Abstract
Objectives
The current study aim to investigate the effects of SPI on Wnt/β-catenin signaling in the liver of obese rats, as well as the roles of this pathway in regulating the hepatic fat accumulation.
Design and Methods
Obese and lean Zucker rats were fed diets containing either casein or SPI as protein source for 17 weeks. Histology and biochemical analysis, real-time PCR, Western blot, immunostaining, short interfering RNA assay were performed for liver samples.
Results
Our study showed that fat content was significantly lowered in the liver of SPI-fed obese rats, accompanied by a reduction in hepatocellular vacuolation, compared to the casein-fed control. β-catenin protein level in the liver of obese rats was down-regulated compared to the lean group, indicating that the obese genotype exhibits an overall reduction in Wnt signaling. Importantly the repression of β-catenin in the obese rats was alleviated by feeding the SPI diet. siRNA treatment in rat hepatoma cells confirmed that silencing of β-catenin exacerbated fatty acid-induced fat accumulation, which implicated an important function of Wnt/β-catenin signaling in hepaticfat metabolism.
Conclusions
SPI intake restored β-catenin signaling and alleviated hepatic fat accumulation and liver damage in the obese rats.
Keywords: diet, hepatic fat, β-catenin signaling, SPI, obese Zucker rat
Introduction
Obesity is a major risk factor for the development of nonalcoholic fatty liver disease (NAFLD), which represents a spectrum of clinical manifestations, progressing from hepatic steatosis, nonalcoholic steatohepatitis, to fibrosis and cirrhosis 1. NAFLD is characterized by excessive hepatic fat deposition by increased lipid influx 2, de novo lipogenesis 3, impaired mitochondrial β-oxidation 4 and/or reduced lipid export 5. The Obese Zucker rat (OZR) is a preclinical rodent model of NAFLD and is frequently used in obesity-related research 6, 7.
Dietary intake of soy protein has several nutritional and physiological benefits8, including anti-obesity and anti-diabetic properties 9. In addition, soy protein isolate (SPI) is shown to improve lipid metabolism in obese rodents 10, as well as to reduce lipogenic activities and hepatic triacylglycerol (TAG) accumulation 11. In OZR rats where NAFLD naturally occurs 7, SPI is shown to modify adipose morphology and metabolic profile, as well as to improve inflammatory status 12.
The Wnt/β-catenin signaling pathway is central to adipogenic regulation in adipose tissue/cells 13 and skeletal muscle 14. In particular, Wnt signaling inhibits the adipogenic transcription factors CCAAT/enhancer binding protein α (C/EBPα) and peroxisome proliferator- activated receptor (PPAR) γ, thereby preventing the maturation of preadipocytes. It has also been demonstrated that disruption of the Wnt pathway in myoblasts promotes trans-differentiation of these cells into adipocytes 15. Thus, Wnt signaling is proposed to affect/control lipid deposition through antagonism of fat tissue development. Recent evidence has established a potential link between the Wnt pathway and lipid regulation in liver tissue. In a mouse model it has been demonstrated that hepatic fat accumulation was increased by liver-specific β-catenin knockout 16. Later, Cain et al reported that a reduction in hepatic lipid content in OZR rats fed SPI was associated with lower expression of Wnt signaling intermediates, including Dact1, GSK3β, Wnt5a and Wnt5b 12. However, it is unclear how different components of Wnt signaling, such as β-catenin, are affected by obesity or soy protein. Therefore, in the current study we investigated the interaction between SPI, β-catenin signaling, and hepatic steatosis in OZR rats. In addition, we used a rat hepatoma-based cell culture model to elucidate the specific mechanisms linking Wnt/β-catenin signaling to liver fat accumulation.
Methods
Animal Model and Diet
Six-week old male homozygous lean and obese Zucker rats were purchased from Charles River Laboratories and randomly assigned to diets containing either casein or SPI as protein source for 17 weeks 12. All procedures were approved by the Institutional Animal Care and Use Committee of Southern Illinois University, Carbondale, IL, USA.
Liver histology and Cell staining
Frozen liver samples were embedded in Tissue-Tek OCT compound (VWR) and cut to a thickness of 5 μm in a cryostat at -20°C. All sections were fixed in 70% ethanol and stained with hematoxylin and eosin (H&E) or Oil red O (ORO) solution (Newcomer Supply). Histopathological examination was conducted by a certified pathologist. Oil red O (ORO) staining was quantified using the AxioVision software. The grading scale of histopathological examination used to assess microscopic pathological findings was 0: none, 1: minimal, 2: mild, 3: moderate, 4: marked and 5: severe. Images of ORO staining were obtained using NanoZoomer Slide Scanner and NDP View software (Hamamatsu).
Biochemical analysis of liver tissue
Frozen liver samples were ground in liquid nitrogen and homogenized in 0.3mL saline (0.9% w/v NaCl). Homogenized samples were diluted 5 times with saline. Twenty microliter of the diluted samples were incubated with 20 μL 1% deoxycholate in 37 °C for 5 min and 10 μL of the sample were analyzed using the Thermo Infinity Triglycerides Liquid Stable Reagent (Thermo Fisher Scientific) and a standard reference kit (Verichem Laboratories) to determine the TAG content. Liver non-esterified fatty acid (NEFA) concentration was determined using a commercially available kit (HR-2 Series, Wako Diagnostics). The results were normalized to the amount of total protein in the sample. Extracted serum was analyzed for aspartate aminotransferase (AST) and alanine aminotransferase (ALT) using the Beckman CX4 Chemistry Analyzer.
RNA isolation and real time quantitative RT-PCR
Total RNA was extracted from liver samples using TRI reagent (Sigma-Aldrich). cDNA was synthesized from the total RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) in a Thermal Cycler (Applied Biosystems) at 25 °C for 10 min, 37 °C for 2 h and 85 °C for 5 s. Real time PCR was then performed using SYBR Green fast master mix (Quanta Biosciences) in a 7300 real-time thermal cycler (Applied Biosystems) at 95 °C for 10 min, followed by 35 cycles of 95 °C for 15 s and 60 °C for 1 min. Relative mRNA quantity for the ribosomal protein L7a was measured and used as the internal control. Primers used for PCR are listed in Supplemental Table 1.
Immunofluorescent staining
Frozen liver samples were embedded in Tissue-Tek OCT compound (VWR, Radnor, PA) and cut to a thickness of 5 μm in a cryostat at -20 °C. The sections were fixed in 70% ethanol, permeablized with 0.1% Triton X-100, blocked with Image-iT FX signal enhancer (Invitrogen) for 30 min, incubated with primary antibody (β-catenin, cat # 9587S, Cell Signaling) for 2 h at 37 °C, and followed by an incubation of a goat anti-rabbit antibody (Alexa Fluor 647 1:200 dilution, Invitrogen) for 45 min in a dark chamber. The samples were counterstained with Hoechst 33342 (Invitrogen) for 15 min and then washed with PBS. Coverslip was mounted on the sections using Prolong Gold antifade reagent (Invitrogen). Pictures were taken using the Axiovert 200M equipment (Zeiss).
Cell culture and short interfering RNA (siRNA) assay
The rat hepatoma cell line H4IIEC3 was obtained from American Type Culture Collection and was cultured in Minimum Essential Medium (MEM) supplemented with 10% (v/v) fetal bovine serum (Sigma-Aldrich) and 100 IU/mL Penicillin, and 100 μg/mL Streptomycin (Mediatech). Cells were maintained at 37 °C in 95% humidity and 5% CO2. For transfection, H4IIEC3 cells were seeded onto 6-well plates and incubated overnight in MEM. At the time of transfection, 30 nmol/L of siRNA against scrambled sequence (non-specific siRNA, N/S si, QIAGEN) or 30 nmol/L of siRNA against rat β-catenin (β-catenin si, Integrated DNA Technologies) was prepared in 200 μL serum-free media (SFM). The siRNA solution was incubated with 5 μL DharmaFect#4 (in 200 μL SFM, Dharmacon) at room temperature for 20 min to allow the formation of transfection complex. Each well of the 6-well plates was replenished with 1.6 mL antibiotic-free MEM before the transfection complex was added (400 μL/well for 6-well). Two days after transfection, cells were treated with MEM supplemented with 450 μmol/L of NEFA (containing 150 μmol/L of bovine serum albumin, BSA, Sigma-Aldrich) or 150 μmol/L of BSA alone for 24 h. Total RNA and protein was isolated and analyzed by quantitative real-time RT-PCR and western blot, respectively.
Protein extraction and western blot analysis
Liver tissue or cells were lysed with 1X Laemmli Buffer [62.5 mmol/L Tris–HCl, pH 6.8, 2% SDS, 10% Glycerol v/v, 0.01% Bromophenol blue, 5% 2-mercaptoethanol, 1x protease inhibitors (Roche) and 1x phosphatase inhibitors (Sigma-Aldrich)]. Lowry assay was performed to determine protein concentration. Samples containing 30 μg of protein were resolved on a 10% SDS-PAGE gel by electrophoresis and transferred to a PVDF membrane (0.2 μm, Bio-Rad) using wet transfer protocol. The membrane was then blocked in 10% milk in TBS/T (30 mmol/L Tris base pH 7.6, 200 mmol/L NaCl and 0.1% Tween 20) for 1 h at room temperature. The membranes were incubated with primary antibodies in 10% non-fat dry milk at a 1:2000 dilution at room temperature for 3 h. SuperSignal West Dura Extended Duration Substrate kit (Thermo Scientific) was used to detect the signal and the images were captured and analyzed by a Chemi Doc XLR system (Bio-Rad). Actin and fast green stained non-specific bands were used as a loading control to normalize the protein data.
Statistical analysis
Results are expressed as interaction means (diet × genotype) ± standard error of mean (SEM) in figures. Post hoc comparison among groups was tested using the Fisher's least significant difference (LSD, SAS v. 9.1.2, SAS Institute) when the P value of interaction of main effects (diet × genotype) was less than 0.15. Individual bars with different letters differ (P<0.05). Correlation between hepatocellular vacuolation and AST or ALT was determined by Spearman's rank correlation coefficient analysis (Spearman's rho). Student t-test was conducted to make the comparison in cell culture study.
Results
Liver histology and functions
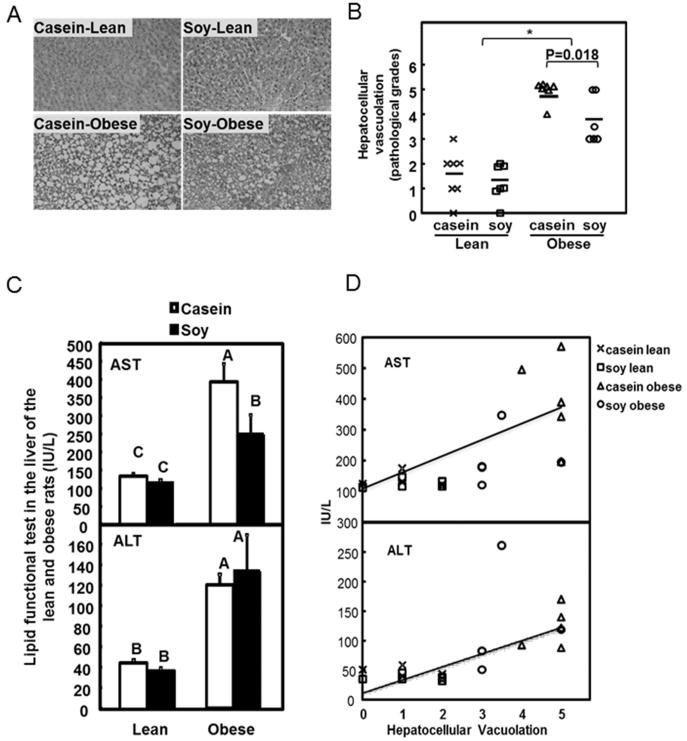
At the end of the 17 weeks, no or minimal to mild centrilobular hepatocellular vacuolation was observed in lean rats (Figure 1A). In obese rats, moderate to severe (soy) and marked to severe (casein) micro- and/or macro-hepatocellular vacuolation was observed. Overall, obese rats had a significant higher vacuolation when compared to lean rats (Figure 1B, P<0.05; Supplemental Table 2, genotype effect, P<0.0001). Particularly in the obese rats, the average grading score decreased from 4.86 (5 being the most severe) in control group to 3.75 in soy group (P=0.018), showing a significant decrease in severity of hepatocellular vacuolation. This suggested that SPI-fed rodents had improved pathological conditions in liver (Supplemental Table 2, diet effect, P=0.035).
Figure 1. Histological and physiological assays in the liver of lean and obese rats.
A. Representative images of H&E staining in casein group (left panels) and soy group (right panels) and in lean (top panels) and obese (bottom panels) animals. No or minimal hepatocellular vacuolation was noted in lean rats (top panels); Moderate vacuolation in an obese rat with soy protein (lower right panel); Severe vacuolation in an obese rat fed with casein (lower left panel). Objective, 20x.
B. Scattered plot of hepatocellular vacuolation counting. Each symbol represents one individual sample. Horizontal lines represent the mean values. *P<0.05, n=6~7 individual animals per group.
C. AST and ALT levels were measured in the liver of lean and obese rats. Data are presented as the mean ± SEM, n= 6. Individual bars with different letters differ (P<0.05).
D. Correlation of AST and ALT with hepatocellular vacuolation. Each symbol represents one individual sample. ×: lean rats fed casein; □: lean rats fed soy; △: obese rats fed casein; ○: obese rats fed soy. Regression line was calculated after the analysis.
Aberrant AST and ALT levels are indicators of liver disease and hepatocyte damage 17. Data from the present study AST and ALT were significantly increased in the obese rats when compared to the lean rats (P<0.05, Figure 1C, Supplemental Table 2, genotype effect, P<0.0001). There is also a significant reduction of AST in soy-fed obese rats comparing to the control (P<0.05; Supplemental Table 2, genotype*diet interaction, P=0.087). Both AST and ALT levels are positively correlated with hepatocellular vacuolation (P<0.001, Figure 1D. Spearman's rho=0.77 and 0.76 for AST and ALT respectively). ALT was not significantly affected by the dietary treatment. This indicated that the improvement of liver condition by dietary soy may be closely associated with the reduced AST level.
Hepatic fat assessment
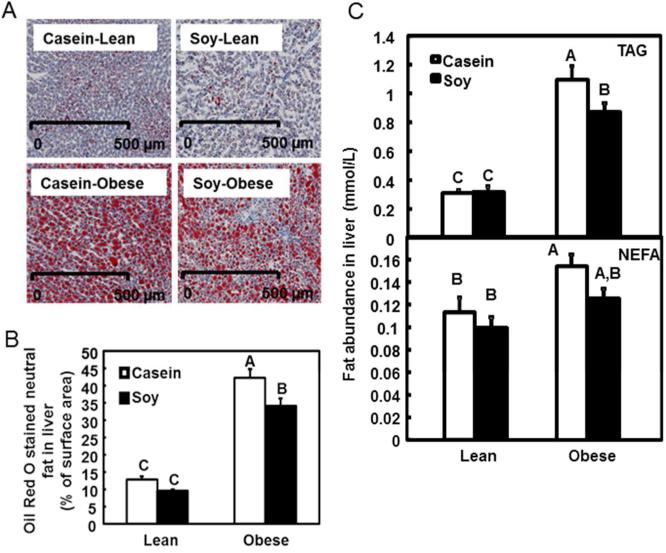
Quantification of ORO staining represents the amount of neutral lipid (Figure 2A and 2B). Obese rats showed 3.3 fold more fat in liver compared to the lean rats (Figure 2B, P<0.05; Supplemental Table 2, genotype effect, P=0.0179). Furthermore, the soy-based diet significantly reduced hepatic fat in the obese rats compared to the obese rats fed the casein diet (Figure 2B, P<0.05; diet effect, P=0.011).
Figure 2. Analysis of hepatic fat.
A. Representative images of Oil Red O staining. Scale bar: 500 μm.
B. Quantification of Oil Red O staining is expressed as percentage of total surface area in the unit of cm2 using computer-assisted image quantification. The open bars represent values of the rats on casein diet while the solid bars on soy diet. Values are presented as the mean ± SEM, n = 10. Individual bars with different letters differ (P<0.05). .
C. TAG (top) and NEFA (bottom) levels were measured in the liver. Raw value was normalized to relative tissue protein amount. Data are presented as the mean ± SEM, n= 6. Individual bars with different letters differ (P<0.05).
Liver samples were further examined for TAG and NEFA content. TAG level was 4 fold higher in obese rats fed control diet (Figure 2C, P<0.05; Supplemental Table 2, genotype effect, P<0.0001). Similarly, NEFA content was ~40% higher in the control group (Figure 2C, P<0.05; genotype effect, P=0.0053). Overall, obese rats showed greater lipid accumulation than lean rats. TAG and NEFA concentrations of the lean rats were not affected by dietary treatment. However, dietary soy significantly reduced TAG level in the obese rats when compared to the obese rats in the control (Figure 2C, P<0.05; diet effect, P=0.098).
Expression of lipid metabolism-related genes
It was demonstrated that SPI attenuated metabolic syndrome through the regulation of Pparα, Srebp1-c and Lxrα expression in liver 18. Our analysis showed that Srebp1-c mRNA increased 2.76 fold in the obese rats when compared to the lean rats fed the casein diet (Table, P<0.05). This was downregulated by dietary soy (P<0.05). Pparα expression was increased in obese rats (P<0.05, Supplemental Table 2, genotype effect, P=0.0043) but was not affected by dietary treatment. On the other hand, Lxrα expression was not significantly affected by genotype or diet. In addition, a marker of hepatic fat content, Adrp 19 increased 4 folds in the obese rats compared to the lean groups but was reduced significantly by SPI (P<0.05, Table). These findings are consistent with the ORO staining and TAG data showing a significant genotype and diet effect on liver fat accumulation. These results also indicate that additional factors other than Pparα, Srebp1-c, and Lxrα may be involved in the regulation of hepatic steatosis.
| Liver Tissue Transcript | Lean | Obese | ||
|---|---|---|---|---|
| Casein | Soy | Casein | Soy | |
| LXRα | 8.46±0.49 | 8.18±1.18 | 10.07±0.88 | 9.83±0.85 |
| PPARα | 0.93±0.07 | 0.88±0.1 | 1.27±0.19 | 1.34±0.1 |
| SREBP1-c | 0.5±0.06c | 0.36±0.04c | 1.38±0.13a | 1.09±0.06b |
| ADRP | 0.38±0.04c | 0.36±0.05c | 1.6±0.17a | 1.05±0.21b |
| Wnt 1 | 0.02±0.009 | 0.08±0.02 | 0.04±0.02 | 0.05±0.02 |
| Wnt 2 | 0.61±0.08 | 0.62±0.1 | 0.28±0.04 | 0.44±0.07 |
| Wnt 2b | 0.95±0.12a,b | 0.94±0.04a,b | 1.11±0.12a | 0.73±0.09b |
| Wnt 3a | 0.008±0.003 | 0.01±0.008 | 0.02±0.01 | 0.008±0.004 |
| Wnt 4 | 0.17±0.02b | 0.17±0.03b | 0.28±0.02a | 0.15±0.007b |
| Wnt 8b | 0.15±0.05a | 0.38±0.12a | 0.19±0.05a | 0.15±0.05a |
| Wnt 9a | 0.51±0.13b | 0.30±0.22b | 1.27±0.14a | 0.67±0.08b |
| Wnt 9b | 0.89±0.06a | 1.13±0.10a | 0.90±0.11a | 0.61±0.07b |
| Wnt 11 | 0.16±0.05a | 0.33±0.10a | 0.33±0.10a | 0.11±0.06a |
| SFRP 1 | 0.23±0.04a | 0.3±0.05a | 0.31±0.05a | 0.18±0.04b |
| SFRP 2 | 0.07±0.02 | 0.1±0.02 | 0.06±0.007 | 0.06±0.01 |
| SFRP 4 | 0.04±0.01a,b | 0.08±0.02b | 0.03±0.007a | 0.007±0.001b |
| SFRP 5 | 0.05±0.02a,b | 0.11±0.04a | 0.08±0.03a,b | 0.03±0.006b |
| DKK1 | 0.02±0.01b | 0.2±0.05a,b | 0.16±0.08a | 0.02±0.01b |
All mRNA analysis data are normalized as the ratio to the internal control L7a and expressed as means ± SEM, n=6.
Letters are assigned by post hoc comparison. Values with different letter assignment differ (p<0.05).
Expression of Wnt genes in liver tissues
mRNA abundance of selected Wnt ligands and antagonists 20 were analyzed to evaluate the involvement of the signaling pathway in fat accumulation in liver. Overall, Wnt signaling genes tested showed limited and selective responses to genotype and dietary treatments. In particular, Wnt4 and Wnt9a showed a significant increase in the obese rats compared to the lean rats and the soy diet reduced the levels in the obese rats to that comparable to the obese rats in the control group (Table, P<0.05). Wnt2 showed modest decrease in the obese rats compared to the lean rats (genotype effect, P=0.0023) but did not show significant effect from diet. Therefore, we further analyzed transcript abundance of selected Wnt antagonists including secreted frizzled-related protein (Sfrp) 1, 4, 5 and Dickkopf-1 (Dkk1). In general, the gene expression of the antagonists was not affected greatly by genotype but in the obese rats, SPI lowered the expression of Sfrp1, 4, 5, and DKK1 when compared to casein diet (Table). Repression of these antagonists potentially contributes to the effect of soy diet on β-catenin observed in this study.
β-catenin protein level in the liver
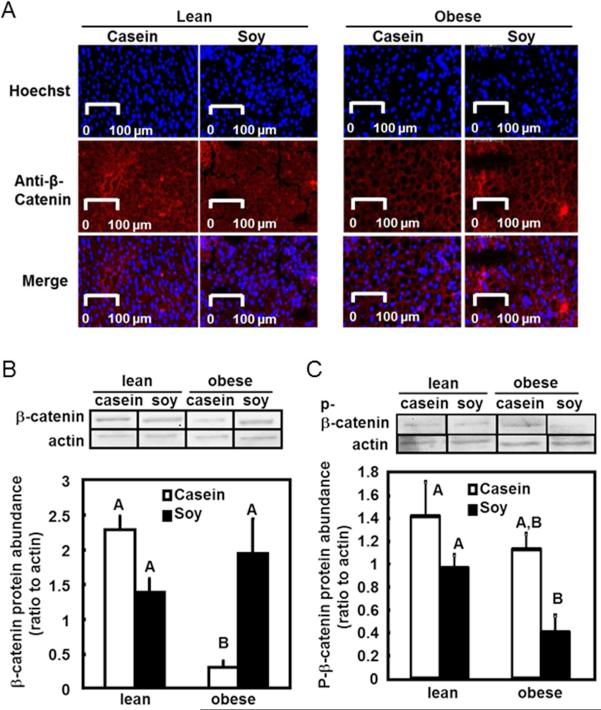
As above-mentioned, analysis of upstream Wnt signals and their antagonists gave complicated and limited information regarding overall Wnt signaling. We therefore examined expression of the key intracellular mediator of the Wnt signaling pathway, β-catenin (Figure 3A). Results showed that liver from the obese rats had a 7-fold reduction of total β-catenin protein compared to that of lean rats (P<0.05, Figure 3B). Importantly, dietary soy protein restored the β-catenin protein level in liver of obese rats compared to casein (P<0.05). Degrading form of β-catenin (p-β-catenin; Figure 3C) was not affected by either genotype or diet, indicating that the change of β-catenin protein observed was likely due to the regulation of β-catenin expression rather than degradation.
Figure 3. Protein abundance of β-catenin in liver.
A. Immunofluorescent staining of β-catenin. Protein content of β-catenin in liver was analyzed by immunofluorescent staining using an antibody against β-catenin protein and an Alexa Fluor 647-labeled secondary antibody (red, middle panel). Nuclei were counterstained with Hoechst 33342 fluorescent stain (blue, top panel). The two pictures were merged to show the cytoplasmic location of β-catenin staining (merge).
B. Hepatic β-catenin protein level in whole cell extract was quantified using western blot analysis. The upper panel shows representative blots from western blot analysis using antibodies against β-catenin and the loading control actin. The amount β-catenin protein was normalized to actin as the relative protein abundance. The relative β-catenin protein was presented as the mean ± SEM, n= 4. Individual bars with different letters differ (P<0.05).
C. Phosphorylated β-catenin (p-β-catenin) protein level was measured using western blot and the quantifications are presented as described in Figure 3B.
NEFA treatment and β-catenin knockdown in H4IIEC3 cell line
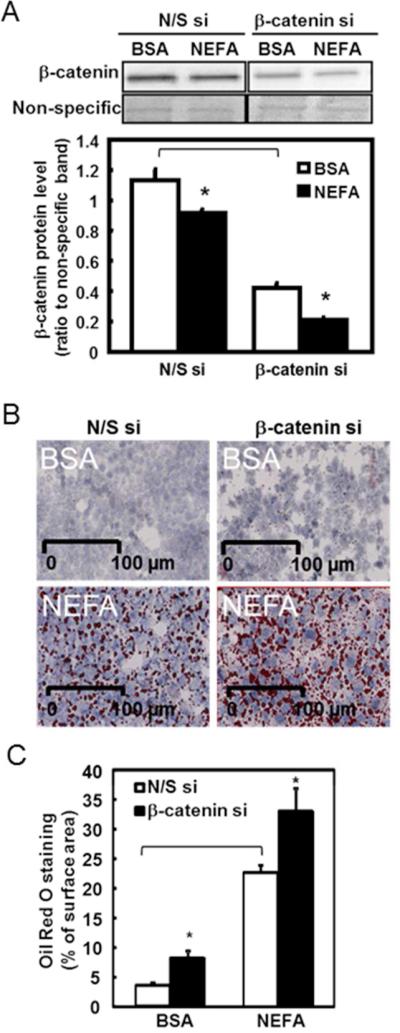
siRNA against β-catenin was used to examine the effects from the reduction of β-catenin in H4IIEC3 rat hepatoma cells. In addition to the reduction of β-catenin by siRNA, NEFA treatment further decreased β-catenin content in both N/S and β-catenin siRNA groups (P<0.05, Figure 4A). Reduced β-catenin alone induced cellular fat accumulation (P<0.05, β-catenin si compared to N/S si, Figure 4B, 4C), although NEFA treatment further induced more pronounced total fat accumulation compared to BSA control.
Figure 4. β-catenin si and NEFA treatment in H4IIEC3 cells.
H4IIEC3 hepatoma cells were cultured and treated with β-catenin si prior to the NEFA treatment.
A. β-catenin protein level was measured by western blot analysis. The upper panel shows a representative western blot of β-catenin. A fast green stained non-specific band was used as the loading control. The lower panel shows the quantification of relative β-catenin protein as the mean ± SEM, n= 3. *P<0.05; Bracket, P<0.05 comparing N/S si to β-catenin si in BSA treatment.
B. Representative images of Oil Red O staining in N/S si (left panel) and β-catenin si (right panel) in both control (BSA, top panel) and NEFA (bottom panel) treated cells. The nucleus was stained in blue and the neutral lipid droplets were stained in red. Scale bar: 100 μm.
C. Quantification of Oil Red O staining in N/S and β-catenin si is presented as the percentage of total surface area in the unit of cm2 using computer-assisted image quantification. The open bars represent the control N/S si group. The solid bars represent the β-catenin si group. Data are presented as the mean ± SEM, n = 10. *P<0.05; Bracket, P<0.05 comparing N/S si to β-catenin si in BSA treatment.
Discussion
The present study demonstrated that SPI reduced hepatic fat accumulation and markers of liver dysfunction in the OZR rats. Hepatic Wnt/β-catenin signaling was down-regulated in the obese animals, and soy protein in the diet markedly corrected this effect as evident from the increased protein level of β-catenin. This study illustrated, for the first time, that dietary soy alleviates hepatic fat accumulation in the obese rats, potentially through the repression of Wnt antagonists and restoration of intracellular β-catenin signaling.
Wnt/β-catenin signaling pathway is reportedly involved in obesity in bone 21, colon 22, adipose tissue 23, and the placenta 24. In our study, obese animals exhibited decreased β-catenin signaling in the liver and an overall increase in hepatic fat. Specifically, loss of the central component of the Wnt signaling, β-catenin, is reported to affect multiple aspects of liver development and functions, including increased risk of hepatocarcinogenesis 25, defective cholesterol metabolism and increased susceptibility to steatohepatitis 16. The current study is novel to report the repression of β-catenin during hepatic fat accumulation in obese animals.
Wnt signaling pathway was shown to inhibit the development of fat cells thereby blocking adipogenesis in cell culture 15. For instance, expression of Wnt10b decreases fat deposits and reduces obesogenic activities 26. Further, conditional deletion of β-catenin in mesenchymal cells during embryogenesis results in a progressive switch from muscle cells to adipocytes 27. In the present study in liver, the restored/increased intracellular β-catenin level appeared to be a major effect from SPI in the obese rats. We observed less response from the Wnt signaling genes and more inverse correlation between β-catenin level and Wnt antagonists. Expressions of Wnt antagonists, including Dkk1 28, Sfrp 1 29 and 5 30 were shown to induce lipid accumulation and increase total adiposity. Hence, the downregulation of Wnt antagonists in the liver of the obese rats by SPI is potentially associated with the restoration of β-catenin level and therefore the reduction of hepatic fat accumulation. Obese individuals with NAFLD showed inhibited regeneration capacity through reduced proliferation of hepatocytes 31. Thus, it is likely that dietary soy reverses the repression of β-catenin and promotes cell proliferation, leading to attenuated fat accumulation. Future studies are needed to further investigate the mechanisms involving the Wnt antagonists in β-catenin during hepatic fat accumulation.
H4IIEC3 rat hepatoma cells represent an excellent model for studying cellular mechanisms of hepatic fat accumulation in obesity, as NEFA treatment in these cells similarly results in marked intracellular lipid accumulation. In the present study, NEFA treatment reduced β-catenin level, confirming the observation made in the obese rat model. Direct knockdown of β-catenin in hepatoma cells also increased lipid accumulation. These results suggest that lipid accumulation is associated with reduced intracellular β-catenin in both liver tissue and hepatoma cells. There have been reports indicating interactions between β-catenin and other signaling pathways in different hepatic cell types during cell-to-cell communications 32-34. Therefore further investigations of β-catenin signaling and interactions with other signaling pathways during hepatic fat accumulation may illustrate the mechanisms of the protective effects from dietary soy.
Soy protein conveys a variety of health-beneficial effects, including alleviation of some symptoms related to obesity 12, 35. It was proposed that unique amino acid composition and bioactive components such as saponins and isoflavonoids are potentially responsible for these effects 36-39. Early study reported that genistein, one of the major soy isoflavones, represses adipogenic differentiation via activating Wnt/β-catenin signaling pathway 40. This suggested that the upregulation of Wnt/β-catenin signaling pathway reported in the present study may be attributed to these components in SPI. Further experiments are needed to identify the functional component(s) that has been working through Wnt signaling to regulate the lipid metabolism. The restoration of hepatic β-catenin level by SPI in the obese rats contributes to the protection against hepatic fat accumulation and could be developed into a potential strategy for the attenuation of hepatic steatosis and NAFLD.
Our results demonstrated that SPI in obese rats significantly reduced hepatic steatosis. Furthermore, reduction of AST by SPI in the obese rats is correlated with the reduction of liver hepatocellular vacuolation. Future experiments are needed to examine the potential effects on these parameters from the necrosis/degeneration of cells in the heart, kidney and muscle.
In conclusion, the establishment of connections between dietary soy, hepatic fat accumulation, and β-catenin signaling in liver is of major significance, in that it provides a potential mechanism of dietary effect on liver patho-physiology. We propose for the first time that dietary soy attenuates hepatic fat accumulation in a β-catenin-dependent manner, which also supports the utilization of soy as a potentially effective dietary therapy. The clinical significance and practical applications for using soy to reduce fatty liver remain to be further elucidated.
Supplementary Material
‘What is already known about this subject’.
Soy protein isolate (SPI) was shown to have anti-obesity and hepato-protective effects.
The Wnt/β-catenin pathway is an important regulator of liver homeostasis.
‘What this study adds’.
Soy protein intake significantly reduced fat accumulation in the obese Zucker rats;
Wnt signaling in liver is suppressed in the obese rats and is upregulated by soy protein diet;
Silencing of β-catenin further confirmed the involvement of Wnt signaling in controlling fat accumulation in liver.
Acknowledgements
Authors would like to thank members of Chen and Pan laboratories at University of Illinois at Urbana-Champaign for their technical assistance and critical comments on the manuscript. JD and WB designed and conducted the animal study. DZ and HC designed the experiments and wrote the manuscript. DZ and HW carried out the experiments. DZ collected and analyzed the data. SL performed histology analysis. This study was supported in part by Illinois Soybean Association, a NIH grant (CA136067) and a grant from the Research Board at the University of Illinois at Urbana-Champaign to HC.
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- NEFA
non-esterified fatty acid
- OZR
Obese Zucker rats
- SPI
Soy protein isolate
- TAG
Triacylglycerol
Footnotes
Conflict of Interest
All authors declare that there is no conflict of interest in the present study.
References
- 1.Chiang DJ, Pritchard MT, Nagy LE. Obesity, diabetes mellitus, and liver fibrosis. Am.J.Physiol Gastrointest.Liver Physiol. 2011;300:G697–G702. doi: 10.1152/ajpgi.00426.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miquilena-Colina ME, Lima-Cabello E, Sanchez-Campos S, et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut. 2011;60:1394–402. doi: 10.1136/gut.2010.222844. [DOI] [PubMed] [Google Scholar]
- 3.Dorn C, Riener MO, Kirovski G, et al. Expression of fatty acid synthase in nonalcoholic fatty liver disease. Int.J.Clin.Exp.Pathol. 2010;3:505–14. [PMC free article] [PubMed] [Google Scholar]
- 4.Rector RS, Thyfault JP, Uptergrove GM, et al. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J.Hepatol. 2010;52:727–36. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charlton M, Sreekumar R, Rasmussen D, Lindor K, Nair KS. Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology. 2002;35:898–904. doi: 10.1053/jhep.2002.32527. [DOI] [PubMed] [Google Scholar]
- 6.Hunt CE, Lindsey JR, Walkley SU. Animal models of diabetes and obesity, including the PBB/Ld mouse. Fed.Proc. 1976;35:1206–17. [PubMed] [Google Scholar]
- 7.Argiles JM. The obese Zucker rat: a choice for fat metabolism 1968-1988: twenty years of research on the insights of the Zucker mutation. Prog.Lipid Res. 1989;28:53–66. doi: 10.1016/0163-7827(89)90007-6. [DOI] [PubMed] [Google Scholar]
- 8.Badger TM, Ronis MJ, Hakkak R, Rowlands JC, Korourian S. The health consequences of early soy consumption. J Nutr. 2002;132:559S–65S. doi: 10.1093/jn/132.3.559S. [DOI] [PubMed] [Google Scholar]
- 9.Mezei O, Banz WJ, Steger RW, Peluso MR, Winters TA, Shay N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J Nutr. 2003;133:1238–43. doi: 10.1093/jn/133.5.1238. [DOI] [PubMed] [Google Scholar]
- 10.Davis J, Iqbal MJ, Steinle J, et al. Soy protein influences the development of the metabolic syndrome in male obese ZDFxSHHF rats. Horm Metab Res. 2005;37:316–25. doi: 10.1055/s-2005-861487. [DOI] [PubMed] [Google Scholar]
- 11.Iritani N, Nagashima K, Fukuda H, Katsurada A, Tanaka T. Effects of dietary proteins on lipogenic enzymes in rat liver. J Nutr. 1986;116:190–7. doi: 10.1093/jn/116.2.190. [DOI] [PubMed] [Google Scholar]
- 12.Cain J, Banz WJ, Butteiger D, Davis JE. Soy protein isolate modified metabolic phenotype and hepatic Wnt signaling in obese Zucker rats. Horm Metab Res. 2011;43:774–81. doi: 10.1055/s-0031-1287855. [DOI] [PubMed] [Google Scholar]
- 13.Li HX, Luo X, Liu RX, Yang YJ, Yang GS. Roles of Wnt/beta-catenin signaling in adipogenic differentiation potential of adipose-derived mesenchymal stem cells. Mol Cell Endocrinol. 2008;291:116–24. doi: 10.1016/j.mce.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Du M, Yin J, Zhu MJ. Cellular signaling pathways regulating the initial stage of adipogenesis and marbling of skeletal muscle. Meat.Sci. 2010;86:103–9. doi: 10.1016/j.meatsci.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–3. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 16.Behari J, Yeh TH, Krauland L, et al. Liver-specific beta-catenin knockout mice exhibit defective bile acid and cholesterol homeostasis and increased susceptibility to diet-induced steatohepatitis. Am J Pathol. 2010;176:744–53. doi: 10.2353/ajpath.2010.090667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SH. Current status of liver disease in Korea: nonalcoholic fatty liver disease. The Korean journal of hepatology. 2009;15(Suppl 6):S34–9. doi: 10.3350/kjhep.2009.15.S6.S34. [DOI] [PubMed] [Google Scholar]
- 18.Ronis MJ, Chen Y, Badeaux J, Badger TM. Dietary soy protein isolate attenuates metabolic syndrome in rats via effects on PPAR, LXR, and SREBP signaling. J Nutr. 2009;139:1431–8. doi: 10.3945/jn.109.107029. [DOI] [PubMed] [Google Scholar]
- 19.Motomura W, Inoue M, Ohtake T, et al. Up-regulation of ADRP in fatty liver in human and liver steatosis in mice fed with high fat diet. Biochem Biophys Res Commun. 2006;340:1111–8. doi: 10.1016/j.bbrc.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 20.Zeng G, Awan F, Otruba W, et al. Wnt'er in liver: expression of Wnt and frizzled genes in mouse. Hepatology. 2007;45:195–204. doi: 10.1002/hep.21473. [DOI] [PubMed] [Google Scholar]
- 21.Chen JR, Lazarenko OP, Wu X, et al. Obesity reduces bone density associated with activation of PPARgamma and suppression of Wnt/beta-catenin in rapidly growing male rats. PloS one. 2010;5:e13704. doi: 10.1371/journal.pone.0013704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Brooks RS, Ciappio ED, et al. Diet-induced obesity elevates colonic TNF-alpha in mice and is accompanied by an activation of Wnt signaling: a mechanism for obesity-associated colorectal cancer. J Nutr Biochem. 2011 doi: 10.1016/j.jnutbio.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alligier M, Meugnier E, Debard C, et al. Subcutaneous adipose tissue remodeling during the initial phase of weight gain induced by overfeeding in humans. J.Clin.Endocrinol.Metab. 2012;97:E183–E92. doi: 10.1210/jc.2011-2314. [DOI] [PubMed] [Google Scholar]
- 24.Strakovsky RS, Pan YX. A Decrease in DKK1, a WNT Inhibitor, Contributes to Placental Lipid Accumulation in an Obesity-Prone Rat Model. Biol Reprod. 2012;86:81. doi: 10.1095/biolreprod.111.094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang XF, Tan X, Zeng G, et al. Conditional beta-catenin loss in mice promotes chemical hepatocarcinogenesis: role of oxidative stress and platelet-derived growth factor receptor alpha/phosphoinositide 3-kinase signaling. Hepatology. 2010;52:954–65. doi: 10.1002/hep.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aslanidi G, Kroutov V, Philipsberg G, et al. Ectopic expression of Wnt10b decreases adiposity and improves glucose homeostasis in obese rats. Am J Physiol Endocrinol Metab. 2007;293:E726–36. doi: 10.1152/ajpendo.00248.2007. [DOI] [PubMed] [Google Scholar]
- 27.Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev.Biol. 2005;288:276–83. doi: 10.1016/j.ydbio.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 28.Christodoulides C, Laudes M, Cawthorn WP, et al. The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J.Cell Sci. 2006;119:2613–20. doi: 10.1242/jcs.02975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar S, Leontovich A, Coenen MJ, Bahn RS. Gene expression profiling of orbital adipose tissue from patients with Graves' ophthalmopathy: a potential role for secreted frizzled-related protein-1 in orbital adipogenesis. J.Clin.Endocrinol.Metab. 2005;90:4730–5. doi: 10.1210/jc.2004-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koza RA, Nikonova L, Hogan J, et al. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet. 2006;2:e81. doi: 10.1371/journal.pgen.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang SQ, Lin HZ, Mandal AK, Huang J, Diehl AM. Disrupted signaling and inhibited regeneration in obese mice with fatty livers: implications for nonalcoholic fatty liver disease pathophysiology. Hepatology. 2001;34:694–706. doi: 10.1053/jhep.2001.28054. [DOI] [PubMed] [Google Scholar]
- 32.Nejak-Bowen K, Kikuchi A, Monga SP. Beta-catenin-NF-kappaB interactions in murine hepatocytes: A complex to die for. Hepatology. 2012 doi: 10.1002/hep.26042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trierweiler C, Blum HE, Hasselblatt P. The Transcription Factor c-Jun Protects against Liver Damage following Activated beta-Catenin Signaling. PloS one. 2012;7:e40638. doi: 10.1371/journal.pone.0040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian J, Niu M, Zhai X, Zhou Q, Zhou Y. beta-Catenin pathway is required for TGF-beta1 inhibition of PPARgamma expression in cultured hepatic stellate cells. Pharmacol Res. 2012;66:219–25. doi: 10.1016/j.phrs.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Iritani N, Hosomi H, Fukuda H, Tada K, Ikeda H. Soybean protein suppresses hepatic lipogenic enzyme gene expression in Wistar fatty rats. J Nutr. 1996;126:380–8. doi: 10.1093/jn/126.2.380. [DOI] [PubMed] [Google Scholar]
- 36.Nagata Y, Ishiwaki N, Sugano M. Studies on the mechanism of antihypercholesterolemic action of soy protein and soy protein-type amino acid mixtures in relation to the casein counterparts in rats. J Nutr. 1982;112:1614–25. doi: 10.1093/jn/112.8.1614. [DOI] [PubMed] [Google Scholar]
- 37.Sugano M, Goto S, Yamada Y, et al. Cholesterol-lowering activity of various undigested fractions of soybean protein in rats. J Nutr. 1990;120:977–85. doi: 10.1093/jn/120.9.977. [DOI] [PubMed] [Google Scholar]
- 38.Cederroth CR, Vinciguerra M, Gjinovci A, et al. Dietary phytoestrogens activate AMP-activated protein kinase with improvement in lipid and glucose metabolism. Diabetes. 2008;57:1176–85. doi: 10.2337/db07-0630. [DOI] [PubMed] [Google Scholar]
- 39.Naaz A, Yellayi S, Zakroczymski MA, et al. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology. 2003;144:3315–20. doi: 10.1210/en.2003-0076. [DOI] [PubMed] [Google Scholar]
- 40.Kim MH, Park JS, Seo MS, Jung JW, Lee YS, Kang KS. Genistein and daidzein repress adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells via Wnt/beta-catenin signalling or lipolysis. Cell Prolif. 2010;43:594–605. doi: 10.1111/j.1365-2184.2010.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.