Abstract
Objective
Free fatty acids (FFAs) are increased in visceral fat and contribute to insulin resistance through multiple mechanisms, including c-Jun N-terminal kinase (JNK) activation and expression of TNFα. Given that IGF-I-mediated proliferation is impaired in omental compared to subcutaneous (sc) preadipocytes, we investigated IGF-I anti-inflammatory action in preadipocytes from sc and omental adipose tissue.
Methods
Preadipocytes isolated from abdominal sc and omental fat of obese subjects were studied in primary culture. Cells were exposed to FFAs with or without IGF-I pretreatment followed by analysis of cytokine expression and JNK phosphorylation. Lentivirus infection was used to express a constitutively active AKT (myr-AKT) in omental preadipocytes.
Results
FFAs increased expression of TNFα, IL-6 and MCP-1 in sc and omental preadipocytes. IGF-I pretreatment reduced FFA-induced JNK1 phosphorylation and TNFα expression in sc but not omental preadipocytes. Treatment with the JNK1/2 inhibitor SP600125 reduced FFAinduced expression of TNFα. FFAs and MALP-2, a specific TLR2/6 ligand, but not specific ligands for TLR4 and TLR1/2, increased JNK1 phosphorylation. IGF-I completely inhibited MALP-2-stimulated phosphorylation of JNK1. Expression of myr-AKT in omental preadipocytes inhibited FFA-stimulated JNK1 phosphorylation.
Conclusions
IGF-I attenuates FFA-induced JNK1 phosphorylation and TNFα expression through activation of AKT in human subcutaneous but not omental preadipocytes.
Keywords: subcutaneous, omental, preadipocyte, IGF-I, FFA, JNK, TNFα
INTRODUCTION
Obesity develops through adipocyte hyperplasia (increased proliferation and differentiation of preadipocytes) and hypertrophy (increased lipid storage), but morbidities such as cardiovascular disease are associated with excess visceral fat, insulin resistance and chronic inflammation(1). Visceral and subcutaneous (sc) fat are active metabolic tissues but with significant differences in adipogenesis, adipokine production and inflammation(2;3). In fact, accumulating evidence indicates that chronic inflammation in visceral adipose tissue, including accumulation of macrophages and production of cytokines such as TNFα and IL-6, leads to insulin resistance and its associated morbidities(4).
Free-fatty acids (FFAs), originating from increased lipolysis of visceral hypertrophic adipocytes, contribute to the inflammatory milieu in visceral fat through the family of Toll-like receptors (TLR)(5). TLRs expressed in adipose tissue mediate both innate immunity and inflammation (6). TLRs are overexpressed in human omental compared to sc fat tissue(7), with TLR4 expression highest in adipocytes(8). Human adipocytes and preadipocytes express TLR1, TLR2, TLR3, TLR4 and TLR6(9). FFAs activate TLR4 and TLR2 partnered with TLR1 or TLR6(5). Activation of TLR4, TLR2/1 and TLR2/6 leads to stimulation of specific signaling pathways followed by expression of certain cytokines, including TNFα, IL-6 and MCP-1(8;9).
Preadipocytes make up a significant portion of cells in white adipose tissue, providing a stable pool of adipocyte precursors for adipogenesis(10). However, it has become increasingly clear that preadipocytes are also secretory cells that have a “macrophage-like” phenotype (11;12) and produce a number of cytokines, including TNFα, IL-6 and MCP-1(13). Preadipocytes exhibit depot-specific differences in gene expression(14;15), proliferation(16), differentiation(2) and fatty acid handling(17). We have previously shown that IGF-I activation of AKT is impaired in human omental compared to sc preadipocytes from obese subjects (18), suggesting intrinsic depot-specific differences in response to IGF-I.
In 3T3-L1 adipocytes, a mixture of saturated and unsaturated FFAs increased the expression of TNFα through the activation of the JNK pathway(19), and the fatty acid palmitate induced the expression of IL-6 more than TNFα through the NFκB pathway(20). In human preadipocytes, the potent TLR4 agonist LPS stimulated TNFα, IL-6 and MCP-1 through the NFκB and ERK pathways(21). Although FFAs have been shown to impair IGF-I signaling in 3T3-L1 preadipocytes (22), evidence that IGF-I inhibits FFA-induced TNFα expression in 3T3- L1 adipocytes (23) suggests crosstalk between IGF-I and FFA-mediated inflammatory pathways.
Given the numerous differences in preadipocyte development and function between visceral and sc fat depots, including our previous observation that IGF-I action is impaired in omental preadipocytes(18), we hypothesized that the inflammatory characteristics of preadipocytes and the role of IGF-I as an anti-inflammatory agent would be different between depots. In this study, we characterize FFA-induced inflammatory pathways and cytokine expression in IGF-I-treated human preadipocytes isolated from abdominal sc and omental adipose tissue.
METHODS and PROCEDURES
Materials
Tissue culture reagents were purchased from GibcoBRL (Life Technologies, Grand Island, NY). Buffer reagents were purchased from Sigma (St. Louis, MO). Enhanced chemiluminescence reagents were purchased from Amersham Life Science (Arlington Heights, IL). Polyvinylidene fluoride membranes were purchased from Bio-Rad Laboratories (Hercules, CA). Antibodies for Western blot analysis were obtained from Cell Signaling Technology (Beverly, MA). Human recombinant IGF-I was obtained from GroPep (Adelaide, Australia). FFA mixture of oleic, linoleic, arachadonic, lauric and myristic acids (Sigma) in 0.5% BSA was used as described(19). Specific TLR agonists LPS (TLR4 agonist), Pam3CSK4 (TLR1/2 agonist) and MALP-2 (TLR2/6 agonist) were purchased from InvivoGen (San Diego, CA). MAPK inhibitors for ERK, p38 and JNK were purchased from Cell Signaling Technology (Danvers MA). The AKT 1/2 kinase inhibitor was purchased from Sigma (#A6730).
Isolation and culture of human preadipocytes
Fat tissue was obtained during intraabdominal surgery from 16 obese but nondiabetic subjects [mean (SD) age 44.5 yr (2.6); body mass index 54 kg/m2 (7.1); 66% female, 100% Caucasian; fasting glucose 120.8 mg/dl (3.7); and fasting insulin 17.3 µU/ml (3.4)] who had given informed consent. The protocol was approved by the Boston University Medical Center and Mayo Clinic Foundation Institutional Review Boards for Human Research. All subjects fasted 10 h before surgery. Subjects with malignancies or taking thiazolidinediones or steroids were excluded. Abdominal sc (outside the fascia superficialis), mesenteric, and greater omental fat were obtained in parallel. Fat tissue was digested, filtered, centrifuged, treated with an erythrocyte lysis buffer and plated. Replating was done after 18 hours and then cells were frozen. Aliquots of donor-specific sc and omental preadipocytes identified only by number were thawed in parallel and passaged three to five times before use in experiments. Since endothelial cells and macrophages are less sensitive to trypsin than preadipocytes, replating and passaging leave them behind in differential plating. Furthermore, macrophages do not divide. This method yields essentially pure preadipocyte populations with less than 5 macrophages per million cells by microscopy irrespective of depot origin as described previously (2). We have also demonstrated that cultures of preadipocytes prepared using this method are free of macrophage or endothelial-specific markers using Affymetrix U133A arrays(24).
Experiments compared sc and omental preadipocytes in parallel from a single donor, and repeat experiments used sc and omental cells in parallel from different donors. Cell monolayers were cultured in α modified Eagle’s medium containing 10% heat-inactivated fetal bovine serum (FBS) and antibiotics, used at 50–70% confluence, and placed in serum-free medium with 0.1% BSA overnight before treatment and analysis.
Cytokine and TLR gene expression by real-time RT-PCR
Total RNA was isolated using TriReagent (Molecular Research, Inc., Cincinnati, OH). cDNA was synthesized from 2µg total RNA with TaqMan Reverse Transcription reagents (Applied Biosystems, Framingham, MA) and assayed using Power SYBR Green RT-PCR Reagents kit (Applied Biosystems) and ABI Prism thermal cycler model 7500 (Applied Biosystems) with 28S as an internal control. Relative expression of PCR products was calculated using the ΔΔCt method, and melt curve analysis was performed to assess the specificity of each PCR.
Primers based on published sequences for TLRs (9) and commercially-available primers for TNFα, IL-6 and MCP-1 were purchased from Integrated DNA Technologies (Coralville, IA).
Western blot analysis
Total cellular lysates were obtained using RIPA buffer [0.15 mM NaCl, 0.05 mM TrisHCl (pH 7.2), 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate] with 0.2% Triton X-100. Proteins were resolved by SDS-PAGE, transferred to polyvinylidene fluoride membranes, blocked in 1% BSA in Tris-buffered saline with 0.1% Triton X-100, and probed with primary antibody at 1:1000 dilution. The blots were washed extensively, probed with secondary antibody for 1 h at room temperature, and then washed again. Specific binding was visualized using enhanced chemiluminescence followed by detection with a ChemiDoc-It Imaging System, UPV Labworks (Upland, CA).
Lentivirus expression of myristoylated AKT
Full length myr-AKT1 in the pUSEamp vector was purchased from Millipore (Billerica, MA), subcloned into the pLenti-His vector (Applied Biological Materials, Richmond BC Canada) and then sent back to Applied Biological Materials to be packaged into lentivirus, propagated and purified. High-titer replication-incompetent myr-AKT-lentivirus and Lenti-GFP (Applied Biological Materials) was stored at −20 C until use.
Low-passage omental preadipocytes were cultured in 6-well plates at ~50% confluence and incubated with 1 ml/well viral supernatant in 1% FBS and 8µg/ml polybrene overnight. The following day, viral supernatant was removed and replaced with complete growth medium for 72 hours. Cells that received Lenti-GFP were monitored by microscopy, and if >80% were positive, then monolayers were serum-starved overnight for experiments the following morning.
Statistical analysis
Data are expressed as mean ± SEM. Differences were analyzed with Student’s t test or ANOVA using Prism version 4 (GraphPad Software, Inc., San Diego, CA). A p value <0.05 was considered statistically significant.
RESULTS
FFA-induced cytokine expression in sc and omental preadipocytes
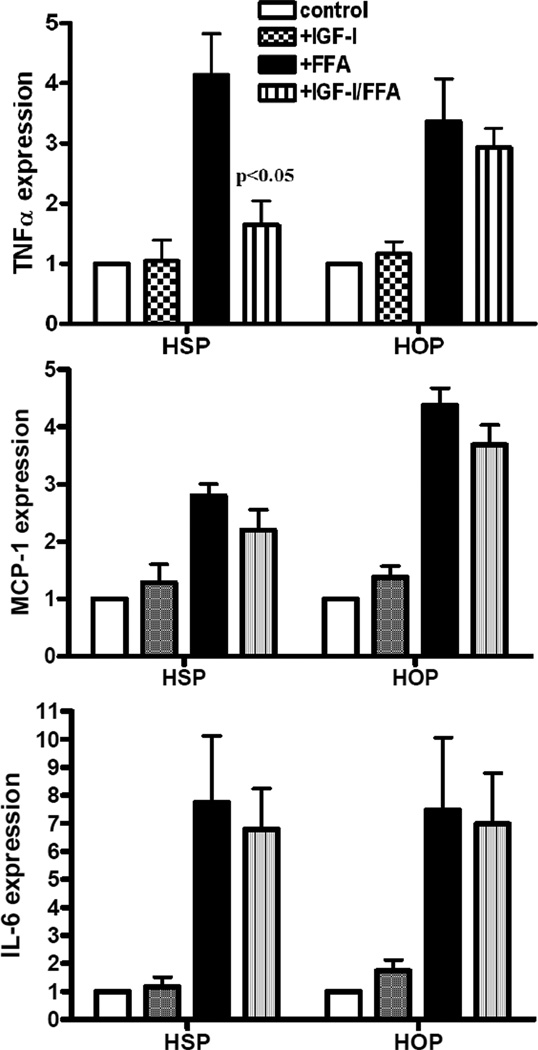
Given our previous observations that IGF-I-mediated proliferation is impaired due to aberrant AKT signaling in omental compared to sc human preadipocytes(18), we hypothesized that the inflammatory characteristics of preadipocytes or the role of IGF-I as an anti-inflammatory agent would be different between depots. In order to determine the effect of FFAs on human preadipocytes from different depots, we treated sub-confluent preadipocytes from sc and omental fat tissue with a mixture of saturated and unsaturated FFAs composed of the common dietary fats oleic, linoleic, arachadonic, lauric and myristic acids for 4 hours with or without IGF-I pretreatment for 1 hour and then analyzed RNA for TNFα, IL-6 and MCP-1 expression (Figure 1). FFAs increased expression of all 3 cytokines in preadipocytes from both depots. IL-6 increased 7 fold whereas TNFα and MCP-1 increased 3–4 fold. There were no statistical differences in TNFα, IL-6 or MCP-1 expression between depots (Figure 1, closed bars).
Figure 1.
FFAs increase cytokine expression in human preadipocytes. Human sc (HSP) and omental (HOP) preadipocytes were serum-starved for 12 hours then pretreated with or without 10 nM IGF-I for 1 hr followed by 0.1mM FFAs for 4 hours prior to RNA isolation. RNA was analyzed by quantitative RT-PCR using Syber green and primers for TNFα, MCP-1 and IL-6. Data are presented as mean +SE, n= 3, p<0.05 in FFA-treated compared to IGF-I plus FFA-treated.
IGF-I treatment alone had no effect on cytokine expression; however, IGF-I treatment significantly decreased FFA-induced TNFα expression in human sc but not omental preadipocytes (Figure 1, striped bars). IGF-I pretreatment had no effect on FFA-induced MCP-1 or IL-6 expression in either human sc or omental preadipocytes.
FFA activation of TLR signaling pathways in human preadipocytes
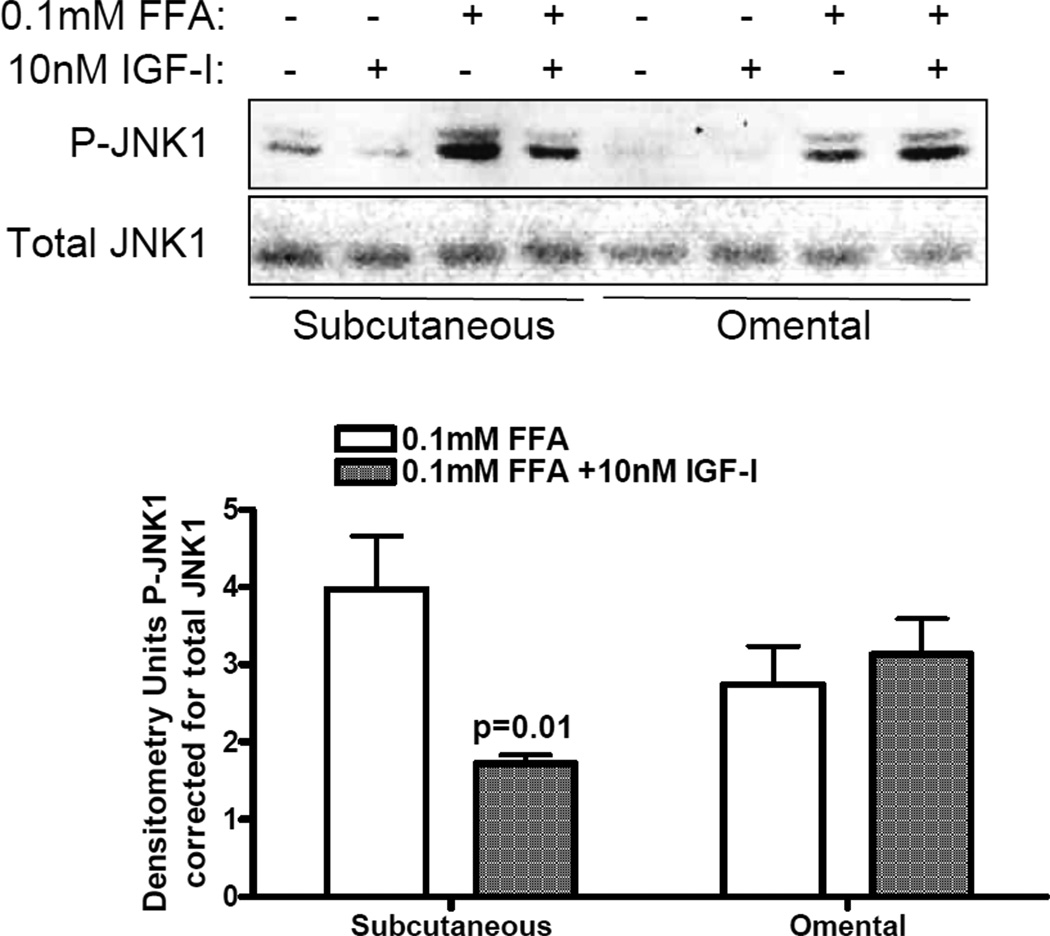
This mixture of FFAs has been shown to activate the stress kinases JNK and IKK and to induce TNFα expression via JNK activation in adipocytes(19), therefore we examined JNK and IKK phosphorylation by FFAs in the presence or absence of IGF-I. Figure 2 shows a representative Western blot of phospho-JNK and total JNK using antibodies that recognize both JNK1 and JNK2 isoforms. FFAs increase phosphorylation of predominantly JNK1 in both sc and omental preadipocytes, and there appears to be negligible JNK2 present in total lysates. IGF-I pretreatment reduced FFA-induced JNK1 phosphorylation by ~50% in sc but not omental preadipocytes, consistent with reduction of FFA-induced TNFα expression. However, FFAs at concentrations ranging 0.1mM to 1mM did not increase the phosphorylation of IKKα or IKKβ, and IKK and NFκB were not detectable in total lysates by Western blot (data not shown).
Figure 2.
IGF-I attenuates JNK activation by FFAs in sc but not omental preadipocytes. Human sc (HSP) and omental (HOP) subconfluent cells were serum-starved overnight then pretreated with 10nM IGF-I for 1hr followed by 0.1mM FFAs for 20 min. Cell lysates were analyzed by WB using phospho-JNK and total JNK antibodies. A representative blot is shown. Data are presented as mean + SE of densitometry analyses of FFA-treated compared to IGF-I plus FFA treatment, p=0.01, n=3.
Both murine and human adipose tissue (6;9;25) express numerous members of the TLR family. We confirmed expression of TLR1, TLR2, TLR4 and TLR6 in sc and omental preadipocytes with no differences in TLR expression between depots (data not shown). We also found no effect of IGF-I treatment for 24 hours or FFA treatment for 4 hours on expression of any of these TLRs (data not shown). FFA treatment of preadipocytes overnight resulted in significant apoptosis (data not shown), so RNA could not be analyzed for TLR expression.
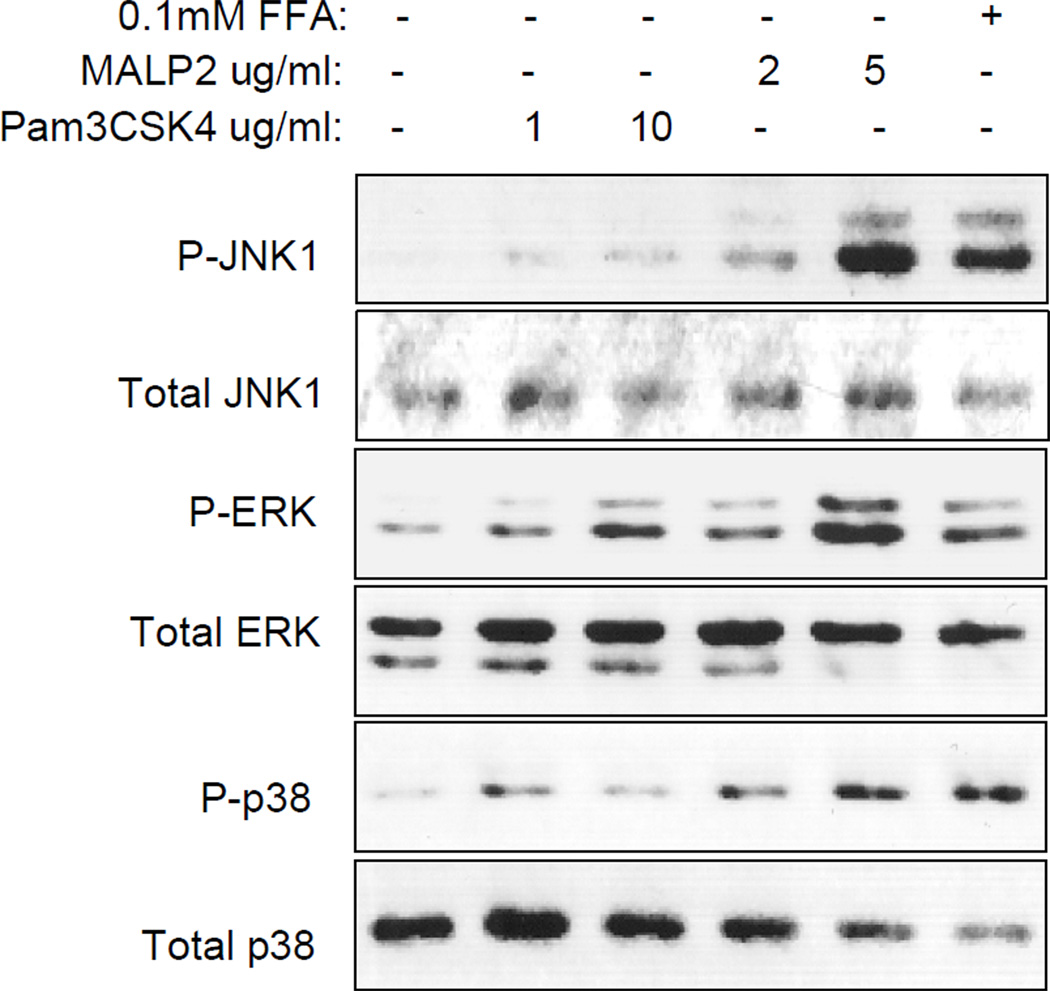
Given that IGF-I inhibition of FFA-induced JNK1 phosphorylation and TNFα expression only occurs in human sc preadipocytes, we focused on characterizing the TLR signaling pathways mediating effects of FFAs in sc preadipocytes. Following preliminary experiments to optimize concentration and time of ligand treatment, we compared phosphorylation of the ERK, JNK and p38 pathways by FFAs and specific TLR ligands, including LPS (TLR4), macrophage-activating lipopeptide-2 (MALP-2; TLR2/6) and Pam3CSK4 (TLR1/2). Figure 3 shows a representative Western blot analyzing cell lysates from sc preadipocytes treated with FFAs, MALP-2 or Pam3CSK4. We found that FFAs increase phosphorylation of all three MAP kinase pathways as does MALP-2 but Pam3CSK4 activates predominantly ERK with barely detectable p38 phosphorylation. LPS also increased phosphorylation of ERK and p38 with negligible phosphorylation of JNK1 (data not shown). These results suggest that of the TLRs expressed in human sc preadipocytes, TLR2/6 is the major activator of JNK1.
Figure 3.
FFA and MALP-2, but not Pam3CSK4 increase phosphorylation of JNK. Human sc preadipocytes were serum-starved overnight and treated with Pam3CSK4 1µg/ml or 10µg/ml, MALP-2 2µg/ml or 5µg/ml or FFAs 0.1mM for 20 minutes followed by WB analysis of cell lysates using phospho and total JNK, ERK and p38 antibodies. Representative blot is shown; this was repeated twice with similar results.
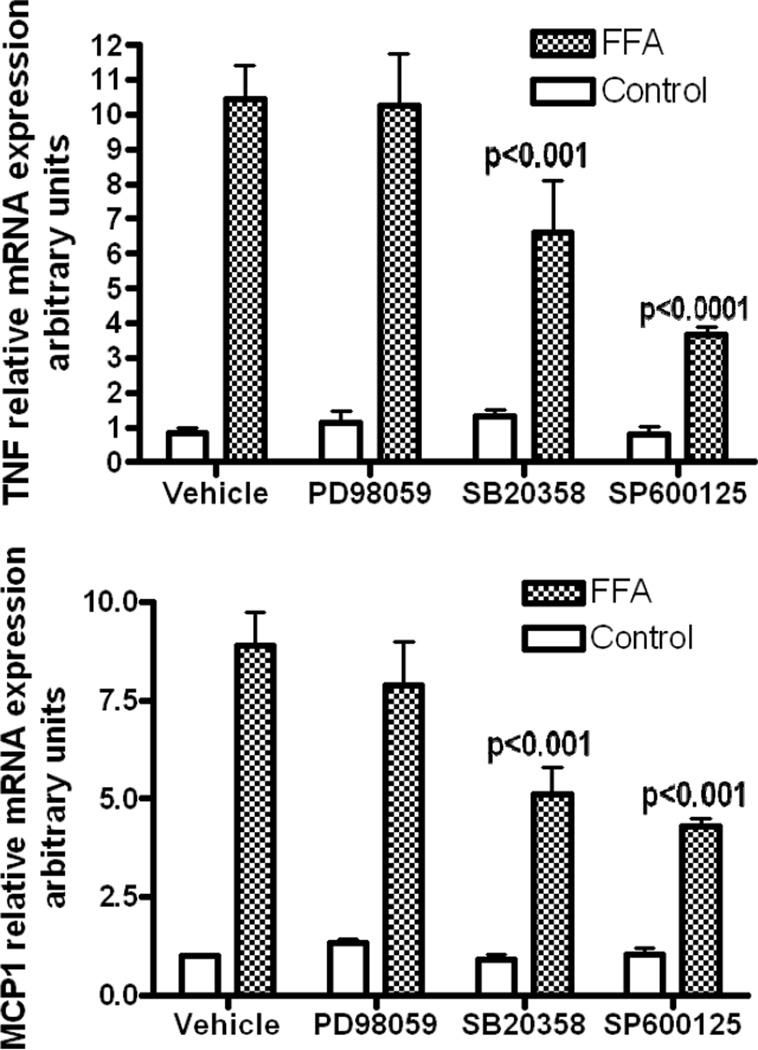
In order to confirm that the JNK pathway is critical to FFA-mediated TNFα expression, we analyzed FFA-induced expression of TNFα, MCP-1, and IL-6 in the presence of specific MAP kinase inhibitors. Human sc preadipocytes were pretreated with various inhibitors prior to FFA treatment and then RNA analyzed by quantitative RT-PCR (Figure 4). In the presence of p38 (SB20358) and JNK (SB600125) inhibitors, FFA-induced TNFα expression decreased by 37% (p<0.001) and 65% (p<0.0001) respectively, while MCP-1 expression decreased by 42.6% (p<0.001) and 51.7% (p<0.001) respectively. FFA-induced IL-6 expression decreased only in the presence of the p38 inhibitor (results not shown). These results suggest that FFAs do not signal through ERK to induce TNFα, MCP-1 and IL-6, but that JNK and p38 are important pathways for FFA-induction of TNFα and MCP-1 expression.
Figure 4.
FFA-induced cytokine expression is decreased by p38 and JNK inhibition. Human sc preadipocytes were serum-starved for 12 hours then pretreated for 1 hour with control, 50µM ERK inhibitor PD098059, 50µM p38 inhibitor SB20358 or 50µM JNK inhibitor SP600125 prior to 0.1mM FFAs for 4 hours. RNA was isolated for analysis by quantitative RT-PCR using Syber green and primers for A. TNFα expression: data are presented as mean +SE, n=3, p<0.001 FFA-treated compared to SB20358 plus FFAs; p<0.0001 FFA-treated compared to SP600125 plus FFAs and B. MCP-1 expression: data are presented as mean +SE, n=3, p<0.001 FFA-treated compared to SB20358 plus FFAs; p<0.001 FFA-treated compared to SP600125 plus FFAs.
IGF-I inhibits TLR2/6 activation of JNK1
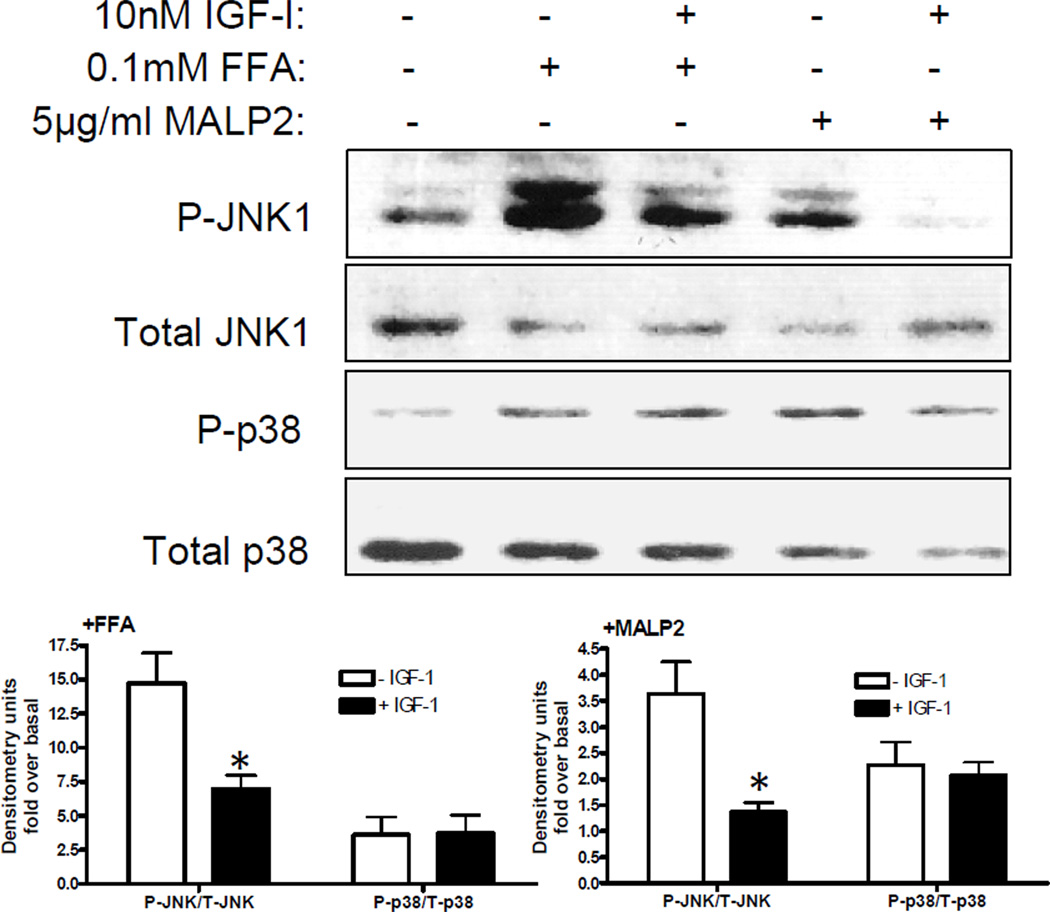
Given that FFAs and MALP-2 phosphorylate JNK1 and p38 and that FFA-induced TNFα expression requires JNK and p38 activation, we tested the hypothesis that the attenuation of FFA-induced JNK1 activation by IGF-I occurs through crosstalk with TLR 2/6. We pretreated sc preadipocytes with IGF-I for 1hr, then with the specific TLR2/6 agonist MALP-2 or FFAs followed by Western blot analysis of phospho and total JNK and p38 (Figure 5). IGF-I partially inhibited FFA activation of JNK1 and not p38 but IGF-I completely blocked MALP-2 activation of JNK1 with no effect on p38. Others have shown that like FFAs, LPS specific activation of TLR4 induces TNFα expression in human preadipocytes(21), but we found no effect of IGF-I on LPS-stimulated JNK1 phosphorylation (data not shown). These results suggest that IGF-I attenuation of JNK1 activation by FFAs in sc preadipocytes occurs through crosstalk with TLR2/6-activated JNK1 pathway.
Figure 5.
IGF-I decreases FFA and MALP-2-stimulated JNK phosphorylation. Human sc preadipocytes were serum-starved overnight and then pretreated with 10nM IGF-I for 1hr followed by control, MALP-2 5µg/ml or FFAs 0.1mM for 20 minutes. Total cell lysates were analyzed by WB using phospho and total JNK and p38 antibodies. A representative blot is shown and data are presented as mean + SE of densitometry analyses of fold over basal (phospho-JNK and phospho-p38 corrected for total JNK and total p38) of 3 experiments; *p<0.05.
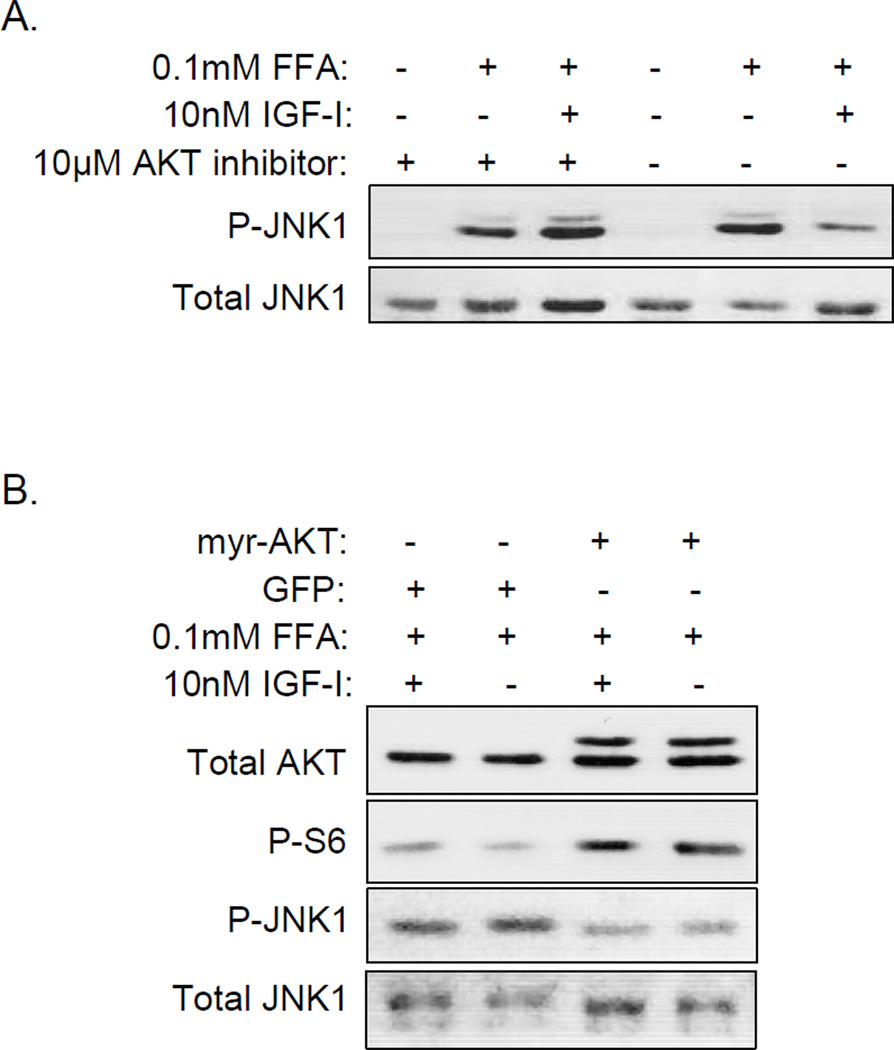
AKT inhibits FFA-stimulated JNK1 phosphorylation
Other studies have shown that IGF-I inhibition of JNK requires AKT(26), so given our previous findings that IGF-I activation of AKT is impaired in omental preadipocytes(18), we hypothesized that IGF-I attenuates JNK phosphorylation in sc but not omental preadipocytes through AKT activation. First, we pretreated sc preadipocytes with a specific chemical inhibitor of AKT(27), exposed them to IGF-I and FFAs and then analyzed JNK1 phosphorylation (Figure 6A). Inhibition of AKT prevented IGF-I attenuation of FFA-activated JNK1, suggesting that AKT mediates IGF-I action on JNK1. In order to obtain more direct evidence of AKT action on JNK1, we infected omental preadipocytes with lentivirus expressing a constitutively active form of AKT, myristoylated AKT(28) (myr-AKT) or GFP as a control and then treated the cells with FFAs with or without IGF-I pretreatment (Figure 6B). We found that omental cells expressing myr-AKT had increased S6 phosphorylation confirming AKT activity and reduced FFAstimulated JNK1 phosphorylation compared to GFP control cells, confirming inhibition of JNK1 by AKT.
Figure 6.
AKT regulates JNK phosphorylation in both sc and omental preadipocytes. A. Human sc preadipocytes were serum-starved overnight, pretreated with 10µM AKT inhibitor or its diluent DMSO for 1 hour then treated with 10nM IGF-I for 1 hour followed by 0.1mM FFAs for 20 minutes prior to generation of cell lysates for analysis by WB using phospho and total JNK antibodies. This experiment was repeated using cells from a different donor with similar results. B. Human omental cells were transduced with lentivirus containing GFP or myr-AKT, allowed to recover for 3 days, serum-starved overnight, treated with or without 10nM IGF-I followed by 0.1mM FFAs for 20 minutes. Total cell lysates were analyzed by WB using total AKT, phospho-S6, total and phospho JNK antibodies. This experiment was repeated using cells from a different donor with similar results.
DISCUSSION
We investigated the effects of a mixture of FFAs on primary cultures of preadipocytes isolated from abdominal sc and omental fat of obese subjects in order to test the hypothesis that FFA-induced inflammatory effects in preadipocytes would differ between depots. FFA uptake into human omental preadipocytes is greater than sc preadipocytes (17), suggesting there may be a difference in FFA action between depots. There is more evidence that the inflammatory properties of adipocytes differ between omental and subcutaneous depots. For example, human omental adipocytes secrete more IL-6 than sc adipocytes (29) and expression of the TNFα receptor-1 is higher in human omental than sc adipocytes (30). However, we found no differences in the FFA-induced expression of TNFα, MCP-1 and IL-6 or expression of the TLR family members TLR1, TLR2, TLR4 or TLR6 between sc and omental preadipocytes. A weakness of our studies is that cytokine protein concentrations were not measured in culture medium, so we cannot address whether cytokine secretion, like gene expression, was similar between depots. We found FFA-induced expression of IL-6 was greater than TNFα and MCP-1 in preadipocytes, similar to greater palmitate-induced IL-6 than TNFα expression in adipocytes (20). LPS-induced IL-6 expression has also been shown to be higher in preadipocytes than adipocytes(21;31).
Our results provide indirect evidence that FFAs increase TNFα expression through TLR2/6 activation of the JNK1 pathway in preadipocytes from obese subjects. We did not study preadipocytes from fat depots of normal weight subjects nor did we perform rigorous metabolic testing in our obese subjects to definitively exclude prediabetes or diabetes. Despite these limitations, we suspect these results reflect preadipocytes regardless of the degree of obesity or glycemia, because they are similar to findings in 3T3-L1 adipocytes that FFA-induced TNFα requires JNK activation(19). However, important differences in FFA signaling exist between preadipocytes and adipocytes. We found JNK1 to be the predominant JNK isoform, but unlike adipocytes(8;19), we detected no activation of IKKβ or NKκB in preadipocytes. ERK phosphorylation occurs through FFA activation of three TLRs, but unlike adipocytes(32), ERK does not have a role in expression of TNFα, MCP-1 or IL-6 in preadipocytes. Our observations indicate that, similar to adipocytes(33;34), the p38 pathway is critical for IL-6 and important for TNFα and MCP-1 expression in preadipocytes.
Given our previous observation that IGF-I regulation of proliferation is impaired in omental preadipocytes from these same subjects(18), we also suspected that the anti-inflammatory action of IGF-I would be reduced in omental preadipocytes. Evidence of anti-inflammatory action of IGF-I is limited. IGF-I inhibits TNFα and IL-6 expression in vascular smooth muscle cells (35), and IGF-I inhibits FFA-induced TNFα expression in 3T3-L1 adipocytes(23). We found that IGF-I reduced FFA-induced JNK1 activation and TNFα expression in human sc but not omental preadipocytes. IGF-I had no significant effect on FFA-induced MCP-1 or IL-6 in preadipocytes from either depot. In addition, IGF-I attenuated only JNK1 and not p38 phosphorylation by FFA and the TLR2/6 ligand MALP-2, suggesting the mechanism of IGF-I action is restricted to the JNK1 pathway downstream of TLR2/6 activation.
IGF-I attenuation of the JNK pathway has been described in a number of cell types, including 293 cells(26), islets(36), neuroblastoma cells (37) and now preadipocytes. In all cases, IGF-I inhibited JNK activation through AKT activation. Consistent with these observations, we were able to inhibit FFA-stimulated JNK1 in omental preadipocytes by restoring AKT activity through expression of a constitutively activated AKT. The mechanism of JNK inhibition has been shown to involve AKT regulation of JNK-interacting protein 1 (JIP1), a scaffold protein that is required for JNK activation(38;39). In neurons, AKT1 binding to JIP1 prevents JNK activation(39), and in adipose tissue, JIP1 is essential to JNK activation (40). This mechanism may explain our findings that IGF-I-activated AKT attenuates JNK1 phosphorylation in human sc but not omental preadipocytes.
Our observations extend previous studies of FFA action in adipose tissue, including significant FFA-induced cytokine expression by preadipocytes(9). To our surprise, FFA stimulation of TNFα, IL-6 and MCP-1 was not different between depots, but we did not measure cytokine protein secretion. Like other studies, we found similar expression of TLRs in sc and omental preadipocytes(7). Although we did not directly compare preadipocytes and adipocytes, we found many similarities in FFA action between preadipocytes and adipocytes (8;19;32), including inhibition of FFA-induced TNFα expression in adipocytes by IGF-I(23). Our results reinforce the significance of the preadipocyte as an inflammatory cell, confirm the role of AKT in regulating JNK1 and support an emerging role of IGF-I as anti-inflammatory. We conclude that impaired anti-inflammatory action of IGF-I in omental preadipocytes may contribute to the chronic inflammation in visceral adipose tissue.
Acknowledgments
Supported in part by NIH RO1DK59339 (C.M.B.), Rhode Island Hospital Department of Pediatrics (C.M.B.), NIH R01DK080746 (H.X.), NIH R01AG13925 and NIH P01AG031736 (J.L.K.), the Noaber Foundation (J.L.K.) and the Ted Nash Foundation (J.L.K.).
Footnotes
The authors have nothing to disclose.
Disclosure
Competing interests: the authors have no competing interests and no disclosures to make.
Reference List
- 1.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 2.Tchkonia T, Giorgadze N, Pirtskhalava T, Tchoukalova Y, Karagiannides I, Forse RA, DePonte M, Stevenson M, Guo W, Han J, Waloga G, Lash TL, Jensen MD, Kirkland JL. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1286–R1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- 3.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, Lee WH, Fitzgerald KA, Hwang DH. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 6.Pietsch J, Batra A, Stroh T, Fedke I, Glauben R, Okur B, Zeitz M, Siegmund B. Toll-like receptor expression and response to specific stimulation in adipocytes and preadipocytes: on the role of fat in inflammation. Ann N Y Acad Sci. 2006;1072:407–409. doi: 10.1196/annals.1326.021. [DOI] [PubMed] [Google Scholar]
- 7.Poulain-Godefroy O, Le Bacquer O, Plancq P, Lecoeur C, Pattou F, Fruhbeck G, Froguel P. Inflammatory role of Toll-like receptors in human and murine adipose tissue. Mediators Inflamm. 2010:823486. doi: 10.1155/2010/823486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vitseva OI, Tanriverdi K, Tchkonia TT, Kirkland JL, McDonnell ME, Apovian CM, Freedman J, Gokce N. Inducible Toll-like receptor and NF-kappaB regulatory pathway expression in human adipose tissue. Obesity (Silver Spring) 2008;16:932–937. doi: 10.1038/oby.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp A, Buechler C, Neumeier M, Weigert J, Aslanidis C, Scholmerich J, Schaffler A. Innate immunity and adipocyte function: ligand-specific activation of multiple Toll-like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obesity (Silver Spring) 2009;17:648–656. doi: 10.1038/oby.2008.607. [DOI] [PubMed] [Google Scholar]
- 10.Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J, Bernard S, Arner P. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59:105–109. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cousin B, Munoz O, Andre M, Fontanilles AM, Dani C, Cousin JL, Laharrague P, Casteilla L, Penicaud L. A role for preadipocytes as macrophage-like cells. FASEB J. 1999;13:305–312. doi: 10.1096/fasebj.13.2.305. [DOI] [PubMed] [Google Scholar]
- 12.Charriere G, Cousin B, Arnaud E, Andre M, Bacou F, Penicaud L, Casteilla L. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278:9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- 13.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 14.Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I, Gerry N, Forse RA, Tchoukalova Y, Jensen MD, Pothoulakis C, Kirkland JL. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 15.Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, Kahn CR. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61:1691–1699. doi: 10.2337/db11-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tchkonia T, Tchoukalova YD, Giorgadze N, Pirtskhalava T, Karagiannides I, Forse RA, Koo A, Stevenson M, Chinnappan D, Cartwright A, Jensen MD, Kirkland JL. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol Endocrinol Metab. 2005;288:E267–E277. doi: 10.1152/ajpendo.00265.2004. [DOI] [PubMed] [Google Scholar]
- 17.Caserta F, Tchkonia T, Civelek VN, Prentki M, Brown NF, McGarry JD, Forse RA, Corkey BE, Hamilton JA, Kirkland JL. Fat depot origin affects fatty acid handling in cultured rat and human preadipocytes. Am J Physiol Endocrinol Metab. 2001;280:E238–E247. doi: 10.1152/ajpendo.2001.280.2.E238. [DOI] [PubMed] [Google Scholar]
- 18.Cleveland-Donovan K, Maile LA, Tsiaras WG, Tchkonia T, Kirkland JL, Boney CM. IGF-I activation of the AKT pathway is impaired in visceral but not subcutaneous preadipocytes from obese subjects. Endocrinology. 2010;151:3752–3763. doi: 10.1210/en.2010-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen MT, Satoh H, Favelyukis S, Babendure JL, Imamura T, Sbodio JI, Zalevsky J, Dahiyat BI, Chi NW, Olefsky JM. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280:35361–35371. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- 20.Ajuwon KM, Spurlock ME. Palmitate activates the NF-kappaB transcription factor and induces IL-6 TNFalpha expression in 3T3-L1 adipocytes. J Nutr. 2005;135:1841–1846. doi: 10.1093/jn/135.8.1841. [DOI] [PubMed] [Google Scholar]
- 21.Chung S, LaPoint K, Martinez K, Kennedy A, Boysen SM, McIntosh MK. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology. 2006;147:5340–5351. doi: 10.1210/en.2006-0536. [DOI] [PubMed] [Google Scholar]
- 22.Guo W, Wong S, Xie W, Lei T, Luo Z. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am J Physiol Endocrinol Metab. 2007;293:E576–E586. doi: 10.1152/ajpendo.00523.2006. [DOI] [PubMed] [Google Scholar]
- 23.Kubota Y, Unoki H, Bujo H, Rikihisa N, Udagawa A, Yoshimoto S, Ichinose M, Saito Y. Low-dose GH supplementation reduces the TLR2 and TNF-alpha expressions in visceral fat. Biochem Biophys Res Commun. 2008;368:81–87. doi: 10.1016/j.bbrc.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 24.Tchkonia T, Giorgadze N, Pirtskhalava T, Thomou T, DePonte M, Koo A, Forse RA, Chinnappan D, Martin-Ruiz C, von Zglinicki T, Kirkland JL. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55:2571–2578. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- 25.Batra A, Pietsch J, Fedke I, Glauben R, Okur B, Stroh T, Zeitz M, Siegmund B. Leptin-dependent toll-like receptor expression and responsiveness in preadipocytes and adipocytes. Am J Pathol. 2007;170:1931–1941. doi: 10.2353/ajpath.2007.060699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okubo Y, Blakesley VA, Stannard B, Gutkind S, Le Roith D. Insulin-like growth factor-I inhibits the stress-activated protein kinase/c-Jun N-terminal kinase. J Biol Chem. 1998;273:25961–25966. doi: 10.1074/jbc.273.40.25961. [DOI] [PubMed] [Google Scholar]
- 27.Barnett SF, Defeo-Jones D, Fu S, Hancock PJ, Haskell KM, Jones RE, Kahana JA, Kral AM, Leander K, Lee LL, Malinowski J, McAvoy EM, Nahas DD, Robinson RG, Huber HE. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem J. 2005;385:399–408. doi: 10.1042/BJ20041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 29.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 30.Good M, Newell FM, Haupt LM, Whitehead JP, Hutley LJ, Prins JB. TNF and TNF receptor expression and insulin sensitivity in human omental and subcutaneous adipose tissue--influence of BMI and adipose distribution. Diab Vasc Dis Res. 2006;3:26–33. doi: 10.3132/dvdr.2006.003. [DOI] [PubMed] [Google Scholar]
- 31.Harkins JM, Moustaid-Moussa N, Chung YJ, Penner KM, Pestka JJ, North CM, Claycombe KJ. Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob mice. J Nutr. 2004;134:2673–2677. doi: 10.1093/jn/134.10.2673. [DOI] [PubMed] [Google Scholar]
- 32.Kopp A, Buechler C, Bala M, Neumeier M, Scholmerich J, Schaffler A. Toll-like receptor ligands cause proinflammatory and prodiabetic activation of adipocytes via phosphorylation of extracellular signal-regulated kinase and c-Jun N-terminal kinase but not interferon regulatory factor-3. Endocrinology. 2010;151:1097–1108. doi: 10.1210/en.2009-1140. [DOI] [PubMed] [Google Scholar]
- 33.Fain JN, Bahouth SW, Madan AK. Involvement of multiple signaling pathways in the postbariatric induction of IL-6 and IL-8 mRNA and release in human visceral adipose tissue. Biochem Pharmacol. 2005;69:1315–1324. doi: 10.1016/j.bcp.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Tchivileva IE, Tan KS, Gambarian M, Nackley AG, Medvedev AV, Romanov S, Flood PM, Maixner W, Makarov SS, Diatchenko L. Signaling pathways mediating beta3-adrenergic receptor-induced production of interleukin-6 in adipocytes. Mol Immunol. 2009;46:2256–2266. doi: 10.1016/j.molimm.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, Song YH, Titterington J, Delafontaine P. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:2684–2690. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- 36.Aikin R, Maysinger D, Rosenberg L. Cross-talk between phosphatidylinositol 3-kinase/AKT and c-jun NH2-terminal kinase mediates survival of isolated human islets. Endocrinology. 2004;145:4522–4531. doi: 10.1210/en.2004-0488. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Yang HJ, Xia YY, Feng ZW. Insulin-like growth factor 1 protects human neuroblastoma cells SH-EP1 against MPP+-induced apoptosis by AKT/GSK-3beta/JNK signaling. Apoptosis. 2010;15:1470–1479. doi: 10.1007/s10495-010-0547-z. [DOI] [PubMed] [Google Scholar]
- 38.Levresse V, Butterfield L, Zentrich E, Heasley LE. Akt negatively regulates the cJun N-terminal kinase pathway in PC12 cells. J Neurosci Res. 2000;62:799–808. doi: 10.1002/1097-4547(20001215)62:6<799::AID-JNR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 39.Kim AH, Yano H, Cho H, Meyer D, Monks B, Margolis B, Birnbaum MJ, Chao MV. Akt1 regulates a JNK scaffold during excitotoxic apoptosis. Neuron. 2002;35:697–709. doi: 10.1016/s0896-6273(02)00821-8. [DOI] [PubMed] [Google Scholar]
- 40.Jaeschke A, Czech MP, Davis RJ. An essential role of the JIP1 scaffold protein for JNK activation in adipose tissue. Genes Dev. 2004;18:1976–1980. doi: 10.1101/gad.1216504. [DOI] [PMC free article] [PubMed] [Google Scholar]