Abstract
Wnt signaling is a hallmark of all embryonic development with multiple roles at multiple developmental time points. Wnt signaling is also important in the development of several organs, one of which is the inner ear, where it participates in otic specification, the formation of vestibular structures, and the development of the cochlea. In particular, we focus on Wnt signaling in the auditory organ, the cochlea. Attempting to dissect the multiple Wnt signaling pathways in the mammalian cochlea is a challenging task due to limited expression data, particularly at proliferating stages. To offer predictions about Wnt activity, we compare cochlear development with that of other biological systems such as Xenopus retina, brain, cancer cells and osteoblasts. Wnts are likely to regulate development through crosstalk with other signaling pathways, particularly Notch and FGF, leading to changes in the expression of Sox2 and proneural (pro-hair cell) genes. In this review we have consolidated the known signaling pathways in the cochlea with known developmental roles of Wnts from other systems to generate a potential timeline of cochlear development.
Keywords: Wnt, PCP, cochlea, β-catenin, hair cell, retina
1. Introduction
1.1 Inner ear development
The vertebrate inner ear is a highly complex labyrinth generated through an intimate association of epithelial tissues with the surrounding mesenchyme. The mammalian vestibular system of the inner ear contains 5 organs responsible for sensing changes in angular acceleration or gravity, while the auditory system consists of the coiled cochlea that houses the organ of Corti for detecting sound. Both the vestibular and auditory parts originate from the otic placode, a small patch of surface head ectoderm that acquires otic fate under the influence of surrounding signals during the neurulation stage of embryogenesis. The otic placode invaginates and fuses to form a fluid-filled otic vesicle. Neuroblasts delaminate from the vesicle to take up residence adjacent to the otic ectoderm, eventually becoming the vestibular and cochlear (spiral) cranial ganglia. Meanwhile, the simple epithelium of the otic vesicle is sculpted into a set of chambers and ducts through directed outgrowths, epithelial fusions, and focal hotspots of programmed cell death. The adjacent mesenchymal tissue elaborates an additional fluid compartment surrounding the otic epithelium. These chambers and the associated ganglia are encapsulated and protected by a cartilaginous cover, the otic capsule, which eventually ossifies. This complex morphology optimally stimulates the opening of mechanically gated ion channels located on the sterecociliary bundles protruding from the apical surface of hair cells localized on discrete sensory organs.
Thus far, there are well-established links between members of the Wnt signaling pathways and otic induction, dorsal-ventral axial specification of the otocyst, planar orientation of the stereocilia, and chondrogenesis of the otic capsule. More recently, evidence is emerging for additional roles for Wnts in the establishment and patterning of the cochlear sensory epithelium, and in the proliferative capacity of otic stem cells. This review will focus primarily on Wnt signaling during development of the mammalian cochlea and will draw parallels between the signaling pathways used by this organ and by the retina. The reader is invited to consult several recent reviews that offer additional coverage of Wnt functions in the developing inner ear [1, 2].
1.2 Wnt signaling pathways
Wnts are secreted glycoproteins that are important for a wide array of processes throughout embryonic development, including cell fate specification, proliferation, progenitor maintenance, various aspects of planar cell polarity (PCP), and axon guidance. Wnt signaling can be categorized into canonical and non-canonical signaling pathways, although there is increasing evidence for numerous potential extracellular and intracellular intersections of these pathways (Fig. 1) [3]. The canonical Wnt pathway acts through Fzd receptors and Dishevelled, culminating in the transcriptional activation of genes regulated by TCF/LEF transcription factors, with β-catenin serving as a major second messenger. Non-canonical Wnt signaling pathways are known to influence cell-cell rearrangements (that occur during convergent-extension movements) or cytoskeletal reorganizations (such as those that take place during stereociliary bundle formation and axon outgrowth). These pathways either use calcium as a second messenger or modulate Jnk kinase [4]. Wnts can also signal through the Ryk receptor tyrosine kinase to repel axons or through Fzd receptors to attract axons in the central nervous system [5].
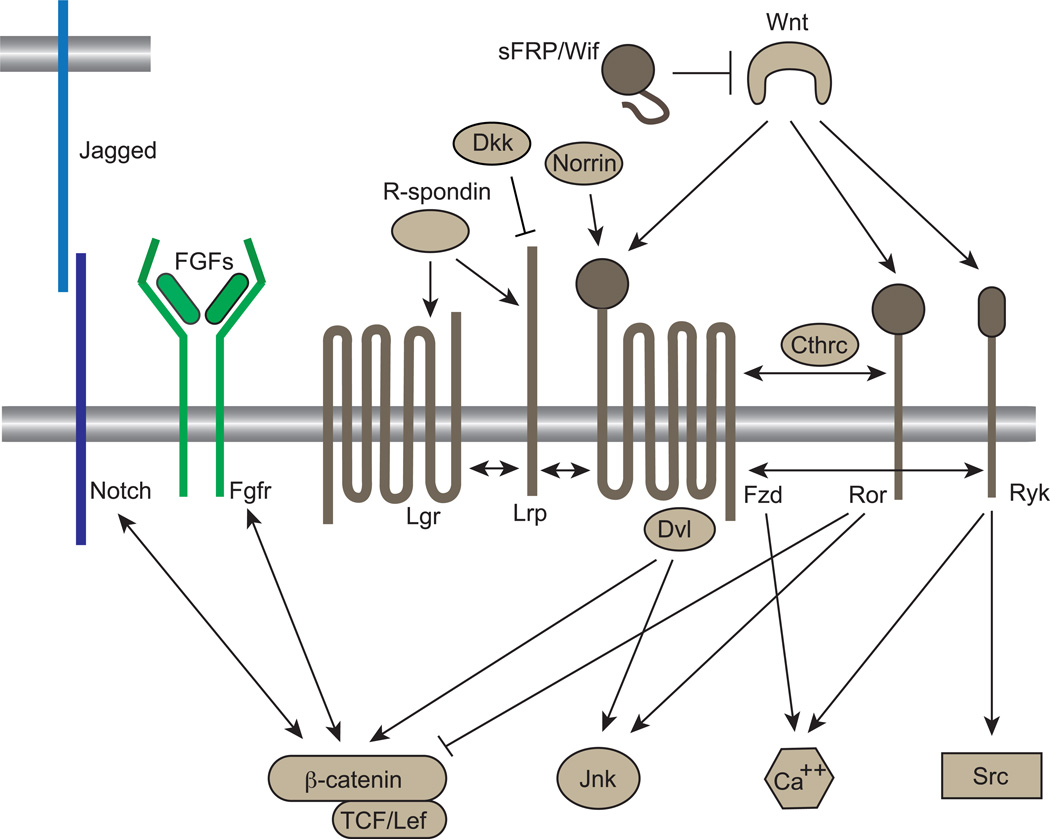
Figure 1.
Wnt signaling pathways. Canonical vs. non-canonical Wnt signaling is dependent on ligand, receptor and co-receptor interactions. Wnt ligands can activate both canonical pathways by binding to Fzd receptors and non-canonical pathways through co-receptors such as Ror and Ryk. Ror can inhibit β-catenin activity while activating Jnk. R-spondins directly bind to Lgr and Lrp co-receptors that interact with Fzd to stimulate/augment β-catenin signaling. Norrin can also activate the canonical pathway, independent of Wnts. Wnt Inhibitors such as sFRP/Wif also regulate Wnt signaling by sequestering Wnt ligands, while Dkk interacts with Lrp to inhibit Wnt signaling. The Notch and Fgfr signaling pathways have been found to act both, upstream and downstream of Wnt/β-catenin signaling. Figure modified from [3].
1.3 Wnt signaling during otic induction and axial polarity of the chicken and mouse inner ears
During early embryonic development, Wnts are known to be important for establishing the anteroposterior body axis. Wnt inhibition anteriorizes while Wnt activation posteriorizes the embryo [6]. At the anterior (head) end of the embryo, the dorsal ectoderm acquires an anterior neural fate (the future brain), which then sends signals to the adjacent ectoderm to become a continuous field of pre-placodal cells. Various signaling molecules emanate from the germ layers of the head to influence the segregation of pre-placodal ectoderm into its component parts-olfactory, lens, trigeminal, otic and epibranchial placodes. For example, in the development of the inner ear, the initial formation of the otic-epibranchial placode (OEP) relies on fibroblast growth factors (FGFs) secreted from the endoderm, mesoderm and/or neural ectoderm to specify Pax2-positive ectoderm [7–9]. Next, high Wnt signaling to the OEP from adjacent hindbrain induces otic fate medially, whereas low Wnt signaling coupled with high FGF signaling restricts epibranchial fate to the lateral OEP territory [2, 10–12]. Thus, while Wnt inhibition was initially required to form the anterior half of the embryo, Wnt activation later becomes important to direct development of the pre-placodal field.
As the otic placode invaginates to form an otic vesicle, Wnt signaling plays a prominent role in establishing the dorsoventral axis. Wnt signaling from the dorsal hindbrain regulates the expression of Wnt target genes such as Dlx5/6, Hmx2/3 and Gbx2 in the dorsal portion of the otocyst [13–16]. These genes are preeminent in the formation of the endolymphatic duct and semicircular canals in the vestibular system. On the other hand, Shh signaling from the notochord or floor plate (in chick) becomes important in specifying the ventral structures of the otocyst such as the saccule and the cochlea [17, 18].
2.1 The emergence of cell types in the mouse cochlea
While there is overwhelming evidence that Wnts play a dominant role in the formation of the dorsal structures of the inner ear, their importance in the development of the cochlea and the organ of Corti remains an active area of investigation. We will extend our discussion beyond experimental evidence to speculate how Wnt signaling may intersect with other known signaling pathways to regulate cochlear cell fate and patterning, taking clues from other model systems. In the mouse otocyst at embryonic day (E) 10.5–11, cochlear duct formation initiates as an evagination of the ventral portion of the otic vesicle. As the cochlear duct elongates, the cells at the apical end are the first to exit the cell cycle, on E12.5, but are the last to differentiate. The differentiation of sensory cells initiates at the mid-base on about E14.5 and progresses outward toward both the extreme base and the apex over a period of 1–3 days [19, 20]. The “neural" cells of the organ of Corti are the mechanosensory hair cells that relay electrical signals to the spiral ganglion. The “non-neural” cells of the organ of Corti, the supporting cells, provide structural and trophic support for hair cells and thus, are important for long-term hair cell survival.
Cochlear development involves cell state transitions from progenitor to prosensory to proneural to a fully differentiated state (Fig. 2). The transcription factor, Sox2, marks precursor cells in all states up to, but excluding, their specification into hair cells. Sox2 is a member of the SoxB1 HMG box family of transcription factors and is frequently referred to as a stem cell marker. In the organ of Corti, only cells that assume the alternative fate of a supporting cell will retain Sox2 expression as they differentiate. The association of Sox2 with the progenitor state, and a requirement for its down-regulation to initiate neuronal differentiation, is well known throughout the nervous system. Here we wish to draw attention to a parallel between the progressive development of mammalian cochlear cells and those of the Xenopus retina. The retinal progenitors go through a comparable sequence of state changes, terminating in both Sox2-negative (neuronal) and Sox2-positive (glial) fates. The Sox2-positive cells in the retina become Müller glia, which, like the supporting cells of the organ of Corti, serve a supportive function for retinal neurons (including the photoreceptors). Temporal changes in the responsiveness of retinal cells to perturbations in Wnt signaling thus offer a template for understanding comparable events in cochlear development, and will be reviewed first.
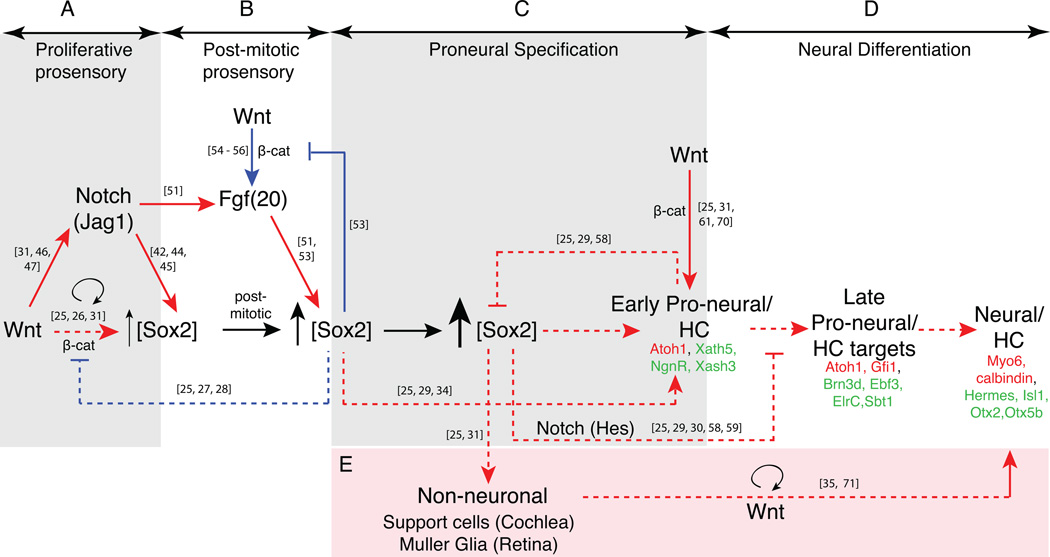
Figure 2.
A model of a developmental timeline with intersecting Wnt, Notch and FGF pathways in the vertebrate cochlea is compared and contrasted with vertebrate retina. Wnt signaling, together with Notch, stimulates proliferation and low-level Sox2 expression during the proliferative prosensory phase of development (A). Wnt signaling can stimulate FGF-mediated elevation of Sox2 levels in post-mitotic prosensory cells (B). We speculate that intermediate Sox2 levels will induce proneural expression, whereas the highest levels of Sox2 will push cells to adopt a non-neuronal fate (C) and will block neural differentiation via Notch signaling (D). Wnt signaling also can reprogram non-neuronal cells to proliferate and adopt neural fates (E). Pathways represented with a red line denote signaling pathways in the mouse cochlea/ chick inner ear. Pathways represented with a dashed line denote signaling in Xenopus/ mouse retina, while blue lines denote pathways from other biological systems (see text for details). Hair cell genes and retinal genes are colored in red and green, respectively.
2.3 Wnt signaling in the developing Xenopus retina
Among the most well-known and robust effects of canonical (β-catenin-mediated) Wnt signaling is the stimulation of cell proliferation, not only throughout normal development, but also as an underlying cause of cancer and other human diseases [21]. During the early stages of retinal development, progenitor cell proliferation is regulated by Wnts [22–24]. Forced activation of canonical Wnt signaling in Xenopus retina enhances cell division [25, 26], confirming that the progenitors are Wnt responsive and that the endogenous level of Wnt activation is probably not saturating. Wnt activity promotes both proliferation and Sox2 expression [25, 26], as shown in Figure 2. However, Sox2 over-expression alone is insufficient to induce proliferation and can, in fact, feedback to inhibit β-catenin [25]. The molecular basis of the repressive activity of Sox2 on the Wnt pathway has been studied in other systems. Specifically, SoxB1 transcription factors directly repress β-catenin activity through protein-protein interactions via their C-terminal domains and independent of their DNA binding domains, thus providing a negative feedback mechanism to down-regulate Wnt/β-catenin activity [25, 27, 28].
In the course of normal development, retinal progenitors transition from a proliferative state to a Sox2-positive, non-proliferative state before encountering a bifurcating fate choice. Cells that maintain Sox2 expression are fated to become Müller glia, not neurons. Thus, when Sox2 is over-expressed, cells are diverted from the neural fate and more Müller glia are generated [25].
The alternative choice of a neural fate is a gradual process: early and late proneural states can be identified by gene expression markers before cells finally begin to express differentiation markers that are characteristic of the various neuronal and photoreceptor cell types in the mature retina (Fig. 2). Enhanced Wnt activation or Sox2 gene delivery does not affect the expression of early proneural genes, such as Xath5, NgnR and Xash3. Instead, these manipulations prevent cells from transitioning to a late proneural stage: both Wnt and Sox2 can repress the expression of downstream proneural targets such as Brn3d, Ebf3, ElrC and Sbt1 [25]. The block of late proneural targets by Sox2 is mediated by Notch signaling [25, 29, 30]. As evidence, chemical inhibition of the Notch pathway allows more retinal precursors to express late proneural target genes. This transition from an early to a late proneural state comes at the expense of Sox2. Therefore, after an initial requirement for Sox2 expression to transition cells out of the proliferative state and into the early proneural stage, there arises a need for a negative-feedback mechanism to down-regulate Sox2 expression so that the neural program can proceed. This negative feedback is mediated by at least one of the early proneural proteins, Xath5 [25]. Whether Wnt signaling contributes to either glial or neural cell fate decisions before or after the Notch input, or whether Wnt function can all be explained by its ability to promote a Sox2-positive stem-cell state, is unresolved. Next, we will examine the similarities between retinal development and cochlear development.
2.4.1 Wnt signaling in the proliferative prosensory cochlea
We were drawn to the parallels between retinal and cochlear development for several reasons. First, Sox2 plays a prominent role in the establishment of prosensory identity in the cochlea. Second, Sox2-positive, postmitotic cells confront a bifurcating cell fate choice between supporting cells, which maintain Sox2 expression, and hair cells, which begin to express proneural genes. Third, this cell fate decision is strongly influenced by Notch signaling, with evidence of feedback inhibition on Sox2, as discussed below. Also, recent evidence suggests that Wnts can influence cochlear progenitors not only during early development, like the retina, but also postnatally. During cochlear development, we will see 3 potential windows of opportunity for Wnt/β-catenin signaling. We begin by reviewing the embryonic stages.
In the cochlea, the early stages of prosensory formation can be broken down into 2 phases: a proliferative and a post-mitotic progenitor phase. Cochlear proliferation is still ongoing throughout the cochlea at E12.5, a time when the culture and delivery of bioactive substances is manageable in vitro. The laboratory of Alain Dabdoub used this approach to interrogate the system for Wnt responsiveness and activity. Indeed, Wnt activation by LiCl dramatically expanded the domain of proliferating Sox2-positive cells in the E12.5 explanted cochlea [31]. This confirms that cochlear progenitors are Wnt-responsive. Like the retina, these results further imply that Wnt/β-catenin activity, if present as a normal feature of cochlear development, is not saturating.
The question then arises as to whether either cell proliferation or the initiation of Sox2 expression are normally regulated by intermediate levels of endogenous Wnt signaling in the cochlea. There are two ways to demonstrate this, as discussed below: (1) confirm expression of Wnt-regulated genes in or near the sensory primordium; and (2) reveal phenotypic changes in cell numbers and/or cell fates in response to Wnt inhibition. The former can be addressed using Wnt-reporters, such as the TCF/Lef:H2B-GFP mouse line, to map β-catenin-mediated gene transcription. In these mice, GFP expression is robust in the Sox2-positive domain of the cochlea at E12.5 and in the non-vestibular parts of the otocyst at E10.5 [31, 32]. Sox2 is expressed by cochlear progenitors where it overlaps spatially and temporally with expression of the Notch ligand, Jagged1 [33]. One perspective suggests that Jagged1 expression serves to maintain Sox2 expression in the neurosensory field of the early otocyst [34]. While we are aware that β-catenin, Jagged1 and Sox2 are expressed in these earlier stages, we focus our attention on murine cochlear development from E12 onwards.
Between E11.5 and E13.5 in the mid-base of the cochlea, Sox2 and Jagged1 gradually retreat into separate domains, with only a small margin of overlap. Sox2 continues to mark the future organ of Corti, while Jagged1 occupies the future greater epithelial ridge domain on the medial (neural) side of the cochlear duct. Sox2 expression is within the GFP expression domain in the Wnt reporter mouse. Furthermore, both of these patterns resemble that of Lgr5, a known Wnt target gene [31, 35, 36]. Confounding the search for ongoing Wnt signaling in the E11.5-E12.5 cochlea using reporter genes is a curious finding: genetic fate mapping indicates that some dorsal otic progenitors are displaced ventrally and may contribute to the cochlea, including its prosensory domain [14]. Thus, we have to consider the possibility that Lgr5 expression could instead be the remnant of earlier Wnt signaling that occurred outside the cochlea. Despite this caveat, we should consider the possible functional consequences of having ongoing expression of genes such as Lgr5. Lgr5 is highly expressed in the stem cells of several tissues and is often regarded as an adult stem cell marker [37]. On E13.5, when the segregation of the Jagged1 vs. Sox2 domains is nearly complete in the mid-basal turn of the cochlea, the cells of the future organ of Corti pull out of division under the control of the cell cycle inhibitor, p27kip1 [20]. Shortly thereafter, Sox2 will decline in hair cell precursors.
Currently lacking for the mouse is direct evidence for cochlear expression of Wnt ligands at the correct time and place to promote cell proliferation and induce Sox2 expression. Lgr5 associates with Frizzled (Fzd) receptors to augment Wnt signaling via R-spondins, which are Lgr/ Lrp receptor ligands (Fig. 1). Thus, Lgr5 is poised to serve as a potential Wnt receptor to mediate the observed proliferative response to Wnts in the E12.5 cochlea [38]. Unfortunately, there is a dearth of knowledge on the expression patterns of Wnt ligands, R-spondins and Wnt receptors in the mouse cochlea during the prosensory phase. In the chick basilar papilla, both Wnt7a and Wnt9a are expressed at a comparable stage of prosensory development at E4-5. Fzd1, 2, 4, 7, 8 and 10 receptors are simultaneously expressed in the prosensory region [39].
If endogenous ligands of the Wnt-signaling pathway are acting on cochlear progenitors, then blocking this pathway should prove detrimental. It is thus noteworthy that pharmacological inhibition of Wnt signaling at E12.5 decreased prosensory formation in the mouse cochlea [31]. This result resembles the effect of blocking the Notch pathway: pharmacological inhibition of Notch signaling at E12.5 suppresses prosensory formation [40]. This is further supported by the lack of prosensory formation and Sox2 expression in Jagged1 knockout mice [41]. Recent work in the adult mouse hippocampus reveals an Rbpj-binding site on the Sox2 promoter, suggesting a mechanism by which the Notch pathway could directly activate Sox2 transcription [42], since Rbpj interacts with the Notch intracellular domain to regulate transcription [43]. Whether Notch directly up-regulates Sox2 expression in the cochlea is still unknown. Recent reports of Notch activation resulting in the formation of ectopic sensory patches support a potential mechanism for direct activation [44, 45]. So how might Wnts feed into the known prosensory role of the Notch pathway? Wnts can augment Notch signaling in cancer cells [46, 47] and they also do so during the formation of the otic placode, earlier in development, by up-regulating components of the Notch pathway [12]. In a similar vein, Wnt activation increases Jagged1 expression in E12.5 cochlea [31]. Together these data suggest that Wnt could indeed promote Notch’s early prosensory role and thereby enhance Sox2 expression (Fig. 2A), although we cannot rule out that Wnt may have a more direct effect on Sox2 regulation. Interestingly, Wnt and Notch cooperatively regulate proliferation during tumorigenesis [48]. Since the inhibition of either Wnt or Notch signaling prevents prosensory formation, there is a high probability that crosstalk between these pathways normally regulates proliferation in the cochlea.
Is there evidence of a critical window of sensitivity to Wnts in the cochlear prosensory organ? Progenitors appear to adopt an early proliferative state followed by a Sox2-positive non-proliferative, competent state before they differentiate. Wnt/β-catenin activation robustly stimulates proliferation of cochlear explants at E12.5, whereas treatment at E13.5 does not: an expanded Sox2 domain is still observed, but it contains fewer proliferating cells that are restricted to the lateral domain [31]. The Wnt reporter mouse at E12.5 also shows the highest levels of nuclear GFP in the prosensory domain. Therefore, the period up to E13.5 may represent a window of opportunity for endogenous Wnts to influence the size of the prosensory domain by regulating both its proliferation and Sox2 expression (Fig. 2A).
2.4.2 Development in the post-mitotic prosensory cochlea
The developing cochlea enters terminal mitosis from E13.5 onwards. Earlier we discussed that Wnt activation in E13.5 cochlea expanded the Sox2-positive prosensory domain with limited proliferation in the prosensory domain. Thus, at E13.5 we see a post-mitotic phase of prosensory formation. In this phase, Sox2 levels are increased to consolidate the transition toward an early proneural state. We will digress for a moment to discuss the roles of Sox2. In some cases, Sox2 is permissive to proneural formation, while in others, antagonistic. We speculate that levels of Sox2 might be essential in elucidating the multiple and contradictory roles of Sox2. There is evidence that small increases in Sox2 levels (within two-fold or less) can trigger mouse embryonic stem cells to differentiate [49]. During foregut endoderm formation, Sox2 has multiple dose-dependent roles in patterning and differentiation [50]. There appears to be a similar dose-dependent role of Sox2 in the cochlea. Based on these findings, we propose a model where the levels of Sox2 determine cell state transitions. For simplicity, we show progenitors transitioning through 3 different states with varying Sox2 levels: low, intermediate and high (Fig 2A-C).
What factors regulate the elevation of Sox2 during the prosensory phase of cochlear development? One potential candidate is Fgf20, which partially mediates prosensory formation [51]. Fgf20 is transiently expressed between E13.5-E15 and up-regulates Sox2 expression in post-mitotic precursors (Fig. 2B) [51, 52]. These cells are portrayed with intermediate levels of Sox2 signaling (Fig. 2B). During cartilage formation, FGF activity is preceded by a requirement for Wnt/β-catenin to expand the mesenchymal proliferative pool, and this proliferative Wnt activity is then repressed as cells transition to the osteoblast lineage [53]. In a manner remniscent of the Xenopus retina, elevated Sox2 levels in the osteoblast lineage feedback to inhibit Wnt activity by direct interaction of the C-terminal of Sox2 with β-catenin [25, 27, 28, 53]. The reader is reminded that Fgf20 expression in the cochlea occurs after Wnt-mediated proliferation, where proliferating cells have relatively lower Sox2 levels (Fig. 2A) [49]. Fgf20 was found to be acting downstream of Notch’s prosensory induction in the cochlea [51]. However, in cancer cells, Fgf20 is a known target positively regulated by Wnt signaling. In zebrafish, fgf20a expression is induced early during fin regeneration and is rapidly down-regulated in Dkk-1 over-expressing fins during regeneration. Such a rapid response (3 hours after amputation) suggests direct regulation of fgf20a by Wnt/β-catenin [54]. In zebrafish, Wnt/β-catenin interacts with the FGF pathway to regulate proneuromast migration during the development of the lateral line primordium. Here once again, Wnt/β-catenin has been shown to stimulate FGF production [55]. Since Wnt activation expanded the Sox2 domain, we postulate that together with Notch, Wnt/β-catenin may be involved in promoting Fgf20-mediated Sox2 elevation within the post-mitotic cochlea (Fig. 2B) [56]. It is an intriguing notion that Wnts may help titrate Sox2 levels in the cochlea.
2.5 Choosing between proneural (hair cell) and non-neural (supporting cell) fates
Although Wnts can act early on the prosensory lineage in the cochlea, its affect on Sox2 expression raises the possibility that it could influence the next fate decision progenitors face: to be a hair cell or a supporting cell. Here we examine the ability of Sox2 to influence and repress proneural genes as cells transition from intermediate Sox2 levels to high Sox2 levels (Fig. 2B-C).
First we will examine the role of Sox2 in proneural differentiation. Both the overexpression of Sox2 and the forced activation of Wnt/β-catenin prevented progenitors from fully differentiating [25, 57]. In both cases, cells expressed early proneural genes; however the expression of late proneural target genes was blocked. These data suggest that to a certain extent, Wnts and Sox2 can stimulate early proneural gene expression before inhibiting further differentiation. Sox2 has been shown to have both activator and repressor activity. For example, studies in the chick inner ear revealed the presence of Sox2 transcriptional activating sites on the Atoh1 (an early hair cell marker) promoter and suggested that Sox2 perhaps aids in the initial activation of early proneural basic helix-loop-helix (bHLH) genes [34]. In our model, we show cells that express intermediate levels of Sox2 can influence early proneural gene expression. On the other hand, Sox2 can also antagonize Atoh1 activity and other late proneural target genes [25, 58]. We speculate that these cells have a higher level of Sox2 activity (Fig. 2C). Experimentally blocking the Notch pathway releases cells from their progenitor state (or even supporting cell state), allowing them to undergo proneural differentiation (Fig. 2C, D) [25, 29, 30, 59]. The downstream effectors for Notch signaling are well studied: Hes proteins are Notch effectors with repressor activity that block transcription of proneural genes such as Atoh1 [59, 60]. Therefore, Notch signaling likely moderates neural differentiation to prevent all cells from adopting a similar cell fate. Once early proneural gene expression has been initiated, the proneural bHLH proteins counteract Sox2 by repressing its transcriptional activity, thus allowing the progression of neural differentiation [25, 29, 58]. Wnt activation also promoted early proneural gene expression [25]. While it is not known whether this occurs via Sox2 or independent of Sox2, there is some preliminary evidence that β-catenin up-regulates Atoh1 by interacting with its 3’ enhancer region in neural progenitors (Fig. 2C)[61]. This alludes to a direct mechanism for hair cell differentiation and will be re-visited later on.
Next, we will examine the specification of non-neuronal cells. Wnt activation increases expression of Prox1, a supporting cell marker of the lateral compartment, in cochlear explant cultures [31]. Similarly, forced expression of Sox2 also increases Prox1 expression in the cochlea [58]. Prox1, like Sox2, must be rapidly down-regulated prior to hair cell formation in the organ of Corti [58, 62, 63]. Therefore, the over-expression of Sox2 in the cochlea forces cells to adopt a supporting cell fate. Similarly in the Xenopus retina, Sox2 over-expression promoted Müller glia formation (Fig. 2C) [25].
At each of these stages--proliferative prosensory, postmitotic prosensory and proneural specification—there are opportunities for endogenous Wnts to influence cell fate specification. Low levels of Sox2, possibly activated by Wnt signaling, suggest that progenitors remain in a proliferative stem-cell state. Intermediate levels of Sox2 are achieved by Fgf20 stimulation (also possibly regulated by Wnts) to increase Sox2 levels to a non-proliferative precursor state that can easily differentiate into hair cells and supporting cells. Cells with very high levels of Sox2 are blocked from expressing proneural target genes and adopt a supporting cell fate in the cochlea or a Müller glial fate in the retina (Fig. 2C). In essence, Wnt signaling may aid in regulating Sox2 levels, by employing different pathways. Negative feedback loops help cells transition from one phase in development to another.
What are the potential Wnt ligands that are expressed in the cochlea? Wnt5a and Wnt7a are expressed in the mouse cochlear duct at E14.5. Wnt receptors: Fzd 1, 2, 3, 4, and 6, Ryk, Ror2 and Lgr5, are expressed in the mouse cochlea [36, 64–68] (Fig. 3A). Wnt4, 5, 6 and Wnt11 are expressed in the chick cochlear duct at E5-7, a time when the prosensory cells are pulling out of division [39]. At the same time, Fzd1, 2, 4, 7, 8, 9 and 10 are expressed at comparable stages of development when proneural specification is taking place [39]. The temporal expressions of Fzd receptors that could regulate Wnt/β-catenin activity from prosensory to proneural stages of differentation are unknown in the mouse cochlea. Fzd10 is another candidate gene that has been linked to non-syndromic deafness [69].
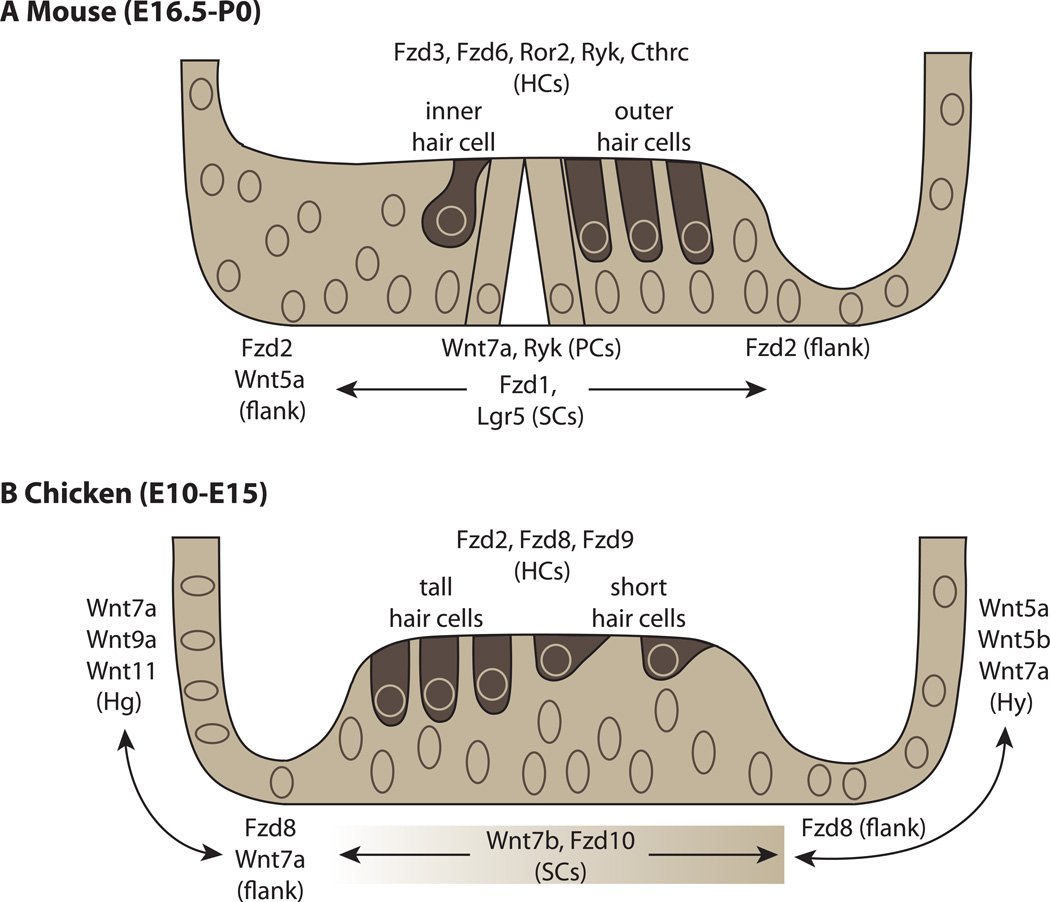
Figure 3.
Expression patterns of Wnt ligands and receptors in the mouse and chick cochlear ducts.(A) Expression in the mouse cochlea from E16.5-P0. Fzd3, Fzd 6, Ror2, Ryk and Cthrc are expressed in hair cells (HCs) [64, 66, 83] while Fzd1 and Lgr5 are expressed in support cells (SCs) [65, 35, 36]. Wnt5a is expressed in the greater epithelial ridge on the neural side of the organ of Corti, while Fzd2 flanks the hair cells on both sides of the organ [65, 67]. (B) Expression in the chick cochlea from E10-E15 [39]. Fzd2 and Fzd9 are high in hair cells while Fzd8 is much weaker in comparison to its expression on the immediate flanks of the sensory organ in clear cells (on the neural side) and border cells (on the abneural side). Wnt7b and Fzd10 are expressed in the SCs in a gradient with stronger expression on the abneural side. Several Wnts are expressed in non-sensory cells types including the homogene cells (Hg) and the hyaline cells (Hy). To focus on regional or cell-type-specific expression patterns, Wnt receptors/ligands/inhibitors with ubiquitous and/or exceptionally weak expression at these time points have been omitted. Details of omitted genes can be found in [39].
2.6 Lgr5 as a high fidelity adult stem cell marker
In both the chicken and the mouse cochleas, Wnts are able to increase hair cell numbers by enlarging the prosensory pool of progenitors [31, 70]. More surprising, perhaps, is that Wnts can also promote hair cell fates long after proliferation has ceased and hair cell differentiation is well underway, at least in vitro. Notably, cochlear expression of Lgr5 continues postnatally and well into adulthood (at least up to 60-days in mice). At postnatal stages, Lgr5 becomes restricted to a subset of supporting cells located within the organ of Corti (inner pillar cells and third-row Deiter’s cells) and beyond it (inner border cells) [35, 36].
Two different groups asked whether Lgr5-positive cells retain their ability to respond to Wnt/β-catenin signaling in the postnatal cochlea. Lgr5-GFP cells can be isolated from postnatal cochlea by fluorescence cell sorting, expanded as neurosphere cultures, and treated with Wnt inhibitors. Treated cells show a marked decrease in proliferation. Thus, Lgr5-positive cells maintain their proliferative capacity, and this is dependent on Wnts (Fig. 2E) [71]. Müller glia show a similar Wnt-mediated capacity to proliferate [25, 72].
Since the entire prosensory domain is Lgr5-positive during development, it must be the case that Lgr5-expressing cells can give rise to both hair cells and supporting cells. Does Lgr5 expression mark an adult stem cell with the ability to generate hair cells? Interestingly, the Lgr5-GFP reporter mouse shows a transient co-localization of Lgr5 with Myo6-positive hair cells during development [36]. Lgr5-positive cells from postnatal cochlea maintained their responsiveness to Wnt3a and R-spondin-1 and differentiated into Myo6-positive hair cells (Fig. 2E) [35, 71]. It would seem that, in the postnatal cochlea, Lgr5 stays true to its reputation as an adult stem cell marker and that Wnt signaling can reprogram Lgr5-positive cells to proliferate and undergo neural differentiation. Myo6 is a late hair cell marker relative to Atoh1. In fact, isolated Lgr5 cells with activated β-catenin from P2 cochleas differentiated into Atoh1-positive cells at a much higher rate than into Myo6-positive cells, supporting a role for Atoh1 regulation by Wnt/β-catenin signaling (Fig. 2C) [35]. There is precedence from studies on neural progenitors for direct activation of Atoh1 by TCF/LEF/β-catenin [61]. An open question is what the normal function of the Lgr5-positive cells might be in adult cochleas, and whether or not their proliferation is held in check in vivo by either the absence of Wnt ligands or the presence of repressors of Wnt signaling.
2.7 Temporal Switch from canonical to non-canonical Wnt signaling in the cochlea
So far we have discussed canonical signaling, but there is also non-canonical Wnt signaling in the cochlea. It is important to keep in mind that whether a Wnt ligand is involved in a canonical vs. a non-canonical pathway is dependent on the spatial and temporal expression of ligands, receptors and co-receptors [3]. The Wnt reporter appears to have similar expression patterns to Lgr5 [31, 36]. In E14.5 cochlea, there is a down-regulation of Wnt activity at the base, while some expression remains in the apex, suggesting a base to apex sweep. By E14.5, β-catenin localizes at the membrane, implying that Wnt signaling may be transitioning from canonical to PCP signaling. What are the potential ligands that could be involved in this transition? Wnt4 is one potential ligand that can redirect stable β-catenin from the nucleus to the membrane and participate in cadherin-mediated cell adhesion instead of transcriptional regulation. Wnt4 has been shown to strongly interact with Fzd6 [73]. The Fzd6 mutant has been shown to have defects in planar cell polarity (PCP) in the cochlea [66]. While it is unknown whether Wnt4 is expressed in the mouse cochlea, it is expressed in the non-sensory regions of the chick cochlear duct [39]. Wnt5a is expressed at E14.5 in mouse cochlea [67]. The onset of Wnt5a expression coincides with the down regulation of Wnt reporter expression. Wnt5a can potentially participate in canonical Wnt signaling via Fzd4 and its LRP5 co-receptor, as well as in non-canonical Wnt signaling via Ror2 [74, 75]. In addition to activating the PCP pathway, Wnt5a/Ror2 can also inhibit the canonical pathway [74], possibly influencing the downregulation of Wnt activity signaling observed at E14.5 [31]. Alternatively, pull-down experiments of the Ryk receptor suggests possible interaction with proteins in both the Wnt/β-catenin and PCP pathways [76]. However in the cochlea, Ryk has only been linked with PCP signaling thus far [64]. Later in development, low-level Wnt reporter activity is still maintained at E17 in pillar cells and in the third-row of Deiter’s cells, similar to Lgr5 expression [31, 35, 36]. Thus, these data, together with evidence in colon cancer cells, suggest a potential alternation between (or even simultaneous) canonical and non-canonical signaling [77].
2.8 PCP signaling
PCP is the term used to identify the coordinated orientation and alignment of cells within the plane of an epithelium. PCP proteins are molecules that underlie PCP processes, with many but not all of the players being evolutionarily conserved among invertebrates and vertebrates. In the developing inner ear, at least two independent PCP events are noteworthy: the polarized alignment of hair cell stereociliary bundles and the elongation of the cochlear duct by convergent extension. Convergent extension is a process by which cytoskeletal changes allow the intercalation and positional changes of cells within the plane of the epithelium, ultimately converting a broad, short array into a thinner, longer array of cells. The gradual orientation of stereociliary bundles also depends on PCP proteins. Hair cell bundles are composed of actin-rich stereocilia that are arranged in rows of ascending heights. A single microtubule-based primary cilium (called the kinocilium) is positioned at the vertex of each V- or W-shaped cochlear hair cell bundle in the mammalian cochlea. Uni-directional deflection of these bundles along the PCP axis is generated following vertical displacement of the basilar membrane in response to sound. This displacement opens mechanosensory channels located on the stereocilia, leading to an influx of positive ions that depolarizes the hair cells [78]. Thus, the precise and uniform orientations of these hair cell bundles are critical for auditory detection.
The misorientation of hair cell bundles and cochlear elongation defects are common phenotypes in mutants of the Wnt/PCP pathway. The PCP pathway regulates the Rac-Rho signaling to modulate cytoskeletal rearrangements required to position the kinocilia and stereocilia [79, 80]. Secreted Wnt inhibitors can be added to developing cochlear explants to probe for the function of Wnt signaling in the establishment of PCP. Indeed, the inhibitors Sfrp1, Wif1 and Frzb (sFRP3) induce PCP defects [67, 81].
Frzb is expressed lateral to the sensory domain. Addition of Frzb to wild-type cochlea explants suppresses cochlear elongation and influences bundle misorientation, indicating the requirement of active Wnt signaling in PCP. Pre-treatment with Wnt5a prior to Frzb rescues both bundle orientation and convergent extension, indicating a potential role of Wnt5a in both processes [67]. Indeed, Wnt5a mutants show bundle orientation defects as well as cochlear elongation defects. The cochlea of Wnt5a mutants is wider and shorter in length than that of wild-type mice [67]. Beginning at E14.5, Wnt5a is expressed medially to the sensory domain (Fig. 3A) [67]. Ror2 is a receptor tyrosine kinase with an extracellular Frizzled-like cysteine-rich domain that interacts with the Wnt5a ligand (Fig. 1) [82]. In-situ expression analysis shows Ror2 localization in hair cells, where Fzd6 is also expressed (Fig. 3). Ror2 knockout mice show bundle orientation defects in the organ of Corti [83]. Along with Wnt5a, Ror2 mediates non-canonical Wnt signaling and activates the PCP pathway [83, 84]. Fzd1, 2, 3 and 6 are other receptors that have also been linked to PCP signaling in the mouse cochlea [65, 66].
Wnt7a is expressed in the sensory domain at E14 and is strongly expressed at E16 in the cells that are fated to become pillar cells (Fig. 3A). Here it participates in outer hair cell specification. However, the Wnt7a mutants do not show alterations in bundle orientation. This could be due to genetic redundancy, or it could be that Wnt7a is not a primary regulator of PCP signaling in the cochlea. The addition of Wnt7a-conditioned media to explants caused hair cell bundles to misorient, suggesting that Wnt7a has the potential to direct bundle orientation and confirms that an asymmetric source of Wnt (such as Wnt5a; see Fig. 3A) may offer directional information across the cochlear epithelium to fine-tune bundle orientation [81]. Wnt5a, 5b, 7a, 7b, 9a, Fzd8 and 10 are expressed in the chick cochlear duct at comparable ages of E10-E15 when PCP signaling is likely to occur (Fig. 3B) [39].
2.9 Wnt regulation of axon outgrowth in the cochlea
Wnts can function to either attract or repel axon outgrowth in the central nervous system [5]. In the developing chicken inner ear, Wnts are primarily expressed on the flanks of the prosensory domains, but rarely within them (see Fig. 3B). This led us to hypothesize that Wnts might serve to confine axons to their sensory targets by serving as repellents. We used both in vitro and in vivo methods to challenge statoacoustic ganglion neurons from the chicken with Wnts1, 4, 5a, 6 or 7b, but found axon outgrowth to be unchanged [85]. Wnt7a may be an exception: preliminary data show that 2.5 µg/ml of Wnt7a added to cultures of embryonic day 4–5 statoacoustic ganglia can enhance axon outgrowth within 24 hours (unpublished observations) [85]. Interestingly, Wnt7a is transiently expressed in the prosensory basilar papilla on embryonic day 4 [86] just as auditory fibers invade the organ [87, 88]. These observations will require additional followup experiments to reveal whether Wnt7a guides auditory axons in vivo.
2.10 Wnt-independent β-catenin signaling in the cochlea
Dissecting the various roles of Wnts is challenging and we cannot rely solely on the expression patterns of Wnt ligands and receptors to reveal the underlying mechanisms [3]. A specific Wnt ligand can activate canonical Wnt signaling in one situation and non-canonical signaling in another. Downstream pathways are dependent on which receptors and co-receptors are present at any given time in development. The presence of Wnt inhibitors adds another layer of complexity. There are also Wnt-independent factors that can activate β-catenin. For example, Norrin activates β-catenin signaling and has a high affinity for the Fzd4 receptor, both of which are expressed in the inner ear (Fig. 1). Norrin is expressed in the vascularized region of the spiral limbus, and the lateral wall adjacent to the stria vascularis of postnatal and adult mouse cochlea [89]. Fzd4 is expressed strongly in inner hair cells and weakly in outer hair cells. Fzd4-null mice show progressive hearing loss due to sensory hair cell loss, later in development [68, 90]. The main mode of action for Norrin/Fzd4 signaling may be in vascular development and the maintenance of the stria vascularis [90, 91].
3. Conclusions
The cochlea is a complex structure that involves crosstalk of several pathways from otic placode formation up to postnatal development. Wnts are widely known for their role in stem cell proliferation. However, there are several lines of evidence that show the involvement of Wnts beyond their mitogenic capacity. There is evidence for both canonical and non-canonical Wnt signaling in the cochlea. Apart from the crosstalk of multiple signaling pathways, there is also crosstalk within the Wnt network between ligands, receptors, co-receptors, inhibitors and additional regulators that are important in modulating canonical vs. non-canonical signaling. Dissecting the multiple roles of Wnt signaling is hampered by limited information on the temporal and spatial expressions of ligands and Wnt receptors throughout cochlear development. Such information is urgently needed.
Drawing inferences from several other systems, particularly Xenopus retina, we propose 3 sequential roles of canonical Wnt/β-catenin in the cochlea and a fourth additional role in reprogramming non-neuronal cells to proliferate. The first window of opportunity is at E12.5 when Wnt activation can increase progenitor proliferation. The second occurs at E13.5, when Wnt activation increases Sox2 expression in progenitors that have exited the cell cycle. This may take place through an Fgf20 signaling pathway. The third observation suggestive of Wnt signaling in the cochlea is that Wnt activation increases hair cell formation. And finally, Wnt signaling can reprogram non-neuronal cells (supporting cells) to re-enter the cell cyle and differentiate into neural cells (hair cells). Since Wnts may participate at several different stages of development, there are a series of negative feedback loops that periodically abrogate Wnt signaling and/or Sox2 expression and a feedforward block (via Notch) to help the progression of development. These events are summarized in Figure 2.
Non-canonical Wnt signaling is important in coordinating the elongation of the cochlear duct as well as patterning the organ of Corti via PCP signaling. Wnt5a is the best-described Wnt ligand involved, with several potential receptors linked to both canonical and non-canonical pathways. We propose a possible switch from canonical to non-canonical Wnt signaling. As helpful as it has been comparing cochlear development with other systems, it is important to collectively analyze the temporal expression patterns of ligands, receptors and co-receptors to confirm our speculations on the multiple roles of Wnt signaling in cochlear development.
Highlights.
Wnt activation stimulates proliferation during early prosensory formation
Fgf20-mediated Sox2 elevation could be titrated by Wnt signaling
High Sox2 levels block neural formation via Notch and force non-neuronal fates
Wnts can reprogram support cells to proliferate and differentiate into hair cells
Wnts regulate hair cell bundle orientation and convergent extension in the cochlea
Acknowledgments
We thank Alain Dabdoub and Fernando Giraldez for valuable comments on the manuscript. Work in the Fekete lab related to Wnt signaling in the cochlea is funded by NIH grant DC002756.
Abbreviations
- bHLH
basic helix-loop-helix
- FGF
fibroblast growth factors
- PCP
planar cell polarity
- OEP
otic-epibranchial placode
- Fzd
Frizzled receptor
- E
embryonic day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Vidhya Munnamalai, Email: vmunnama@purdue.edu.
Donna M. Fekete, Email: dfekete@purdue.edu.
References
- 1.Groves AK, Fekete DM. Shaping sound in space: the regulation of inner ear patterning. Development. 2012;139:245–257. doi: 10.1242/dev.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladher RK, O'Neill P, Begbie J. From shared lineage to distinct functions: the development of the inner ear and epibranchial placodes. Development. 2010;137:1777–1785. doi: 10.1242/dev.040055. [DOI] [PubMed] [Google Scholar]
- 3.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 4.Nusse R. Wnt signaling. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou Y, Lyuksyutova AI. Morphogens as conserved axon guidance cues. Curr Opin Neurobiol. 2007;17:22–28. doi: 10.1016/j.conb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Ladher RK, Wright TJ, Moon AM, Mansour SL, Schoenwolf GC. FGF8 initiates inner ear induction in chick and mouse. Genes Dev. 2005;19:603–613. doi: 10.1101/gad.1273605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarez Y, Alonso MT, Vendrell V, Zelarayan LC, Chamero P, Theil T, et al. Requirements for FGF3 and FGF10 during inner ear formation. Development. 2003;130:6329–6338. doi: 10.1242/dev.00881. [DOI] [PubMed] [Google Scholar]
- 9.Wright TJ, Mansour SL. Fgf3 and Fgf10 are required for mouse otic placode induction. Development. 2003;130:3379–3390. doi: 10.1242/dev.00555. [DOI] [PubMed] [Google Scholar]
- 10.Ladher RK, Anakwe KU, Gurney AL, Schoenwolf GC, Francis-West PH. Identification of synergistic signals initiating inner ear development. Science. 2000;290:1965–1967. doi: 10.1126/science.290.5498.1965. [DOI] [PubMed] [Google Scholar]
- 11.Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–875. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- 12.Jayasena CS, Ohyama T, Segil N, Groves AK. Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development. 2008;135:2251–2261. doi: 10.1242/dev.017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Z, Cantos R, Patente M, Wu DK. Gbx2 is required for the morphogenesis of the mouse inner ear: a downstream candidate of hindbrain signaling. Development. 2005;132:2309–2318. doi: 10.1242/dev.01804. [DOI] [PubMed] [Google Scholar]
- 14.Riccomagno MM, Takada S, Epstein DJ. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 2005;19:1612–1623. doi: 10.1101/gad.1303905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parr BA, Shea MJ, Vassileva G, McMahon AP. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 1993;119:247–261. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Grimmer JF, Van De Water TR, Lufkin T. Hmx2 and Hmx3 homeobox genes direct development of the murine inner ear and hypothalamus and can be functionally replaced by Drosophila Hmx. Dev Cell. 2004;7:439–453. doi: 10.1016/j.devcel.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Bok J, Bronner-Fraser M, Wu DK. Role of the hindbrain in dorsoventral but not anteroposterior axial specification of the inner ear. Development. 2005;132:2115–2124. doi: 10.1242/dev.01796. [DOI] [PubMed] [Google Scholar]
- 18.Riccomagno MM, Martinu L, Mulheisen M, Wu DK, Epstein DJ. Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 2002;16:2365–2378. doi: 10.1101/gad.1013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;(Suppl 220):1–44. [PubMed] [Google Scholar]
- 20.Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- 21.Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol. 2009;129:1614–1627. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denayer T, Locker M, Borday C, Deroo T, Janssens S, Hecht A, et al. Canonical Wnt signaling controls proliferation of retinal stem/progenitor cells in postembryonic Xenopus eyes. Stem Cells. 2008;26:2063–2074. doi: 10.1634/stemcells.2007-0900. [DOI] [PubMed] [Google Scholar]
- 23.Kubo F, Takeichi M, Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development. 2003;130:587–598. doi: 10.1242/dev.00244. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi M, Tonou-Fujimori N, Komori A, Maeda R, Nojima Y, Li H, et al. Histone deacetylase 1 regulates retinal neurogenesis in zebrafish by suppressing Wnt and Notch signaling pathways. Development. 2005;132:3027–3043. doi: 10.1242/dev.01881. [DOI] [PubMed] [Google Scholar]
- 25.Agathocleous M, Iordanova I, Willardsen MI, Xue XY, Vetter ML, Harris WA, et al. A directional Wnt/beta-catenin-Sox2-proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development. 2009;136:3289–3299. doi: 10.1242/dev.040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Raay TJ, Moore KB, Iordanova I, Steele M, Jamrich M, Harris WA, et al. Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron. 2005;46:23–36. doi: 10.1016/j.neuron.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Zorn AM, Barish GD, Williams BO, Lavender P, Klymkowsky MW, Varmus HE. Regulation of Wnt signaling by Sox proteins: XSox17 alpha/beta and XSox3 physically interact with beta-catenin. Mol Cell. 1999;4:487–498. doi: 10.1016/s1097-2765(00)80200-2. [DOI] [PubMed] [Google Scholar]
- 28.Kan L, Israsena N, Zhang Z, Hu M, Zhao LR, Jalali A, et al. Sox1 acts through multiple independent pathways to promote neurogenesis. Dev Biol. 2004;269:580–594. doi: 10.1016/j.ydbio.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- 30.Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 31.Jacques BE, Puligilla C, Weichert RM, Ferrer-Vaquer A, Hadjantonakis AK, Kelley MW, et al. A dual function for canonical Wnt/beta-catenin signaling in the developing mammalian cochlea. Development. 2012;139:4395–4404. doi: 10.1242/dev.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrer-Vaquer A, Piliszek A, Tian G, Aho RJ, Dufort D, Hadjantonakis AK. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ss-catenin signaling in the mouse. BMC Dev Biol. 2010;10:121. doi: 10.1186/1471-213X-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohyama T, Basch ML, Mishina Y, Lyons KM, Segil N, Groves AK. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J Neurosci. 2010;30:15044–15051. doi: 10.1523/JNEUROSCI.3547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neves J, Uchikawa M, Bigas A, Giraldez F. The prosensory function of Sox2 in the chicken inner ear relies on the direct regulation of Atoh1. PLoS One. 2012;7:e30871. doi: 10.1371/journal.pone.0030871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi F, Kempfle JS, Edge AS. Wnt-responsive lgr5-expressing stem cells are hair cell progenitors in the cochlea. J Neurosci. 2012;32:9639–9648. doi: 10.1523/JNEUROSCI.1064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chai R, Xia A, Wang T, Jan TA, Hayashi T, Bermingham-McDonogh O, et al. Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea. J Assoc Res Otolaryngol. 2011;12:455–469. doi: 10.1007/s10162-011-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuijers J, Clevers H. Adult mammalian stem cells: the role of Wnt Lgr5 and Rspondins. EMBO J. 2012;31:2685–2696. doi: 10.1038/emboj.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 39.Sienknecht UJ, Fekete DM. Comprehensive Wnt-related gene expression during cochlear duct development in chicken. J Comp Neurol. 2008;510:378–395. doi: 10.1002/cne.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayashi T, Kokubo H, Hartman BH, Ray CA, Reh TA, Bermingham-McDonogh O. Hesr1 and Hesr2 may act as early effectors of Notch signaling in the developing cochlea. Dev Biol. 2008;316:87–99. doi: 10.1016/j.ydbio.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehm O, Goritz C, Covic M, Schaffner I, Schwarz TJ, Karaca E, et al. RBPJkappadependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci. 2010;30:13794–13807. doi: 10.1523/JNEUROSCI.1567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- 44.Hartman BH, Reh TA, Bermingham-McDonogh O. Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proc Natl Acad Sci U S A. 2010;107:15792–15797. doi: 10.1073/pnas.1002827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan W, Jin Y, Stanger B, Kiernan AE. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc Natl Acad Sci U S A. 2010;107:15798–15803. doi: 10.1073/pnas.1003089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X, Stoeck A, Lee SJ, Shih Ie M, Wang MM, Wang TL. Jagged1 expression regulated by Notch3 and Wnt/beta-catenin signaling pathways in ovarian cancer. Oncotarget. 2010;1:210–218. doi: 10.18632/oncotarget.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodilla V, Villanueva A, Obrador-Hevia A, Robert-Moreno A, Fernandez-Majada V, Grilli A, et al. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc Natl Acad Sci U S A. 2009;106:6315–6320. doi: 10.1073/pnas.0813221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fre S, Pallavi SK, Huyghe M, Lae M, Janssen KP, Robine S, et al. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci U S A. 2009;106:6309–6314. doi: 10.1073/pnas.0900427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kopp JL, Ormsbee BD, Desler M, Rizzino A. Small increases in the level of Sox2 trigger the differentiation of mouse embryonic stem cells. Stem Cells. 2008;26:903–911. doi: 10.1634/stemcells.2007-0951. [DOI] [PubMed] [Google Scholar]
- 50.Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, et al. Multiple dosedependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munnamalai V, Hayashi T, Bermingham-McDonogh O. Notch prosensory effects in the Mammalian cochlea are partially mediated by fgf20. J Neurosci. 2012;32:12876–12884. doi: 10.1523/JNEUROSCI.2250-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayashi T, Ray CA, Bermingham-McDonogh O. Fgf20 is required for sensory epithelial specification in the developing cochlea. J Neurosci. 2008;28:5991–5999. doi: 10.1523/JNEUROSCI.1690-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mansukhani A, Ambrosetti D, Holmes G, Cornivelli L, Basilico C. Sox2 induction by FGF and FGFR2 activating mutations inhibits Wnt signaling and osteoblast differentiation. J Cell Biol. 2005;168:1065–1076. doi: 10.1083/jcb.200409182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, et al. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- 55.Aman A, Piotrowski T. Wnt/beta-catenin and Fgf signaling control collective cell migration by restricting chemokine receptor expression. Dev Cell. 2008;15:749–761. doi: 10.1016/j.devcel.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 56.Chamorro MN, Schwartz DR, Vonica A, Brivanlou AH, Cho KR, Varmus HE. FGF-20 and DKK1 are transcriptional targets of beta-catenin and FGF-20 is implicated in cancer and development. EMBO J. 2005;24:73–84. doi: 10.1038/sj.emboj.7600460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kubo F, Takeichi M, Nakagawa S. Wnt2b inhibits differentiation of retinal progenitor cells in the absence of Notch activity by downregulating the expression of proneural genes. Development. 2005;132:2759–2770. doi: 10.1242/dev.01856. [DOI] [PubMed] [Google Scholar]
- 58.Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, et al. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci U S A. 2008;105:18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- 60.Kageyama R, Ohtsuka T, Kobayashi T. Roles of Hes genes in neural development. Dev Growth Differ. 2008;50(Suppl 1):S97–S103. doi: 10.1111/j.1440-169X.2008.00993.x. [DOI] [PubMed] [Google Scholar]
- 61.Shi F, Cheng YF, Wang XL, Edge AS. Beta-catenin up-regulates Atoh1 expression in neural progenitor cells by interaction with an Atoh1 3' enhancer. J Biol Chem. 2010;285:392–400. doi: 10.1074/jbc.M109.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bermingham-McDonogh O, Oesterle EC, Stone JS, Hume CR, Huynh HM, Hayashi T. Expression of Prox1 during mouse cochlear development. J Comp Neurol. 2006;496:172–186. doi: 10.1002/cne.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirjavainen A, Sulg M, Heyd F, Alitalo K, Yla-Herttuala S, Moroy T, et al. Prox1 interacts with Atoh1 and Gfi1, and regulates cellular differentiation in the inner ear sensory epithelia. Dev Biol. 2008;322:33–45. doi: 10.1016/j.ydbio.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 64.Macheda ML, Sun WW, Kugathasan K, Hogan BM, Bower NI, Halford MM, et al. The Wnt receptor Ryk plays a role in mammalian planar cell polarity signaling. J Biol Chem. 2012;287:29312–29323. doi: 10.1074/jbc.M112.362681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu H, Smallwood PM, Wang Y, Vidaltamayo R, Reed R, Nathans J. Frizzled 1 and frizzled 2 genes function in palate, ventricular septum and neural tube closure: general implications for tissue fusion processes. Development. 2010;137:3707–3717. doi: 10.1242/dev.052001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, et al. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Huso D, Cahill H, Ryugo D, Nathans J. Progressive cerebellar, auditory, and esophageal dysfunction caused by targeted disruption of the frizzled-4 gene. J Neurosci. 2001;21:4761–4771. doi: 10.1523/JNEUROSCI.21-13-04761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blanton SH, Liang CY, Cai MW, Pandya A, Du LL, Landa B, et al. A novel locus for autosomal dominant non-syndromic deafness (DFNA41) maps to chromosome 12q24-qter. J Med Genet. 2002;39:567–570. doi: 10.1136/jmg.39.8.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stevens CB, Davies AL, Battista S, Lewis JH, Fekete DM. Forced activation of Wnt signaling alters morphogenesis and sensory organ identity in the chicken inner ear. Dev Biol. 2003;261:149–164. doi: 10.1016/s0012-1606(03)00297-5. [DOI] [PubMed] [Google Scholar]
- 71.Chai R, Kuo B, Wang T, Liaw EJ, Xia A, Jan TA, et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci U S A. 2012;109:8167–8172. doi: 10.1073/pnas.1202774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007;27:4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bernard P, Fleming A, Lacombe A, Harley VR, Vilain E. Wnt4 inhibits beta-catenin/TCF signalling by redirecting beta-catenin to the cell membrane. Biol Cell. 2008;100:167–177. doi: 10.1042/BC20070072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Amerongen R, Fuerer C, Mizutani M, Nusse R. Wnt5a can both activate and repress Wnt/beta-catenin signaling during mouse embryonic development. Dev Biol. 2012;369:101–114. doi: 10.1016/j.ydbio.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berndt JD, Aoyagi A, Yang P, Anastas JN, Tang L, Moon RT. Mindbomb 1, an E3 ubiquitin ligase, forms a complex with RYK to activate Wnt/beta-catenin signaling. J Cell Biol. 2011;194:737–750. doi: 10.1083/jcb.201107021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bordonaro M, Tewari S, Cicco CE, Atamna W, Lazarova DL. A switch from canonical to noncanonical Wnt signaling mediates drug resistance in colon cancer cells. PLoS One. 2011;6:e27308. doi: 10.1371/journal.pone.0027308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dabdoub A, Kelley MW. Planar cell polarity and a potential role for a Wnt morphogen gradient in stereociliary bundle orientation in the mammalian inner ear. J Neurobiol. 2005;64:446–457. doi: 10.1002/neu.20171. [DOI] [PubMed] [Google Scholar]
- 79.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol. 2012;28:627–653. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- 81.Dabdoub A, Donohue MJ, Brennan A, Wolf V, Montcouquiol M, Sassoon DA, et al. Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development. 2003;130:2375–2384. doi: 10.1242/dev.00448. [DOI] [PubMed] [Google Scholar]
- 82.Masiakowski P, Carroll RD. A novel family of cell surface receptors with tyrosine kinase-like domain. J Biol Chem. 1992;267:26181–26190. [PubMed] [Google Scholar]
- 83.Yamamoto S, Nishimura O, Misaki K, Nishita M, Minami Y, Yonemura S, et al. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008;15:23–36. doi: 10.1016/j.devcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 84.Yamamoto H, Yoo SK, Nishita M, Kikuchi A, Minami Y. Wnt5a modulates glycogen synthase kinase 3 to induce phosphorylation of receptor tyrosine kinase Ror2. Genes Cells. 2007;12:1215–1223. doi: 10.1111/j.1365-2443.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 85.Fantetti KN, Zou Y, Fekete DM. Wnts and Wnt inhibitors do not influence axon outgrowth from chicken statoacoustic ganglion neurons. Hear Res. 2011;278:86–95. doi: 10.1016/j.heares.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sienknecht UJ, Fekete DM. Mapping of Wnt, frizzled, and Wnt inhibitor gene expression domains in the avian otic primordium. J Comp Neurol. 2009;517:751–764. doi: 10.1002/cne.22169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hemond SG, Morest DK. Formation of the cochlea in the chicken embryo: sequence of innervation and localization of basal lamina-associated molecules. Brain Res Dev Brain Res. 1991;61:87–96. doi: 10.1016/0165-3806(91)90117-2. [DOI] [PubMed] [Google Scholar]
- 88.Whitehead MC, Morest DK. The growth of cochlear fibers and the formation of their synaptic endings in the avian inner ear: a study with the electron microscope. Neuroscience. 1985;14:277–300. doi: 10.1016/0306-4522(85)90178-2. [DOI] [PubMed] [Google Scholar]
- 89.Ye X, Smallwood P, Nathans J. Expression of the Norrie disease gene (Ndp) in developing and adult mouse eye, ear, and brain. Gene Expr Patterns. 2011;11:151–155. doi: 10.1016/j.gep.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 91.Rehm HL, Zhang DS, Brown MC, Burgess B, Halpin C, Berger W, et al. Vascular defects and sensorineural deafness in a mouse model of Norrie disease. J Neurosci. 2002;22:4286–4292. doi: 10.1523/JNEUROSCI.22-11-04286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]