SUMMARY
The 6th OARSI Workshop on Imaging in Osteoarthritis combined with the 3rd OA Biomarkers Workshop is the first to bring together the imaging and molecular biomarker communities to focus on clinical validation and qualification of osteoarthritis biomarkers. The workshop was held in Hilton Head, SC, USA, from June 12–14, 2012; 138 attendees participated, including representatives from academia, pharmaceutical and MRI industries, FDA, and NIH. Presentations and discussions raised awareness, consolidated knowledge, and identified strategies to overcome challenges for the development and application of imaging and biochemical biomarkers in OA research studies and clinical trials.
CONCLUSIONS
The OA research communities need to work alongside regulatory agencies across the world, to qualify and validate new chemical and imaging biomarkers for future research and clinical trials.
Keywords: Imaging workshop, Osteoarthritis
Magnetic resonance imaging (MRI) of knee joints has contributed significantly to the change in perception of osteoarthritis (OA) from wear and tear disease limited to radiographic changes in bone and loss of joint space related to cartilage, to a multi-tissue, whole organ, complex disease with many phenotypes [1]. Aging, obesity, injuries, and an adverse mechanical environment from joint malalignment, can all contribute to OA incidence and progression. The disease may also proceed via different metabolic pathways influenced by race, genetics, and gender.
OA is a symptomatic disease associated with characteristic changes in synovial tissue structures. Imaging modalities should reflect this complex phenotype. Although, MRI is a holistic structural assessment modality that provides measures that are the most direct and valid measure of joint status, and the most responsive measure of disease progression [1], the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have yet to accept MRI as an imaging endpoint for OA in clinical trials. Currently, the quantitative measurement of radiography-based joint space width (JSW) is the only accepted imaging endpoint in disease modification efficacy OA trials. Development of disease-modifying therapy has been slowed because radiography has limited capacity to detect clinically meaningful changes in joint morphology that accompany disease progression.
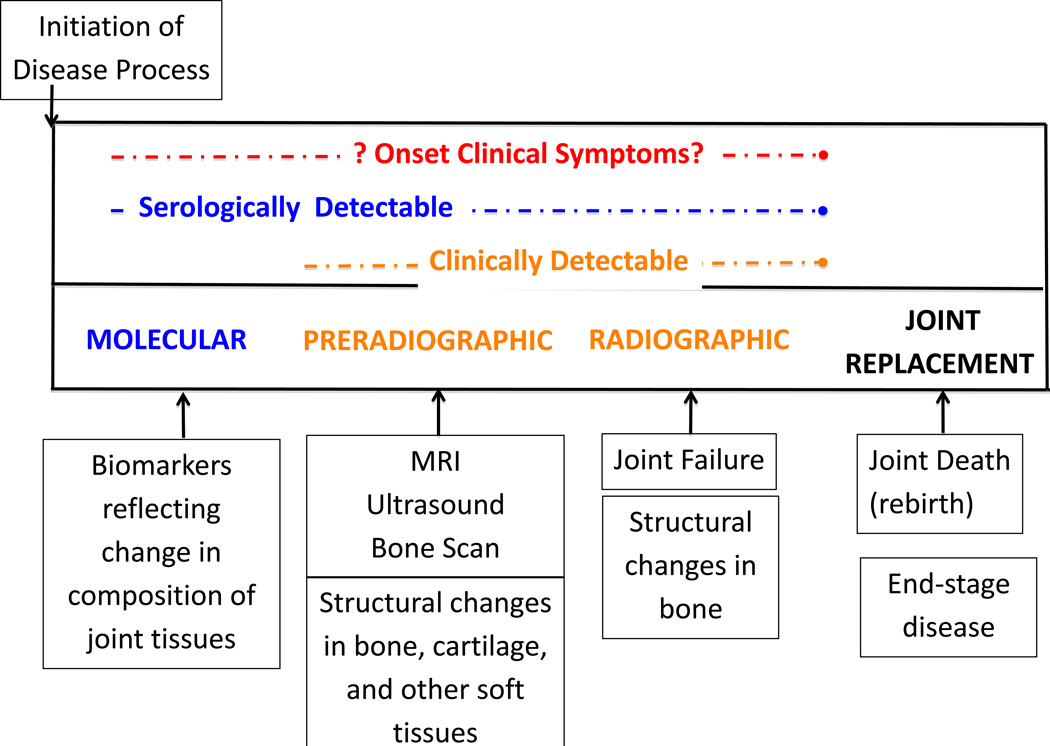
The utility of any disease-related biomarker is a function of how well the marker links disease biology and pathology with clinical outcomes. Pain, which along with JSW is a common endpoint in OA clinical trials, appears to at least partly derive from joint tissue alterations (including synovitis, effusion, meniscal pathology, and bone marrow lesions) but associations are generally moderate. Not only do these changes go undetected on conventional radiographs, but pain often antedates radiographic manifestations of disease (Figure 1). Moreover, the structural OA disease process and related pain are dynamic. Such variation in disease progression requires biomarkers that can reflect morphological and pathological changes in joints, beginning in the earliest stages of OA development and progression and throughout the course of disease (see Figure 1). For example, biochemical markers may reflect ultra-structural changes in joint tissue metabolism very early in the disease process prior to any apparent change in imaging appearance on either radiographs or MRI. Similarly, short-term (weeks/months) variation in symptoms is unlikely to be reflected in poorly responsive endpoints such as radiographs.
Figure 1.
The natural history of osteoarthritis and the purported roles of biomarkers during the disease process. Original attributed to V Kraus (originally presented at OARSI Congress 2009: Kraus, VB. 2009. Clinical perspective on the role of biomarkers and the diagnosis and monitoring of OA. Osteoarthritis Cartilage Sept 17 (Suppl 1): S1.) can also be found in [5].
The OA biomarker community has been addressing these and other issues in a series of workshops [2]. Most recently, the OARSI Biomarkers Workshop III – Imaging Biomarker Validation and Quantification organized in conjunction with the 6th International Workshop on Osteoarthritis Imaging, was held July 12 – 14 on Hilton Head Island, South Carolina. More than 138 scientists from academia, the government, and the private sector convened for the event.
Research presented at the three-day meeting highlighted progress in the field to validate MRI as a biomarker with prognostic and/or diagnostic capabilities.
Meeting participants endorsed a plan to facilitate the use of MRI as an endpoint in large-scale, multi-center interventional clinical trials with extended follow-up lasting one and two years, to ascertain the efficacy and safety of different interventions using MRI-based measures as a biomarker of efficacy. In discussing the population on which to base such trials, participants agreed that such a study should, in one instance, focus on recently injured joints; this study paradigm facilitates defining a pathway to OA development and progression because the inciting event and time can be clearly identified (a summary of issues under discussion can be found in Table 1). Meeting participants also emphasized that such trials should utilize imaging technology that could detect differences in the morphological and compositional makeup of an injured joint; detect differences between the injured and uninjured joint; and monitor changes in morphological and compositional makeup over time.
Table 1.
Designing the Optimal Trials for Understanding OA. Discussion and Future Directions
| Discussion Points | Suggestions |
|---|---|
| Determine imaging modalities for longitudinal multi-center injury trials. | Use T2 as basis assessment of joint morphology and then T1rho as background if this modality can be standardized. |
| Establish standard measurement parameters on imaging (a core-set). | For example of cartilage composition, cartilage lesions and cartilage thickness, shape measures need to be collected to determine effect and which best predicts long term clinical outcome |
| Determine best strategy for regulatory bodies to generate different guidance criteria for different phases of clinical trial intervention. | Include MRI with all the other endpoints in early phase trials (especially Phase 2), and then determine if you can use it later as a primary endpoint based on the study. Modify requirement for 30–50% reduction in JSW as a threshold for evidence of successful structural modification as no single drug will impact readily this parameter. |
| Identify which structural changes are most specifically associated with clinical endpoints in (knee) OA, and hence need to be treated. | Reach a consensus on standardized clinical endpoint so studies are easier to compare. Explore utility of virtual total joint replacement (TJR) as a potential endpoint in future clinical trials. |
| Determine histopathological relationship among structural changes to cartilage and subchondral bone and 'soft' tissues of the articular organ that can be seen on MRI. (Joint tissue- MRI structure correlation) | Match treatable pathology with appropriate imaging methodology. |
| Monitor patients for damage in other joints especially when treated by highly effective analgesics; any damage needs to be detected early. | Some suggested methods of determining risk include asking patients about pain levels; radiographic screening; joint specific biochemical markers. |
| Determine whether quantitative MRI, either morphometric, compositional or semiquantitative as an endpoint, is a suitable method to be used in cartilage repair trials and for long term follow up. Establish imaging criteria that will label cartilage repair procedures successful from a radiological and histological standpoint. |
Need to measure local changes in collagen/GAG and quantitative MRI for morphology suitability but success of the intervention is determined by the long term outcome for the whole joint. MRI (as opposed to arthroscopy) may not be the most suitable outcome for assessing the boundary between native and repaired cartilage. Be mindful of what one considers successful, as it depends on the patients' expectations. |
Identifying the appropriate imaging modality and parameters will be critical for ensuring responsive, reproducible and reliable outcomes. OA typically progresses very slowly so that the disease can remain at the same structural and clinical level of severity for many years. Without accurate MRI technology, a bone marrow lesion (BML) can be confused with a contusion or subchondral insufficiency fracture, and what looks like a meniscal tear or cartilage lesions can simply be an artifact.
An MRI protocol that can assess cartilage quality (e.g. matrix composition) along with morphology and shape measures may help distinguish pathology from normal reparative processes (e.g. increase in cartilage thickness seen after ACL injury could be pathological swelling or adaptive hypertrophy) [3]. Imaging modalities that rely on intravenous contrast administration, which would be useful in assessing synovitis or cartilage proteoglycan (dGEMRIC),may be difficult to use in the current clinical trials and epidemiological studies since they add complexity to the study design and prolong the procedure time; contrast injection necessitates ascertaining adequate renal function prior to injection in order to avoid the very rare contrast medium-induced nephropathy, and entails a waiting period prior to image acquisition.
Some of the main drivers in MRI research have been a pursuit of improved metrics for OA trials and their acceptance by regulatory agencies. Although quantitative measurement of cartilage thickness on MRI may have high validity, carrying it forward for trials and clinical use depends on a response from the FDA and the EMA (amongst other world-wide regulatory authorities). In 2010, OARSI submitted an analysis to the FDA in which the use of MRI in osteoarthritic joints was detailed. The FDA is actively working to address recommendations necessary to approve MRI parameters as endpoints in clinical trials and this may be facilitated by a formal request for biomarker qualification.
In the opinion of some of the meeting attendees, the imaging biomarkers that may be best suited for quantitative measurement of cartilage composition on MRI assessed are T2 and potentially T1rho. Both T2 and T1rho can be implemented and assessed using a software package that could be purchased and standardized across sites [4]. However, there are challenges regarding the reproducibility of T2 and T1rho, and thus these modalities will need to be further standardized before implementation across machines and sites.
In all likelihood, any imaging modality utilized as a biomarker will be used in conjunction with biochemical measures. Towards that end, OARSI, as part of an initiative with the Foundation of NIH, has initiated a study using Osteoarthritis Initiative samples to generate data on 12 urine and serum OA biomarkers related to cartilage and bone turnover. Although there’s still a need for more specific validation regarding origin, these biochemical measures will be correlated with imaging measures (or outcomes). Urine sampling is not invasive and is particularly valuable for collagen biomarkers; but biomarker levels need to be normalized to urine creatinine to account for the varying hydration states of the individuals. This initiative will provide the opportunity to compare a large cadre of imaging and biochemical markers and to evaluate the potential synergy for these different types of markers singly, and in combination, to reflect disease status and progression.
The joint itself can be viewed as a “test tube,” with the synovial fluid providing access to proximal information regarding joint tissue metabolites that can be correlated with imaging or histological outcomes. Disadvantages to synovial fluid sampling are several, including: discomfort to the patient, dislike of the procedure by practitioners, requirement for ancillary imaging (ultrasound or computed tomography) for sampling of some joints (such as the hip) and short half-life of some biomarkers in the joint (e.g. the brief half-life of hyaluronic acid in the joint suggests that the concentration of hyaluronic acid changes over minutes). Although there are disadvantages to sampling synovial fluid, in a research setting it can provide the most proximal quantitative data through biomarker analyses of the disease process and thereby can be invaluable for providing biological insights in the disease.
Biochemical biomarkers may also help categorize who is at risk and who may benefit from screening for OA by helping detect signal changes linked to OA. Recognizing those patients who will progress rapidly will prove critical in the effort to accurately stage the disease and thus identify people most at risk and most likely to benefit from therapeutic intervention.
There is a critical need for imaging methods to evaluate DMOAD activity in a reasonable timeframe, with reasonable sample size, at a reasonable cost. Setting a framework to evaluate OA biomarkers that include potential for surrogacy as a major emphasis in modifiable disease pathways affecting patient outcomes will identify and advance biomarkers with the greatest promise.
Acknowledgements
The authors wish to thank science writer Jeanne Erdmann for help in preparation of this manuscript. Ms Erdmann was supported by general funds for the meeting.
Support for the meeting was provided by NIAMS Grant number: 5U13AR057296-03. Support was also provided by the Arthritis Foundation, MERCK Serono, Sanofi, Fidia, Orthopedic Research Society, Pfizer, Bioclinica, Boston Imaging Core Lab, Novartis, Piramal Healthcare, QMetrics, Bioiberica, Bioventus, Chondrometrics, Optasia Medical and VirtualScopics.
David Hunter is funded by an Australian Research Council Future Fellowship with support from the FNIH OA Biomarkers Consortium.
Felix Eckstein is CEO and co-owner of Chondrometrics GmbH. He provides consulting services to MerckSerono, Novartis, Sanofi Aventis, Perceptive, Bioclinica and Abbot.
Virginia Kraus is funded by grants from the Foundation for NIH, NIH/NIA Claude D Pepper 5P30 AG028716 and NIH/NIAMS 5P01-AR050245.
Elena Losina is funded by grants from NIAMS R01 AR053112, K24 AR057827, P60 AR47782. Linda Sandell receives royalties from Millipore.
Ali Guermazi is the President of Boston Imaging Core Lab (BICL) LLC. He is a consultant to Facet solutions, Merckserono, Genzyme, Novartis, and Stryker.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
All authors were involved in collecting data, reviewing the literature and drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published.
David Hunter, MBBS, PhD, FRACP, and Ali Guermazi, MD, PhD, served as meeting chairs. The scientific program committee was comprised of Felix Eckstein, MD, Virginia Byers Kraus, MD, Elena Losina, PhD, and Linda Sandell, PhD.
Reference List
- 1.Hunter DJ, Zhang W, Conaghan PG, Hirko K, Menashe L, Reichmann WM, et al. Responsiveness and reliability of MRI in knee osteoarthritis: a meta-analysis of published evidence. Osteoarthritis Cartilage. 2011;19:589–605. doi: 10.1016/j.joca.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter DJ, Eckstein F. From joint anatomy to clinical outcomes in osteoarthritis and cartilage repair: summary of the fifth annual osteoarthritis imaging workshop. Osteoarthritis Cartilage. 2011;19:1263–1269. doi: 10.1016/j.joca.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Frobell RB. Change in cartilage thickness, posttraumatic bone marrow lesions, and joint fluid volumes after acute ACL disruption: a two-year prospective MRI study of sixty-one subjects. J Bone Joint Surg Am. 2011;93:1096–1103. doi: 10.2106/JBJS.J.00929. [DOI] [PubMed] [Google Scholar]
- 4.Mosher TJ, Zhang Z, Reddy R, Boudhar S, Milestone BN, Morrison WB, et al. Knee Articular Cartilage Damage in Osteoarthritis: Analysis of MR Image Biomarker Reproducibility in ACRIN-PA 4001 Multicenter Trial. Radiology. 2011;258:832–842. doi: 10.1148/radiol.10101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwoh C. Epidemiology of osteoarthritis. In: Newman A, Cauley J, editors. The Epidemiology of Aging. Springer; 2012. [Google Scholar]