Abstract
Little is known about the effects of muscle and tendon unloading on the molecular response of the rotator cuff. Tendon unloading following rupture of one of the rotator cuff tendons can induce alterations in muscle physiology and tendon structure, which can subsequently affect reparability and healing potential. The goals of the current study were to determine the effect of mechanical unloading on gene expression and morphology of (1) healthy supraspinatus tendons and muscles, and (2) supraspinatus tendons and muscles after acute injury and repair. Mechanical unloading was achieved by tenotomy and/or botulinum toxin A (BTX) chemical denervation in a rat rotator cuff model of injury and repair. Gene expression profiles varied across regions of the muscle, with the greatest changes seen in the distal aspect of the muscle for most genes. Myogenic and adipogenic genes were upregulated in muscle when unloaded (tenotomy and BTX). Tendon injury, with and without repair, resulted in upregulation of fibrosis- and tendon-specific gene expression. The expression of scleraxis, a transcription factor necessary for tendon development, was upregulated in response to injury and repair. In summary, tendon detachment and repair had the greatest effect on tendon gene expression, while unloading had the greatest effect on muscle gene expression.
Keywords: rotator cuff injury, healing, gene expression, supraspinatus, rodent
INTRODUCTION
The rotator cuff provides stability and motion of the glenohumeral joint.1 The prevalence of rotator cuff tear is high; approximately 30% of the population over the age of 60 years has a cuff tear.2 Rotator cuff repair is one of the most commonly performed orthopedic surgical procedures, with over 75,000 repairs performed each year in the United States.3 Healing after rotator cuff repair, however, is a well-known clinical challenge, with the reported failure rates ranging from 30 to 94%.4,5 Chronic rotator cuff tears are associated with the development of significant degenerative changes in the rotator cuff muscles such as atrophy, fat accumulation, and fibrosis.6,7 This muscle degeneration is known to have a negative influence on the outcome of a rotator cuff repair.
Tissue unloading resulting from a rotator cuff tendon tear has deleterious effects on the structure and function of cuff musculotendinous units and has been shown to lead to increased muscle atrophy, intramuscular fatty accumulation, and passive stiffness.5,8,9 Detachment of a tendon from its bony insertion leads to poor collagen organization within the tendon10 and muscle retraction7, which may subsequently result in the traction of the nerve innervating the muscle11. This can further exacerbate the degeneration of the rotator cuff muscles and tendons. The loading environment therefore has a significant effect on muscle and tendon physiology in both the healthy and injured rotator cuff.12 Previously, it was shown that rotator cuff tendon injury and muscle denervation in a chronic degenerative setting leads to the accumulation of intra- and inter-myocellular fatty accumulation13,autophagy14 of the muscles, and increased expression of adipogenic and myogenic markers.13 Traction injury to the suprascapular nerve may occur in conjunction with retraction of a massive rotator cuff tear.15 While isolated or concomitant tendon/nerve injury may contribute to varying magnitudes of degenerative changes to the musculotendinous unit, the molecular mechanisms of each patho-physiological process has not yet been determined.
The purpose of the current study was to determine the effects of unloading on gene expression of healthy and injured supraspinatus tendons and muscles in a well-established rat model of rotator cuff disease.16,17 With regard to muscle response, we hypothesized that mechanical unloading via tenotomy and/or BTX in healthy and injured rotator cuffs would lead to downregulation of myogenic regulatory factors (MRFs) and markers in muscle, upregulation of adipogenic transcription factors and markers in muscle, and impaired muscle regeneration. With regard to tendon response, we hypothesized that mechanical unloading via tenotomy and/or BTX in healthy and injured rotator cuffs would lead to decreased expression of collagen and collagen-inducing growth factors and reduced scarring and inflammation.
METHODS
Animal injury models
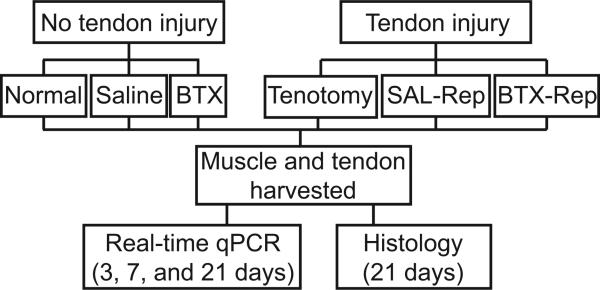
All animal procedures were approved by the institutional Animal Studies Committee. Adult male Sprague-Dawley rats (N=110) weighing 400-450 grams were used in this study. All animals were anesthetized using isoflurane with an oxygen carrier by nose cone and divided into five experimental groups (N=20/group; Figure 1). Surgeries were performed under sterile techniques and described in detail in the supplemental material. For the complete muscle unloading group (BTX), chemical denervation of the supraspinatus muscle was effectuated via intramuscular injection of botulinum toxin A (9U/kg) diluted in physiological saline (100 μL total volume) into the supraspinatus muscle belly. A second group (Saline) was used as an injection control. In order to simulate injury to the rotator cuff tendon, a third group of animals was subjected to tenotomy of the supraspinatus tendon (Tenotomy group).13 A fourth group was used to simulate a repaired tendon injury; animals were subjected to tenotomy of the supraspinatus tendon and subsequent repair (SAL-Repair group).17 A fifth group of animals received a similar tendon injury followed by repair along with chemical denervation using BTX (9 U/kg, BTX-Repair group). All animals receiving tendon injury were treated with subcutaneous bupivacaine post-operatively (0.1%). The contralateral shoulders were left untreated in all five groups. A sixth group of animals was used as uninjured, age-matched controls (Normal group, N=10). Postoperative animal care was administered by an animal care technician.
Figure 1.
Schematic of study design. BTX = chemical denervation via botulinum-toxin; SAL-Rep = Tenotomy/repair + injection of Saline; BTX-Rep = Tenotomy/repair + injection of BTX.
Real-time PCR
For gene expression studies, animals were sacrificed at 3, 7, and 21 days after injury (N=5 per time-point per group). Immediately following sacrifice, supraspinatus muscles and tendons were dissected and extramuscular fat globules were removed. Muscles were then weighed for wet muscle mass and subsequently divided into thirds (proximal, middle, and distal). All samples were frozen immediately using liquid nitrogen, stored at -80°C, and later processed for quantitative PCR using methods described in the supplemental material. Gene expression levels were examined in muscle for MRFs (myogenin, myogenic differentiation-1 [myoD1], myf5 and myf6); myosin heavy chains (myh4 and myh7); adipogenic transcription factors and markers (CCAAT/enhancer binding protein-α [c/ebpα] and peroxisome proliferator-activated receptor-γ [pparγ], and leptin). Gene expression levels were examined in tendon for extracellular matrix markers (collagen I [col1a1] and collagen III [col3a1]; growth factors (transforming growth factor-β1 [tgf-β1] and tgf-β3); and differentiation marker (scleraxis). Expression levels of genes of interest relative to GAPDH (2-ΔCt) were compared using a two-way ANOVA (factors: time, group) with repeated measures (muscle region) to determine region-specific differences for gene expression as well as the effect of time and injury on gene expression, excluding the Normal group. If regional differences in expression were not present, relative expression levels were averaged across regions and a two-way ANOVA (factors: time, group) was used to determine differences for gene expression, excluding the Normal group. Post-hoc analyses were performed using Fisher's protected least squared difference. To compare relative gene expression of experimental groups to the Normal group, a one-way ANOVA was used with Fisher's protected least squared difference as a post-hoc analysis. Alpha levels of p < 0.05 were considered statistically significant.
Histology-based assays
A separate group of animals from each group (N=3 per time point per group) were used for histological analysis at 21 days. Sections were stained with Masson's trichrome to examine fibrocartilage morphology, picrosirius red to examine tendon morphology under polarized light, and hematoxylin and eosin to examine muscle morphology. Sections were evaluated qualitatively for tendon organization, fibroblast accumulation, and myocyte regeneration by a single, blinded observer (MK).
RESULTS
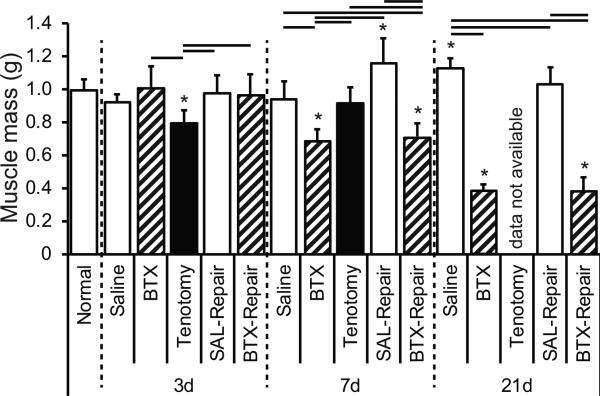
Chemical denervation and subsequent muscle unloading, observed in both the BTX and BTX-Repair groups, led to significantly decreased muscle mass at 7 and 21 days following BTX injection compared to all other groups, with 61% and 62% decreases in mass by 21 days for BTX and BTX-Repair, respectively, compared to the normal group (Figure 2). Tenotomy alone led to a significant (30%) decrease in muscle mass at 3 days compared to normal muscle, but this decrease was not observed at 7 days (Figure 2). At 21 days, muscle weights were not obtained for the Tenotomy only group.
Figure 2.
Supraspinatus muscle mass (in grams) for Normal, SAL, BTX, Tenotomy, SAL-Repair, and BTX-Repair groups at 3, 7, and 21 days post-injury. Muscle mass significantly decreased via denervation. Data not available for Tenotomy at 21days. * = sig. less than Normal.
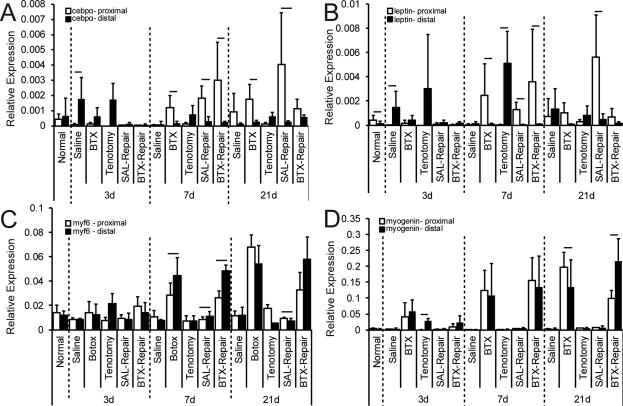
Gene expression profiles varied significantly across muscle regions (i.e., proximal to distal) for several genes in multiple experimental groups (Figure 3). The proximal regions of the muscles demonstrated a higher level of expression across injury groups compared to the distal regions for all adipogenic genes (pparγ, leptin (Figure 3A & B, respectively, and c/ebpα). Therefore, only the proximal regions were compared between groups and time points for adipogenic genes. Following regional comparisons of myogenic genes, some expression profiles (e.g. myf5 and myf6) presented differences between proximal and distal muscle. In these cases, the trend of gene expression did not influence relative expression comparisons between groups. For genes that did not demonstrate a region-specific difference in gene expression following injury (i.e., myogenin, myod1, myh4, and myh7), and for genes whose expression pattern trends were similar between proximal and distal muscle (i.e. myf5 and myf6), the expression profiles were averaged across regions.
Figure 3.
Regional comparisons of gene expression for adipogenic markers, (A) cepbα and (B) leptin, and the myogenic marker, (C) myf6, which demonstrate region-dependent expression, and myogenic markers, (D) myogenin, which does not demonstrate region-dependent expression. * = significantly different than Normal for same region. Bar indicates significant differences between proximal and distal regions for the same experimental condition and time point (p<0.05).
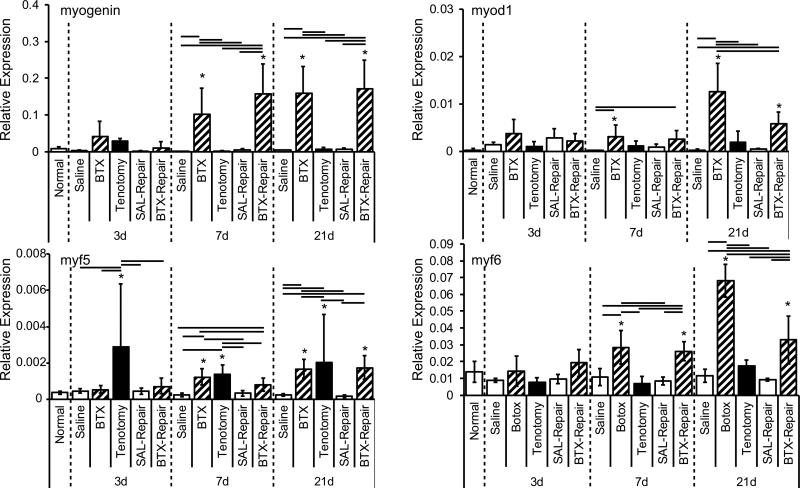
Chemical denervation via BTX injection had a dramatic effect on the expression of MRFs in the supraspinatus muscle. Specifically, myogenin, myod1, myf5 and myf6 were all significantly upregulated in muscle-unloaded groups (BTX and/or BTX-Repair) compared to the normal group at 7 and 21 days (Figure 4; p < 0.01 for myod1, myf5 and myf6 and p≤0.02 for myogenin). Upregulation of MRFs was significantly higher at 21 days than 3 and/or 7 days in each group for each factor. A significant effect of time was also associated with expression of myogenic markers investigated in this study (Figure 4; p = 0.007 for myf5; p = 0.002 for myogenin; and p < 0.0001 for myod1, and myf6). Tenotomy resulted in increased myf5 expression at all time points compared to the Normal group (Figure 4; p<0.001). Myosin heavy chain IIB, myh4, showed significant changes following tenotomy (Figure S1). At all three time points, myh4 was significantly upregulated in the Tenotomy group compared to all other treatment groups. There was abrupt upregulation of myh4 at 7 days, and this upregulation gradually diminished over the next two later time points (Figure S1).
Figure 4.
Myogenic regulatory factors myogenin and myod1 (top row), and myf5 and myf6 (bottom row), were significantly upregulated due to chemical denervation at 7 and 21days post-injury. * = significantly different than Normal; bars = significant differences between groups.
Expression of c/ebp-α, leptin, and ppar-γ demonstrated increased expression patterns following both muscle and tendon injury and repair in the proximal region of muscle (Figure S2). At 7 days, SAL-Repair demonstrated significantly greater expression of c/ebp-α compared to Normal and all other groups (Figure S2). At 21 days, SAL-Repair resulted in significant upregulation of both c/ebp-α and leptin compared to Normal and most other groups (Figure S2). Expression of lipid storage and glucose metabolism activator, ppar-γ, demonstrated a significant increase in the BTX-Repair group at 7 days compared to Normal and all other groups (Figure S2).
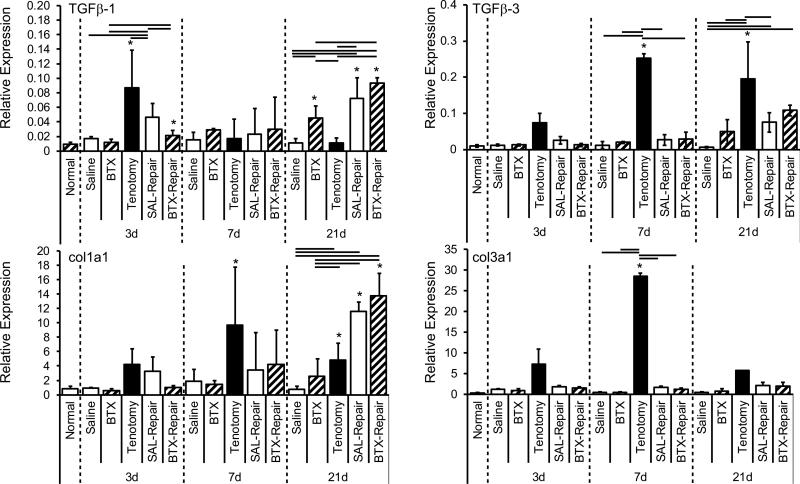
Tendon injury with and without repair resulted in a significant upregulation of fibrosis-specific genes, particularly tgf-β1 and -β3, col1a1, and col3a1 (Figure 5; p < 0.05). In the SAL-Repair group, expression of both tgf-β1 and -β3 demonstrated a significant increase over time, while Tenotomy alone resulted in peak expression of tgf-β1 at 3 days and tgf-β3 at 7 days. Interestingly, at 3 days, the gene expression profiles of tgf-β1 in the Tenotomy group were significantly higher than for SAL-Repair and BTX-Repair (p ≤ 0.006). This trend disappeared at 7 days, and at 21 days; tgf-β1 expression in tendons from the Tenotomy group was significantly lower than all other groups (Figure 5). Chemical denervation of the muscle via BTX injection resulted in only few significant changes in tendon mRNA expression. Of the tendon-specific genes investigated in this study, only tgf-β1 (Figure 5) and scleraxis (Figure S3) showed a significant increase in expression patterns in the BTX group (at 7 days for Scleraxis 21 days for tgf-β1) compared to Normal. Scleraxis demonstrated significantly increased gene expression at 7 and 21 days following tendon injury with and without chemical denervation by BTX injections compared to the saline-injected group (Figure S3).
Figure 5.
Fibrogenic and extracellular matrix genes were significant upregulated following tendon injury with and without nerve injury. tgf-β1 and -β3 (top row), and col1a1 and col3a1 (bottom row) were significantly increased due to tendon injury with and without repair. * = significantly different than Normal; bars = significant differences between groups.
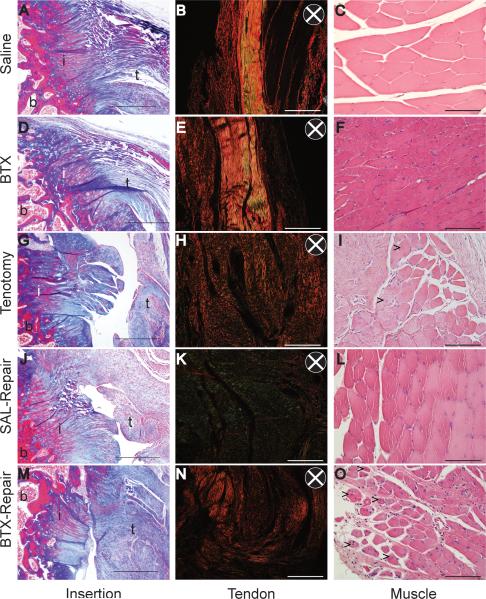
Fibrocartilage at the supraspinatus tendon insertion was intact and congruent in the Saline and BTX groups (Figure 6A and D), but it was deficient with an impaired transitional zone following tendon injury (Tenotomy, SAL-Repair and BTX-Repair; Figure 6G, J, and M). There was no continuity between tendon and bone for the Tenotomy group (Figure 6M). Histological findings suggested increased cellularity at the tendon stump for the Tenotomy group after 21 days of tendon injury (Figure 6). An increase in fibrous scar tissue was also observed for Tenotomy, Saline Repair, and BTX-Repair groups near the insertion. In the tendon, a qualitative decrease in collagen fiber organization was observed following tendon and/or muscle injury (Figure 6). Collagen fiber appeared well alignment in intact tendons following treatment with either Saline or BTX (Figure 6B and E), indicated by increased light intensity in polarized, picrosirius red stained images; however, organization was drastically reduced following injury with and without repair (Tenotomy, SAL-Repair and BTX-Repair; Figure 6H, K, and N). Signs of muscle regeneration, as indicated by centrally located nuclei in myocytes, were observed for the BTX-Repair and Tenotomy groups (Figure 6O). Qualitatively, a decrease in myostructure organization, decreased myocyte size, and increased cellularity were observed for both muscle-unloaded groups (BTX and BTX-Repair groups), as well as the Tenotomy group (Figure 6F and O).
Figure 6.
Representative histological sections of the tendon-to-bone insertion (left column, Masson's trichrome), tendon organization (middle column, picrosirius red under polarized light), and muscle morphology (right column, hematoxylin and eosin) of Saline (A-C), BTX (D-F), Tenotomy (G-I), SAL-Repair (J-L), and BTX-Repair (M-O) at 21 days post-injury. Arrows in I and O indicate cell nuclei clustering in myocytes. B = bone, I = insertion, T = tendon. Scale bar = 500μm (left column) and 100μm (middle and right columns). The direction of the polarizing filters is shown in the insets of B, E, H, K, and N.
DISCUSSION
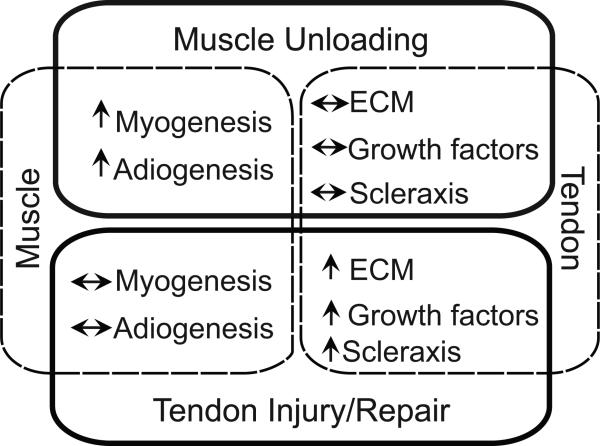
Perturbations to tendon and muscle loading led to time-dependent and loading-specific changes in the expression of regulatory factors associated with myogenesis and fibrogenesis. Chemical denervation, the mechanism of severe muscle unloading used in this study, led to changes mostly localized to the muscle (e.g., increased myogenic expression and morphological adaptations to muscle) whereas tendon injury led to changes primarily in the tendon (e.g., increased fibrogenic expression and disorganized tendon morphology), even in the presence of repair (Figure 7). Chronic unloading potentially leads to irreversible changes in rotator cuff tissue, which has severe implications clinically.
Figure 7.
Tendon injury and repair had the greatest effect on tendon gene expression and tendon morphology, while muscular unloading had the greatest effect on muscle gene expression and skeletal muscle morphology.
Gene expression changes were more dramatic on the proximal end compared to the distal end of the supraspinatus muscles for adipogenic-specific genes. It has previously been shown that fatty accumulation occurs following tendon detachment at both the extramuscular and intramuscular level13, and repair following chronic detachment does not necessarily reverse fatty degenerative changes18. The detachment of tendon from bone has previously demonstrated an effect on localized fatty accumulation in a rabbit model for rotator cuff injury19-21. This is the first study to our knowledge that investigates the distribution of gene expression patterning specifically of skeletal muscle following rotator cuff unloading, injury, and repair. In this study, region-specific variability in gene expression was primarily observed for adipogenic factors and only a few myo-specific genes (e.g. myf5 and myf6). Others have investigated regional changes in fatty degeneration, demonstrating an increased accumulation of fat in the distal region compared to the proximal region of the supraspinatus muscle for rabbit19, canine9, and rat20 models. Previously, Itiogawa et al. found that massive rotator cuff injury without repair in a rodent model led to increased expression of adipogenic genes in the distal region compared to the middle region20. In that study, it may be possible that the influence of a massive tear involving multiple tendons (supraspinatus, infraspinatus, and subscapularis) without repair more severely influenced muscle degenerative patterns than an isolated supraspinatus tendon injury, as was performed in the present study. Additionally, it is possible that dissection techniques differed between the two studies. In the present study, we were concerned with expression of adipogenic and myogenic genes specifically within the skeletal muscle, and thus globules of extramuscular fat were removed at time of dissection. Maintaining the pocket of extramuscular fat that accumulates primarily at the distal end of muscle following injury likely influences the expression patterns of adipogenic genes, as tissue homogenate would be a mixture of both adipose and skeletal muscle, and this method may have biased previous work investigating the propensity of adipogenesis following rotator cuff unloading. The present study attempted, to the best of ability, to isolate skeletal muscle only and exclude extraneous adipose tissue at time of dissection.
In previous studies using a rodent model for rotator cuff injury, Kim et al. demonstrated that skeletal muscle unloading via BTX led to comparable degenerative changes in skeletal muscle pathology to that of suprascapular neurotomy13. Muscular adaptations to these nerve injuries mimicked the fatty accumulation and degeneration seen clinically in human rotator cuffs21. A benefit of using BTX instead of neurotomy is the potential for reversal of nerve injury following rotator cuff repair. This approach is therefore more clinically relevant, as reversal of paralysis would be expected following surgical repair and release of nerve traction on the acromial notch11. The present study attempted to characterize acute, tissue-specific changes in gene expression profiles that likely initiate chronic degenerative changes following rotator cuff injury.
The observed increases in the expression of MRF genes in muscle following paralysis, as well as myocellular morphological adaptations that indicate muscular regeneration (e.g., centrally located nuclei) were confounded by decreased muscle mass, implying an impaired attempt of molecular cues to rescue muscle degeneration following denervation. Muscle unloading via neurotomy and chemical denervation has been associated with increased myogenic markers in the developing rotator cuff22,23. Additionally, the role of these markers in adult rotator cuff muscle degeneration and repair has been previously elucidated13. In vitro experiments have shown distinct time-dependent expression patterns of MRFs (early-acting factors, myod1 and myf5, demonstrating involvement in myoblast formation and satellite cell proliferation during fusion, and late-acting factors, myogenin and myf6, expressed during muscle differentiation)24. While MRFs are known to play roles in myocellular differentiation during development, the specificity of each molecule to regenerative or degenerative changes has only recently begun to be understood.25-27Myogenin has been shown to play a role in denervation-induced atrophy28, and others have demonstrated an increased expression of MRFs as early as 16 hrs following denervation in adult murine skeletal muscle29. The present study demonstrates a potentially delayed MRF expression in adult skeletal muscle following denervation, with distinct upregulation in myogenin, myod1, and myf6 at 7d, but not at 3d, following chemical denervation with and without tendon injury. The delayed increase in MRF expression may be a result of delayed myogenesis or impaired reinnervation of the muscle. In the present study, the model of denervation used (i.e., chemical denervation) may lead to differing expression patterns of MRFs than what is observed in other models of nerve injury, such as nerve crush or neurotomy29, due to the effect of BTX on neuromuscular junctions. MRFs have been shown to influence subunit expression of acetylcholine receptors of neuromuscular junctions30,31, and denervation may regulate myogenin expression via prolonged denervation28. Nonetheless, the transcriptional timing of these molecules likely has implications on skeletal muscle remodeling, atrophy, and degeneration following muscle unloading and tendon injury, and can provide insight into the remodeling process associated with rotator cuff injury and repair.
Upregulation of adipogenic factors and markers in this study suggest the critical role of mechanical loading on muscle homeostasis. In the case of tendon injury with concomitant repair and denervation, muscle retraction via tenotomy was only present for a short duration (on the order of 15 min) prior to suture repair. However, the effect of tendon injury and repair may exacerbate the effect of chemical denervation (i.e., Botox treatment) on the muscle. Inflammation may play a role in muscle fatty accumulation and atrophy14, and previous work has demonstrated increased inflammation following tendon injury and repair7,16,18. Although potential mechanisms of inflammation were not tested in our study, it is clear that muscle abnormalities following chemical denervation with tenotomy and repair results in a different myocellular response than just denervation or tenotomy alone, as illustrated histologically. Additionally, increased expression of leptin, a marker produced by mature adipocytes, suggests upregulation of its expression corresponding to the accumulation of adipose tissue in muscle, as seen in chronic rotator cuff disease.9 While induction of large rotator cuff injuries in a rat model have demonstrated dramatic increases in adipogenic factors13, regulation of these factors in isolated muscle/tendon injuries were not as dramatic in the present study. The time points investigated and unloading/injury mechanisms implicated in this study likely did not induce strong adipogenic differentiation signaling, as has been previously observed in more severe, chronic rotator cuff pathologies that lead to fatty accumulation13. Additionally, the intrinsic adipogenesis of skeletal muscle may not be as dramatic as the accumulation of adipose on the periphery of the muscle belly. A “gradient-like” accumulation of fat has been previously reported to ensue following rotator cuff injury, with increasing concentration of fat from the proximal to the distal end of the supraspinatus muscle19. However, this dramatic accumulation of fat most likely occurs in the space previously occupied by skeletal muscle following atrophy19. It is possible that the expression of intramuscular adipogenic genes in the proximal region has been obscured in previous studies because of the maintainance of extramuscular fat at the time of dissection20.
As has been reported in other models9, 17, the healing process following tendon injury illustrated in this study was characterized by significant and time-dependent upregulation in growth factors and ECM genes, specifically tgf-β1, -β3, col1a1, and col3a1. The initial expression of tgf-β1 and col1a1 following tendon injury with muscle loading was observed as early as 3 days, suggesting an acute response of the tendon injury with muscle activation, whereas tendon injury with muscle denervation did not induce changes in remodeling gene expression until 7 days. Additionally, the delay in expression of tgf-β3 following tendon injury with repair may suggest an impaired capacity of the tendon to properly remodel in the adult injury model. Interestingly, tenotomy alone resulted in increased tgf-β3 expression as early as 3 days; however, scar-formation related expression (col3a1) were also upregulated at early time points in the injury-only scenario. It is likely that acute tendon injury with innervated muscle leads to altered muscle stimulation and/or altered stress distributions at the tendon, which is no longer capable of carrying its original, physiological load. Acute release of tgf-β has been implicated following tendon injury32, implying a quick regulation of tgf-β following damage.
There are several limitations to this study. First, we only investigated a limited number of genes and their expression patterns at three time points. The purpose of this study was to provide a temporal description of gene expression patterns under repair and unloading conditions. The current study illustrated changes in gene expression following rotator cuff muscle unloading and tendon injury, and will aid in the design of future studies to explore targeted interventions for rotator cuff pathologies. Gene expression assays offer a snapshot of expression at a particular time point; alterations in expression patterns may fluctuate depending on environmental and physiological factors. The current study quantitatively examined many of the known regulators of tendon/muscle function and injury response13,17,24. Many of these processes and molecular responses that have previously been examined by us13,17,24 and others33-35 in other injury and unloading scenarios. However, we did not investigate some pathways that have recently been elucidated to be involved with rotator cuff pathology following prolonged rotator cuff injury (e.g., inflammation- and autophagy-related)14. A second limitation of our study is the lack of rigorous assessment of protein expression (e.g., via western blot or immunohistochemistry). Observed changes at the transcriptional level are not necessarily translated into protein production. Despite these limitations, results from the current study can aid in the generation of mechanistic hypotheses and therapeutic interventions in future studies. A third limitation is that we only investigated morphological changes at the latest time point (21 days), as alterations to the muscle and tendon were expected to be greatest at this time. It is possible that muscle adaptations may have already been completed and recovery may be initiated at this time point. However, this is unlikely, as evidenced by previous studies investigating skeletal muscle morphology following 2-3 wks of unloading36. As our denervation method was via chemical denervation with BTX, it is possible that the effects of chemical denervation had begun to wear off by 21 days. Others have shown that efficacy of a single injection of BTX in adult skeletal muscle to last more than 21 days in mice37 and more than 2 months in rabbits38. Lastly, we may be limited by the use of the rodent model to identify potential targets of clinically relevant adaptations following rotator cuff injury. While the rodent model has been extensively used and is a well-established model to investigate rotator cuff tendon injury and repair16,17,39, it has yet been entirely validated for chronic rotator cuff degeneration.
In conclusion, we demonstrated that tendon injury and repair had the greatest effect on tendon gene expression and tendon morphology, while muscular unloading had the greatest effect on muscle gene expression and skeletal muscle morphology (Figure 7). These finding have clinical relevance to the treatment of rotator cuff tears, as chronic changes pose a negative prognosis on healing and restoration of function after surgical treatment. Further studies will target genes associated with tendon and muscle degeneration in an effort to enhance rotator cuff tendon-to-bone healing.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by the Orthopedic Research & Education Foundation, the National Institutes of Health (R01 ARAR057836), and the Washington University Musculoskeletal Research Center (NIH P30 AR057235).
REFERENCES
- 1.Soslowsky LJ, Carpenter JE, Bucchieri JS, Flatow EL. Biomechanics of the rotator cuff. Orthop Clin North Am. 1997;28(1):17–30. doi: 10.1016/s0030-5898(05)70261-3. [DOI] [PubMed] [Google Scholar]
- 2.Lehman C, Cuomo F, Kummer FJ, Zuckerman JD. The incidence of full thickness rotator cuff tears in a large cadaveric population. Bull Hosp Jt Dis. 1995;54(1):30–31. [PubMed] [Google Scholar]
- 3.Vitale MA, Vitale MG, Zivin JG, et al. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181–187. doi: 10.1016/j.jse.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Harryman DT, Mack LA, Wang KY, et al. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982–989. [PubMed] [Google Scholar]
- 5.Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A(2):219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs B, Weishaupt D, Zanetti M, et al. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8(6):599–605. doi: 10.1016/s1058-2746(99)90097-6. [DOI] [PubMed] [Google Scholar]
- 7.Goutallier D, Postel JM, Bernageau J, et al. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. [PubMed] [Google Scholar]
- 8.Galatz LM, Griggs S, Cameron BD, Iannotti JP. Prospective longitudinal analysis of postoperative shoulder function : a ten-year follow-up study of full-thickness rotator cuff tears. J Bone Joint Surg Am. 2001;e 83-A(7):1052–1056. [PubMed] [Google Scholar]
- 9.Safran O, Derwin KA, Powell K, Iannotti JP. Changes in rotator cuff muscle volume, fat content, and passive mechanics after chronic detachment in a canine model. J Bone Joint Surg Am. 2005;87(12):2662–2670. doi: 10.2106/JBJS.D.02421. [DOI] [PubMed] [Google Scholar]
- 10.Charousset C, Bellaïche L, Kalra K, Petrover D. Arthroscopic repair of full-thickness rotator cuff tears: is there tendon healing in patients aged 65 years or older? Arthroscopy. 2010;26(3):302–309. doi: 10.1016/j.arthro.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Costouros JG, Porramatikul M, Lie DT, Warner JJ. Reversal of suprascapular neuropathy following arthroscopic repair of massive supraspinatus and infraspinatus rotator cuff tears. Arthroscopy. 2007;23(11):1152–1161. doi: 10.1016/j.arthro.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Iannotti JP, Naranja RJ, Gartsman GM. Surgical treatment of the intact cuff and repairable cuff defect: Arthroscopic and open techniques. In: Norris TR, editor. Orthopaedic Knowledge Update: Shoulder and Elbow. American Academy of Orthopaedic Surgeons; Rosemont IL: 1994. pp. 151–155. [Google Scholar]
- 13.Kim H, Galatz L, Lim C, et al. The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elbow Surg. 2012;21(7):847–858. doi: 10.1016/j.jse.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gumucio J, Davis M, Bradley J, et al. Rotator cuff tear reduces muscle fiber specific force production and induces macrophage accumulation and autophagy. J Orthop Res. 2012 doi: 10.1002/jor.22168. E-Pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boykin RE, Friedman DJ, Higgins LD, Warner JJP. Suprascapular Neuropathy. J Bone Joint Surg Am. 2010;92(13):2348–2364. doi: 10.2106/JBJS.I.01743. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter JE, Thomopoulos S, Flanagan CL, et al. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg. 1998;7(6):599–605. doi: 10.1016/s1058-2746(98)90007-6. [DOI] [PubMed] [Google Scholar]
- 17.Thomopoulos S, Hattersley G, Rosen V, et al. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J Orthop Res. 2002;20(3):454–463. doi: 10.1016/S0736-0266(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto F, Uhthoff HK, Trudel G, Loehr JF. Delayed tendon reattachment does not reverse atrophy and fat accumulation of the supraspinatus--an experimental study in rabbits. Journal of Orthopaedic Research. 2002;20(2):357–363. doi: 10.1016/S0736-0266(01)00093-6. [DOI] [PubMed] [Google Scholar]
- 19.Rubino LJ, Stills HF, Jr., Sprott DC, Crosby LA. Fatty infiltration of the torn rotator cuff worsens over time in a rabbit model. Arthroscopy. 2007;23(7):717–722. doi: 10.1016/j.arthro.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Itiogawa Y, Kishimoto KN, Sano H, et al. Molecular mechanism of fatty degeneration in rotator cuff muscle with tendon rupture. J Orthop Res. 2011;29:861–866. doi: 10.1002/jor.21317. [DOI] [PubMed] [Google Scholar]
- 21.Steinbacher P, Tauber MK, S, Stoiber W, Resch H, Sanger A. Effects of rotator cuff ruptures on the cellular and intracellular composition of the human supraspinatus muscle. Tissue & Cell. 2010;42(1):37–41. doi: 10.1016/j.tice.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Kim HM, Galatz LM, Das R, et al. Musculoskeletal deformities secondary to neurotomy of the superior trunk of the brachial plexus in neonatal mice. J Orthop Res. 2010;28(10):1391–1398. doi: 10.1002/jor.21128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das R, Rich J, Kim HM, et al. Effects of botulinum toxin-induced paralysis on postnatal development of the supraspinatus muscle. J Orthop Res. 2011;29(2):281–288. doi: 10.1002/jor.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dedieu S, Mazeres G, Cottin P, Brustis JJ. Involvement of myogenic regulator factors during fusion in the cell line C2C12. Int J Dev Biol. 2002;46(2):235–241. [PubMed] [Google Scholar]
- 25.Koishi K, Zhang M, Mclennan IS, Harris AJ. MyoD protein accumulates in satellite cells and is neurally regulated in regenerating myotubes and skeletal muscle fibers. Dev Dyn. 1995;202:244–254. doi: 10.1002/aja.1002020304. [DOI] [PubMed] [Google Scholar]
- 26.Ishido M, Kami K, Masuhara M. In vivo expression patterns of MyoD, p21, and Rb proteins in myonuclei and satellite cells of denervated rat skeletal muscle. Am J Physiol Cell Physiol. 2004;287:C484–C493. doi: 10.1152/ajpcell.00080.2004. [DOI] [PubMed] [Google Scholar]
- 27.Moresi V, Williams A, Meadows E, et al. Myogenin an dclass II HDACs control neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell. 2010;143(1):35–45. doi: 10.1016/j.cell.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macpherson P, Wang X, Goldman D. Myogenin regulates denervation-dependent muscle atrophy in mouse soleus muscle. J Cell Biochem. 2011;112(8):2149–2159. doi: 10.1002/jcb.23136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buonanno A, Apone L, Morasso M, Beers R, Brenner H, Eftimie R. The MyoD family of myogenic factors is regulated by electrical activity: isolation and characterization of a mouse Myf-5 cDNA. Nucleic Acids Res. 1992;20(3):539–544. doi: 10.1093/nar/20.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prody C, Merlie J. A developmental and tissue-specific enhancer in the mouse skeletal muscle acetylcholine receptor alpha-subunit gene regulated by myogenic factors. J Biol Chem. 1991;266:22588–22596. [PubMed] [Google Scholar]
- 31.Ma J, Shen J, Garrett J, Lee C, Li Z, Elsaidi GA, Ritting A, Hick J, Tan KH, Smith T, Smith B, Komen L. Gene expression of myogenic regulatory factors, nicotinic acetylcholine receptor subunits, and GAP-43 in skeletal muscle following denervation in a rat model. Journal of Orthopaedic Research. 2007;25:1489–1505. doi: 10.1002/jor.20414. [DOI] [PubMed] [Google Scholar]
- 32.Manning CN, Kim HM, Sakiyama-Elbert S, et al. Sustained delivery of transforming growth factor beta three enhances tendon-to-bone healing in a rat model. J Orthop Res. 2011;29(7):1099–1105. doi: 10.1002/jor.21301. [DOI] [PubMed] [Google Scholar]
- 33.Frey E, Regenfelder F, Sussmann P, Zumstein M, Gerber C, Born W, Fuchs B. Adipogenic and myogenic gene expression in rotator cuff muscle of the sheep after tendon tear. J Orthop Res. 2009;27(4):504–509. doi: 10.1002/jor.20695. [DOI] [PubMed] [Google Scholar]
- 34.Hamada K, Tomonaga A, Gotoh M, Yamakawa H, Fukuda H. Intrinsic healing capacity and tearing process of torn supraspinatus tendons: in situ hybridization study of alpha 1 (I) procollagen mRNA. Journal of Orthopaedic Research. 1997;15(1):24–32. doi: 10.1002/jor.1100150105. [DOI] [PubMed] [Google Scholar]
- 35.Jamali AA, Afshar P, Abrams RA, Lieber RL. Skeletal muscle response to tenotomy. Muscle Nerve. 2000;23(6):851–862. doi: 10.1002/(sici)1097-4598(200006)23:6<851::aid-mus3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 36.Ishihara A, Oishi Y, Roy R, Edgerton V. Influence of two weeks of non-weight bearing on rat soleus motoneurons and muscle fibers. Aviat Space Environ Med. 1997;68(5):421–425. [PubMed] [Google Scholar]
- 37.Rogozhin A, Pang K, Bukharaeva E, et al. Recovery of mouse neuromuscular junctions from single and repeated injections of botulinum neurotoxin A. J Physiol. 2008;586(13):3163–3182. doi: 10.1113/jphysiol.2008.153569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korfage J, Wang J, Lie S, Langenbach G. Influence of botulinum toxin on rabbit jaw muscle activity and anatomy. Muscle Nerve. 2012;45(5):684–691. doi: 10.1002/mus.23229. [DOI] [PubMed] [Google Scholar]
- 39.Soslowsky LJ, Carpenter JE, et al. Development and use of an animal model for investigations on rotator cuff disease. Journal of Shoulder & Elbow Surgery. 1996;5(5):383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.