Abstract
Cognitive deficits have been reported in children who experienced early neglect, especially children raised in institutionalized settings. Previous research suggests early neglect may differentially affect the directional organization of white matter in the prefrontal cortex (PFC). This may be one mechanism to explain cognitive deficits associated with neglect. To test this idea, properties of white matter and neurocognitive performance was assessed in children who suffered early neglect and those raised in typical environments (n=63, Mean Age=11.75 years). As predicted, prefrontal white matter microstructure was affected, consistent with more diffuse organization, in children that suffered early neglect and this was related to neurocognitive deficits. Such findings underscore how early adversity may affect the PFC and explain cognitive deficits associated with neglect.
Keywords: stress, prefrontal cortex, brain development, early neglect, maltreatment, neuroimaging, frontal lobe, cognitive development, cognitive neuroscience, critical period, sensitive period, chronic stress, executive functioning, neural plasticity
A growing body of research investigating the sequelae of early childhood neglect underscores that such adverse experiences can compromise cognitive development (Fox, Levitt, & Nelson, 2010). Yet, little is currently known about the mechanisms through which early experience influences later cognitive functioning in humans. Early post-natal experience plays a key role in aspects of neurobiological development such as changes in synaptic production, axonal pruning, and myelination in the brain, especially the prefrontal cortex (PFC; for review, see Petrosini et al., 2009). The PFC has been linked to cognitive development (Diamond, 2002), has one of the most protracted developmental timelines in the brain (Lenroot & Giedd, 2006), and therefore may be particularly vulnerable to the effects of early experience. In addition, experimental research in non-human animals, where the early social environment has been controlled or manipulated, suggests that the PFC may be especially vulnerable to early neglect (Helmeke et al., 2009; Sanchez et al., 1998). A growing body of research in children who had suffered maltreatment also underscores that the PFC may be uniquely affected by such early life stress (Davidson & McEwen, 2012; Hanson et al., 2010).
Some evidence for the effects of early childhood neglect on cognitive development comes from research examining children raised in institutional settings, where a lack of toys or stimulation, unresponsive caregiving, and an overall dearth of individualized care and attention are common (Rutter, 1998). Starting life in such settings has been associated with pervasive cognitive deficits (Beckett, Castle, Rutter, & Sonuga-Barke, 2010; Kreppner, et al., 2010). The effects of this type of early childhood neglect also appear to be quite persistent, as significant deficits have been reported even after neglected children have transitioned into enriched environments where caregivers are typically highly stable, well educated, and supportive (Hellerstedt et al., 2008).
Here, we examine the relation between directional organization of white matter in the PFC and cognitive development among children who experienced early neglect and then move into normative caregiving environments. Prefrontal white matter supports increased speed of information processing (Asato, Terwilliger, Woo, & Luna, 2010; Nagy, Westerberg, & Klingberg, 2004), develops greatly in the first two years of life (Gao, et al., 2008). This is at the same time when aberrant caregiving in our sample is ongoing and may be a central mechanism for the cognitive deficits associated with early neglect. Children raised in institutionalized settings, an environment where neglect is common, display decrements in PFC-dependent neurocognitive tasks (Bos et al., 2009; Pollak et al., 2010) and aberrant white matter organization in the PFC (Behen et al., 2009; Eluvathingal et al., 2006; Govindan et al. 2009). These emerging bodies of research, though suggestive, have not connected brain alterations with differences in cognitive development related to neglect. Other regions such as the amygdala that may be affected by early adversity (for review see Tottenham & Sheridan, 2009) are not likely to be strongly related to cognitive decrements associated with early neglect.
To fully interrogate this potential mechanism, we collected diffusion-tensor imaging (DTI) data along with neurocognitive assessments in children who had suffered early neglect along with comparison children who had not suffered any type of early adversity. DTI is a non-invasive imaging method that is highly sensitive to white matter directional organization as this novel form of MRI can measure the local microstructural characteristics of water diffusion. Other MRI methods (e.g., voxel-based morphometry) only measure anatomical volume, charting how large or small portions of the brain are. We specifically focused on a DTI-derived measure of the white matter organization- fractional anisotropy (FA). FA describes the directionality of water diffusion and is modulated by microstructural properties of white matter, including fiber density, axonal diameter and myelination. During childhood and adolescence, FA increases across the whole-brain (Li & Noseworthy, 2002; Mukherjee et al., 2001) and specific change in white matter FA during childhood and adolescence have been associated with cognitive functions (Bava et al., 2010). Additional research found FA was related to myelination in childhood (Dubois et al. 2008), with greater FA being related to higher rates of myelination. Examination of FA can be an important tool in tracking the course and timing of normative brain development in children and adolescents (for review, see Chanraud, Zahr, Sullivan & Pfefferbaum, 2010). Lower FA may be either indicative of white matter being more diffusely organized, connecting to portions of the brain in an equivalent fashion or reflect reduced myelinated axons. Either more diffuse white matter connections, lower axonal density or reduced myelination would likely impede the role of the prefrontal cortex in brain function.
We specifically posit that early neglect may disrupt basic organizational properties of the brain leading to different patterns of prefrontal white matter organization, as indexed by lower FA in children who had suffered early neglect compared to comparison children who had been raised in typical family environments. We predicted that lower directional organization of white matter in these same prefrontal white matter bundles would be associated with poorer neurocognitive performance in the children that suffered early neglect, as this region has been related to aspects of cognitive development. To test the specificity of our effects, structural MRIs were also collected in the same subjects to examine the relation between volumetric changes in the brain and the effects of interest. No associations between volumetric properties, early neglect, and cognitive development were hypothesized.
Method
Participants
As shown in Table 1, sixty-three adolescents (25 who had experienced early neglect and 38 comparison children who had not experienced neglect) between the ages of 9 and 14 years of age (Mean Age= 141.63 +/− 20.8 months) participated in this study. We selected this age range because this development period is marked by major regressive and progressive brain changes (for review, see Lenroot & Giedd, 2006). The average amount of time spent in institutional care by children in the early neglect group was 29.52 (+/− 16.681) months, with a range from 3 to 64 months. Children were on average 38.08 (+/− 22.69) months when they adopted, with a range of 3 to 92 months. Children in the early neglect group were adopted from Romania (n=11), Russia (n=8), China (n=3), and Bulgaria (n=2). Parents for one child did not report the country of origin. As a comparison group, we selected children without a history of early neglect from families with similar current socioeconomic statuses as the adoptive families. This strategy has been suggested and employed by other research groups (e.g, Roberts & Scott, 2009; Rutter, Dunn, Plomin, & Simonoff, 1997). This was done to ensure similar current family environments, as the children who experienced early neglect were typically adopted into middle or high socioeconomic status households. Groups did not differ on age, pubertal stage, sex, or socioeconomic status as measured by the Hollingshead inventory as shown in Table 1.
Table 1.
Group Demographic Information.
| Sample | Group Comparisons | ||
|---|---|---|---|
|
| |||
| Comparison | Early Neglect | ||
| Age (months) | 142.43 +/− 21.6 | 140.42 +/− 19.87 | F=.139, p=.711 |
| Puberty (Tanner Stage) | 1.487 +/− 1.19 | 1.500 +/− 1.17 | F=.002, p=.966 |
| Sex | 23 males, 12 females | 15 males 13 females | χ2=.958, p=.328 |
| Socioeconomic Status (Hollingshead Index) | 55.72 +/− 6.03 | 53.48 +/− 8.19 | F=1.52, p=.223 |
Note: Pubertal stage ranged from 1–5, with higher scores indicating more adult development. Socioeconomic Status ranged from 0–66 with lower scores corresponding to lower parental education and less prestigious parental occupations.
Procedures
Families visited the lab and completed an MRI, a pubertal examination, a fetal alcohol exposure screening in one visit. Before enrolling in the study, informed consent and assent was obtained from parents and children. High-resolution T1-weighted structural brain images and diffusion tensor volumes were collected for all participants. Pubertal examinations were completed during a physical examination by experienced pediatric nurse practitioners. The clinical examination for girls assessed breast development with brief palpitation and visual examination of pubic hair. Boys were assessed via visual inspection of pubic hair and an orchidometer was used to measure testicular size (Genentech, 1997). Interobserver reliability between the nurse practitioners was good (K =0.88). Puberty was rated ranging from 1 (no development) to 5 (adult development) (Marshall & Tanner, 1969, 1970). Children who suffered early neglect did not differ from comparison children in terms of pubertal development (Early neglect Group: 1.5 +/− 1.175; Comparison Children: 1.487 +/− 1.194) as shown in Table 1.
We screened children for fetal alcohol exposure because this is a heightened risk factor in the countries where children resided prior to adoption. Digital pictures of all participants were analyzed using Fetal Alcohol Syndrome Facial Photographic Analysis Software (Astley, 2003). This system assesses (1) distance between the endocanthion and exocanthion landmarks, (2) philtrum smoothness, and (3) upper lip thinness. This screening tool has demonstrated very high sensitivity and specificity for prenatal alcohol exposure (Astley et al., 2002). A medical geneticist who specializes in fetal alcohol syndrome also reviewed facial photographs of all participants. No children from either group had signs of fetal alcohol exposure or fetal alcohol syndrome.
A subsample of families also returned for a follow-up visit that involved neurocognitive testing. Children’s neurocognitive functioning was assayed using the Cambridge Neuropsychological Test Automated Battery (CANTAB). This well-validated test battery is a computerized series of neurocognitive tests that cover a wide range of cognitive domains. The CANTAB has been used extensively with children and adolescents (Luciana & Nelson, 2002). The CANTAB is computerized for standardized administration and the tasks do not require verbal responses. Forty-eight participants (21 who had experienced early neglect and 27 comparison children) were able to return to complete the CANTAB. Groups did not differ on age (F=.166, p=.686), puberty (F=.017, p=.897), or gender (χ2=.001, p=.917).
Children completed four CANTAB subtests: Paired Associates Learning (PAL), Intra-Extra Dimensional Set Shift (IED), Stockings of Cambridge (SOC), and Spatial Working Memory (SWM). These tests were chosen based on previous research reports noting issues with attention, inhibitory control, and planning in children who experienced early neglect (Bos et al., 2009; Pollak et al., 2010).
The PAL subtest assesses visual memory and learning. During this task, subjects are presented with a series of boxes on a computer screen. The contents of the boxes are then shown in randomized order, with one or more of them containing an abstract pattern. The patterns are then presented in the middle of the screen one at a time, and subjects are then required to select the box containing the respective pattern’s correct location. The total number of errors made by subjects is tracked as the task progresses, while the difficulty of the trials increases. The IED subtest probed set-sorting and then shifting. During this test, participants are shown items with two different dimensions: solid color-filled shapes and white lines. Simple stimuli consist of only one of these dimensions, whereas more complex stimuli include both, specifically the overlay of white lines upon solid shapes. Two different simple stimuli are presented and participants must learn a sorting rule, which is then altered. These rule shifts are initially “intradimensional” (colored shapes are the only relevant dimension) and then become “extradimensional” (white lines become the only relevant dimension). The total number of errors during this task is collected and used as the major measurement of performance. The SOC is a variant of the Tower of London or Tower of Hanoi spatial planning test. Participants are shown two displays, each of which contains three colored balls. The displays are presented in such a way that they can be perceived as stacks of colored balls held in stockings, or socks, suspended from a beam. The subject is then instructed to move the balls in the lower display to match the pattern in the upper display. The balls can be only moved one at a time. Test performance is measured by the correct number of matches each subject completes in the minimum number of moves. The SWM subtest examines the ability to retain spatial information and to manipulate items in working memory. For this task, individuals much touch boxes by a process of elimination, find one blue “token” in each of a quantity of boxes and use them to fill an empty column on the right side of the screen. A participant’s score is based upon total errors (touching boxes that have already been found to be empty and revisiting boxes that have already been revealed to contain a token).
Neuroimaging on all subjects was performed using a 3.0 Tesla General Electric SIGNA (Waukesha, WI) MRI system with a quadrature birdcage head coil. The scanning protocol consisted of both high-resolution 3D T1-weighted imaging (described below) and diffusion tensor imaging. Diffusion tensor imaging was performed using a cardiac-gated, diffusion-weighted, spin-echo, single-shot, EPI pulse sequence. Diffusion tensor encoding was achieved using twelve optimum non-collinear encoding directions (obtained by minimum energy numerical optimization) with a diffusion weighting of 1114 s/mm2 and a non-DW T2-weighted reference image. Other imaging parameters were TE = 78.2 ms, 3 averages (NEX: magnitude averaging), and an image acquisition matrix of 120 × 120 over a field of view of 240 × 240 mm2. The cerebrum was covered using 39 contiguous 3-mm thick axial slices. The acquired voxel size of 2 × 2 × 3 mm was interpolated to 0.9375 mm isotropic dimensions (256 ×256 in plane image matrix). High order shimming and fieldmap images were collected, in order to minimize field inhomogeneity and image artifacts. Field-map images were collected using a pair of non-EPI gradient echo images at two echo times: TE1 = 8 ms and TE2 = 11 ms. Before scanning, participants were oriented to the MRI through the use of a mock-MRI simulator.
Measures
Measures of early neglect
Adopted parents of children who suffered early neglect completed several questionnaire measures, including background data (e.g., parent education) and pre-adoption information (e.g., length of time in institution, conditions in the institution, age at adoption).
White matter microstructure
Diffusion-tensor images were collected to specifically assess white matter microstructural properties. These images were corrected for eddy current related distortion and head motion via FSL software suite (http://www.fmrib.ox.ac.uk/fsl/) and distortions from field inhomogeneities were corrected using custom software algorithms based upon the field map method described by Jezzard and Clare (1995) before performing a non-linear tensor estimation using CAMINO (Cook et al., 2006).
After these steps, linear and non-linear normalization was performed using DTI-ToolKit (DTI-TK; http://www.nitrc.org/projects/dtitk), a spatial normalization & atlas construction toolkit (Zhang et al., 2007). This was to allow for voxel-wise analyses (across the whole brain) of white matter integrity. The DTI-TK tool suite was specifically designed for manipulating diffusion-tensor images and was recently validated as the best performing normalization method among eight publicly available methods (Wang et al., 2011). Particularly important for pediatric imaging research, all spatial normalization was done using study-specific templates. Such an approach minimizes sources of variability (e.g., possible over-warping pediatric imaging data to adult diffusion-tensor templates) and yields high sensitivity at the voxel level.
We constructed a study-specific template based on children who had suffered neglect and also children who had not suffered such neglect. This template was produced through iterative averaging and registering of subjects using rigid, affine and lastly diffeomorphic registrations. The resulting template was unbiased towards any single subject and captured the average diffusion properties of the population at each voxel with a diffusion tensor. Subsequently, the template was “shape-corrected” to ensure that it also represents the average shape of the population. Voxelwise fractional anisotropy (FA) were then calculated for all diffusion tensor volumes diffeomorphically registered to our study specific template. In template space, these files were smoothed using a 5mm full-width, half-maximum Gaussian filter.
Voxelwise group t-tests and whole brain regressions were next conducted in FMRISTAT (Worsley et al., 2002). Total white matter and pubertal status were entered into a linear regression model as nuisance variables. An initial statistical threshold of t(59) = 2.916, p < 0.005 uncorrected, was used in examining any possible group brain differences. In addition to examining group differences, voxelwise correlations were also conducted with CANTAB z-scores. An initial statistical threshold of t(18) = 3.197, p < 0.005 uncorrected, was used in examining any possible group brain differences. These analyses were only conducted within the sample that suffered early neglect, as significant group differences were detected on the neurocognitive variables of interest. These correlations were then used in a logical AND conjunction analysis to identify the brain regions that were different between groups and also related to neurocognitive performance for the children who suffered early neglect.
This analytic approach has been used in a number of research studies (Nichols et al., 2006); such an approach is a straightforward way to find brain regions different between groups and also correlated with behavioral outcomes, with minimal statistics. This was done to minimize ‘non-independence errors’ that sometimes occurs in brain imaging research. In brief, statistical bias can be introduced by selecting data using a first statistical test and then applying a second non-independent statistical test to those same data. In our sample, there is a behavioral difference in neurocognitive performance. If we select voxels in the brain that are significantly different between groups and then simply correlate our group differences with group behavioral differences, this may violate numerous statistical assumptions and distort our results (for additional discussion, see Kriegeskorte, Simmons, Bellgowan, & Baker, 2009).
Brain structure
T1-weighted images were collected to measure brain volume. These images were first corrected for field inhomogeneity. Whole brain masks were then drawn by hand, to exclude extraneous aspects of the brain (e.g., dura matter, skull). Each individual brain was then registered to our template using Symmetric Normalization (SyN). This algorithm allows for large deformations, but also constrains the deformations to be physically reasonable. Jacobian determinants of the deformation field indicate the fractional volume expansion and contraction at each voxel, quanitified the magnitude of regional volume alterations required to match the template. Jacobian determinants were then log transformation to make the distribution closer to the normal distribution (Avants & Gee, 2004) and smoothed with 6 mm full-width, half-maximum Gaussian filter.
Our MRI template was study-specific, constructed based on all subjects. Template construction consisted of a multi-resolution strategy (for this study, a four level Gaussian pyramid) as well as the similarity metric for the optimization, along with a maximum number of iterations. We used registration parameters optimized for pediatric neuroimaging data (for additional details, see Hanson et al., 2010). Recent research validated SyN as one of the best available warping algorithms in a recent comparison of 14 nonlinear registration algorithms (Klein et al., 2009). Similar statistical models to the tests employed for analyses for FA were employed for brain volume data (e.g., voxelwise group t-tests, whole brain regressions, and logical AND conjunction analyses).
Also, to specifically probe the diffusion properties of the brain and control for possible differences in white matter, T1-weighted images were segmented via Statistical Parametric Mapping 8 (Wellcome Department of Cognitive Neurology: London, England). Probabilistic segments were output and then checked the accuracy of each subjects’ segmentation. If any errors were present, the bounding box or image matrix was adjusted and MRI images were reprocessed. If after this correction segments still contained errors, they were corrected by hand to remove skull, dura, and other non-brain matter. Post inspection, volumes were thresholded to exclude all voxels below 40% white matter probability and then the number of non-zero voxels was calculated.
Results
Neurocognitive Differences Between Groups
Consistent with previous reports in the literature, children who had suffered early neglect displayed poorer neurocognitive performance compared to comparison children. Specifically, children who had suffered early neglect had more total errors on the IED, PAL, and SWM, compared to children who had not suffered early neglect as shown in Table 2. Children who suffered early neglect also solved fewer problems on the SOC in the minimum number of moves (F=8.797, p=.005) compared to comparison children. Group means and standard deviations for each of these tests are listed in Table 2.
Table 2.
Descriptive Data For Individual CANTAB Measures
| CANTAB Subtest (as index by z-scores) | Sample | Group Comparisons | |
|---|---|---|---|
|
| |||
| Comparison | Early Neglect | ||
| IED (adjusted total errors) | 0.746 +/− 0.61 | 0.296 +/−0.55 | F=6.875, p=.012 |
| PAL (adjusted total errors) | 0.327 +/− 0.32 | 0.096 +/− 0.380 | F=5.13, p=.028 |
| SWM (total errors) | 1.1 +/− 0.588 | 0.261 +/− 0.861 | F=16.084, p<.001 |
| SOC (correct trials in minimum moves) | 0.386 +/− 0.765 | −0.376 +/− 1.015 | F=8.797, p=.005 |
Note: IED- Intra-Extra Dimensional Set Shift; PAL- Paired Associates Learning; SWM- Spatial Working Memory; SOC- Stockings of Cambridge
Group Brain Differences
Comparison children had greater total white matter volumes than children who suffered early neglect (Comparison: 418.59 +/−38.78 mm^3; Early Neglect: 375.59 +/−47.26 mm^3; F=15.566, p<.001). All subsequent analyses controlled for these difference since our hypotheses were specifically focused on the diffusion properties of the brain.
Children who had experienced early neglect showed lower FA values in a number of white matter fibers compared to children who had not suffered such early adversity. Table 3 details regions where lower FA values were found in neglected children. In brief, lower FA was noted in the inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, corticospinal tract, cingulum, anterior corona radiata, and near the middle cerebellar peduncle (all p’s <.005 uncorrected, extent size>250 voxels). Greater FA was also noted in portions of the anterior thalamic radiation and the forceps minor in children who had suffered early neglect compared with comparison subjects, as shown in Table 4 (all p’s <.005 uncorrected, extent size>250 voxels). Brain-behavior correlations for each sample are shown in Tables 5–8. All of these associations correct for variation in total white matter and pubertal status.
Table 3.
Diffusion Tensor Imaging Group Comparisons (Early Neglect < Comparison Sample FA-Values)
| Region (nearest Brodmann area or anatomical landmark) | Extent | Peak of cluster coordinates (x,y,z; in MNI coordinate space) |
|---|---|---|
| Right Retrolenticular part of internal capsule (near Right Optic radiations) | Extent = 1302 voxels | Peak=4.02 at +35.00, −30.00, +1 |
| Left Corticospinal tract (near BA 6) | Extent =613 voxels | Peak=4.25 at −22.00, −20.00, +53.00 |
| Bilateral Cerebral peduncle (near brainstem) | Extent =605 voxels | Peak=3.9 at +6.00, −16.00, −19.00 |
| Left Inferior limb of internal capsule (near putamen) | Extent =544 voxels, | Peak=3.8 at −27.00, −12.00, −5.00 |
| Left Cingulum (near hippocampus) | Extent =360 voxels | Peak at 3.83 at −21.00, −29.00, − +18.00 |
| Anterior corona radiata (between BA 47 & BA 12) | Extent =253 voxels | Peak at 3.06 at +13.00. +52.00, − 12.00 |
| Right Middle cerebellar peduncle | Extent =253 voxels, | Peak=3.1 at +18.00, −26.00, −32.00 |
Note: All regions noted significant at p=.005, uncorrected with an extent of < 250 voxels (1mm3); all results control for differences in pubertal status and total white matter
Table 4.
Diffusion Tensor Imaging Group Comparisons (Early Neglect > Comparison Sample FA-Values)
| Region (nearest Brodmann area or anatomical landmark) | Extent | Peak of cluster coordinates (x,y,z; in MNI coordinate space) |
|---|---|---|
| Right Anterior thalamic radiation (near globus pallidus) | Extent = 490 voxels | Peak=3.72 at +17.00, −3.00, −1.00 |
| Forceps minor (near BA 46 & 9) | Extent =345 voxels | Peak=3.58 at −17.00, +31.00, +21.00 |
Note: All regions noted significant at p=.005, uncorrected with an extent of < 250 voxels (1mm3); all results control for differences in pubertal status and total white matter
Table 5.
Negative Correlations Between Stockings of Cambridge Performance and FA In Children Who Suffered Early Social Neglect
| Region (nearest Brodmann area or anatomical landmark) | Extent | Peak of cluster Coordinates (x,y,z; in MNI coordinate space) |
|---|---|---|
| Right Anterior corona radiata (between BA 47 & BA 12) | Extent = 727 voxels | Peak=6.06 at +12.00, +52.00, − 13.00 |
| Right Superior longitudinal fasciculus, temporal portion (near BA 44 & 45) | Extent =505 voxels | Peak=5.91 at +34.00, +15.00, +21.00 |
| Left Superior longitudinal fasciculus, temporal portion (near BA 21) | Extent =498 voxels | Peak=4.3 at −49.00, −23.00, −11.00 |
| Left Inferior fronto-occipital fasciculus (near BA 44 & 45) | Extent =283 voxels | Peak=4.5 at −43.00, +26.00, +11.00 |
| Left Superior longitudinal fasciculus, temporal portion (near BA 21) | Extent =268 voxels | Peak=4.3 at −43.00, −53.00, −3.00 |
Note: All regions noted significant at p=.005, uncorrected with an extent of < 250 voxels (1mm3); all results control for differences in pubertal status and total matter
Table 8.
Negative Correlations Between Paired Associates Learning Performance And FA In Comparison Children
| Region (nearest Brodmann area or anatomical landmark) | Extent | Peak of cluster Coordinates (x,y,z; in MNI coordinate space) |
|---|---|---|
| Right Inferior longitudinal fasciculus | Extent = 750 voxels | Peak=4.87 at +29, −60, +21 |
| Middle cerebellar peduncle | Extent = 498 voxels | Peak=4.35 at −18, −76, −26 |
Note: All regions noted significant at p=.005, uncorrected with an extent of < 250 voxels (1mm3); all results control for differences in pubertal status and total matter
Brain Regions Different Between Groups And Related To Neurocognitive Functioning
To test our primary hypothesis, we examined the extent to which brain regions that were different between groups were related to children’s neurocognitive functioning. As detailed earlier, these analyses combined two types of statistical maps: 1) group brain differences and 2) correlations between brain variables and neurocognitive functioning. Clusters in three fiber tracts emerged: anterior corona radiata (ACR) in the prefrontal cortex, hippocampal portions of the cingulum (CGH), and the inferior longitudinal fasciculus (ILF). The ACR has been implicated in cognitive control and behavioral regulation (Rothbart et al., 2011). The two later tracts are involved with connecting the temporal lobe and the prefrontal cortex.
For the analyses with the Stockings of Cambridge subtest, a measure of spatial planning, FA in the ACR (peak at x= +15, y= +51, z= −11, extent= 23mm3) and CGH (peak at x= +28, y= −28, z= −18, extent= 54mm3) was different between groups (Early neglect < Comparison Children) and related to subtest performance in the sample that suffered early neglect (ACR cluster r=.748, p<.001; CGH r=.669, r=.002). For the Paired Associated Learning CANTAB subtest a measure of visual learning and memory, FA in the ILF (peak at x= +39, y= −9, z= −25, extent= 23mm3) was different between groups (Early neglect < Comparison Children) and related to task performance in the sample that suffered early neglect (Temporal Lobe Cluster r=.714, r=.001). These results are shown in Figure 1. Figure 2 shows these fiber tracts running through the brain. No brain areas emerged for the conjunction analyses for the SWM and IED subtests.
Figure 1.
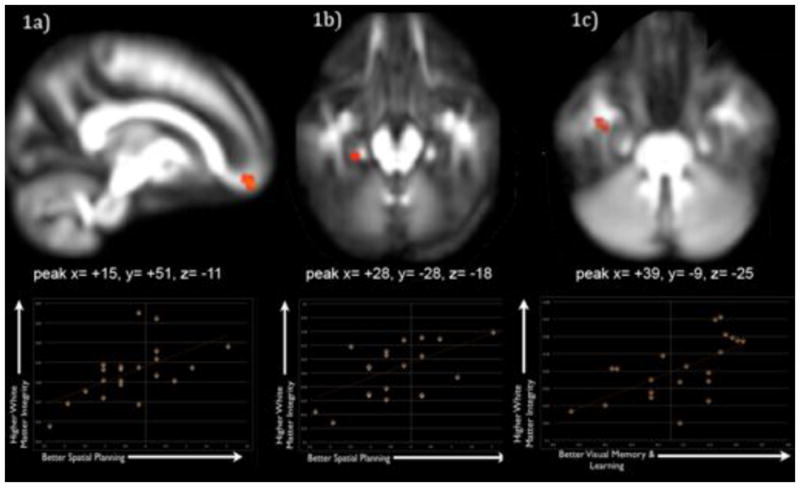
FA in prefrontal (Panel 1a) and temporal cortex (Panel 1b) was related to Spatial Planning. FA in a different portion of Temporal Lobe (Panel 1c) was related to Visual Memory and Learning (All regions FA Early Neglect < Comparison Sample; Higher FA = better performance). Scatterplots below each region show FA in the region on the vertical axis and neurocognitive performance (indexed by z-scores) on the horizontal axis.
Figure 2.
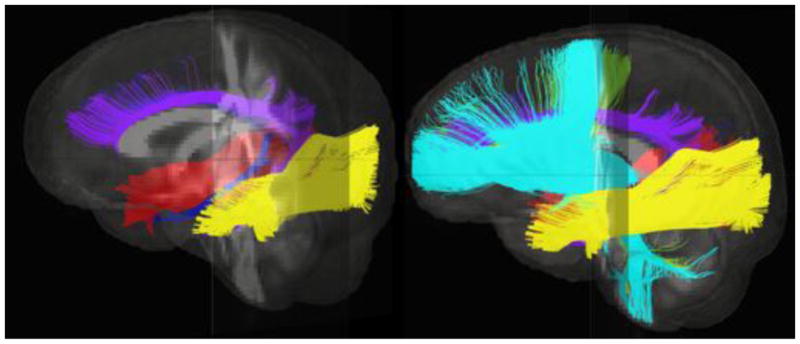
Fiber tracts of interest running through the brain. The inferior longitudinal fasciculus is shown in yellow and red, the cingulum appears in purple and blue, and the anterior corona radiata in turquoise.
Correlations With Characteristics Of Early Neglect
We conducted partial correlations (controlling for total white matter) between the regions that emerged from our logical AND conjunction analyses and characteristics of early neglect (e.g., age at adoption, time spent in institutional care). No significant relation emerged (p<.34 for all correlations) between brain data and variables quantifying early neglect.
Discussion
The primary goal of this study was to precisely map brain connectivity affected by early neglect to the cognitive deficits that have been observed in children who experienced early adversity. Our results indicate that children who suffered early neglect had lower white matter directional organization in the PFC and also lower directional organization in white matter tracts connecting the temporal lobe and the PFC. Individual differences in white matter microstructure, as indexed by FA, was associated with poorer neurocognitive performance among the neglected children. Similar associations were not found when using measures of brain volume rather than FA.
A number of previous research reports have found an association between neurocognitive functioning and early neglect (e.g., Bos et al., 2009; Pollak et al., 2010). This is the first report of a link between such deficits and white matter integrity. Decreased white matter FA in the prefrontal and temporal cortex was associated with decrements in performance on a spatial planning task and a visual learning and memory task in children who suffered early neglect. This provides support for the idea that prefrontal white matter directional organization may be mediating the cognitive deficits conferred by early neglect.
These results are consistent with previous experimental studies in which the early environment in rodents and non-human primates was manipulated. Such research finds that early stress affects development of the PFC (Arnsten et al., 2009). Sanchez and colleagues (1998), for example, found major alterations in prefrontal white matter in rhesus macaques separated at 2 months of age and singly housed until 12 months of age. Similar patterns have been noted in rodents (Oomen et al., 2010; Zhang et al., 2002). In our sample, we found lower total white matter in children who suffered early neglect compared to comparison children. These alterations are present long after the early adversity was suffered, which fits with non-human animal research. Helmeke and colleagues (2009) found parentally deprived rodents had shorter and a reduced number of prefrontal dendrites early in development, exhibited a slight “catch-up” after the deprivation, but ultimately have less complex prefrontal architecture after this deprivation. Our results also fit with previous research on children who had suffered maltreatment, which found lower white matter volumes in maltreated groups compared to non-maltreated children (DeBellis et al., 2002). These alterations may be related to the particularly protracted post-natal developmental timeline of white matter.
The association with white matter directional organization and neurocognitive performance also fits well with a number of past research reports in humans. A large number of studies have reported relations between prefrontal activity and performance during cognitive tasks similar to those featured in this report (Anderson, Albert, & Fincham, 2005; Beaucham, Dagher, Aston, & Doyon, 2003). Similar relations have been reported between measures of functional brain activity in temporal lobe structures including the hippocampus and parahippocampal gyrus and spatial planning task performance (Baker et al., 1996; Dagher et al., 1999). In addition, our data support previous functional imaging work that found successful performance of the PAL relies heavily upon medial temporal lobe connections to the frontal lobe (Aizenstein et al., 2000). This CANTAB subtest examines visual memory and new learning, as children learn the locations of a progressively increasing number of abstract stimuli. Taken together, the data reported here suggests a potential neurobiological mechanism through which early adverse experiences convey risk for impaired cognitive development in children.
In addition to the anterior corona radiata, cingulum, and inferior longitudinal fasciculus, FA values in other brain areas were related to early neglect, but these regions were not related to any behaviors measured for this study (as detailed in Tables 3 & 4). These regions may be related to other negative outcomes associated with early neglect that were not measured in this study (e.g., difficulties with emotional processing or regulation). Early neglect may disrupt white matter organization and result in more diffuse connections between brain regions, as indexed by lower FA. Similar experiences may also cause some areas (e.g., the amygdala) to be connected to other portions of the brain at a higher degree, as indexed by higher FA. Such alterations in the brain may be related to socio-emotional function early in development. Recent work by Wolff and colleagues (2012) noted higher regional FA in children with autism compared to typically-developing controls.
There was variability in the duration and exact timing of the neglect suffered. Reports from adopted parents about these details or other characteristics of neglect did not correlate with the brain-behavior relations reported here. Such data was however retrospective and may not be fully accurate due to recall bias and other factors. Variability also exists in the exact type of life stressors that each child suffered. It is, however, probable that children’s pre-adoption environments fell below the quality needed to sustain normal physical and behavioral development. Parents consistently reported few one-to-one interactions with caregivers, lack of toys or stimulation, and very little linguistic stimulation before 2 years of age. Each of these types of experiences may uniquely affect the brain, or additively influence specific neural circuits. Richer information on the neglect suffered would aid in understanding the effects of early adversity on brain– behavior relations, as the characteristics of child maltreatment have been linked with differential psychological outcomes (Manly et al., 1994).
There are a number of features of the present study that are important to consider in interpreting these data. First, associations between neglect and FA emerged for some but not all neurocognitive tasks. Additional research is needed to understand the nature of cognitive functions that are and are not related to neglect. Subsequent research employing neurocognitive tasks with task-dependent fMRI may provide additional insights and aid in explaining neurocognitive deficits associated with early neglect. Differences in brain activity, along with white matter integrity, may be related to the deficits seen in children who suffered early neglect. Second, we did not have specific information regarding the prenatal history or possible post-natal malnutrition. Malnourishment is often part of the “neglect” experience. However, malnourishment and social neglect appear to have separate effects on brain development, and also may interact. Classic work by Bowlby (1944a, 1944b), Casler (1961), Spitz (1945), and others that studied children who experienced social neglect in situations where nutrition was adequate and found behavioral decrements in social, emotional, and cognitive functioning in these children. Future research with more precise information regarding pre- and post-natal nutrition could aid in understanding the likely complex interaction of malnourishment and parental separation. Although we did not have access to the health records of parents or children, making it difficult to rule out the effects of exposure to teratogens, we used the best measures currently possible to rule out prenatal alcohol exposure. Third, FA values be affected by fibers in the brain crossing and may be less indicative of diffuse connections or lower levels of myelination. Subsequent studies should employ DTI sequences with a greater number of encoding directions to fully rule out this possibility. Finally, we did discover additional brain regions that differed between groups that were not part of our a priori hypotheses. We did not centrally focus on the portions of temporal lobe white matter, as they were unexpected findings. Lower FA in this area may be related to memory processes necessary to complete the neurocognitive tasks we employed here.
A growing body of research indicates that environmental context in interaction with the active engagement by the subject massively shapes neurocognitive functioning and development (Fox, Levitt, & Nelson, 2010). Our data add to this burgeoning field of study. In addition, our results suggest one potential neurobiological mechanism (aberrant organization of prefrontal white matter) that mediates the deleterious effects of early neglect on cognitive functioning. By increasing our understanding of the neurobehavioral consequences of child neglect, and more broadly early adversity in childhood, targeted intervention and training experiences may be developed and tailored to optimize children’s cognitive development.
Table 6.
Negative Correlations Between Paired Associates Learning Performance And FA In Children Who Suffered Early Social Neglect
| Region (nearest Brodmann area or anatomical landmark) | Extent | Peak of cluster Coordinates (x,y,z; in MNI coordinate space) |
|---|---|---|
| Right Middle cerebellar peduncle | Extent = 475 voxels | Peak=3.72 at +16.00, −40.00, −34.00 |
| Right Inferior longitudinal fasciculus (near fusiform) | Extent =314 voxels | Peak=3.58 at +35.00, −54.00, −11.00 |
| Right Inferior longitudinal fasciculus (near BA 20 & 21) | Extent =306 voxels | Peak=5.3 at +42.00, −11.00, −23.00 |
Note: All regions noted significant at p=.005, uncorrected with an extent of < 250 voxels (1mm3); all results control for differences in pubertal status and total matter
Table 7.
Negative Correlations Between Stockings of Cambridge Performance And FA In Comparison Children
| Region (nearest Brodmann area or anatomical landmark) | Extent | Peak of cluster Coordinates (x,y,z; in MNI coordinate space) |
|---|---|---|
| Left inferior longitudinal fasciculus | Extent = 1340 voxels | Peak=4.45 at −37, −45, +29 |
| Left Forceps minor | Extent = 521 voxels | Peak=4.99 at −18, +50, +10 |
| Right inferior fronto-occipital fasciculus | Extent = 490 voxels | Peak=4.03 at +30, +42, +4 |
| Left superior longitudinal fasciculus | Extent = 360 voxels | Peak=4.54 at −31, +8, +24 |
| Right superior longitudinal fasciculus | Extent = 360 voxels | Peak=4.38 at +39, −47, +28 |
| Right superior longitudinal fasciculus | Extent = 352 voxels | Peak=3.92 at +41, −25, +34 |
| Left superior corona radiata | Extent = 253 voxels | Peak=3.62 at −15, +15, +48 |
Note: All regions noted significant at p=.005, uncorrected with an extent of < 250 voxels (1mm3); all results control for differences in pubertal status and total matter
Acknowledgments
This work was supported by the US National Institute of Mental Health (Grants MH61285, MH68858 to SDP and Grant MH84051 to RJD), along with a US National Institute of Drug Abuse Fellowship (DA028087 to JLH). Infrastructure support was provided by the Waisman Center at the University of Wisconsin, through the National Institute of Child Health and Human Development (Grant P30-HD03352).
We thank Michael Anderle, Lisa Angelos, Elizabeth Shirtcliff, and Barbara Roeber for help with this project. Andrew Fox, Terrence Oakes, and Nicole Strang provided helpful discussion of our results. We also thank Elizabeth Zakszewski for help with figure preparation.
References
- Aizenstein HJ, MacDonald AW, Stenger VA, Nebes RD, Larson JK, Ursu S, Carter CS. Complementary category learning systems identified using event-related functional MRI. Journal of Cognitive Neuroscience. 2000;12:977–987. doi: 10.1162/08989290051137512. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Albert MV, Fincham JM. Tracing problem solving in real time: fMRI analysis of the subject-paced Tower of Hanoi. Journal of Cognitive Neuroscience. 2005;17:1261–1274. doi: 10.1162/0898929055002427. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B. White Matter Development in Adolescence: A DTI Study. Cerebral Cortex. 2010;20:2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley SJ. FAS Facial Photographic Analysis Software. Version 1.0.0. Seattle, WA: FAS Diagnostic & Prevention Network, University of Washington; 2003. [Google Scholar]
- Astley SJ, Stachowiak J, Clarren SK, Clausen C. Application of the fetal alcohol syndrome facial photographic screening tool in a foster care population. The Journal of Pediatrics. 2002;141:712–717. doi: 10.1067/mpd.2002.129030. [DOI] [PubMed] [Google Scholar]
- Avants B, Gee JC. Geodesic estimation for large deformation anatomical shape averaging and interpolation. NeuroImage. 2004;23(Suppl 1):S139–50. doi: 10.1016/j.neuroimage.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Baker SC, Rogers RD, Owen AM, Frith CD, Dolan RJ, Frackowiak RS, Robbins TW. Neural systems engaged by planning: a PET study of the Tower of London task. Neuropsychologia. 1996;34:515–526. doi: 10.1016/0028-3932(95)00133-6. [DOI] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Research. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MH, Dagher A, Aston JAD, Doyon J. Dynamic functional changes associated with cognitive skill learning of an adapted version of the Tower of London task. NeuroImage. 2003;20:1649–1660. doi: 10.1016/j.neuroimage.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Beckett C, Castle J, Rutter M, Sonuga-Barke EJ. VI. Institutional deprivation, specific cognitive functions, and scholastic achievement: English and Romanian Adoptee study findings. Monographs of the Society for Research in Child Development. 2010;75:125–142. doi: 10.1111/j.1540-5834.2010.00553.x. [DOI] [PubMed] [Google Scholar]
- Behen ME, Muzik O, Saporta ASD, Wilson BJ, Pai D, Hua J, Chugani HT. Abnormal Fronto-striatal Connectivity in Children with Histories of Early Deprivation: A Diffusion Tensor Imaging Study. Brain Imaging and Behavior. 2009;3:292–297. doi: 10.1007/s11682-009-9071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos KJ, Fox N, Zeanah CH, Nelson CA., III Effects of early psychosocial deprivation on the development of memory and executive function. Frontiers in Behavioral Neuroscience. 2009;3:16–16. doi: 10.3389/neuro.08.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J. Forty-four juvenile thieves: their characters and home life. International Journal of Psychoanalysis. 1944a;25:19–53. [Google Scholar]
- Bowlby J. Forty-four juvenile thieves: their characters and home life (II) International Journal of Psychoanalysis. 1944b;25:107–127. [Google Scholar]
- Casler L. Maternal deprivation: a critical review of the literature. Monographs of the Society for Research in Child Development. 1961;26:1–64. [PubMed] [Google Scholar]
- Chanraud S, Zahr N, Sullivan EV, Pfefferbaum A. MR Diffusion Tensor Imaging: A Window into White Matter Integrity of the Working Brain. Neuropsychology Review. 2010;20:209–225. doi: 10.1007/s11065-010-9129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P, Bai Y, Nedjati-Gilani S, Seunarine K, Hall M, Parker G, Alexander DC. Camino: Open-source diffusion-MRI reconstruction and processing. 14th scientific meeting of the international society for magnetic resonance in medicine; 2006. p. 2759. [Google Scholar]
- Dagher A, Owen AM, Boecker H, Brooks DJ. Mapping the network for planning: a correlational PET activation study with the Tower of London task. Brain : a journal of neurology. 1999;122:1973–1987. doi: 10.1093/brain/122.10.1973. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Mcewen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nature Neuroscience. 2012;15:689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biological Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- Diamond A. Principles of frontal lobe function. New York, NY, US: Oxford University Press; 2002. Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry; pp. 466–503. [Google Scholar]
- Dubois J, Dehaene-Lambertz G, Soares C, Cointepas Y, Le Bihan D, Hertz-Pannier L. Microstructural Correlates of Infant Functional Development: Example of the Visual Pathways. Journal of Neuroscience. 2008;28:1943–1948. doi: 10.1523/JNEUROSCI.5145-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhász C, Muzik O, Maqbool M, Chugani DC, et al. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117:2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Fox S, Levitt P, Nelson C., III How the timing and quality of early experiences influence the development of brain architecture. Child Development. 2010;81:28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W, Chen Y, Gerig G, Smith J, Jewells V, Gilmore J. Temporal and Spatial Development of Axonal Maturation and Myelination of White Matter in the Developing Brain. American Journal of Neuroradiology. 2008;30:290–296. doi: 10.3174/ajnr.A1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genentech. Assessment of pubertal stages, G70577-R1#LF0050. San Francisco: Genentech; 1997. [Google Scholar]
- Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT. Altered Water Diffusivity in Cortical Association Tracts in Children with Early Deprivation Identified with Tract-Based Spatial Statistics (TBSS) Cerebral Cortex. 2010;20:561–569. doi: 10.1093/cercor/bhp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience. 2010;30:7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstedt WL, Madsen NJ, Gunnar MR, Grotevant HD, Lee RM, Johnson DE. The International Adoption Project: population-based surveillance of Minnesota parents who adopted children internationally. Maternal and child health journal. 2008;12:162–171. doi: 10.1007/s10995-007-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmeke C, Seidel K, Poeggel G, Bredy TW, Abraham A, Braun K. Paternal deprivation during infancy results in dendrite- and time-specific changes of dendritic development and spine formation in the orbitofrontal cortex of the biparental rodent Octodon degus. Neuroscience. 2009;163:790–798. doi: 10.1016/j.neuroscience.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Clare S. Sources of distortion in functional MRI data. Human Brain Mapping. 1999;8:80–85. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<80::AID-HBM2>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreppner J, Kumsta R, Rutter M, Beckett C, Castle J, Stevens S, Sonuga-Barke EJ. IV. Developmental course of deprivation-specific psychological patterns: early manifestations, persistence to age 15, and clinical features. Monographs of the Society for Research in Child Development. 2010;75:79–101. doi: 10.1111/j.1540-5834.2010.00551.x. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Li TQ, Noseworthy MD. Mapping the development of white matter tracts with diffusion tensor imaging. Developmental Science. 2002;5:293–300. [Google Scholar]
- Luciana M, Nelson CA. Assessment of neuropsychological function through use of the Cambridge Neuropsychological Testing Automated Battery: performance in 4- to 12-year-old children. Developmental Neuropsychology. 2002;22:595–624. doi: 10.1207/S15326942DN2203_3. [DOI] [PubMed] [Google Scholar]
- Manly JT, Kim JE, Rogosch FA, Cicchetti D. Dimensions of child maltreatment and children’s adjustment: contributions of developmental timing and subtype. Development and Psychopathology. 2002;13:759–782. [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BCP, Almli CR, McKinstry RC. Normal Brain Maturation during Childhood: Developmental Trends Characterized with Diffusion-Tensor MR Imaging. Radiology. 2001;221:349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Nichols EA, Kao YC, Verfaellie M, Gabrieli JD. Working memory and long-term memory for faces: Evidence from fMRI and global amnesia for involvement of the medial temporal lobes. Hippocampus. 2006;16:604–616. doi: 10.1002/hipo.20190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Soeters H, Audureau N, Vermunt L, Van Hasselt FN, Manders EMM, Joels M, et al. Severe Early Life Stress Hampers Spatial Learning and Neurogenesis, but Improves Hippocampal Synaptic Plasticity and Emotional Learning under High-Stress Conditions in Adulthood. Journal of Neuroscience. 2010;30:6635–6645. doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosini L, De Bartolo P, Foti F, Gelfo F, Cutuli D, Leggio MG, Mandolesi L. On whether the environmental enrichment may provide cognitive and brain reserves. Brain Research Reviews. 2009;61:221–239. doi: 10.1016/j.brainresrev.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL, Frenn KA, et al. Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Development. 2010;81:224–236. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JA, Scott KA. Interpreting Assessment Data of Internationally Adopted Children. Topics in Language Disorders. 2009;29:82–99. [Google Scholar]
- Rothbart MK, Sheese BE, Rueda MR, Posner MI. Developing Mechanisms of Self-Regulation in Early Life. Emotion Review. 2011;3:207–213. doi: 10.1177/1754073910387943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Developmental catch-up, and deficit, following adoption after severe global early privation. Journal of child psychology and psychiatry, and allied disciplines. 1998;39:465–476. [PubMed] [Google Scholar]
- Rutter MM, Dunn JJ, Plomin RR, Simonoff EE, Pickles AA, Maughan BB, Ormel JJ, et al. Integrating nature and nurture: implications of person-environment correlations and interactions for developmental psychopathology. Development and Psychopathology. 1997;9:335–364. doi: 10.1017/s0954579497002083. [DOI] [PubMed] [Google Scholar]
- Sánchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Research. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Spitz RA. Hospitalism: an inquiry into the genesis of psychiatric conditions in early childhood. In: Freud A, Hartmann H, Kris E, editors. Psychoanalytic Study of the Child 1. International Universities Press Inc; New York: 1945. [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gupta A, Liu Z, Zhang H, Escolar ML, Gilmore JH, Gouttard S, et al. DTI registration in atlas based fiber analysis of infantile Krabbe disease. NeuroImage. 2011;55:1577–1586. doi: 10.1016/j.neuroimage.2011.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. American Journal of Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC. A general statistical analysis for fMRI data. NeuroImage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
- Zhang H, Avants BB, Yushkevich PA, Woo JH, Wang S, McCluskey LF, Elman LB, et al. High-dimensional spatial normalization of diffusion tensor images improves the detection of white matter differences: an example study using amyotrophic lateral sclerosis. IEEE transactions on medical imaging. 2007;26:1585–1597. doi: 10.1109/TMI.2007.906784. [DOI] [PubMed] [Google Scholar]
- Zhang LX, Levine S, Dent G, Zhan Y, Xing G, Okimoto D, Kathleen Gordon M, et al. Maternal deprivation increases cell death in the infant rat brain. Brain research Developmental brain research. 2002;133:1–11. doi: 10.1016/s0926-6410(01)00118-5. [DOI] [PubMed] [Google Scholar]


