Abstract
Objective
In a large prospective cohort, we examined the relationship of BMI with mortality among blacks and compared the results to those among whites in this population.
Design and methods
The study population consisted of 7,446 non-Hispanic black and 130,598 white participants, ages 49–78 at enrollment, in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. BMI at baseline, BMI at age 20, and BMI change were calculated using self-reported and recalled height and weight. Relative risks were stratified by race and sex and adjusted for age, education, marital status, and smoking.
Results
1,495 black and 18,236 white participants died during follow-up (mean=13 years). Clear J-shaped associations between BMI and mortality were observed among white men and women. Among black men and women, the bottoms of these curves were flatter, and increasing risks of death with greater BMI were observed only at higher BMI levels (≥35.0). Associations for BMI at age 20 and BMI change also appeared to be stronger in magnitude in whites versus blacks, and these racial differences appeared to be more pronounced among women.
Conclusion
Our results suggest that BMI may be more weakly associated with mortality in blacks, particularly black women, than in whites.
Introduction
In the U.S., there are substantial disparities in obesity prevalence across racial and ethnic groups. In 2009–2010, it was estimated that 38.8% of black men and 58.5% of black women, as compared with 36.2% of white men and 32.2% of white women, were obese 1. Evidence suggests that differences in obesity between black and white populations has increased in the past decade2–3 and is likely to continue to increase in the future4. It is of great public health importance to understand how racial and ethnic differences in the burden of obesity might contribute to health disparities.
In the white population, it has been well-established that there is a J-shaped relation between body mass index (BMI) and all-cause mortality, with recent studies showing that mortality is the lowest at BMI 22.5–25.0 kg/m2 and increases monotonically with increasing levels of BMI above 25.0 5–6. However, the shape of relation between BMI and mortality in the black population is less clear. Several early studies have suggested that the association for higher BMI values may be weaker among blacks than whites, especially for black women 7–10. However, in a recent study conducted within a large U.S. cohort of black women 6, Boggs et al. found a J-shaped association between BMI and mortality that was largely similar to that in the white population 11. This and other studies have suggested that the racial difference in the BMI-mortality association may differ according to factors such as sex, age, and education 10–11.
BMI in young adulthood and subsequent weight changes may additionally affect health later in life. Previous studies, conducted in predominantly white populations, have linked excess body weight at young ages to elevated mortality 12–14. Recent results from the Atherosclerosis Risk in Communities (ARIC) study showed a positive association between BMI at age 25 and all-cause mortality in African-American women, although the authors did not examine the association of BMI change 15. More studies are needed to explore the health effects of weight at young adulthood and long-term weight change in the black population and compare these effects to those in the white population.
To further clarify the BMI-mortality association in the black population, we investigated the relationship of BMI at baseline and at age 20, as well as BMI change during this period, with total and cause-specific mortality among black men and women in a large U.S. cohort. To investigate racial differences in these associations, we directly compared the results among blacks to those among whites in this population.
Methods
Study population
The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial is a randomized controlled, multi-center trial designed to evaluate selected methods for the early detection of prostate, lung, colorectal, and ovarian cancers16–17. Briefly, between November 1993 and June 2001, over 150,000 men and women aged 49–78 were enrolled from 10 study sites and were randomly assigned to receive either specific cancer screening regime or standard care. Of the 149,980 study participants who completed the self-administered baseline risk factor questionnaire, we excluded those who reported to be neither non- Hispanic black nor non-Hispanic White (n=9,696), did not provide information on height or weight (n=1,723), were not followed up for mortality (n=8), or reported extremely low or high BMI values (below 15 or above 50 kg/m2, n=509). The analytic cohort consisted of 3,278 non-Hispanic black men, 4,168 non-Hispanic black women, 64,162 non-Hispanic white men, and 66,436 non-Hispanic white women. The study protocol was approved by the Institutional Review Board of the National Cancer Institute and the participating centers. All participants provided written information consent upon enrollment.
Exposure assessment
In the baseline risk factor questionnaire, participants were asked to report their current height (in feet and inches), current weight (in pounds), and weight at age 20 (in pounds). We calculated BMI at baseline and at age 20 as the weight at these respective ages in kilograms (kg) divided by current height in meters (m) squared. We additionally calculated the change in BMI between age 20 and baseline for each participant. Other information ascertained from the baseline questionnaire included demographic characteristics; lifestyle factors, such as physical activity, alcohol intake, and smoking history; and medical history, including diabetes, hypertension, heart attack, stroke, and cancer.
Mortality ascertainment
Information on deaths and cause of deaths were obtained through periodic linkage of the study population to the National Death Index. We used the International Classification of Diseases, Ninth Revision [ICD-9] to define death due to cardiovascular diseases (ICD-9 codes 390–459) or cancer (ICD-9 codes 140–208). Study staff retrieved death certificates and relevant medical records to confirm deaths occurring during follow-up and to verify the underlying cause of death in a uniform and unbiased manner 17.
Statistical analysis
Age-standardized mortality rates (number of deaths/1,000 person-years) were calculated by direct standardization using five-year age categories. Multivariable-adjusted hazard ratios (HRs) and two-sided 95% confidence intervals (CIs) were calculated using Cox proportional hazards models via the SAS PROC PHREG procedure (SAS 9.3; SAS Institute, Cary, North Carolina). Person-years were calculated from the date of completion of the baseline questionnaire until the date of death, the last date of follow-up, the 13th anniversary of randomization, or December 31, 2009, whichever occurred first.
In multivariable-adjusted models, we included age at baseline (continuous), education (less than high school, high school, post-high school, some college, college graduate, and postgraduate), marital status (married, widowed, divorced, separated, and never married), smoking status (never, former, and current smoker), pack-years (>0–10, >10–20, >20–30, and >30), and years since quitting (<5, 5–<10, 10–<20, 20–<30, and 30+). For each covariate, missing values (generally <5%) were put into a separate group. Because further adjustment for physical activity, alcohol drinking, and intervention status had little influence on the results (i.e., <5% change in the HRs for BMI), these variables were not included in the final models. In the main analysis, BMI at baseline was categorized into 7 groups: 15.0–<22.5, 22.5–<25.0, 25.0–<27.5, 27.5–<30.0, 30.0–<35.0, 35.0–<40.0, and 40.0–50.0. In analyses of cause-specific mortality and subgroup analyses, we used consolidated categories and continuous values of BMI to preserve statistical power, and participants with BMI values less than 20 were excluded to account for the elevated risks of death observed in this group. Due to the generally lower values of BMI at age 20, we used the following 5 categories for analyses: 15.0–<20, 20–<22.5, 22.5–<25.0, 25.0–<27.5, 27.5–50. BMI change between age 20 and baseline was categorized into 4 groups (<0, 0–<5, 5–<10, and 10+). In multivariable-adjusted models of BMI change, we additionally adjusted for BMI at age 20. Tests for linear trend across BMI categories were performed by modeling median values in each category as a continuous variable. Tests for interaction were performed using the likelihood-ratio test, comparing the fit of models having a cross-product term between BMI and the covariate of interest to that of models without this term. Tests for heterogeneity were performed using the Mantel-Haenszel test. To assess the impact of reporting error in self-reported BMI on our results, we used race- and sex-specific regression models to calculate adjusted BMI using the self-reported BMI for each participant (BMI adjusted=−2.03+1.07 BMI self-reported + 0.0062 age for black men, BMI adjusted=−0.88+1.00 BMI self-reported + 0.0273 age for white men, BMI adjusted=− 1.10+1.02 BMI self-reported + 0.0174 age for black women, and BMI adjusted=−0.51+1.02 BMI self-reported + 0.0117 age for white women). We generated these equations using data from participants ages 49 years and older in the U.S. National Health and Nutrition Evaluation Survey (NHANES), 1999–2006,18 by regressing BMI calculated from measured height and weight on BMI calculated from self-reported height and weight, adjusting for age.
Results
During an average of 13 years of follow up, we identified 1,495 deaths in blacks and 18,236 in whites. Table 1 describes study characteristics of black and white men and women. The mean age at baseline was 62 for all race and sex groups. Compared with whites, both black men and women were less likely to be college educated and married and more likely to be current smokers and have a history of diabetes, hypertension, heart attack, and/or stroke. The mean baseline BMI was higher in blacks than in whites, and this race difference was more pronounced in women than in men. The distribution of BMI at age 20 was similar across categories of race and sex.
Table 1.
Demographic and lifestyle characteristics of non-Hispanic black and white participants in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial.
| Non-Hispanic black | Non-Hispanic white | |||
|---|---|---|---|---|
|
| ||||
| Men (n=3,278) | Women (n=4,168) | Men (n=64,162) | Women (n=66,436) | |
| No. of deaths | 881 | 614 | 11557 | 6682 |
| BMI (kg/m2) at baseline, mean (SD) | 27.9 (4.7) | 30.1 (6.0) | 27.5 (4.1) | 26.9 (5.3) |
| BMI (kg/m2) at baseline, % | ||||
| 15–<18.5 | 1 | 1 | 0 | 1 |
| 18.5–<25 | 26 | 19 | 26 | 41 |
| 25–<30 | 44 | 36 | 51 | 35 |
| 30–<35 | 22 | 25 | 18 | 16 |
| 35–50 | 8 | 19 | 5 | 8 |
| BMI (kg/m2) at age 20, mean (SD) | 22.7 (3.3) | 21.6 (3.7) | 23.0 (3.0) | 21.1 (2.8) |
| BMI (kg/m2) at age 20, % | ||||
| 15–<18.5 | 6 | 16 | 5 | 11 |
| 18.5–<25 | 71 | 71 | 72 | 81 |
| 25–<30 | 21 | 10 | 21 | 6 |
| 30–<50 | 2 | 3 | 2 | 1 |
| Age, mean (range) | 62 (53–74) | 62 (54–77) | 62 (49–78) | 62 (50–78) |
| Education, % | ||||
| Less than high school | 22 | 16 | 7 | 1 |
| High school | 21 | 21 | 18 | 28 |
| Post-high school | 11 | 11 | 12 | 13 |
| Some college | 24 | 25 | 21 | 23 |
| College graduate | 10 | 12 | 19 | 15 |
| Postgraduate | 12 | 15 | 23 | 15 |
| Marital Status, % | ||||
| Married | 65 | 40 | 84 | 71 |
| Widowed | 6 | 24 | 4 | 13 |
| Separated | 5 | 5 | 1 | 1 |
| Never married | 5 | 5 | 3 | 3 |
| Never married | 5 | 5 | 3 | 3 |
| Smoking, % | ||||
| Never | 28 | 50 | 37 | 56 |
| Former | 48 | 34 | 52 | 35 |
| Current | 24 | 16 | 11 | 9 |
| Disease history, % | ||||
| Diabetes | 19 | 17 | 8 | 5 |
| Hypertension | 55 | 61 | 33 | 32 |
| Heart attack | 12 | 8 | 14 | 5 |
| Stroke | 5 | 5 | 3 | 2 |
| Any cancer | 2 | 6 | 2 | 7 |
Abbreviations: BMI, Body-mass index
Age-standardized mortality rates were lowest between 27.5 and 29.9 in black men and 25.0 and 27.4 in black women, whereas the lowest mortality rates among white participants were generally found between 22.5 and 24.9. Black men and women had higher age-standardized mortality rates in all BMI categories when compared with their white counterparts (Table 2).
Table 2.
Hazard ratios (HRs) and 95% confidence intervals (Cis) for death from any cause according to body mass index (BMI) at baseline, by race and sex.
| BMI, kg/rn 2 | Non-Hispanic black
|
Non-Hispanic white
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. of deaths | Age-standardized mortality rate a | Multivariable-adjusted HR (95% CI) b | Multivariable-adjusted HR (95% CI) b using adjusted BMI c | No. of deaths | Age-standardized mortality rate a | Multivariable-adjusted HR (95% CI) b | Multivariable-adjusted HR (95% CI) b using adjusted BMI c | |
| MEN | ||||||||
| All men | ||||||||
| 15.0–<22.5 | 124 | 40.8 | 1.35 (1.06, 1.71) | 1.32 (1.04, 1.67) | 1105 | 20 | 1.24 (1.15, 1.33) | 1.29 (1.19, 1.40) |
| 22.5–<25.0 | 160 | 26.9 | ref | ref | 2116 | 14.5 | ref | ref |
| 25.0–<27.5 | 190 | 22.8 | 0.95 (0.77, 1.17) | 0.95 (0.77, 1.19) | 3186 | 14.2 | 0.98 (0.93, 1.04) | 0.95 (0.90, 1.01) |
| 27.5–<30.0 | 149 | 21.4 | 0.91 (0.73, 1.14) | 0.88 (0.70,1.10) | 2233 | 15.2 | 1.04 (0.98, 1.11) | 0.99 (0.94, 1.06) |
| 30.0–<35.0 | 178 | 26.4 | 1.18 (0.95, 1.47) | 1.15 (0.93, 1.43) | 2188 | 18.8 | 1.26 (1.18, 1.34) | 1.17 (1.10, 1.24) |
| 35.0–<40.0 | 61 | 35 | 1.42 (1.06, 1.92) | 1.25 (0.93, 1.67) | 541 | 25.2 | 1.64 (1.49, 1.80) | 1.60 (1.47, 1.75) |
| 40.0–50.0 | 19 | 46.2 | 1.91 (1.18, 3.09) | 1.87 (1.25, 2.79) | 178 | 33.9 | 2.25 (1.93, 2.63) | 2.06 (1.80, 2.37) |
| Healthy d men who never smoked or quit 5+ years before baseline | ||||||||
| 15.0–<22.5 | 31 | 22.4 | 1.30 (0.82, 2.06) | 1.33 (0.84, 2.12) | 433 | 10.9 | 1.25 (1.12, 1.40) | 1.26 (1.11, 1.44) |
| 22.5–<25.0 | 49 | 15.9 | ref | ref | 977 | 9.1 | ref | ref |
| 25.0–<27.5 | 78 | 14.3 | 0.88 (0.61, 1.26) | 0.90 (0.62, 1.31) | 1608 | 9.9 | 1.05 (0.97, 1.14) | 0.99 (0.91, 1.08) |
| 27.5–<30.0 | 63 | 14.1 | 0.87 (0.59, 1.26) | 0.92 (0.63, 1.35) | 1229 | 11.5 | 1.19 (1.09, 1.29) | 1.10 (1.01, 1.19) |
| 30.0–<35.0 | 78 | 17.4 | 1.03 (0.72, 1.48) | 1.03 (0.71, 1.49) | 1127 | 13.7 | 1.37 (1.25, 1.49) | 1.23 (1.13, 1.34) |
| 35.0–<40.0 | 30 | 26.9 | 1.68 (1.06, 2.66) | 1.34 (0.84, 2.16) | 278 | 18.2 | 1.76 (1.53,2.01) | 1.73 (1.53, 1.96) |
| 40.0–50.0 | 11 | 39.5 | 2.42 (1.25, 4.68) | 2.57 (1.48, 4.54) | 97 | 27.7 | 2.62 (2.12, 3.23) | 2.33 (1.93, 2.82) |
|
| ||||||||
| WOMEN | ||||||||
| All women | ||||||||
| 15.0–<22.5 | 72 | 21.4 | 1.64 (1.18, 2.27) | 1.55 (1.07, 2.24) | 1473 | 9.6 | 1.22 (1.13, 1.31) | 1.29 (1.19, 1.40) |
| 22.5–<25.0 | 75 | 12.8 | ref | ref | 1308 | 7.6 | ref | ref |
| 25.0–<27.5 | 102 | 11.6 | 0.93 (0.69, 1.26) | 0.80 (0.58, 1.10) | 1280 | 7.7 | 1.00 (0.93, 1.08) | 0.97 (0.90, 1.05) |
| 27.5–<30.0 | 95 | 12.2 | 1.02 (0.75, 1.38) | 0.84 (0.61, 1.14) | 867 | 8.6 | 1.12 (1.02, 1.22) | 1.07 (0.98, 1.16) |
| 30.0–<35.0 | 143 | 13.4 | 1.10 (0.83, 1.46) | 0.89 (0.67, 1.19) | 1082 | 9.7 | 1.27 (1.17,1.38) | 1.22 (1.13, 1.33) |
| 35.0–<40.0 | 71 | 14.9 | 1.21 (0.87, 1.69) | 1.04 (0.75, 1.44) | 418 | 11.7 | 1.55 (1.39, 1.73) | 1.41 (1.27, 1.57) |
| 40.0–50.0 | 56 | 26.1 | 1.83 (1.29, 2.62) | 1.50 (1.06, 2.14) | 254 | 17.4 | 2.28 (1.99,2.61) | 2.20 (1.95, 2.49) |
| Healthy d men who never smoked or quit 5+ years before baseline | ||||||||
| 15.0–<22.5 | 26 | 11.8 | 1.34 (0.80, 2.26) | 1.51 (0.81, 2.79) | 707 | 6.2 | 1.22 (1.10, 1.35) | 1.27 (1.14, 1.43) |
| 22.5–<25.0 | 32 | 8.6 | ref | ref | 703 | 5.2 | ref | ref |
| 25.0–<27.5 | 50 | 8.3 | 0.92 (0.58, 1.44) | 0.89 (0.55, 1.44) | 717 | 5.6 | 1.06 (0.95,1.17) | 1.05 (0.94, 1.16) |
| 27.5–<30.0 | 54 | 9.4 | 1.05 (0.68, 1.64) | 0.94 (0.59, 1.48) | 499 | 6.3 | 1.19 (1.06, 1.33) | 1.11 (0.99, 1.24) |
| 30.0–<35.0 | 58 | 8.4 | 0.94 (0.61, 1.46) | 0.90 (0.58, 1.40) | 659 | 7.6 | 1.40 (1.26, 1.56) | 1.34 (1.21, 1.49) |
| 35.0–<40.0 | 36 | 10.7 | 1.16 (0.72,1.88) | 1.00 (0.61, 1.63) | 251 | 9.2 | 1.70 (1.47, 1.97) | 1.56 (1.36, 1.79) |
| 40.0–50.0 | 27 | 21.2 | 1.87 (1.11, 3.16) | 1.67 (1.00, 1.14) | 171 | 14.8 | 2.68 (2.27,3.17) | 2.46 (2.10, 2.88) |
Number of deaths per 1,000 person-years, calculated by direct standardization using five-year age categories
Adjusted for age, education, marital status, smoking status, pack-years, and years since quitting smoking for all men and all women. For healthy men and women who never smoked or quit 5+ years before baseline, models were adjusted for age, education, marital status, remote former smoking, previous pack-years, and years since quitting smoking
BMI adjusted for reporting error using external data from NHANES. Adjustment equations: BMI adjusted=−2.03+1.07 BMI self-reported + 0.0062 age for black men, BMI adjusted=−0.88+1.00 BMI self-reported + 0.0273 age for white men, BMI adjusted=−1.10+1.02 BMI self-reported + 0.0174 age for black women, and BMI adjusted=−0.51+1.02 BMI self-reported + 0.0117 age for white women
Healthy was defined as no self-reported history of cancer, heart attack, or stroke at baseline
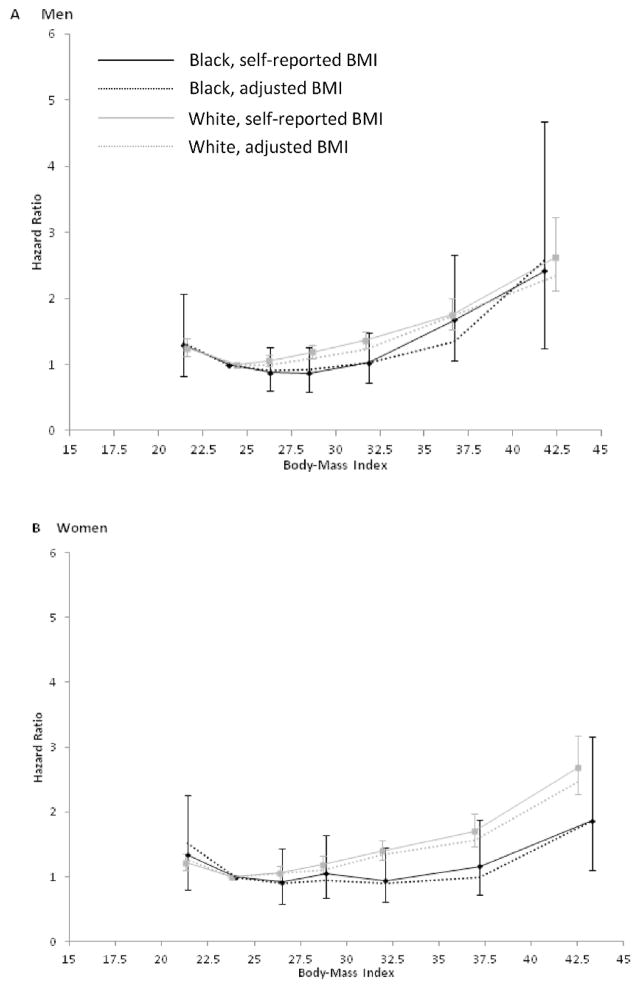
In multivariable-adjusted models using 22.5–<25.0 as the reference category of BMI, we observed J-shaped associations between BMI and mortality in both blacks and whites. Additional exclusion of current smokers, recent quitters (<5 years), and subjects with a history of cancer, heart attack, or stroke at baseline generally yielded slightly weaker associations for the lowest BMI category and slightly stronger positive associations at the higher range of BMI (Table 2, Figure 1). The associations between BMI and total mortality appeared to be stronger, with increasing HRs beginning at lower BMI values, in whites compared to blacks, and the racial difference was more pronounced in women (Table 2, Figure 1). For the highest compared to the reference category of BMI, the magnitudes of the HRs were similar between white (HR=2.62, 95% CI: 2.12, 3.23) and black (HR=2.42, 95% CI: 1.25, 4.68) men but appeared to be stronger for white (HR=2.68, 95% CI: 2.27, 3.17) versus black (HR=1.83, 95% CI: 1.28, 2.61) women (Table 2, Figure 1). After adjusting for reporting error using NHANES data, associations between BMI and mortality generally became slightly more negative, with the exception of the highest category of BMI in black men and the lowest category of BMI in black women, which became more strongly positive.
Figure 1.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for death from any cause according to body mass index (BMI) among healthy a subjects who were not current cigarette smokers or recent quitters, by race and sex. Light colored lines represent whites and dark colored lines represent blacks. Solid lines represent results with self-reported BMI and dotted lines represent results with adjusted b BMI. Squares and circles represent the HR corresponding to the median values in each BMI category. Vertical lines represent the 95% CIs (self-reported BMI alone). Models were adjusted for age, education, marital status, remote smoking status, previous pack-years, and years since quitting smoking. a Healthy was defined as no self-reported history of cancer, heart attack, or stroke at baseline. b BMI adjusted for reporting error using external data from NHANES (1999–2006). Adjustment equations: BMI adjusted=− 2.03+1.07 BMI self-reported + 0.0062 age for black men, BMI adjusted=−0.88+1.00 BMI self-reported + 0.0273 age for white men, BMI adjusted=−1.10+1.02 BMI self-reported + 0.0174 age for black women, and BMI adjusted=− 0.51+1.02 BMI self-reported + 0.0117 age for white women
Nonetheless, the shapes of the BMI curves remained largely similar (Table 2, Figure 1). In a sensitivity analysis, we further excluded all former smokers, which reduced somewhat the excess risk of death observed among those with a low BMI, but otherwise results were similar (data not shown). We also excluded those who died within the first year of follow-up but found that the results were largely unchanged (data not shown).
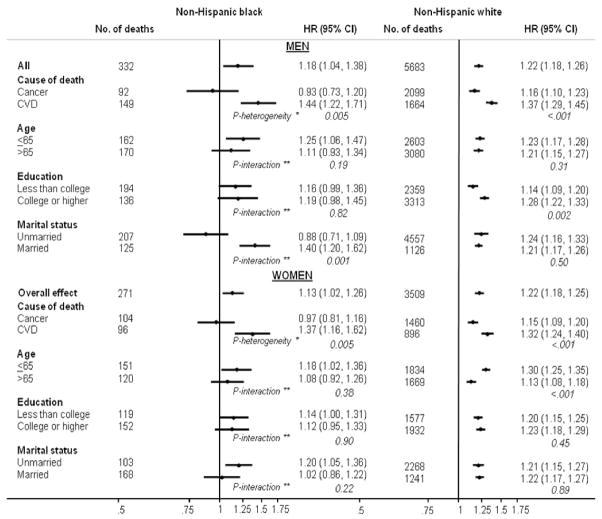
We also examined associations between BMI and cardiovascular disease and cancer mortality among blacks and whites (Figure 2, Supplementary Table 1). Among white men and women, a high BMI was associated with an increased risk of cancer and, to a greater extent, cardiovascular disease mortality. Among black men and women, BMI was positively associated with cardiovascular mortality but not with cancer mortality; however, the number of cancer deaths in these populations was small. We further examined BMI and all-cause mortality for men and women by age, education, and marital status (Figure 2, Supplementary Table 2 and 3). In all subgroups of white men and women, there was a positive and significant association between BMI and risk of death. The association differed according to age in women and education in men, with stronger relationships observed among younger white women (P-interaction <0.001) and more educated white men (P-interaction=0.002). Among blacks, the association was slightly stronger in those who were 65 years or younger at baseline, but the interaction with age was not significant. Education did not modify the BMI-mortality association in black men or women. However, a strong positive association between BMI and mortality was observed among married, but not unmarried, black men (P-interaction=0.001).
Figure 2.
Race- and sex-specific hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between body mass index (for each 5 unit increase) and cause-specific and total mortality (by age, education and marital status) among healthy a subjects who were not current cigarette smokers or recent quitters and whose BMI was in the range of 20–50. Models were adjusted for age, education, marital status, remote smoking status, previous pack-years, and years since quitting smoking. a Healthy was defined as no self-reported history of cancer, heart attack, or stroke at baseline. * P-heterogeneity calculated using the Mantel-Haenszel test for heterogeneity. ** P-interaction calculated using the likelihood ratio test comparing a model with a cross-product term to a model without.
Finally, we examined BMI at age 20 and BMI change between age 20 and baseline in relation to total mortality (Table 3). We found that, compared to the reference group (22.5–<25.0), higher categories of BMI at age 20 were associated with increased total mortality in white men and women, but this association appeared to be weaker in blacks, possibly due to smaller numbers of deaths. Compared to gaining less than five units of BMI between age 20 and baseline, gaining more than 10 units was associated with a significantly elevated risk of death in whites and in black men; however, no association was observed among black women.
Table 3.
Hazard ratios (HRs) and 95% confidence intervals (Cis) for death from any cause according to body mass index (BMI) at age 20 among healthy a subjects who were not current cigarette smokers or recent quitters, by race and sex.
| Non-Hispanic black
|
Non-Hispanic white
|
|||
|---|---|---|---|---|
| No. of deaths | Multivariable- adjusted HR (95% CI) | No. of deaths | Multivariable- adjusted HR (95% CI) | |
| MEN | ||||
| BMI at 20 b | ||||
| 15.0–<20.0 | 59 | 0.94 (0.67, 1.31) | 871 | 0.98 (0.90, 1.06) |
| 20.0–<22.5 | 109 | 0.87 (0.65, 1.16) | 1825 | 0.96 (0.90, 1.03) |
| 22.5–<25.0 | 85 | ref | 1612 | ref |
| 25.0–<27.5 | 55 | 0.97 (0.69, 1.36) | 995 | 1.18 (1.09, 1.27) |
| 27.5–50.0 | 24 | 1.35 (0.85, 2.13) | 408 | 1.46 (1.31,1.63) |
| BMI change c | ||||
| <0 | 23 | 0.93 (0.58, 1.49) | 415 | 1.16 (1.04, 1.29) |
| 0–<5 | 143 | ref | 2758 | Ref |
| 5–<10 | 107 | 0.78 (0.60, 1.01) | 1937 | 1.08 (1.01, 1.14) |
| 10+ | 59 | 1.41 (1.03, 1.93) | 601 | 1.55 (1.41, 1.70) |
|
| ||||
| WOMEN | ||||
| BMI at 20 b | ||||
| 15.0–<20.0 | 100 | 0.86 (0.61, 1.21) | 1131 | 0.88 (0.80, 0.97) |
| 20.0–<22.5 | 91 | 0.81 (0.57, 1.14) | 1581 | 0.94 (0.85, 1.03) |
| 22.5–<25.0 | 50 | ref | 609 | ref |
| 25.0–<27.5 | 12 | 0.58 (0.31, 1.09) | 211 | 1.29 (1.10, 1.51) |
| 27.5–50.0 | 18 | 1.19 (0.69, 2.04) | 139 | 1.61 (1.34,1.93) |
| BMI change c | ||||
| <0 | 13 | 1.59 (0.84, 2.99) | 293 | 1.28 (1.12, 1.46) |
| 0–<5 | 62 | ref | 1414 | ref |
| 5–<10 | 105 | 0.92 (0.67, 1.26) | 1211 | 1.05 (0.97, 1.14) |
| 10+ | 91 | 1.04 (0.75,1.45) | 753 | 1.51 (1.38, 1.64) |
Healthy was defined as no self-reported history of cancer, heart attack, or stroke at baseline
Multivariable-adjusted model included age, education, marital status, remote former smoking, previous pack-years, years since quitting.
Multivariable-adjusted model included variables in b band BMI at 20.
Discussion
In this large prospective U.S. cohort, we found a positive association between BMI and mortality which appeared to be weaker in blacks, particularly black women, compared to whites. When compared with the reference group of a BMI of 22.5–<25.0, mortality increased monotonically with greater BMI in healthy, non-smoking white men and women, which largely confirmed previous findings 5–6. Among healthy, non-smoking black men and women, the bottoms of these curves were flatter, and increasing risks of death with greater BMI were observed only at higher BMI levels (≥35.0). Similarly, associations of BMI at age 20 and BMI change with mortality appeared to be weaker in blacks than in whites.
Our findings for BMI and all-cause mortality may be evaluated against those of several other large prospective studies conducted in the U.S., including the NIH-AARP Diet and Health Study 10, the Cancer Prevention Study-II 9, the Southern Community Cohort Study 19, and the Reasons for Geographic and Racial Differences in Stroke study 20, in which the shape of BMI-mortality curves among black and white men and women were also directly compared within the same cohort. The first three studies used the same reference BMI category that was used in our study and similarly found a weaker association between higher BMI categories and mortality in black women than in white women; among black women, the BMI-mortality association was relatively flat until BMI≥40. For men, the NIH-AARP study found a similar BMI-mortality relationship between blacks and whites, while the Southern Community Cohort Study showed an elevated mortality with higher BMI only among whites. Results from the Cancer Prevention Study were inconclusive due to limited numbers of black men in the extreme BMI categories. In the Reasons for Geographic and Racial Differences in Stroke study, increased mortality was associated with higher BMI in white men and women; however, there was no significant elevation in mortality among overweight and obese black men and women.
Our results differed with those of the Black Women’s Health Study, which reported an association that was largely similar to that found in white women11. This discrepancy may be partly due to the fact that our study included an older population (49–78 years), while the participants in the Black Women’s Health Study were somewhat younger (21–69 years). As shown in our study and others, the association between BMI and mortality tended to be stronger in younger populations than in older populations 6, 9–10. Nevertheless, like the Black Women’s Health Study, the Multiethnic Cohort Study observed similar BMI-mortality associations across racial/ethnic groups 21, and, similar to our study, included participants who were middle-to-older aged (45–75 years). Thus, there may be other differences across studies, such as socioeconomic or demographic factors, that could account for these inconsistencies.
Our findings confirmed those from previous studies showing a positive association of BMI with cancer and cardiovascular mortality in whites 6, 9. We observed a positive association between BMI and cardiovascular, but not cancer, mortality in both black men and women. The results were consistent with those from the Black Women’s Health Study 11. Because only a subset of cancers are obesity-related 22–24, differences in site-specific cancer mortality rates between blacks and whites may partially account for the differences in the BMI-cancer mortality association. However, the relatively small number of cancer deaths in our study precluded us from examining the relationship between BMI and death from specific cancers.
Consistent with previous studies, we found that higher BMI at age 20 was linked to higher mortality in whites; however, this association appeared to be weaker among blacks. Moreover, excess weight gain since age 20 was also associated with higher mortality in whites, and in black men, but not in black women. Although results from the ARIC Study showed significantly elevated mortality among black men and women who had a young-adulthood BMI of over 30 15, we were unable to examine risks of death at higher values of young-adulthood BMI among blacks in this cohort due to the small number of deaths. However, both the ARIC study and our study found possible racial difference across lower values of BMI, with weaker associations found in blacks. These results suggest that there may be lifelong differences in how BMI affects health in blacks versus whites.
Several lines of evidence suggest that underlying physiological distinctions among different race and sex groups may contribute to the observed differences in the BMI-mortality association. The association between BMI and the total level and distribution of body fat may be race- or sex-specific 25–26. For instance, some studies have reported that the slope of the association between BMI and visceral adipose tissue (VAT) is less steep in blacks compared to whites 27–28. Therefore, the same increments in BMI may lead to smaller increments in VAT in the black population. Similar differences have also been observed for associations between BMI and adipokine levels, with several studies reporting a more clearly linear association between BMI and leptin or adiponectin in white women than in black women 29–30. Such racial differences may have important health implications. VAT has been proposed to be a better marker than BMI for certain chronic conditions, such as the metabolic syndrome31, and leptin and adiponectin are critically involved in a variety of physiological or pathogenic pathways including energy regulation, insulin sensitivity, inflammatory response, and tumorigenesis 32–34. Stronger relationships between BMI and these biomarkers in whites may translate into stronger effects of BMI on general health and mortality. On the other hand, BMI may not be an accurate indicator of risk of obesity-related diseases among blacks, and other measures of adiposity may be more appropriate in directly comparing health risks associated with obesity across racial/ethnic groups.
The BMI-mortality association may be modified by socioeconomic factors as well. Both the Black Women’s Health Study and the Cancer Prevention Study-I found that the BMI-mortality association was attenuated in women with lower education levels (less than 12 years of education in the Black Women’s Health Study and less than high school education in the Cancer Prevention Study-I) 8, 11. It was suggested that among people with low socioeconomic status, factors other than BMI, such as chronic psychological stress and limited access to care, are major determinants of health and may mask the effect of BMI on mortality. However, we did not find the association to differ according to education levels in blacks. Instead, we saw a significant interaction between BMI and education level in white men, whereby the BMI-mortality relation was weaker among those who had a less-than-college education. Interestingly, we found that among black men, marital status appeared to be a strong effect modifier for the BMI-mortality association, with a significant and positive association found only in the married men. These findings support that socioeconomic status may modify the BMI-mortality association, but the modifying factor may be population-specific.
Our study’s strengths include the inclusion of both black and white male and female participants, which allowed us to investigate and directly compare the relation between BMI and mortality across these different groups. To reduce the potential for confounding, we examined the effect of excluding subjects whose BMI might have been affected by a previous diagnosis of cancer or cardiovascular disease, as well as current and recent smokers. We found no difference in the results after excluding participants who died within the first year of follow-up, further reducing of the potential for bias due to reverse causality.
There are, however, several limitations of our study. The sample size among blacks in this study was modest and the numbers of deaths were small compared to previous studies on this topic in this and other racial/ethnic groups and limited our ability to detect possible associations, particularly in sub-group analyses. Self-reported weight and height, especially recalled weights, are prone to error. Although validation studies have generally found strong correlations between measured and recalled past weight 35–36, previous studies reported that women and whites are more likely to underreport their weight compared to men and non-whites 37. However the shapes of the BMI and mortality associations were similar after correcting for reporting error in BMI, using race- and sex-specific equations for adjustment. We also lacked more direct measures of central adiposity and visceral fat, which may be independent 36, or even more important 18, 38, risk factors for certain health outcomes compared with general obesity. Lastly, although we controlled for multiple factors, we could not rule out the possibility of residual confounding from other lifestyle factors such as diet.
In conclusion, we found differences in the shape of the BMI-mortality associations between blacks and whites. BMI at baseline and at age 20, as well as the change in BMI during this period, appeared to have a stronger positive association with all-cause mortality in whites than in blacks. We have also identified socioeconomic factors, such as education in white men and marital status in black men, which may be important effect modifiers for the BMI-mortality relationship. Such differences may be rooted in both the distinct biology and socioeconomic environment across populations defined by race/ethnicity and sex. For future studies, utilizing more valid measures of adiposity, particularly more direct measures of abdominal adiposity and visceral fat, may better address the health risks associated with overweight and obesity in the black population.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, National Institutes of Health, Department of Health and Human Services. The authors would like to thank Dr Alan Flint (School of Public Health, Harvard University) for his help with the analysis using NHANES data.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Beydoun MA, Wang Y. Gender-ethnic disparity in BMI and waist circumference distribution shifts in US adults. Obesity (Silver Spring) 2009;17(1):169–76. doi: 10.1038/oby.2008.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 4.Robinson WR, Keyes KM, Utz RL, Martin CL, Yang Y. Birth cohort effects among US-born adults born in the 1980s: foreshadowing future trends in US obesity prevalence. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1. 46 million white adults. N Engl J Med. 2010;363(23):2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens J, Keil JE, Rust PF, Tyroler HA, Davis CE, Gazes PC. Body mass index and body girths as predictors of mortality in black and white women. Arch Intern Med. 1992;152(6):1257–62. [PubMed] [Google Scholar]
- 8.Stevens J, Plankey MW, Williamson DF, et al. The body mass index-mortality relationship in white and African American women. Obes Res. 1998;6(4):268–77. doi: 10.1002/j.1550-8528.1998.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 9.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 10.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–78. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 11.Boggs DA, Rosenberg L, Cozier YC, et al. General and abdominal obesity and risk of death among black women. N Engl J Med. 2011;365(10):901–8. doi: 10.1056/NEJMoa1104119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmans MD, Kromhout D, de Lezenne Coulander C. The impact of body mass index of 78,612 18-year old Dutch men on 32-year mortality from all causes. J Clin Epidemiol. 1988;41(8):749–56. doi: 10.1016/0895-4356(88)90161-8. [DOI] [PubMed] [Google Scholar]
- 13.Jeffreys M, McCarron P, Gunnell D, McEwen J, Smith GD. Body mass index in early and midadulthood, and subsequent mortality: a historical cohort study. Int J Obes Relat Metab Disord. 2003;27(11):1391–7. doi: 10.1038/sj.ijo.0802414. [DOI] [PubMed] [Google Scholar]
- 14.Corrada MM, Kawas CH, Mozaffar F, Paganini-Hill A. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol. 2006;163(10):938–49. doi: 10.1093/aje/kwj114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens J, Truesdale KP, Wang CH, Cai J, Erber E. Body mass index at age 25 and all-cause mortality in whites and African Americans: the Atherosclerosis Risk in Communities study. J Adolesc Health. 2012;50(3):221–7. doi: 10.1016/j.jadohealth.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gohagan JK, Prorok PC, Hayes RB, Kramer BS. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control Clin Trials. 2000;21(6 Suppl):251S–272S. doi: 10.1016/s0197-2456(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 17.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6 Suppl):273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 18.National Health and Nutrition Examination Survey data. Department of Health and Human Services; Hyattsville, MD: 2006. [Google Scholar]
- 19.Cohen SS, Signorello LB, Cope EL, et al. Obesity and All-Cause Mortality Among Black Adults and White Adults. Am J Epidemiol. 2012 doi: 10.1093/aje/kws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakoski SG, Le AH, Muntner P, et al. Adiposity, inflammation, and risk for death in black and white men and women in the United States: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. J Clin Endocrinol Metab. 2011;96(6):1805–14. doi: 10.1210/jc.2010-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SY, Wilkens LR, Murphy SP, Monroe KR, Henderson BE, Kolonel LN. Body mass index and mortality in an ethnically diverse population: the Multiethnic Cohort Study. Eur J Epidemiol. 2012 doi: 10.1007/s10654-012-9695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 23.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335(7630):1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parr CL, Batty GD, Lam TH, et al. Body-mass index and cancer mortality in the Asia-Pacific Cohort Studies Collaboration: pooled analyses of 424,519 participants. Lancet Oncol. 2010;11(8):741–52. doi: 10.1016/S1470-2045(10)70141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson AS, Stanforth PR, Gagnon J, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int J Obes Relat Metab Disord. 2002;26(6):789–96. doi: 10.1038/sj.ijo.0802006. [DOI] [PubMed] [Google Scholar]
- 26.Katzmarzyk PT, Bray GA, Greenway FL, et al. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91(1):7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carroll JF, Chiapa AL, Rodriquez M, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 2008;16(3):600–7. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 28.Camhi SM, Bray GA, Bouchard C, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring) 2011;19(2):402–8. doi: 10.1038/oby.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen SS, Gammon MD, Signorello LB, et al. Serum adiponectin in relation to body mass index and other correlates in black and white women. Ann Epidemiol. 2011;21(2):86–94. doi: 10.1016/j.annepidem.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen SS, Fowke JH, Cai Q, et al. Differences in the association between serum leptin levels and body mass index in black and white women: a report from the Southern Community Cohort Study. Ann Nutr Metab. 2012;60(2):90–7. doi: 10.1159/000336180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 32.Stofkova A. Leptin and adiponectin: from energy and metabolic dysbalance to inflammation and autoimmunity. Endocr Regul. 2009;43(4):157–68. [PubMed] [Google Scholar]
- 33.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2569–78. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 34.Prieto-Hontoria PL, Perez-Matute P, Fernandez-Galilea M, Bustos M, Martinez JA, Moreno-Aliaga MJ. Role of obesity-associated dysfunctional adipose tissue in cancer: a molecular nutrition approach. Biochim Biophys Acta. 2011;1807(6):664–78. doi: 10.1016/j.bbabio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Stevens J, Keil JE, Waid LR, Gazes PC. Accuracy of current, 4-year, and 28-year self-reported body weight in an elderly population. Am J Epidemiol. 1990;132(6):1156–63. doi: 10.1093/oxfordjournals.aje.a115758. [DOI] [PubMed] [Google Scholar]
- 36.Perry GS, Byers TE, Mokdad AH, Serdula MK, Williamson DF. The validity of self-reports of past body weights by U.S. adults. Epidemiology. 1995;6(1):61–6. doi: 10.1097/00001648-199501000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Wen M, Kowaleski-Jones L. Sex and ethnic differences in validity of self-reported adult height, weight and body mass index. Ethn Dis. 2012;22(1):72–8. [PMC free article] [PubMed] [Google Scholar]
- 38.Dobbelsteyn CJ, Joffres MR, MacLean DR, Flowerdew G. A comparative evaluation of waist circumference, waist-to-hip ratio and body mass index as indicators of cardiovascular risk factors. The Canadian Heart Health Surveys. Int J Obes Relat Metab Disord. 2001;25(5):652–61. doi: 10.1038/sj.ijo.0801582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.