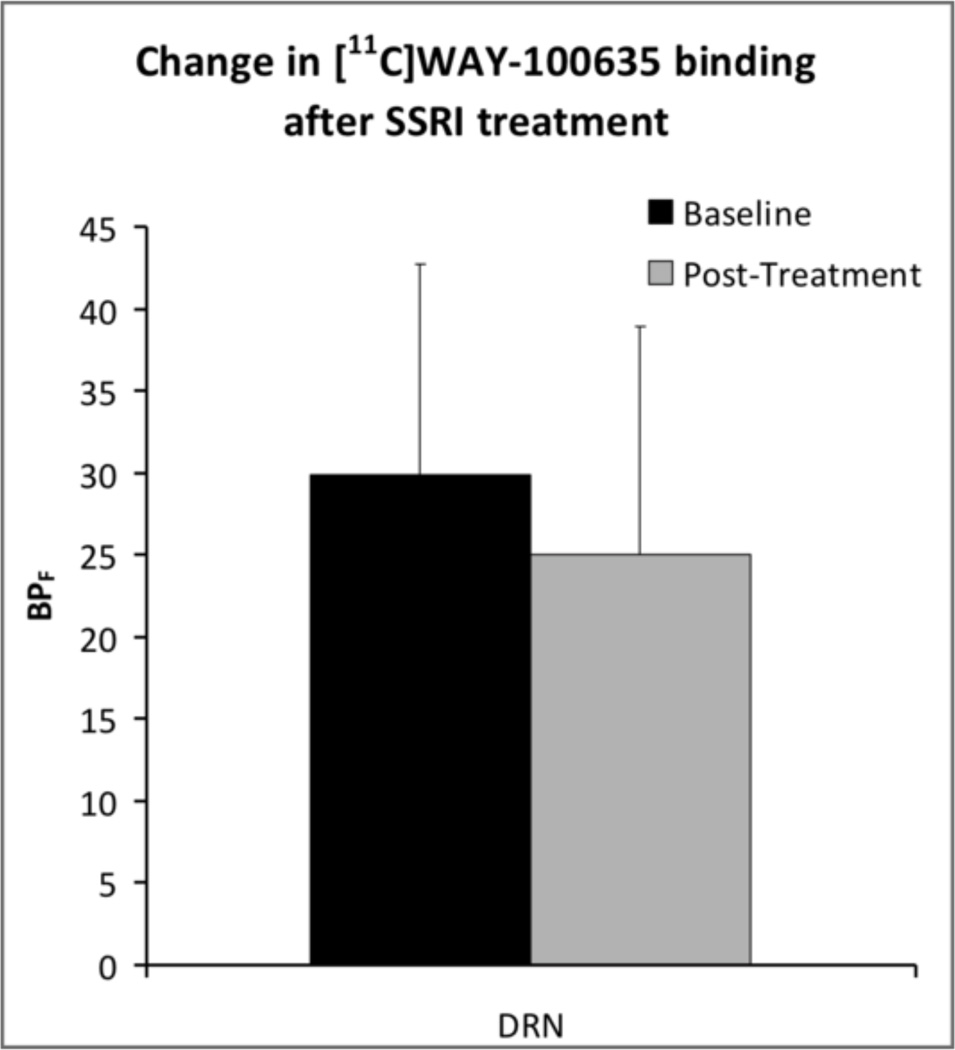
5-HT1A autoreceptor BPF in the raphe was reduced 18% on average after SSRI treatment (df=1,18; F=5.12; p=0.036). Removal of the one bipolar II subject did not appreciably alter results. The free fraction of [11C]WAY-100635 in plasma did not differ between pre- and post-treatment scans (p=0.54 by paired samples T-test), nor did adding free fraction as a covariate alter the main effect of treatment. Reduction in 5-HT1A BPF in the raphe did not correlate with post treatment HDRS scores when covarying for baseline HDRS (df=1,16; F=1.27; p=0.276). Gender was unrelated to the effect of treatment on raphe binding (df=1,17; F=1.10; p=0.31). In post-hoc analyses, we examined the effects of SSRI treatment on binding across all ROIs except the raphe. In contrast to autoreceptors, no significant effect of treatment (df = 1,18; F = 2.458; p = 0.134) or treatment×region interaction (df = 11,396; F = 0.580; p = 0.845) was detected (Figure S1 in Supplement 1).
In secondary analysis, we determine that age at onset of MDD, lifetime number of episodes and length of current episode were unrelated to baseline binding or change in binding with treatment. Because of the small number of subjects, we recoded these variables for analysis purposes as binary, based on approximate medians: young age of onset (<=21 years, n=9), extended current episode (>6 months, n=9), and highly recurrent (>5 episodes, n=6). None of these values were significantly predictive of baseline binding, or change in binding during treatment.
Because BPF data were positively skewed (1.071), all analyses were conducted using log-transformed data. Nevertheless, analysis of raw BPF in the raphé showed a similar effect of SSRI treatment (df=1,18; F=4.59; p=0.046).
DISCUSSION
In the present study, we found that short-term SSRI treatment downregulated 5-HT1A autoreceptor binding in the raphe in not recently treated major depression. The effect in other brain regions was not statistically significant. We have previously reported that elevated 5-HT1A binding is a trait of major depressive disorder (14), since it is present both during an episode of major depression and between episodes of major depression. The current study indicates that, as has been reported in rodent studies (25, 26), a short course of SSRIs may “normalize” a trait abnormality of elevated autoreceptor binding in MDD.
Together with our earlier reports (14), we have proposed a model in which a trait abnormality, elevated raphe 5-HT1A autoreceptor binding in MDD that may result in less serotonin release due to shorter serotonin neuron firing duration, is normalized by SSRI antidepressant treatment. Downregulation of 5-HT1A autoreceptors by SSRI treatment over weeks could enhance serotonin neuronal firing rate and the level of serotonin release. SSRIs then amplify that signal by blocking the serotonin transporter which removes released serotonin from the synaptic cleft.
Two previous studies investigated the effect of SSRI treatment on 5-HT1A binding in depressed subjects. As part of a larger study on MDD, Sargent et al rescanned 10 subjects after a median 14 weeks on SSRI treatment, and found a similar 15–20%, but statistically nonsignificant decrease in BPND (27). No changes in BPND were found in 15 MDD patients scanned before and after a median 9.4 weeks treatment with an SSRI or venlafaxine (28). Our study has a larger sample size, and also other important technical issues differentiate our study from these prior studies.
Both prior studies relied on the simplified reference tissue model (SRTM), and included cerebellar gray matter in the reference tissue, to estimate BPND as the outcome measure. Because BPND is highly sensitive to changes in reference region binding, and because the cerebellar gray matter has specific WAY-100635 binding, any SSRI-induced decreases in cerebellar 5-HT1A would likely obscure similar changes in other regions such as the raphé (13). When we analyze our data using BPND and cerebellar gray matter as the reference region, we also find no significant effect of treatment (10.0% reduction in raphé binding, p = 0.26). Likewise, using BPP as the outcome measure, we find a non-significant 5.3% reduction in binding.
We found the degree of downregulation of 5-HT1A raphe autoreceptors to be unrelated to SSRI antidepressant response. It may be a necessary but insufficient requirement for an antidepressant effect. This effect may endure for possibly as long as years because we previously found that a history of recent antidepressant exposure was associated with binding that was closer to control levels (13). Depressed patients off antidepressants for at least four years have similar 5-HT1A binding to antidepressant-naïve patients. This suggests that the antidepressant effect may have dissipated by four years off medication. The time-course for this possible reversion to untreated MDD elevated autoreceptor binding levels is unknown. However, if this apparent reversal of autoreceptor downregulation matches clinical course, it could prove to be a biomarker for tracking vulnerability to relapse.
The majority of animal studies report functional desensitization, rather than downregulation of binding of the autoreceptor by SSRI administration. Our study, and all previous such studies, have used antagonist PET tracers that cannot distinguish high-affinity and low-affinity agonist binding sites (G protein-coupled and –uncoupled, respectively). Such tracers have the advantage that they are unlikely to be affected by intra-synaptic levels of serotonin. Thus, we do not believe that the effect of SSRIs on intra-synaptic serotonin levels will reduce binding by competing with an antagonist high affinity tracer for the receptor. A future PET study with an agonist tracer could detect desensitization of the autoreceptor at the level of G protein coupling and that might be more sensitive to antidepressant action and more functionally relevant. But such tracers would need to be very high affinity to avoid being affected by intra-synaptic serotonin levels (16).
SSRI-induced autoreceptor downregulation may be unique to the pathophysiological state. This is suggested by the finding that mice selectively bred for ‘helpless’ behavior in the tail suspension test (TST) shows elevated 5-HT1A binding in a variety of brain regions (26). Chronic fluoxetine treatment led to both reduced helplessness in the TST, as well as a normalization of 5-HT1A binding, while having no effect on either parameter in wild type mice. Similarly, elevations in 5-HT1A binding have also been reported in isolation-housed mice relative to group-housed mice (25). Again, chronic SSRI administration led to reductions in 5-HT1A binding in the isolated mice, but not in the group-housed mice.
An association between the C(−1019)G functional polymorphism of the promoter region of the 5-HT1A gene and response of major depression to antidepressants has been reported, such that G allele carriers have a poorer antidepressant response (29, 30). We have previously reported that the G allele of the C-1019G promoter polymorphism in serotonin neurons has an allele-dose relationship with higher 5-HT1A binding in human subjects (15). Genetic ablation of the transcription factor Deaf-1, which binds only to the C allele, leads to increased expression of 5-HT1A and reduced 5-HT content in the raphé, but decreased 5-HT1A expression in the cortex (32). In our study we did not find that G allele carriers had less downregulation of binding (data not shown); however, our small sample, particularly given the uneven distribution of genotypes, is too underpowered to detect such an effect.
Some limitations of this study must be considered. First, the participants in this study reflect comorbidities typically seen in depressed patients. We cannot rule out the possibility that comorbid conditions will affect the action of SSRIs on the autoreceptor. Likewise, while lifetime substance dependence was an exclusion criterion, participants with non-recent substance abuse disorder (with the exception of MDMA use) were included. Subjects were given either paroxetine or citalopram, with the dose determined by the treating physician; additionally, short-acting benzodiazepines were permitted up to 72 hours before the post-treatment scan, provided that use was consistent with pre-enrollment use. A study using a single SSRI with a treatment algorithm might be more informative, although it is difficult recruiting such medication-free subjects into a PET study because past exposure to many SSRIs is so common. We do not believe our results are explained by SSRIs altering the availability of WAY-100635 because the free fraction of tracer in plasma is incorporated into our outcome measure BPF , and no significant difference in free fraction was detected. Likewise, direct competition is highly unlikely, given that neither citalopram nor paroxetine are known to bind to 5-HT1A in detectable levels (33, 34); displacement of tracer by enhanced extracellular 5-HT is also improbable, as WAY-100635 is an extremely potent antagonist of serotonin signaling at 5-HT1A (16).
In conclusion, we find raphé 5-HT1A autoreceptor BPF is reduced 18% by SSRI treatment in depressed patients without a history of recent medication. This study suggests that rodent findings about SSRI action can also be observed in MDD treated with SSRIs and therefore this is a plausible mechanism of action in patients. Future work should seek to replicate this finding, evaluating SSRI desensitization effects autoreceptors with a high affinity agonist PET tracer and characterizing the relationship to longer-term clinical outcome.
Supplementary Material
Figure 1.
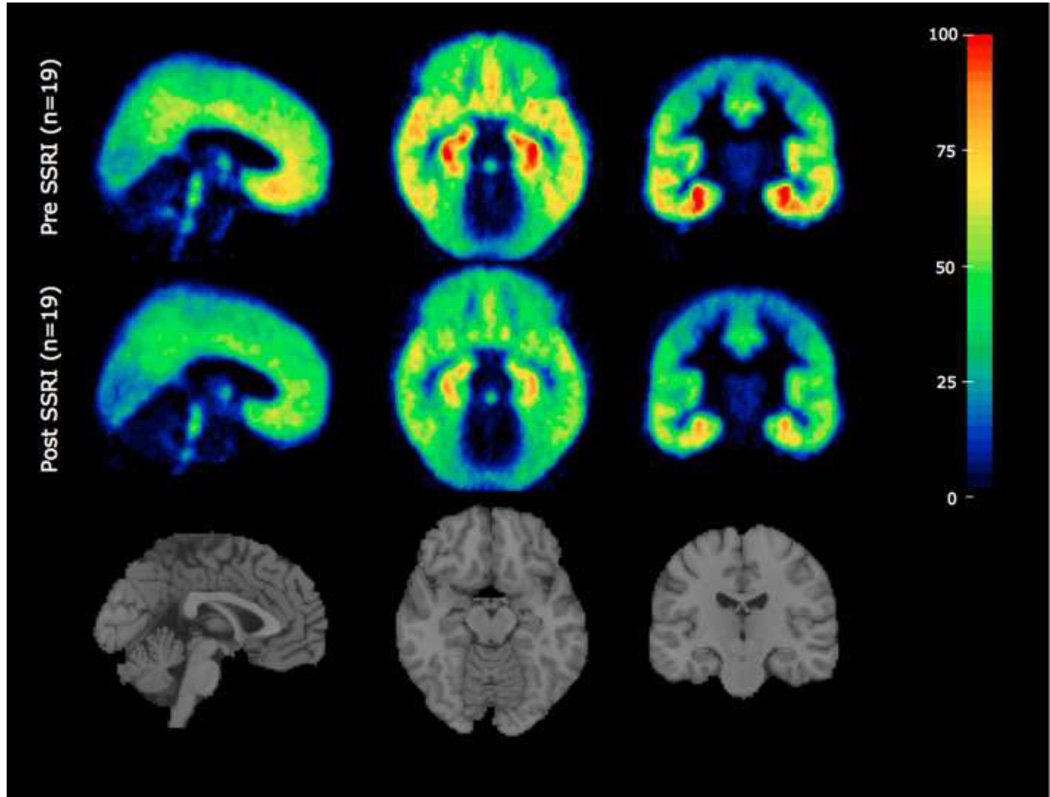
Average 5-HT1A receptor binding of 19 not recently-medicated subjects before and after SSRI treatment. Binding potential maps corrected for free fraction (BPF) were derived from the PET scans of all subjects. Each subject’s BPF map was transformed onto their structural MRI via parameters from a PET to MRI co-registration. BPF maps in MRI space were then transformed into standard MNI space via parameters from an SPM5 MRI to MNI normalization.
Figure 2.
5-HT1A binding in the dorsal raphé nuclei (DRN) is reduced following SSRI treatment in not recently-medicated depressed subjects (df=1,18; F=5.12; p=0.036).
ACKNOWLEDGEMENTS
The authors would like to thank Natalie Hesselgrave for assistance in data analysis. This research was funded by NIMH grant MH040695.
FINANCIAL DISCLOSURES
J. John Mann, M.D. received previous unrelated grants from Novartis and GSK.
Footnotes
The other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new '5-HT' hypothesis of depression: Cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010 doi: 10.1016/j.pnpbp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Zarcone VP, Jr, Berger PA, Brodie KH, Sack R, Barchas JD. The indoleamine hypothesis of depression: an overview and pilot study. Dis Nerv Syst. 1977;38:646–653. [PubMed] [Google Scholar]
- 3.Neumeister A. Tryptophan depletion, serotonin, and depression: where do we stand? Psychopharmacology bulletin. 2003;37:99–115. [PubMed] [Google Scholar]
- 4.Lanfumey L, Hamon M. 5-HT1 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:1–10. doi: 10.2174/1568007043482570. [DOI] [PubMed] [Google Scholar]
- 5.Blier P, de Montigny C, Chaput Y. Modifications of the serotonin system by antidepressant treatments: implications for the therapeutic response in major depression. J Clin Psychopharmacol. 1987;7:24S–35S. [PubMed] [Google Scholar]
- 6.Hamon M, Lanfumey L, Haj-Dahmane S, Jolas T, Kidd E, Bolanos F, et al. Adaptation of central 5-HT1A receptors after chronic antidepressant treatment in rats. Excerpta Medica, International Congress Series. 1991;968:297–300. [Google Scholar]
- 7.De Montigny C, Blier P. Enhancement of 5-HT synaptic efficacy by antidepressant treatments: A tenable unitary theory? Excerpta Medica, International Congress Series. 1991;968:301–304. [Google Scholar]
- 8.Lerer B, Gelfin Y, Gorfine M, Allolio B, Lesch KP, Newman ME. 5-HT1A receptor function in normal subjects on clinical doses of fluoxetine: blunted temperature and hormone responses to ipsapirone challenge. Neuropsychopharmacology. 1999;20:628–639. doi: 10.1016/S0893-133X(98)00106-7. [DOI] [PubMed] [Google Scholar]
- 9.Lesch KP, Mayer S, Disselkamp-Tietze J, Hoh A, Wiesmann M, Osterheider M, et al. 5-HT1A receptor responsivity in unipolar depression. Evaluation of ipsapirone-induced ACTH and cortisol secretion in patients and controls. Biol Psychiatry. 1990;28:620–628. doi: 10.1016/0006-3223(90)90400-v. [DOI] [PubMed] [Google Scholar]
- 10.Navines R, Martin-Santos R, Gomez-Gil E, Martinez de Osaba MJ, Imaz ML, Gasto C. Effects of citalopram treatment on hypothermic and hormonal responses to the 5-HT1A receptor agonist buspirone in patients with major depression and therapeutic response. Psychoneuroendocrinology. 2007;32:411–416. doi: 10.1016/j.psyneuen.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Lesch KP, Hoh A, Schulte HM, Osterheider M, Muller T. Long-term fluoxetine treatment decreases 5-HT1A receptor responsivity in obsessive-compulsive disorder. Psychopharmacology (Berl) 1991;105:415–420. doi: 10.1007/BF02244438. [DOI] [PubMed] [Google Scholar]
- 12.Rausch JL, Johnson ME, Kasik KE, Stahl SM. Temperature regulation in depression: functional 5HT1A receptor adaptation differentiates antidepressant response. Neuropsychopharmacology. 2006;31:2274–2280. doi: 10.1038/sj.npp.1301088. [DOI] [PubMed] [Google Scholar]
- 13.Parsey RV, Ogden RT, Miller JM, Tin A, Hesselgrave N, Goldstein E, et al. Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol Psychiatry. 2010;68:170–178. doi: 10.1016/j.biopsych.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller JM, Brennan KG, Ogden TR, Oquendo MA, Sullivan GM, Mann JJ, et al. Elevated serotonin 1A binding in remitted major depressive disorder: evidence for a trait biological abnormality. Neuropsychopharmacology. 2009;34:2275–2284. doi: 10.1038/npp.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, et al. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59:106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE, et al. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- 17.Parsey RV, Slifstein M, Hwang DR, Abi-Dargham A, Simpson N, Mawlawi O, et al. Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: comparison of arterial and reference tisssue input functions. J Cereb Blood Flow Metab. 2000;20:1111–1133. doi: 10.1097/00004647-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 18.DeLorenzo C, Klein A, Mikhno A, Gray N, Zanderigo F, Mann JJ, et al. Medical Imaging 2009: Image Processing. 1 ed. Lake Buena Vista, FL, USA: SPIE; 2009. A new method for assessing PET-MRI coregistration; pp. 72592W–72598W. [Google Scholar]
- 19.Duvernoy HM, Bourgouin P. The human brain: surface, three-dimensional sectional anatomy with MRI, and blood supply. Springer; 1999. [Google Scholar]
- 20.Talairach J, Tornoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system : an approach to cerebral imaging. Stuttgart: Georg Thieme; 1988. [Google Scholar]
- 21.Parsey RV, Arango V, Olvet DM, Oquendo MA, Van Heertum RL, John Mann J. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J Cereb Blood Flow Metab. 2005;25:785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- 22.Ogden RT, Tarpey T. Estimation in regression models with externally estimated parameters. Biostatistics. 2006;7:115–129. doi: 10.1093/biostatistics/kxi044. [DOI] [PubMed] [Google Scholar]
- 23.Wu S, Comings DE. A common C-1018G polymorphism in the human 5-HT1A receptor gene. Psychiatr Genet. 1999;9:105–106. doi: 10.1097/00041444-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Parsey R, Oquendo M, Ogden R, Olvet D, Simpson N, Huang Y, et al. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59:106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Gunther L, Liebscher S, Jahkel M, Oehler J. Effects of chronic citalopram treatment on 5-HT1A and 5-HT2A receptors in group- and isolation-housed mice. Eur J Pharmacol. 2008;593:49–61. doi: 10.1016/j.ejphar.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Naudon L, El Yacoubi M, Vaugeois JM, Leroux-Nicollet I, Costentin J. A chronic treatment with fluoxetine decreases 5-HT(1A) receptors labeling in mice selected as a genetic model of helplessness. Brain research. 2002;936:68–75. doi: 10.1016/s0006-8993(02)02548-9. [DOI] [PubMed] [Google Scholar]
- 27.Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, et al. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 28.Moses-Kolko EL, Price JC, Thase ME, Meltzer CC, Kupfer DJ, Mathis CA, et al. Measurement of 5-HT1A receptor binding in depressed adults before and after antidepressant drug treatment using positron emission tomography and [11C]WAY-100635. Synapse. 2007;61:523–530. doi: 10.1002/syn.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ou XM, Lemonde S, Jafar-Nejad H, Bown CD, Goto A, Rogaeva A, et al. Freud-1: A neuronal calcium-regulated repressor of the 5-HT1A receptor gene. J Neurosci. 2003;23:7415–7425. doi: 10.1523/JNEUROSCI.23-19-07415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Francois B, Czesak M, Steubl D, Albert PR. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology. 2008;55:977–985. doi: 10.1016/j.neuropharm.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 31.Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 2004;10:575–593. doi: 10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- 32.Czesak M, Le François B, Millar AM, Deria M, Daigle M, Visvader JE, et al. Increased serotonin-1A (5-HT1A) autoreceptor expression and reduced raphe serotonin levels in deformed epidermal autoregulatory factor-1 (Deaf-1) gene knock-out mice. Journal of Biological Chemistry. 2012;287:6615–6627. doi: 10.1074/jbc.M111.293027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cusack B, Nelson A, Richelson E. Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl) 1994;114:559–565. doi: 10.1007/BF02244985. [DOI] [PubMed] [Google Scholar]
- 34.Millan MJ, Gobert A, Lejeune F, Newman-Tancredi A, Rivet JM, Auclair A, et al. S33005, a novel ligand at both serotonin and norepinephrine transporters: I. Receptor binding, electrophysiological, and neurochemical profile in comparison with venlafaxine, reboxetine, citalopram, and clomipramine. J Pharmacol Exp Ther. 2001;298:565–580. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.