Abstract
Objective
To characterize the prevalence of Non-Alcoholic Fatty Liver Disease (NAFLD) by race in a nationally representative sample of the U.S. population and to investigate potential explanatory factors for racial disparities.
Methods
Cross-sectional study of 4,037 non-Hispanic white, 2,746 non-Hispanic black, and 2,892 Mexican-American adults in the Third National Health and Nutrition Examination Survey. NAFLD was defined using ultrasound and with elevated aminotransferases.
Results
Age-adjusted prevalence of NAFLD was highest in Mexican-Americans (21.2%), followed by non-Hispanic whites (12.5%), and was lowest in non-Hispanic blacks (11.6%). Even after adjustment for demographic, lifestyle, adiposity, and metabolic factors, compared to non-Hispanic whites, Mexican-Americans were more likely to have NAFLD (OR: 1.67, 95% CI: 1.26, 2.22). Non-Hispanic blacks were significantly less likely to have NAFLD with elevated aminotransferases (OR: 0.51, 95% CI: 0.27, 0.97). Racial differences were attenuated among those with normal body mass index and/or among “never drinkers.”
Conclusions
In this representative sample of the U.S. population, we found significant racial differences in the prevalence of ultrasound-defined NAFLD (with and without elevated liver enzymes). The racial differences were not fully explained by lifestyle, adiposity and metabolic factors. More works is needed to identify potential contributors.
Keywords: Liver, Race, Ethnicity, Obesity, NHANES III
Nonalcoholic fatty liver disease (NAFLD) is characterized by excessive accumulation of triglycerides in the liver in the absence of excessive alcohol consumption. NAFLD comprises a spectrum of hepatic disorders, ranging from steatosis to steatohepatitis (NASH) and cirrhosis (1, 2). Previous studies have demonstrated differences in NAFLD and NASH prevalence by race/ethnicity, with the highest prevalence found in Hispanics and the lowest prevalence found in blacks (3–11). Reasons for the differences in prevalence by race are not clear, although genetic (12, 13), and metabolic factors (3, 4, 6, 7) have been suggested as contributing factors underlying the differences. Specifically, Hispanics tend to have greater adiposity and insulin resistance compared to whites, which may contribute to their higher reported prevalences (4, 6, 7, 14, 15). Blacks also have greater adiposity and a higher rate of diabetes, but they also tend to have lower levels of triglycerides as compared to Hispanics or whites, which may contribute to their lower reported prevalences (16).
Some prior studies have suggested differences in hepatic steatosis prevalence by gender, with a higher prevalence reported in men compared to women (4, 6, 17), while other studies have found no difference by gender (18). However, among many of studies that reported gender differences, individuals were classified as having hepatic steatosis regardless of alcohol consumption, so it remains unclear if NAFLD prevalence differs by gender (17, 18). Importantly, with the exception of the U.S. population-based Dallas Heart Study (4), previous studies were conducted using small, highly select, predominately clinic-based populations (3, 5, 7–11), so it remains unclear if the reported patterns are representative of the broader U.S. population. Although liver biopsy is the gold standard for the diagnosis of NAFLD, due to the invasive nature of the procedure, liver biopsy is not feasible to do in a healthy community-dwelling population. However, ultrasound provides a non-invasive, albeit less sensitive and less specific method to detect hepatic steatosis and is widely used in clinical practice and research settings (19).
The objectives of this study were to characterize the prevalence of NAFLD and NAFLD with elevated aminotransferases (e.g. NASH) by race and gender in a nationally representative sample of the U.S. population and to assess whether demographic, lifestyle, adiposity-related, and/or metabolic factors explained differences in NAFLD prevalence.
METHODS
Study Population
The Third National Health and Nutrition Examination Survey (NHANES III) was a cross-sectional survey conducted between 1988 and 1994 of the civilian non-institutionalized U.S. population. NHANES III used a complex sampling design to select a representative sample of U.S. persons (20).
Data Collection
Detailed descriptions of the NHANES III data collection and variable definitions are available elsewhere (21). Briefly, individuals participated in a standardized interview and physical examination. We categorized smoking status as: never; former; current. Participants were classified as physically inactive if they answered no to all the questions regarding engaging in any of activities over the last month (jog/run, bicycle, swim, aerobics, dancing, calisthenics, garden/yard work, weight lifting or other sport). Average alcohol consumption was estimated by multiplying the number of drinking days and the number of drinks per day, on average on a drinking day and categorized as: never; former low/moderate (<5 drinks/day); former high (≥5 drinks/day); low current (women ≤1 drink/day; men ≤2 drinks/day); moderate current (women >1 to <5 drinks/day, men >2 to <5 drinks/day); high current (≥5 drinks/day). Total calories consumed per day and percent calories from carbohydrates, protein, and fat were estimated from 24-hour dietary recalls.
We defined diabetes as self-reported physician diagnosis, medication use, fasting plasma glucose ≥126 mg/dl or 2-hour glucose tolerance test ≥200 mg/dl. Hypertension was defined as self-reported physician diagnosis, medication use, systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg. History of cardiovascular disease was defined by self-report of past myocardial infarction, congestive heart failure, or stroke. Body mass index (BMI) was calculated by dividing measured weight in kilograms by measured height in meters squared. Skinfold measurements were taken at 4 locations (triceps, subscapular, suprailiac, thigh) and were summed to get a composite measure of subcutaneous fat. A body composition analyzer for bioelectrical impedance (Valhalla 1990B Valhalla Scientific, San Diego, CA) was used to measure whole-body electrical resistance and reactance (22) and percent body fat was calculated using validated prediction equations (23). Plasma glucose, glycated hemoglobin (HbA1c), triglycerides, HDL cholesterol, antibodies to hepatitis C, and antibodies to hepatitis B core antigen (anti-HBc) were measured using standard methods (24). The following liver tests were measured on the Hitachi 737 Analyzer (Boehringer-Mannheim Diagnostics, Indianapolis, IN): alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), alkaline phosphatase, and total bilirubin.
The physical examination in NHANES III also included ultrasounds of the gallbladder performed using a Toshiba (Tustin, CA) SSA-90A machine using 3.75 and 5.0 Mhz transducers. In 2009–2010, these ultrasounds were re-evaluated for the presence of steatosis within the liver. A more detailed description of the steatosis ultrasound protocol can be found elsewhere (25). Briefly, the following information was recorded: 1) presence of liver-to-kidney contrast, 2) degree of brightness of the liver parenchyma, 3) presence of posterior deep beam attenuation, 4) presence of echogenic walls in the small intrahepatic vessels, and 5) definition of the gallbladder walls. Using a standardized algorithm, liver steatosis was categorized as present (moderate or severe steatosis) or absent (normal or mild steatosis). The intra- and inter-rater reliabilities (kappa statistics) were 0.77 (95% CI 0.73–0.82) and 0.70 (95% CI 0.64–0.76), respectively (25). A systematic review found the overall sensitivity and specificity of ultrasound to detect moderate-severe hepatic steatosis (defined as >5–10% excess hepatic triglyceride content), compared to biopsy, are 84.8% and 93.6%, respectively (19). However, the sensitivity and specificity have been found to be lower among persons with BMI >35.0 kg/m2 (49.1% and 75%, respectively) (26).
NAFLD Definitions
NAFLD was defined as the presence of hepatic steatosis in the absence of elevated alcohol consumption (>2 drinks/day for men; >1 drink/day for women) (27) and in the absence of current use of zydovudine or didanosine, medications that have been shown to induce hepatic steatosis. NAFLD with elevated aminotransferases was defined as the presence of NAFLD and elevated ALT or AST, defined as above the upper limit of normal of the NHANES III laboratory values (ALT: >40 U/L for men and >31 U/L for women; AST: >37 U/L for men and >31 U/L for women), in the absence of hepatitis B, hepatitis C, and transferrin saturation >50%. These definitions for NAFLD have been used previously (28).
Statistical Analysis
This analyses was restricted to 9,675 NHANES III participants who met the following criteria: ≥20 years of age, self-identified as non-Hispanic white, non-Hispanic black or Mexican-American race/ethnicity, and who had complete information on ALT, AST, hepatic ultrasound data and covariates of interest.
Analyses were performed incorporating sampling weights to obtain unbiased estimates from the complex NHANES sampling design. Standard errors (SEs) were obtained using Taylor series linearization (20). Using these methods, our estimates can be considered representative of the non-institutionalized U.S. population aged 20–74 in 1988–1994.
We calculated age-adjusted characteristics of the population stratified by race/ethnicity and gender. Age-adjusted prevalences (95% confidence intervals) for NAFLD and NAFLD with elevated aminotransferases were calculated overall for each race group (non-Hispanic white; non-Hispanic black; Mexican-American) and stratified by race, gender, and BMI category (normal [18.5–24.9 kg/m2]; overweight [25.0–29.9 kg/m2]; obese [30.0–39.9 kg/m2]). We also performed sensitivity analyses stratified by diabetes status and physical activity status (active; inactive). P-values for differences in means were calculated using the Wald F statistic and distributional assumptions for sample means were satisfied. In our main analysis, multivariable logistic regression models were used to examine the independent association between race and NAFLD. We constructed three models: Model 1 was adjusted for demographic and lifestyle factors (age, gender, education, family income, smoking, alcohol consumption, physical activity, and total calories consumed per day). Model 2 included all variables in Model 1 plus measures of adiposity (BMI, waist circumference, and sum of skinfolds). Model 3 included all variables in Model 2 plus metabolic factors (triglycerides, HDL cholesterol, hypertension, diabetes, and HbA1c). To further assess the role of adiposity, we conducted a sensitivity analysis adding percent body fat calculated from bioelectrical impedance data to our models (23) and we conducted sensitivity analyses stratified by BMI category: normal (18.5–24.9 kg/m2) (n=3,802), overweight (25.0–29.9 kg/m2) (n=3,408), and obese (30.0–39.9 kg/m2) (n=2,003). We also conducted a sensitivity analysis restricted to participants that self-identified as “never drinkers” (n=1,462) to exclude the possibility that low alcohol consumption was contributing to steatosis.
All reported p-values are two-sided and p<0.05 was considered statistically significant. Analyses were performed using Stata Version 11 (29).
RESULTS
There were substantial differences in the mean age by race; mean age for non-Hispanic whites was 43.1 years, mean age for non-Hispanic blacks was 39.5 years, and mean age for Mexican-Americans was 36.7 years (p<0.001), therefore all subsequent comparisons are age-adjusted (Table 1). Mexican-Americans had higher levels of ALT (mean: 23.1 U/L) and AST (mean: 24.8 U/L) as compared to non-Hispanic whites and to non-Hispanic blacks (both comparisons, p<0.001). Compared to non-Hispanic whites, non-Hispanic blacks had significantly higher mean AST levels (non-Hispanic whites: 20.7 U/L, non-Hispanic blacks: 22.8 U/L) (p<0.001), but mean ALT values were not significantly different (non-Hispanic whites: 17.2 U/L, non-Hispanic blacks: 16.6 U/L) (p=0.160). Non-Hispanic blacks and Mexican-Americans were more likely to self-identify as “never drinkers” compared to non-Hispanic whites (p<0.001 for both comparisons). For all races, men were more likely to self-identify as either “low current drinkers” or “high current drinkers” compared to women (p<0.001 for all races).
Table 1.
Age-adjusted characteristics of participants stratified by race and gender.
| Non-Hispanic White | Non-Hispanic Black | Mexican-American | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Men (n=1,894) | Women (n=2,143) | Men (n=1,246) | Women (n=1,500) | Men (n=1,441) | Women (n=1,451) | |
|
| ||||||
| Age (years), mean (SE) | 42.8 (0.5) | 43.4 (0.5) | 39.3 (0.4) | 39.7 (0.5) | 36.1 (0.5) | 37.4 (0.4) |
|
| ||||||
| Lifestyle factors | ||||||
| Education <high school, % (SE) | 17.9 (1.4) | 16.0 (1.2) | 33.0 (1.9) | 28.3 (1.7) | 59.6 (2.1) | 57.6 (2.4) |
| Family income <poverty level, % (SE) | 6.2 (0.8) | 9.0 (1.0) | 23.0 (2.1) | 32.9 (2.0) | 30.0 (2.1) | 36.4 (2.0) |
| Cigarette smoking status | ||||||
| Current smoker, % (SE) | 32.3 (1.3) | 27.5 (1.2) | 3.9 (1.6) | 28.3 (1.5) | 23.0 (1.3) | 11.5 (1.4) |
| Former smoker, % (SE) | 32.7 (1.2) | 22.5 (0.9) | 22.4 (1.4) | 14.6 (1.1) | 31.7 (1.3) | 16.9 (1.1) |
| Never smoker, % (SE) | 34.9 (1.3) | 50.0 (1.3) | 38.8 (1.9) | 57.1 (1.7) | 45.2 (1.4) | 71.6 (1.6) |
| Alcohol use status | ||||||
| High current (≥5 drinks/day), % (SE) | 5.5 (0.7) | 1.2 (0.3) | 7.7 (0.7) | 1.9 (0.3) | 5.6 (0.8) | 7.9 (0.3) |
| Moderate current (Women >1 to <5 drinks/day, Men >2 to <5 drinks/day), % (SE) | 7.4 (0.7) | 7.4 (1.0) | 6.7 (0.9) | 3.3 (0.5) | 7.3 (0.9) | 2.2 (0.6) |
| Low current (Women ≤1 drink/day; Men ≤2 drinks/day), % (SE) | 54.6 (2.2) | 42.5 (1.6) | 45.4 (2.1) | 23.5 (1.5) | 51.4 (1.5) | 22.0 (1.8) |
| Former high (≥5 drinks/day), % (SE) | 7.8 (1.0) | 2.2 (0.4) | 8.4 (1.0) | 3.8 (0.6) | 8.6 (0.6) | 2.8 (0.4) |
| Former low/moderate (<5 drinks/day), % (SE) | 20.4 (1.3) | 32.9 (1.5) | 22.3 (1.5) | 42.7 (2.0) | 20.7 (1.2) | 41.0 (1.2) |
| Never, % (SE) | 4.3 (0.5) | 13.8 (1.4) | 9.5 (1.0) | 24.8 (1.9) | 6.5 (0.6) | 31.2 (2.1) |
| Physically inactive, % (SE) | 23.9 (1.4) | 22.7 (1.5) | 23.3 (1.4) | 35.1 (1.9) | 36.3 (1.7) | 43.0 (1.5) |
| Total calories consumed per day (kcal/day), mean (SE) | 2753 (30) | 1822 (20) | 2602 (42) | 1783 (23) | 2559 (43) | 1716 (23) |
| Percent of total calories from fat, % (SE) | 34.6 (0.3) | 33.5 (0.3) | 34.4 (0.3) | 34.1 (0.3) | 32.0 (0.3) | 32.7 (0.3) |
| Percent of total calories from protein, % (SE) | 15.1 (0.1) | 15.2 (0.1) | 15.9 (0.2) | 15.4 (0.2) | 16.0 (0.1) | 15.9 (0.2) |
| Percent of total calories from carbohydrates, % (SE) | 47.9 (0.5) | 50.6 (0.4) | 46.2 (0.4) | 50.2 (0.3) | 49.3 (0.4) | 51.9(0.3) |
|
| ||||||
| Adiposity related factors | ||||||
| Body mass index (kg/m2), mean (SE) | 26.3 (0.1) | 25.6 (0.2) | 26.2 (0.1) | 28.4 (0.2) | 26.9 (0.1) | 27.7 (0.2) |
| <18.5 kg/m2, % (SE) | 1.1 (0.3) | 4.0 (0.5) | 1.3 (0.3) | 3.0 (0.5) | 0.7 (0.3) | 1.4 (0.4) |
| 18.5 – 24.9 kg/m2, % (SE) | 39.5 (1.2) | 52.0 (1.6) | 42.2 (1.4) | 33.1 (1.5) | 32.5 (1.6) | 34.5 (2.1) |
| 25 – 29.9 kg/m2, % (SE) | 42.5 (1.2) | 24.0 (1.0) | 38.9 (1.3) | 29.0 (0.9) | 48.2 (1.9) | 34.3 (1.9) |
| ≥30 kg/m2, % (SE) | 17.0 (0.9) | 19.9 (1.5) | 17.7 (0.9) | 34.8 (1.5) | 18.6 (1.4) | 29.7 (1.3) |
| Waist circumference (cm), mean (SE) | 95.1 (0.2) | 86.5 (0.5) | 91.2 (0.3) | 92.3 (0.5) | 94.7 (0.4) | 91.1 (0.4) |
| Men >102 cm; women >88 cm, % (SE) | 26.0 (1.0) | 39.4 (1.4) | 20.0 (1.1) | 57.6 (1.4) | 25.7 (1.5) | 57.1 (1.7) |
| Percent body fat (kg), % (SE) | 23.4 (0.3) | 33.5 (0.4) | 23.8 (0.4) | 37.4 (0.3) | 25.5 (0.4) | 37.4 (0.3) |
|
| ||||||
| Liver related factors | ||||||
| ALT (U/L), mean (SE) | 20.5 (0.7) | 14.1 (0.4) | 20.9 (0.6) | 12.5 (0.4) | 27.9 (1.0) | 18.3 (0.8) |
| AST (U/L), mean (SE) | 22.5 (0.3) | 19.0 (0.2) | 26.8 (0.5) | 19.2 (0.5) | 28.0 (0.8) | 21.6 (0.6) |
| AST/ALT Ratio, mean (SE) | 1.28 (0.03) | 1.57 (0.03) | 1.53 (0.04) | 1.73 (0.05) | 1.20 (0.03) | 1.39 (0.02) |
| *GGT (U/L), mean (SE) | 33.5 (1.6) | 21.5 (0.9) | 56.2 (2.7) | 36.9 (2.3) | 45.3 (2.7) | 28.4 (1.1) |
| Alkaline phosphatase (U/L), mean (SE) | 81.8 (0.8) | 75.5 (0.9) | 89.2 (1.7) | 84.1 (1.1) | 94.3 (1.1) | 88.6 (1.4) |
| Hepatitis B Seropositivity, % (SE) | 0.5 (0.2) | 0.2 (0.1) | 2.0 (0.4) | 0.6 (0.3) | 0.1 (0.1) | 0.1 (0.1) |
| Hepatitis C Seropositivity, % (SE) | 2.3 (0.5) | 1.0 (0.2) | 6.5 (1.0) | 2.7 (0.6) | 3.9 (0.6) | 2.5 (0.7) |
| Total bilirubin (mg/dL), mean (SE) | 0.74 (0.01) | 0.54 (0.01) | 0.65 (0.01) | 0.46 (0.01) | 0.70 (0.01) | 0.49 (0.01) |
| Use of zydovudine or didanosine, % (SE) | 0.2 (0.01) | 0.0 (0.00) | 0.2 (0.01) | 0.1 (0.01) | 0.0 (0.00) | 0.0 (0.00) |
|
| ||||||
| Metabolic factors | ||||||
| Diabetes, % (SE) | 6.8 (0.6) | 5.9 (0.6) | 8.9 (0.7) | 11.9(0.9) | 8.9 (0.5) | 13.4(0.8) |
| Hypertension, % (SE) | 28.7 (1.2) | 25.1 (1.0) | 35.3 (1.3) | 37.8 (1.2) | 26.1 (1.5) | 28.0 (1.2) |
| History of cardiovascular disease, % (SE) | 5.5 (0.4) | 2.4 (0.3) | 5.7 (0.5) | 5.7 (0.8) | 4.7 (0.5) | 5.0 (0.5) |
| **Fasting glucose (mg/dL), mean (SE) | 101.9 (0.6) | 93.7 (0.5) | 100.7 (1.2) | 102.4 (2.3) | 107.3 (1.2) | 101.6 (1.3) |
| HbA1c, mean (SE) | 5.32 (0.02) | 5.16 (0.03) | 5.66 (0.03) | 5.68 (0.04) | 5.56 (0.03) | 5.50 (0.03) |
| **Serum insulin (pmol/L), mean (SE) | 61.0 (2.4) | 54.6 (1.5) | 60.4 (2.3) | 73.1 (3.0) | 71.6 (3.6) | 73.3 (1.4) |
| Total cholesterol (mg/dL), mean (SE) | 202.5 (1.3) | 203.7 (1.0) | 199.8 (1.7) | 201.6 (0.8) | 205.1 (1.7) | 202.1 (1.4) |
| HDL cholesterol (mg/dL), mean (SE) | 45.0 (0.5) | 56.1 (0.5) | 52.9 (0.6) | 57.0 (0.6) | 45.7 (0.4) | 52.7 (0.5) |
| Triglycerides (mg/dL), mean (SE) | 153.4 (4.0) | 123.5 (2.4) | 120.8 (2.9) | 107.7 (1.9) | 171.4 (3.9) | 145.9 (2.7) |
GGT subsample: n=7,431
Fasting subsample: n=5,966
The overall age-adjusted prevalence of NAFLD by race was 21.2% (95% CI: 18.3%, 24.2%) in Mexican-Americans, 12.5% (95% CI: 11.3%, 13.7%) in non-Hispanic whites and 11.6% (95% CI: 9.7%, 13.5%) in non-Hispanic blacks. Similarly, the overall age-adjusted prevalence of NAFLD with elevated aminotransferases was 6.1% (95% CI: 4.9%, 7.3%) in Mexican-Americans, 2.2% (95% CI: 1.6%, 2.7%) in non-Hispanic whites, and 1.6% (95% CI: 1.1%, 2.1%) in non-Hispanic blacks. Among non-Hispanic whites only, we observed a significant gender difference in the age-adjusted prevalence of NAFLD (men: 15.0% [95% CI: 13.2%, 16.8%]; women: 10.1% [95% CI: 8.8%, 11.4%]). Further adjustment for BMI and waist circumference did not attenuate this gender difference among non-Hispanic whites.
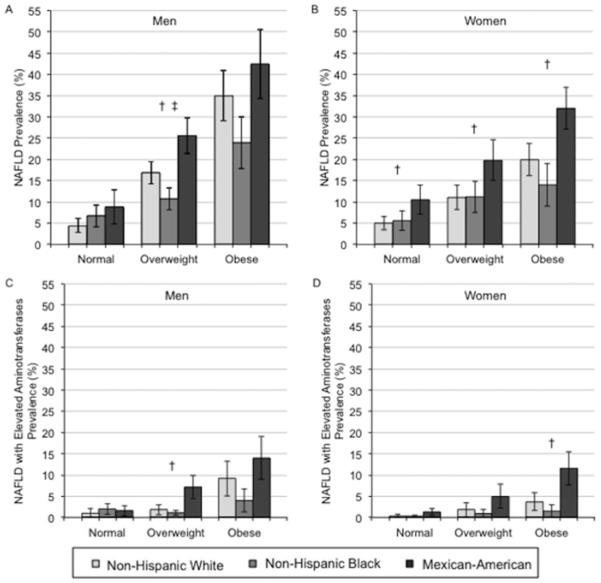
The Figure shows the age-adjusted prevalence (95% CI) of NAFLD (Panels A [men] and B [women]) and NAFLD with elevated aminotransferases (Panels C [men] and D [women]) by race and BMI categories. Among men (Panel A), non-Hispanic blacks had significantly lower age-adjusted prevalence of NAFLD and Mexican-Americans had significantly higher age-adjusted prevalence compared to non-Hispanic whites in the overweight group only. Among women (Panel B), Mexican-Americans had significantly higher age-adjusted prevalence of NAFLD compared to non-Hispanic whites for all BMI categories. Mexican-American men had significantly higher age-adjusted prevalence of NAFLD with elevated aminotransferases compared to non-Hispanic whites in the overweight group only (Panel C). Whereas Mexican-American women had significantly higher age-adjusted prevalence of NAFLD with elevated aminotransferases compared to non-Hispanic whites in the obese group only (Panel D). The gender differences in non-Hispanic whites seen in the overall age-adjusted prevalences of NAFLD were not significant among those in the normal weight category (p>0.05), but remained significant in the overweight and obese categories (p<0.05). In sensitivity analyses stratified by race, BMI category and diabetes status, among persons without diabetes, non-Hispanic blacks had significantly lower and Mexican-Americans had significantly higher age adjusted prevalence of NAFLD compared to non-Hispanic whites in the overweight or obese groups only. No differences were seen among those with diabetes (Appendix Figure 1). In sensitivity analyses stratified by race, BMI category, and physical activity status, among persons who were physically active, Mexican-Americans had significantly higher age adjusted prevalence of NAFLD compared to non-Hispanic whites in the overweight or obese groups only. Among persons who were physically inactive, Mexican-Americans had significantly higher age adjusted prevalence of NAFLD compared to non-Hispanic whites in the overweight group only (Appendix Figure 2).
Figure.
Age-adjusted prevalence (95% confidence interval) for NAFLD* (Panels A [men] and B [women]) and NAFLD with elevated aminotransferases** (Panels C [men] and D [women]) by race and body mass index group.
*NAFLD was defined as the presence of moderate or severe hepatic steatosis by ultrasound in the absence of alcohol consumption >1 drink/day for women and >2 drinks/day for men and in the absence of the current use of zydovudine or didanosine, medications shown to induce hepatic steatosis.
**NAFLD with elevated aminotransferases was defined as the presence of NAFLD and elevated ALT or AST, defined as above the upper limit of normal of the NHANES laboratory values (ALT: >40 U/L for men and >31 U/L for women; AST: >37 U/L for men and >31 U/L for women), in the absence of hepatitis B, hepatitis C, and transferrin saturation >50%.
† p<0.05 comparing Mexican-Americans to non-Hispanic whites
‡ p<0.05 comparing non-Hispanic blacks to non-Hispanic whites
The adjusted odds ratios (95% CIs) for NAFLD and NAFLD with elevated aminotransferases by race are shown in Table 2. Comparing Models 1 to 3, we observed that multiple adjustments did not substantially attenuate the association. In the fully adjusted model, compared to non-Hispanic whites, Mexican-Americans were more likely to have NAFLD (OR: 1.67, 95% CI: 1.26, 2.22) and NAFLD with elevated aminotransferases (OR: 2.84, 95% CI: 1.71, 4.72). Compared to non-Hispanic whites, non-Hispanic blacks were somewhat less likely to have NAFLD (OR: 0.93, 95% CI: 0.70, 1.25), but were significantly less likely to have NAFLD with elevated aminotransferases (OR: 0.51, 95% CI: 0.27, 0.97). Additional adjustment for percent body fat calculated from bioelectrical impedance data did not appreciably alter our results.
Table 2.
Adjusted odds ratios (95% confidence intervals) for NAFLD* and NAFLD with elevated aminotransferases** by race.
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| NAFLD (n cases=1,475) | |||
|
| |||
| Non-Hispanic White | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Non-Hispanic Black | 0.83 (0.65, 1.08) | 0.76 (0.57, 1.00) | 0.93 (0.70, 1.25) |
| Mexican-American | 1.69 (1.31, 2.19) | 1.72 (1.30, 2.26) | 1.67 (1.26, 2.22) |
|
| |||
| NAFLD with elevated aminotransferases (n cases=282) | |||
|
| |||
| Non-Hispanic White | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Non-Hispanic Black | 0.55 (0.34, 0.90) | 0.50 (0.30, 0.82) | 0.51 (0.27, 0.97) |
| Mexican-American | 2.66 (1.62, 4.36) | 2.87 (1.80, 4.56) | 2.84 (1.71, 4.72) |
Model 1: Adjustment for demographic and lifestyle factors (age, gender, education, family income, smoking, alcohol consumption, physical activity, total calories consumed/day)
Model 2: Model 1 + adjustment for adiposity factors (body mass index, waist circumference, sum of skinfolds)
Model 3: Model 2 + adjustment for metabolic factors (triglycerides, HDL cholesterol, hypertension, diabetes, HbA1c)
NAFLD was defined as the presence of moderate or severe hepatic steatosis by ultrasound in the absence of alcohol consumption >1 drink/day for women and >2 drinks/day for men and in the absence of the current use of zydovudine or didanosine, medications shown to induce hepatic steatosis.
NAFLD with elevated aminotransferases was defined as the presence of NAFLD and elevated ALT or AST, defined as above the upper limit of normal of the NHANES laboratory values (ALT: >40 U/L for men and >31 U/L for women; AST: >37 U/L for men and >31 U/L for women), in the absence of hepatitis B, hepatitis C, and transferrin saturation >50%.
In analysis restricted to the 3,802 individuals with normal weight (BMI 18.5–24.9 kg/m2), there were no significant racial differences in the fully adjusted logistic regression model for NAFLD, however Mexican-Americans remained significantly more likely to have NAFLD with elevated aminotransferases (OR: 3.40, 95% CI: 1.29, 7.18). Analyses restricted to the 3,408 overweight individuals (BMI 25.0–29.9 kg/m2) and analyses restricted to the 2,003 obese individuals (BMI 30.0–39.9 kg/m2) were not appreciably different than our main analysis. In analyses restricted to participants who self-identified as “never drinkers” (n=1,462), there were no significant racial differences for either NAFLD or NAFLD with elevated aminotransferases (p>0.05).
CONCLUSIONS
In this large, representative sample of the U.S. population, we confirmed significant racial differences in the prevalence of NAFLD. Mexican-Americans had a significantly higher prevalence of NAFLD and NAFLD with elevated aminotransferases as compared to non-Hispanic whites. Non-Hispanic blacks had significantly lower prevalence of NAFLD with elevated aminotransferases compared to non-Hispanic whites, and tended to have lower prevalence of NAFLD, but this difference was not statistically significant. Among whites only, men had a significantly higher prevalence of NAFLD compared to women. The racial and gender differences were not significant when the population was restricted to individuals with a normal BMI or when the population was restricted to individuals who self-identified as “never drinkers.”
Our results are consistent with previous studies reporting that NAFLD and NASH prevalence is higher among Hispanics and lower among blacks as compared to whites (3–6, 9) and with previous studies reporting a higher prevalence of NAFLD among white men compared to white women (4, 6). Few previous studies have included a large enough sample of black participants to draw conclusions regarding racial differences in NASH or NAFLD (3, 7–9). With the exception of the Dallas Heart Study (4, 6) which is representative of the Dallas, Texas population, previous studies investigating racial differences in NAFLD and NASH were performed in small, highly select, predominately clinic-based study populations (3, 5, 7–11), while our study is representative of the U.S. population.
Although our conclusions are largely consistent with the Dallas Heart Study, there are some differences that should be taken into consideration when comparing NHANES III and the Dallas Heart Study. First, the Dallas Heart Study used proton magnetic resonance spectroscopy to measure hepatic steatosis (4), where we used ultrasound. Ultrasound is less sensitive than proton magnetic resonance spectroscopy in quantifying hepatic steatosis compared to liver biopsy (30). Second, our population is representative of the entire U.S. population and was conducted in 1988–1994, not just one geographic area (Dallas, Texas) conducted in 2000–2002. Although our absolute estimates likely underestimate the true current prevalence of NAFLD because our data is from 1988–1994, the relative racial differences are likely representative. Finally, the Dallas Heart Study did not differentiate alcoholic fatty liver disease (4) from NAFLD in their main analysis, while we restricted the definition of NAFLD to those who consumed <2 drinks/day for men or <1 drink/day for women. However, in the Dallas Heart Study, racial differences persisted in a sensitivity analysis restricted to never drinkers (4).
The higher prevalence of NASH and NAFLD in Hispanics compared to whites might be attributable to a higher prevalence of obesity, metabolic syndrome components and insulin resistance, but blacks also have a high prevalence of obesity and metabolic syndrome components, but may be less insulin resistant for the same degree of obesity as compared to whites (3, 4, 6). However, in our analyses, adding all the components of the metabolic syndrome to the model, the racial differences in NAFLD remained significant. It has been further hypothesized that differences in NAFLD and NASH by race may be due to differences in the distribution of adiposity (e.g. subcutaneous versus visceral) or to differences in triglycerides because blacks have relatively less visceral adipose tissue and lower triglycerides than Hispanics (4, 6). Our results are consistent with those from the Dallas Heart Study (6), where adjustment for triglycerides and subcutaneous fat (skinfold measurements) failed to fully account for the differences in prevalence by race. Additionally, racial differences in our population were largely attenuated when we restricted our population to individuals with a normal BMI. These results are consistent with the Dallas Heart Study (6), where additional adjustment for visceral fat content nearly abolished the racial differences. The Dallas Heart Study also found no difference in the presence of hepatic steatosis among non-obese individuals (4). Our results are also consistent with a study by Lomonaco et al. that matched Hispanic and Caucasian participants on adiposity measured by dual energy x-ray absorptiometry and found no significant racial differences in biopsy proven NASH and insulin resistance (measured by insulin clamp) (7). Together, these results are consistent with the hypothesis that differences in the distribution of adipose tissue may be driving the racial differences in NAFLD because the racial differences are not seen until a threshold level of adiposity exists.
Similarly, we found that racial differences were not significant when the population was restricted to individuals who self-identified as “never drinkers.” There are a few hypotheses that could explain this observation: first, there is differential reporting of alcohol consumption by race, second, even low/moderate levels of alcohol consumption may increase the risk for NAFLD/NASH in Mexican-Americans as compared to blacks and whites (31), and third, minor differences in low-level alcohol consumption between races (alcohol as a confounder) may be contributing to the racial differences.
Our finding of a significant gender difference among non-Hispanic whites is also consistent with findings from the Dallas Heart Study (4, 6). Similar to our results, they found that the gender differences in whites were explained by differences in low amounts of alcohol consumption. Differences in low-level alcohol consumption between men and women (alcohol as a confounder) may be driving the gender differences seen among non-Hispanic white participants. Indeed, in our population men were more likely to self-identify as “low current drinkers” (men ≤2 drinks/day; women ≤1 drink/day) compared to women (men: 55%, women 43%). Alternatively, differences in the hepatic metabolism of alcohol between men and women may be contributing to the gender differences in non-Hispanic whites.
Certain limitations of this study should be considered when interpreting our results. Although ultrasonography is widely used to detect hepatic steatosis in clinical settings and large population-based studies (sensitivity: 85%, specificity: 94% (19)), we were not able to distinguish histologically between NAFLD and NASH in our population. However, we were able to perform an analysis looking at hepatic steatosis with elevated aminotransferases, but this analysis also has limitations. Although the sensitivity of elevated aminotransferases for the presence of hepatic inflammation is low, aminotransferases are often measured clinically as an initial screening test for liver dysfunction (19). Although we had several measurements of adiposity including BMI, waist circumference, and the skinfolds, we did not have a direct measure of visceral adiposity, which has been postulated to be most closely associated with NAFLD and NASH (6). Our stratified analyses had less power than our main analysis and should be interpreted cautiously. However, our study also has a number of important strengths. The large NHANES III study population is representative of the entire U.S. population and the participants are well characterized by interview, examination, laboratory, and hepatic ultrasound data that were collected by trained staff following standardized protocols. The large sample size enabled stratified analyses, adjustment for multiple risk factors, and sensitivity analyses in subsets of the population.
In conclusion, in this representative sample of the U.S. population, we found significant racial differences in the prevalence of NAFLD, and significant gender differences in NAFLD among non-Hispanic whites. Our results suggest that the burden of NAFLD is high among Mexican-American and lowest among non-Hispanic blacks. In our stratified analyses, we attempted to more fully control for strong confounders of the association between NAFLD and race, namely obesity and alcohol consumption. While we did not observe statistically significant differences in the prevalence of NAFLD across race/ethnic group among the normal weight group or “never drinkers”, we cannot rule out the possibility that ultrasound is not sensitive enough to detect smaller amounts of hepatic fat and thus we have limited ability to examine differences at the low end of the distribution. In the Dallas Heart study, the prevalence of NAFLD defined as >5.5% liver fat by magnetic resonance, was not statistically significant different between non-obese white versus non-obese Hispanic (20% vs. 26%, p=0.12), but non-obese blacks had a significantly lower prevalence (11%). More work is needed to identify factors that may contribute to the observed racial discrepancies.
What is already known about this subject?
Nonalcoholic fatty liver disease (NAFLD) prevalence differs by race.
Reasons for racial differences in the prevalence of NAFLD remain unclear.
Previous studies did not have the opportunity to examine racial differences in NAFLD prevalence using a nationally representative study population.
What does this study add?
Racial differences in the prevalence of NAFLD exist in a nationally representative sample of the U.S. population.
The racial differences were not fully explained by several lifestyle, adiposity and metabolic factors. More works is needed to identify potential contributors.
ACKNOWLEDGEMENTS
A.L.C.S. was supported by NIH/NIDDK training grant T32 DK062707. E.S. was supported by NIH/NIDDK grants K01 DK076595 and R01 DK089174. J.M.C. and M.L. were supported by grant R01 DK083393 from NIH/NIDDK. M.L. was also supported by the American Diabetes Association.
Appendix Figure 1.
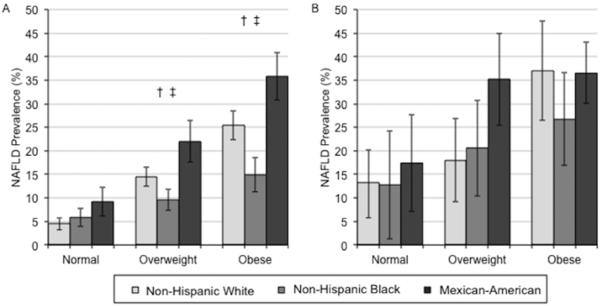
Age-adjusted prevalence (95% confidence interval) for NAFLD* by race and body mass index group among participants without diabetes (Panel A) and among participants with diabetes (Panel B).
*NAFLD was defined as the presence of moderate or severe hepatic steatosis by ultrasound in the absence of alcohol consumption >1 drink/day for women and >2 drinks/day for men and in the absence of the current use of zydovudine or didanosine, medications shown to induce hepatic steatosis.
† p<0.05 comparing Mexican-Americans to non-Hispanic whites
‡ p<0.05 comparing non-Hispanic blacks to non-Hispanic whites
Appendix Figure 2.
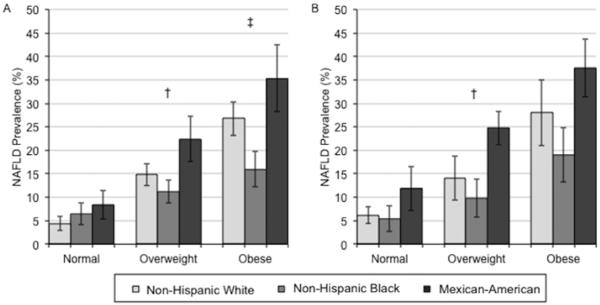
Age-adjusted prevalence (95% confidence interval) for NAFLD* by race and body mass index group among physically active participants (Panel A) and among physically inactive participants (Panel B).
*NAFLD was defined as the presence of moderate or severe hepatic steatosis by ultrasound in the absence of alcohol consumption >1 drink/day for women and >2 drinks/day for men and in the absence of the current use of zydovudine or didanosine, medications shown to induce hepatic steatosis.
† p<0.05 comparing Mexican-Americans to non-Hispanic whites
‡ p<0.05 comparing non-Hispanic blacks to non-Hispanic whites
Footnotes
No potential conflicts of interest relevant to this article were reported. All participants provided written informed consent. NHANES III data is publically available at http://www.cdc.gov/nchs/nhanes/nh3data.htm.
REFERENCES
- 1.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98(5):960–7. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 2.Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11(1):1–16. vii. doi: 10.1016/j.cld.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Bambha K, Belt P, Abraham M, Wilson LA, Pabst M, Ferrell L, et al. Ethnicity and nonalcoholic fatty liver disease. Hepatology. 2012;55(3):769–80. doi: 10.1002/hep.24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell SH, Harris DM, Patrie JT, Hespenheide EE. Is NASH underdiagnosed among African Americans? The American journal of gastroenterology. 2002;97(6):1496–500. doi: 10.1111/j.1572-0241.2002.05795.x. [DOI] [PubMed] [Google Scholar]
- 6.Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49(3):791–801. doi: 10.1002/hep.22726. PMCID: 2675577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lomonaco R, Ortiz-Lopez C, Orsak B, Finch J, Webb A, Bril F, et al. Role of ethnicity in overweight and obese patients with nonalcoholic steatohepatitis. Hepatology. 2011;54(3):837–45. doi: 10.1002/hep.24483. [DOI] [PubMed] [Google Scholar]
- 8.Tabibian JH, Lazo M, Durazo FA, Yeh HC, Tong MJ, Clark JM. Nonalcoholic fatty liver disease across ethno-racial groups: do Asian-American adults represent a new at-risk population? Journal of Gastroenterology and Hepatology. 2011;26(3):501–9. doi: 10.1111/j.1440-1746.2010.06443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41(2):372–9. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- 10.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140(1):124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 11.Mohanty SR, Troy TN, Huo D, O'Brien BL, Jensen DM, Hart J. Influence of ethnicity on histological differences in non-alcoholic fatty liver disease. Journal of Hepatology. 2009;50(4):797–804. doi: 10.1016/j.jhep.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics. 2008;40(12):1461–5. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagenknecht LE, Palmer ND, Bowden DW, Rotter JI, Norris JM, Ziegler J, et al. Association of PNPLA3 with non-alcoholic fatty liver disease in a minority cohort: the Insulin Resistance Atherosclerosis Family Study. Liver international : official journal of the International Association for the Study of the Liver. 2011;31(3):412–6. doi: 10.1111/j.1478-3231.2010.02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13(4):511–31. doi: 10.1016/j.cld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Caldwell SH, Ikura Y, Iezzoni JC, Liu Z. Has natural selection in human populations produced two types of metabolic syndrome (with and without fatty liver)? J Gastroenterol Hepatol. 2007;22(Suppl 1):S11–9. doi: 10.1111/j.1440-1746.2006.04639.x. [DOI] [PubMed] [Google Scholar]
- 16.Zoratti R. A review on ethnic differences in plasma triglycerides and high-density-lipoprotein cholesterol: is the lipid pattern the key factor for the low coronary heart disease rate in people of African origin? European Journal of Epidemiology. 1998;14(1):9–21. doi: 10.1023/a:1007492202045. [DOI] [PubMed] [Google Scholar]
- 17.Bellentani S, Tiribelli C, Saccoccio G, Sodde M, Fratti N, De Martin C, et al. Prevalence of chronic liver disease in the general population of northern Italy: the Dionysos Study. Hepatology. 1994;20(6):1442–9. doi: 10.1002/hep.1840200611. [DOI] [PubMed] [Google Scholar]
- 18.Nomura H, Kashiwagi S, Hayashi J, Kajiyama W, Tani S, Goto M. Prevalence of fatty liver in a general population of Okinawa, Japan. Jpn J Med. 1988;27(2):142–9. doi: 10.2169/internalmedicine1962.27.142. [DOI] [PubMed] [Google Scholar]
- 19.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology. 2011;54(3):1082–90. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Analytical and Reporting Guidelines: The Third National Health and Nutrition Examination Survey, NHANES III (1988–1994) National Center For Health Statistics, Center for Disease Control and Prevention; Hyatsville, MD: 1996. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes3/nh3gui.pdf. [Google Scholar]
- 21.National Center for Health Statistics; Center for Disease Control and PRevention, editor. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Department of Health and Human Services publication; Washington, D.C.: 1994. [Google Scholar]
- 22.Kuczmarski RJ. Bioelectrical impedance analysis measurements as part of a national nutrition survey. The American journal of clinical nutrition. 1996;64(3 Suppl):453S–8S. doi: 10.1093/ajcn/64.3.453S. [DOI] [PubMed] [Google Scholar]
- 23.Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnson CL, Heymsfield SB, et al. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26(12):1596–609. doi: 10.1038/sj.ijo.0802167. [DOI] [PubMed] [Google Scholar]
- 24.Laboratory procedures used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. U.S. Department of Health and Human Services, Center for Disease Control and Prevention, National Center for Environmental Health, National Center for Health Statistics; Bethesda, MD: 1996. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/labman.pdf. [Google Scholar]
- 25.Third National Health and Nutrition Examination Survey: Hepatic Steatosis Ultrasound Images Assessment Procedures Manual. National Center For Health Statistics, Center for Disease Control and Prevention; Hyattsville, MD: 2011. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes3/Hepatic_Steatosis_Ultrasound_Procedures_Manual.pdf. [Google Scholar]
- 26.Mottin CC, Moretto M, Padoin AV, Swarowsky AM, Toneto MG, Glock L, et al. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obesity Surgery. 2004;14(5):635–7. doi: 10.1381/096089204323093408. [DOI] [PubMed] [Google Scholar]
- 27.Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54(1):344–53. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stata . Stata Statistical Software: Release 11. StataCorp; College Station, TX: 2009. [Google Scholar]
- 30.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative Assessment of Liver Fat with Magnetic Resonance Imaging and Spectroscopy. J Magn Reson Imaging. 2011;34(4) doi: 10.1002/jmri.22580. spcone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart SH. Racial and ethnic differences in alcohol-associated aspartate aminotransferase and gamma-glutamyltransferase elevation. Archives of Internal Medicine. 2002;162(19):2236–9. doi: 10.1001/archinte.162.19.2236. [DOI] [PubMed] [Google Scholar]