Abstract
Cannabinoid CB1 receptor antagonists reduce body weight in rodents and humans, but their clinical utility as anti-obesity agents is limited by centrally mediated side effects. Here, we describe the first mixed CB1 antagonist/CB2 agonist, URB447 ([4-amino-1-(4-chlorobenzyl)-2-methyl-5-phenyl-1H-pyrrol-3-yl](phenyl)methanone), which lowers food intake and body-weight gain in mice without entering the brain or antagonizing central CB1-dependent responses. URB447 may provide a useful pharmacological tool for investigating the cannabinoid system, and might serve as a starting point for developing clinically viable CB1 antagonists devoid of central side effects.
Keywords: URB447, Peripheral cannabinoid antagonism, Anti-obesity agents, Pyrrolic CB-antagonists
Cannabinoid type I (CB1) receptors play essential roles in the control of energy balance. Their activation by agonist drugs stimulates feeding in both rodents and humans,2 while CB1 receptor blockade counters these effects.3 Clinical studies have shown that selective CB1 antagonists such as rimonabant or taranabant lower body weight and improve waist circumference, high-density lipoprotein cholesterol, triglycerides and insulin sensitivity in overweight and obese patients.4 These actions are accompanied, however, by centrally mediated adverse events, including nausea, anxiety, and depression, which limit the clinical utility of CB1 antagonism as an anti-obesity strategy.4
CB1 antagonists are thought to reduce food intake by occupying receptors located in brain circuits involved in the regulation of feeding and motivated behaviors.5 Emerging evidence indicates, however, that CB1 receptors present outside the central nervous system (CNS) also contribute to this response.6 Consistent with a peripheral site of action for these drugs, the neurotoxin capsaicin abolishes the anorexic effects of systemically administered rimonabant in rats, while rimonabant fails to alter feeding behavior when is administered centrally to mice.7 Anatomical data indicate that CB1 receptors are present on vagal sensory afferent neurons,8 where their expression is regulated by the feeding state.9 Thus, peripheral CB1 receptors may represent an important mechanism by which CB1 antagonists regulate food intake and energy balance.
Despite the large number of CB1 receptor antagonists/inverse agonists developed thus far,10 compounds characterized by limited access to the CNS are still lacking. A triazole derivative, LH-21 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-hexyl-1H-1,2,4-tria-zole), was reported to be a neutral CB1 antagonist with no brain penetrability,11 but its plasma-to-brain concentration ratio has been recently reported to be near 1:1 in rats after systemic administration.12
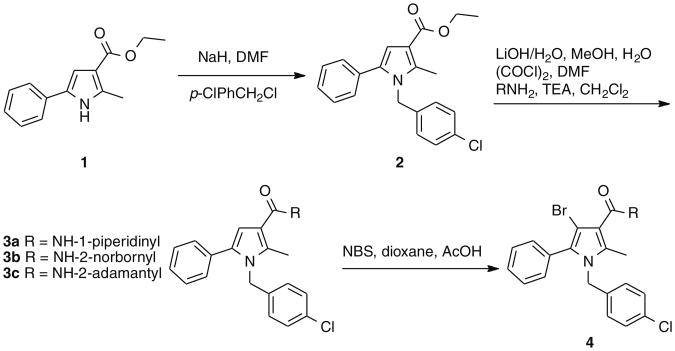
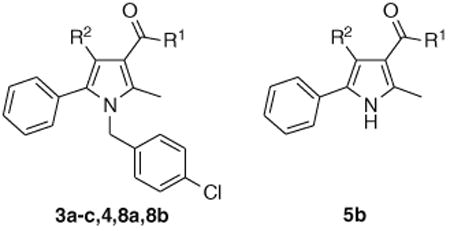
In the present study, we describe a compound that mimics the anorexic and anti-obesity effects of the prototypical CB1 antagonist, rimonabant, without antagonizing centrally mediated CB1-responses. This compound, URB447, belongs to a class of pyrrole-based cannabimimetics previously described by us.13 We observed that the introduction of a p-chlorobenzyl group onto position 1 and of a 1-naphthoyl group onto position 3 of a 2,5-dimethylpyrrole scaffold afforded a compound that displayed partial agonism at CB1 receptor. Moreover, position 4 resulted tolerant to substitution, at least by a bromine atom. To develop antagonists from this pyrrole-based chemical scaffold we introduced a phenyl ring in position 5, to mimic the substituted 5-phenyl ring on the pyrazole scaffold of rimonabant and SR144528, two selective CB1 and CB2 antagonists, respectively. The group at position 3 and the substituent at position 4 were then modulated to test the effect of lipophilicity variation on receptor binding affinity. Compounds 3a–c, 4, 5b, 8a and 8b (Table 1) were thus synthesized and tested for their ability to displace binding of the CB agonist [3H]WIN55,212-2 from rat cerebellar membranes (which mostly contain CB1 subtype) and from CHO-K1 cells overexpressing human CB2 receptors.
Table 1. Structural formulas and IC50 (nM) values for rat CB1a,c or human CB2b,c receptors.
| ||||
|---|---|---|---|---|
|
| ||||
| Compound | R1 | R2 | CB1 (nM) | CB2 (nM) |
| 3a | NH-1-piperidinyl | H | >1000 | >1000 |
| 3b | NH-2-norbornyl | H | >1000 | 420 ± 18 |
| 3c | NH-2-adamantyl | H | 70 ± 10 | 11 ±4 |
| 4 | NH-2-adamantyl | Br | >1000 | 123 ± 29 |
| 5b | C6H5 | NH2 | >1000 | >1000 |
| 8a | C6H5 | H | >1000 | 498 ± 32 |
| 8b URB447 | C6H5 | NH2 | 313 ± 72 | 41 ± 23 |
Inhibition of [3H]WIN55,212-2 binding:
Rat cerebellar membranes.
CHO-K1 cells overexpressing human CB2 receptors. Values are means of four experiments; standard deviation is given.
IC50 values of rimonabant are 5.2 ± 1.8 nM18 (Ki = 1.98 ± 0.36 nM)19 for CB1 and >1000 nM for CB2.20s
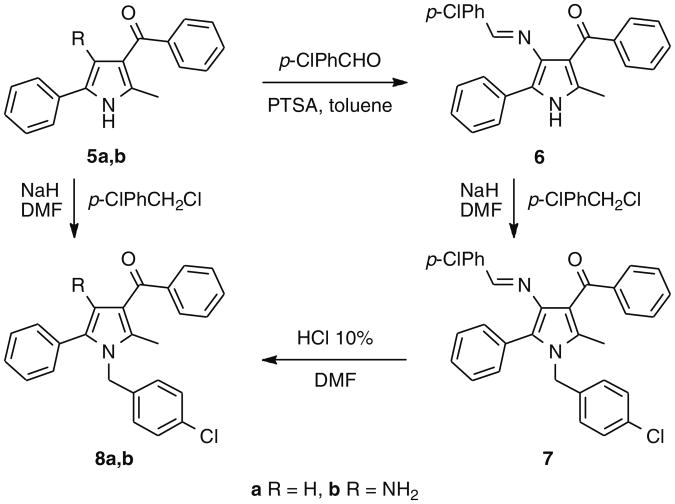
The synthesis of compounds 3a–c started with the alkylation of 2-methyl-5-phenyl-1H-pyrrol-3-carboxylic acid ethyl ester (1)14, followed by hydrolysis of the ester group of 2 and amidation via acyl chloride. Compound 4 was obtained by bromination of 3c with N-bromosuccinimide (Scheme 1). To prepare 8b (URB447), (4-amino-2-methyl-5-phenylpyrrol-3-yl)(phenyl)methanone (5b)15 was converted to the corresponding imine (6) by condensation with 4-chlorobenzaldehyde and p-toluensulphonic acid in the presence of toluene. The imine was N1-alkylated with 4-chlorobenzylchlo-ride and hydrolyzed to afford the desired compound16 (Scheme 2). Compound 8a was synthesized by alkylation of (2-methyl-5-phenyl-1H-pyrrol-3-yl)(phenyl)methanone (5a)17 (Scheme 2).
Scheme 1.
Scheme 2.
The exploration started from the 3-benzoyl derivative 8a, a weak CB2 ligand, in which a polar fragment was introduced either replacing the ketone function by an amide (3a–c, 4) or with an amino group in position 4 (8b, URB447). The amide derivatives showed interesting affinity data for the adamantyl derivative 3c, but, differently from what observed in the previous series of pyrroles,13 introduction of a bromine in position 4 resulted in a significant decrease in CB1 receptor binding affinity. Interestingly, the 3-benzoyl-4-amino derivative, URB447, showed significant affinity for both receptor subtypes, indicating that the polar amino group can produce favorable interactions at the two binding sites. Finally, the role of the N1-p-chlorobenzyl group is confirmed by the inactivity of compound 5b.
In rat cerebellar membrane preparations, URB447 (100 nM–30 μM) inhibited GTPγS binding induced by the CB agonist WIN55,212-2 (1 μM) (EC50 = 4.9 ± 0.8 μM). In the absence of WIN-5512-2, URB447 (100 nM–10 μM) failed to inhibit basal GTPγS binding (data not shown), suggesting that the compound acts as neutral antagonist at CB1 receptors.
In Hek-293 cells stably expressing mouse CB2 receptors, URB447 (1 μM) inhibited cAMP accumulation induced by the β-adrenergic agonist isoproterenol (10 μM) (cAMP levels in pmol/well: basal, 18 ± 2.4; URB447, 5.9 ± 1.2; isoproterenol, 56.2 ± 6; isoproterenol plus URB447,14.3 ± 1.2; n = 4). Similar results were obtained with a higher concentration of URB447 (10 μM, data not shown). The results suggest that URB447 acts as an agonist at CB2 receptors.
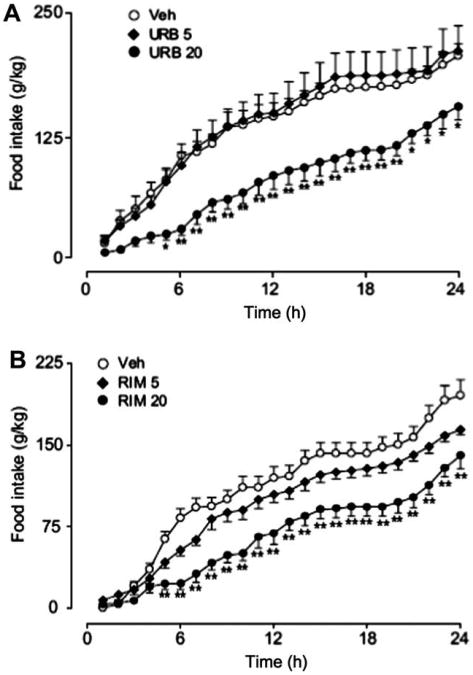
To determine the effects of URB447 on food intake, we administered the drug (5 or 20 mg-kg−1, i.p.) or its vehicle to Swiss mice 30 min before dark onset and monitored feeding for 24 h. Vehicle or a low-dose of URB447 (5 mg-kg−1, i.p.) had no effect on food intake, whereas a high-dose of URB447 (20 mg-kg−1, i.p.) drastically reduced total food consumption (Fig. 1A). This effect was mimicked by rimonabant (20 mg-kg−1, i.p.) (Fig. 1B). The reduction in food intake was not likely to be caused by non-specific behavioral effects, as URB447 (20 mg-kg−1, i.p.) did not alter motor activity (data not shown). The results indicate that URB447 reduces free feeding in mice.
Figure 1.
Effects of vehicle (open circles), URB447 (URB) or rimonabant (RIM) (each at 5 mg-kg−1, closed diamonds, or 20 mg-kg−1, closed circles, i.p.) on cumulative food intake in male Swiss mice (n = 12). Results were analyzed using a 2-way repeated measures ANOVA followed by a Bonferroni post hoc test. *P < 0.05, **P< 0.01 vs vehicle. Error bars represent SEM.
To identify potential sites of action of URB447, we measured drug levels in various tissues after systemic administration (20 mg-kg−1, i.p.). Plasma URB447 levels peaked 30 min after i.p. injection (Cmax 596 ± 117 nM) and maximal tissue levels were reached 15 min post-injection in liver (Cmax 4.3 ± 0.7 nmol/g) and white adipose fat (Cmax 42 ± 12.2 nmol/g). Strikingly, URB447 was not detected in brain tissue at any time after administration (detection limit in the assay was 10 pmol/g) (data not shown). These findings suggest that URB447 does not penetrate the brain, and that its anorexic effects result from the engagement of peripheral CB receptors. To further test this possibility, we asked if URB447 selectively prevents peripheral effects of CB agonists.
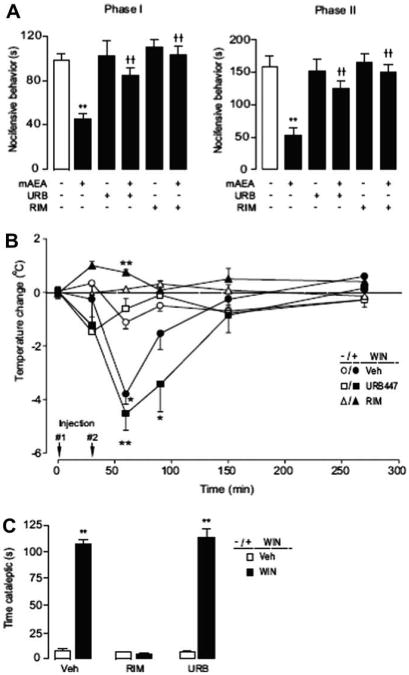
The stable anandamide analogue (R)-methanandamide inhibits nocifensive behavior in the formalin plantar test (Fig. 2A) through a peripheral mechanism.21 Pretreatment with either URB447 (20 mg-kg−1, i.p.) or rimonabant (2 mg-kg−1, i.p.) completely blocked the analgesic effects of methanandamide (Fig. 2A), suggesting that URB447 inhibits peripheral CB1-mediated analgesia. We next induced centrally mediated hypothermia in Swiss mice by administering a single dose of the CB agonist WIN55,212-2 (3 mg-kg−1, i.p.). 30 min after WIN55,212-2 administration the mice developed maximal hypothermia, which was attenuated by pretreatment with rimonabant (2 mg-kg−1, i.p.), but not URB447 (20 mg-kg−1, i.p.) (Fig. 2B). To investigate the effects of URB447 on catalepsy, another central effect of CB1 agonists, we administered URB447 (20 mg-kg−1, i.p.) or rimonabant (5 mg-kg−1, i.p.) 30 min prior to a single injection of vehicle or WIN55,212-2 (5 mg-kg−1) and scored cataleptic behavior 30 min later. Mice treated with WIN55,212-2 exhibited a significant increase in the time spent cataleptic in the ring catalepsy test (Fig. 2C). This response was unaffected by URB447, but was reversed by pretreatment with rimonabant (Fig. 2C). These findings indicate that URB447 selectively prevents peripherally mediated CB responses in mice.
Figure 2.
(A) Effects of vehicle, URB447 (URB, 20 mg-kg−1, i.p.) or rimonabant (RIM, 2 mg-kg−1, i.p.) administered 30 min before intraplantar formalin co-injected with or without (R)-methanandamide (mAEA, 50 μg, i.p.) on first (0–15 min) and second (15–45 min) phase formalin-evoked nociception in Swiss mice (n = 8–13). (B) Effects of a 30 min pretreatment (injection #1) of vehicle, URB447 (20 mg-kg−1, i.p.) or rimonabant (2 mg-kg−1, i.p.) on WIN-55,212 (3 mg-kg−1, i.p., injection #2) –induced hypothermia (n = 5). (C) Effects of vehicle, URB447 (URB, 20 mg-kg−1, i.p.) or rimonabant (RIM, 5 mg-kg−1, i.p.) administered 30 min before vehicle or WIN55,212-2 (5 mg-kg−1, i.p.) on cataleptic behavior measured 30 min after WIN55,212-2 treatment (n = 6). Results were analyzed using a 1-way or 2-way repeated measures ANOVA followed by Bonferroni post hoc tests as appropriate. *P< 0.05, **P< 0.01 vs vehicle; ††P< 0.01 vs mAEA. Error bars represent SEM.
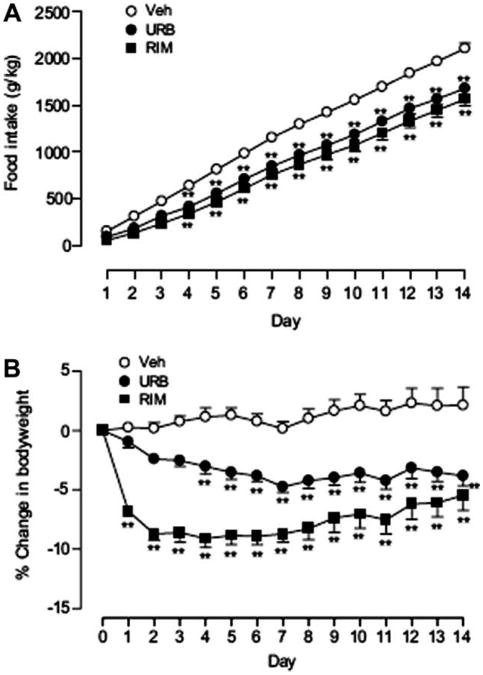
To test whether URB447 inhibits body-weight gain, we treated genetically obese ob/ob mice once daily with URB447, rimonabant (each at 20 mg-kg−1, i.p.) or vehicle for 2 weeks. In keeping with our previous results (Fig. 1), we found that URB447 produced a significant reduction in the amount of food consumed throughout the treatment period (Fig. 3A). This effect was accompanied by a significant attenuation of body-weight gain (Fig. 3B) and was similar in magnitude to that of rimonabant (Fig. 3A and 3B).
Figure 3.
Effects of vehicle, URB447 (URB, 20 mg-kg−1, i.p.) or rimonabant (RIM, 20mg-kg−1, i.p.) administered daily for 2 weeks on (A) food intake or (B) body weight (expressed as % change from day 0) in ob/ob mice (n = 8). Results were analyzed using a 2-way repeated measures ANOVA followed by a Bonferroni post hoc test. **P < 0.01 vs vehicle. Error bars represent SEM.
Contrary to the present findings, URB447 would be expected to cross the blood–brain barrier by passive diffusion,22 considering that its molecular weight (MW = 401), lipophilicity (estimated LogP = 6.39) and polar surface area (PSA = 48.02) are too similar to those of rimonabant (MW = 464, LogP = 6.01, PSA = 50.1).23 The unexpected lack of CNS penetration for URB447 may then be due to high clearance by efflux transporters (e.g., P-gp, MRP1-6 or BCRP)24 or by rapid metabolism by P450 cyrochromes in the astrocytes.25
In conclusion, in the present study we have identified URB447 as the first peripherally restricted mixed CB1 antagonist/CB2 agonist.
We have shown that URB447 reduces food intake and body-weight gain in mice with an efficacy comparable to that of the standard of reference rimonabant. Furthermore, URB447 reversed the analgesic effect of (R)-methanandamide without entering the brain or antagonizing CB1 receptors in the CNS. Indeed, URB447 failed to inhibit WIN55,212-2 induced hypothermia or catalepsy. The ability of URB447 to reduce food intake suggests that this CNS-impermeant antagonist produces anorexia by blocking CB1 receptors in peripheral organs such as the gastrointestinal tract, in which endocannabinoids may act as local orexigenic hormones.7,9 We cannot exclude the possibility, however, that CB2 receptor activation might contribute to the effects of URB447. Indeed, the CB2 agonist JWH133 was shown to improve glucose tolerance after a glucose load26 and ameliorate liver disease27, suggesting a possible role of CB2 in metabolic regulation. Irrespective of these speculations, our findings are clinically relevant because rimonabant and other brain-permeant CB1 antagonist exert serious psychiatric side effects, which limit their clinical usefulness in anti-obesity therapy.
Supplementary Material
Acknowledgments
The Italian Ministry of Instruction, University and Research, Universities of Urbino “Carlo Bo” and Parma, and the Center of Drug Discovery at the University of California, Irvine supported this work. The authors thank Fariba Oveisi for technical assistance.
Footnotes
See Ref. 1.
Supplementary data: Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bmcl.2008.12.059.
Contributor Information
Giorgio Tarzia, Email: giorgio.tarzia@uniurb.it.
Daniele Piomelli, Email: piomelli@uci.edu.
References and notes
- 1.A provisional patent application of the subject of this publication was filed. University of California: Irvine; 2007. [Google Scholar]
- 2.Cota D, Marsicano G, Lutz B, Vicennati V, Stalla GK, Pasquali R, Pagotto U. Int J Obes Relat Metab Disord. 2003;27:289. doi: 10.1038/sj.ijo.0802250. [DOI] [PubMed] [Google Scholar]
- 3.Williams CM, Kirkham TC. Pharmacol Biochem Behav. 2002;71:341. doi: 10.1016/s0091-3057(01)00694-3. [DOI] [PubMed] [Google Scholar]
- 4.Aronne LJ, Isoldi KK. Am J Cardiol. 2007;100:18P. doi: 10.1016/j.amjcard.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Kirkham TC. Behav Pharmacol. 2005;16:297. doi: 10.1097/00008877-200509000-00004. [DOI] [PubMed] [Google Scholar]
- 6.(a) Storr MA, Sharkey KA. Curr Opin Pharmacol. 2007;7:575. doi: 10.1016/j.coph.2007.08.008. [DOI] [PubMed] [Google Scholar]; (b) Pagotto U, Cervino C, Vicennati V, Marsicano G, Lutz B, Pasquali R. Int J Obes. 2006;30(Suppl. 1s):S39. doi: 10.1038/sj.ijo.0803277. [DOI] [PubMed] [Google Scholar]
- 7.Gómez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, Rodríguez de Fonseca F. J Neurosci. 2002;22:9612. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coutts AA, Irving AJ, Mackie K, Pertwee RG, Anavi-Goffer S. J Comp Neurol. 2002;448:410. doi: 10.1002/cne.10270. [DOI] [PubMed] [Google Scholar]
- 9.Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. J Neurosci. 2004;24:2708. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jagerovic N, Fernandez-Fernandez C, Goya P. Curr Top Med Chem. 2008;8:205. doi: 10.2174/156802608783498050. [DOI] [PubMed] [Google Scholar]
- 11.Pavon FJ, Bilbao A, Hernández-Folgado L, Cippitelli A, Jagerovic N, Abellán G, Rodríguez-Franco MI, Serrano A, Macias M, Gómez R, Navarro M, Goya P, Rodríguez de Fonseca F. Neuropharmacology. 2006;51:358. doi: 10.1016/j.neuropharm.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Chen RZ, Frassetto A, Lao JZ, Huang RRC, Xiao JC, Clements MJ, Walsh TF, Hale JJ, Wang J, Tong X, Fong TM. Eur J Pharmacol. 2008;584:338. doi: 10.1016/j.ejphar.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 13.Tarzia G, Duranti A, Tontini A, Spadoni G, Mor M, Rivara S, Plazzi PV, Kathuria S, Piomelli D. Bioorg Med Chem. 2003;11:3965. doi: 10.1016/s0968-0896(03)00413-9. [DOI] [PubMed] [Google Scholar]
- 14.Aoyagi Y, Mizusaki T, Ohta A. Tetrahedron Lett. 1996;37:9203. [Google Scholar]
- 15.Tarzia G, Panzone G. Ann Chim. 1974;64:807. [Google Scholar]
- 16.To a solution of 7 (0.261 g, 0.5 mmol) in DMF (2.5 mL), 10% HCl (1.3 mL) was added slowly (exothermic reaction) at room temperature. After stirring at room temperature for 6 h, the mixture was poured onto 2 N Na2CO3 and extracted (CH2Cl2). The organic layer was dried (Na2SO4) and concentrated. Purification of the residue by column chromatography (cyclohexane EtOAc 8:2) and recrystallization gave URB447 as a yellow solid. Yield 66% (0.132 g). Mp 128–130 °C (EtOAc-petroleum ether). MS (EI): m/z 400 (M+), 275 (100). 1H NMR (CDCl3): δ 1.82 (s, 3H); 4.29 (br s, 2H); 4.97 (s, 2H); 6.84 (d, 2H); 7.24–7.51 (m, 10H); 7.68 (m, 2H) ppm. IR (nujol): 3444, 3359, 1605, 1595 cm−1. Anal. calcd for C25H21ClN2O (400.90): C, 73.84; H, 5.48; N, 6.62. Found: C, 74.03; H, 5.21; N, 6.90.
- 17.Sprio V, Petruso S. Ann Chim. 1973;63:245. [Google Scholar]
- 18.Petitet F, Jeanntaud B, Capet M, Doble A. Biochem Pharmacol. 1997;54:1267. doi: 10.1016/s0006-2952(97)00384-5. [DOI] [PubMed] [Google Scholar]
- 19.Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D, Ferrara P, Soubrié P, Brelière JC, Le Fur G. FEBS Lett. 1994;350:240. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 20.Govaerts SJ, Hermans E, Lambert DM. Eur J Pharm Sci. 2004;23:233. doi: 10.1016/j.ejps.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Calignano A, La Rana G, Giuffrida A, Piomelli D. Nature. 1998;394:277. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 22.Gleeson MP. J Med Chem. 2008;51:817. doi: 10.1021/jm701122q. [DOI] [PubMed] [Google Scholar]
- 23.LogP and PSA values were calculated by ACD/Labs 8.0. Advanced Chemistry Development Inc.; Toronto, Canada: [Google Scholar]
- 24.Loescher W, Potscha H. NeuroRx. 2005;2:86. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer RP, Gelhaus M, Knoth R, Volk B. Curr Drug Metab. 2007;8:297. doi: 10.2174/138920007780655478. [DOI] [PubMed] [Google Scholar]
- 26.Bermudez-Silva FJ, Sanchez-Vera I, Suárez J, Serrano A, Fuentes E, Juan-Pico P, Nadal A, Rodríguez de Fonseca F. Eur J Pharmacol. 2007;565:207. doi: 10.1016/j.ejphar.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 27.Lotersztajn S, Texeira-Clerc F, Julien B, Deveaux V, Ichigotani Y, Manin S, Tran-Van-Nhieu J, Karsak M, Zimmer A, Mallat A. Br J Pharmacol. 2008;153:286. doi: 10.1038/sj.bjp.0707511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.