Abstract
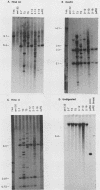
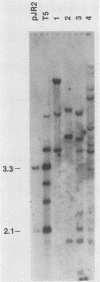
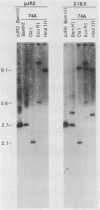
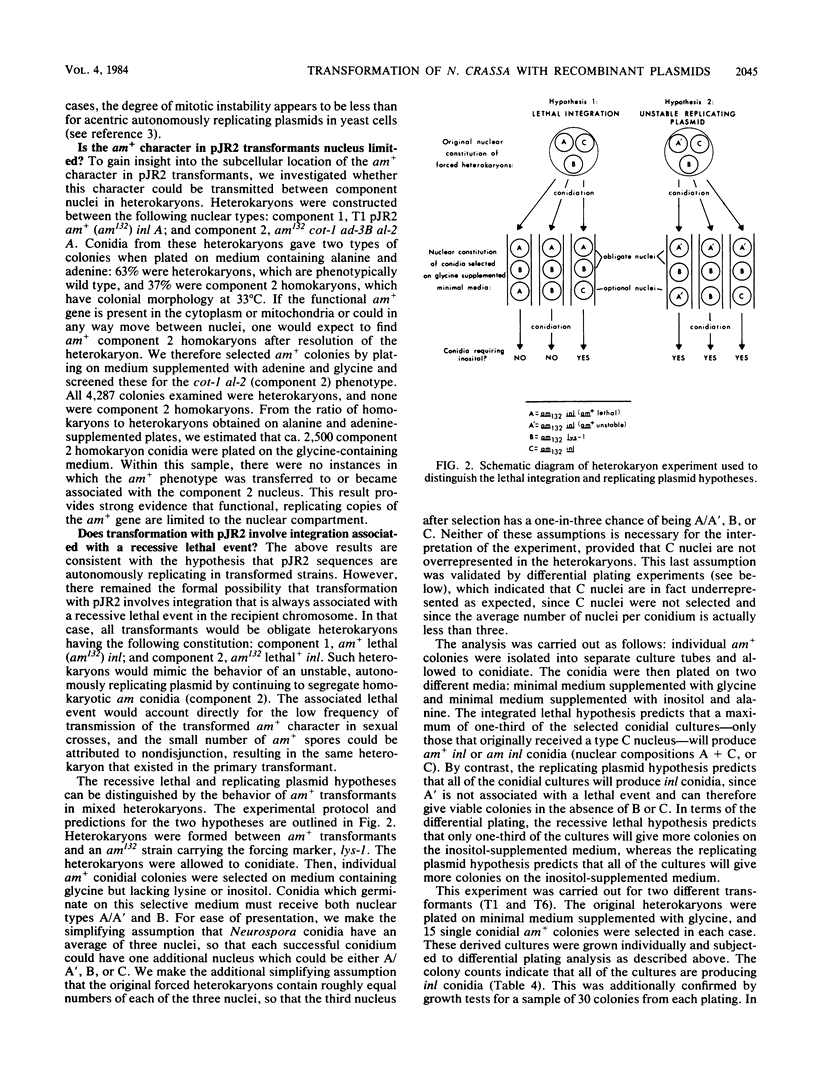
We have characterized Neurospora crassa transformants obtained with plasmid pJR2, which consists of the Neurospora glutamate dehydrogenase (am) gene cloned in pUC8 and an am132 host strain which contains a deletion encompassing the cloned fragment. Every one of 33 transformants tested showed extreme meiotic instability: less than 1 or 2% am+ progeny were obtained in initial or successive backcrosses between am+ transformants and am132 or in intercrosses between am+ progeny. Furthermore, am+ progeny from backcrosses gave a high proportion of auxotrophic (am) mitotic segregants during vegetative growth. These results indicate that the am+ character is not stably integrated into chromosomal DNA in any of the transformants tested. Nuclear DNAs from six transformants were analyzed by Southern hybridization. All six transformants contained sequences homologous to pJR2. Four showed restriction fragments expected for unmodified pJR2, but most showed additional bands. Southern blots of undigested DNAs showed that the plasmid sequences are present predominantly in high-molecular-weight form (larger than 20 kilobases). Southern blots showed that auxotrophic (am) progeny from a backcross to am132 had lost restriction bands corresponding to free plasmid but retained additional bands, apparently integrated into chromosomal DNA in a nonfunctional manner. Considered together, these results are most reasonably interpreted as follows: recombinant plasmids containing the am+ gene can replicate autonomously in N. crassa, the free plasmids are present in oligomeric or modified form or both, and plasmid sequences also integrate at multiple sites in the deletion host but in a nonfunctional manner. An alternate interpretation--that tandem repeats of the plasmid are integrated into chromosomal DNA but eliminated during meiosis--cannot be completely excluded. However, stable integration of the am gene can be obtained under a variety of other conditions, viz., using the am gene cloned in a phage lambda vector (J. A. Kinsey and J. A. Rambosek, Mol. Cell. Biol. 4:117-122, 1984), using derivatives of pJR2, or using pJR2 to transform a frameshift mutant.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Buxton F., Patel V., Giles N. H., Vapnek D. 5'-Untranslated sequences of two structural genes in the qa gene cluster of Neurospora crassa. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1955–1959. doi: 10.1073/pnas.79.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballance D. J., Buxton F. P., Turner G. Transformation of Aspergillus nidulans by the orotidine-5'-phosphate decarboxylase gene of Neurospora crassa. Biochem Biophys Res Commun. 1983 Apr 15;112(1):284–289. doi: 10.1016/0006-291x(83)91828-4. [DOI] [PubMed] [Google Scholar]
- Case M. E., Schweizer M., Kushner S. R., Giles N. H. Efficient transformation of Neurospora crassa by utilizing hybrid plasmid DNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5259–5263. doi: 10.1073/pnas.76.10.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case M. E. Transformation of Neurospora crassa utilizing recombinant plasmid DNA. Basic Life Sci. 1982;19:87–100. doi: 10.1007/978-1-4684-4142-0_10. [DOI] [PubMed] [Google Scholar]
- Coddington A., Fincham J. R., Sundaram T. K. Multiple active varieties of Neurospora glutamate dehydrogenase formed by hybridization between two inactive mutant proteins in vivo and in vitro. J Mol Biol. 1966 Jun;17(2):503–512. doi: 10.1016/s0022-2836(66)80160-2. [DOI] [PubMed] [Google Scholar]
- Collins R. A., Stohl L. L., Cole M. D., Lambowitz A. M. Characterization of a novel plasmid DNA found in mitochondria of N. crassa. Cell. 1981 May;24(2):443–452. doi: 10.1016/0092-8674(81)90335-4. [DOI] [PubMed] [Google Scholar]
- Geever R. F., Case M. E., Tyler B. M., Buxton F., Giles N. H. Point mutations and DNA rearrangements 5' to the inducible qa-2 gene of Neurospora allow activator protein-independent transcription. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7298–7302. doi: 10.1073/pnas.80.23.7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautala J. A., Conner B. H., Jacobson J. W., Patel G. L., Giles N. H. Isolation and characterization of nuclei from Neurospora crassa. J Bacteriol. 1977 May;130(2):704–713. doi: 10.1128/jb.130.2.704-713.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- James A. A., Morrison P. T., Kolodner R. Isolation of genetic elements that increase frequencies of plasmid recombinants. Nature. 1983 May 19;303(5914):256–259. doi: 10.1038/303256a0. [DOI] [PubMed] [Google Scholar]
- Khoury G., Gruss P. Enhancer elements. Cell. 1983 Jun;33(2):313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Kinnaird J. H., Fincham J. R. The complete nucleotide sequence of the Neurospora crassa am (NADP-specific glutamate dehydrogenase) gene. Gene. 1983 Dec;26(2-3):253–260. doi: 10.1016/0378-1119(83)90195-6. [DOI] [PubMed] [Google Scholar]
- Kinnaird J. H., Keighren M. A., Kinsey J. A., Eaton M., Fincham J. R. Cloning of the am (glutamate dehydrogenase) gene of Neurospora crassa through the use of a synthetic DNA probe. Gene. 1982 Dec;20(3):387–396. doi: 10.1016/0378-1119(82)90207-4. [DOI] [PubMed] [Google Scholar]
- Kinsey J. A., Hung B. S. Mutation at the am locus of Neurospora crassa. Genetics. 1981 Nov-Dec;99(3-4):405–414. doi: 10.1093/genetics/99.3-4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey J. A., Rambosek J. A. Transformation of Neurospora crassa with the cloned am (glutamate dehydrogenase) gene. Mol Cell Biol. 1984 Jan;4(1):117–122. doi: 10.1128/mcb.4.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGILVERY R. W., MOKRASCH L. C. Purification and properties of fructose-1, 6-diphosphatase. J Biol Chem. 1956 Aug;221(2):909–917. [PubMed] [Google Scholar]
- Mishra N. C., Tatum E. L. Non-Mendelian inheritance of DNA-induced inositol independence in Neurospora. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3875–3879. doi: 10.1073/pnas.70.12.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargang F. E., Bell J. B., Stohl L. L., Lambowitz A. M. A family of repetitive palindromic sequences found in Neurospora mitochondrial DNA is also found in a mitochondrial plasmid DNA. J Biol Chem. 1983 Apr 10;258(7):4257–4260. [PubMed] [Google Scholar]
- Perkins D. D., Radford A., Newmeyer D., Björkman M. Chromosomal loci of Neurospora crassa. Microbiol Rev. 1982 Dec;46(4):426–570. doi: 10.1128/mr.46.4.426-570.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambosek J. A., Kinsey J. A. Fine Structure Mapping of the am (Gdh) Locus of Neurospora. Genetics. 1983 Oct;105(2):293–307. doi: 10.1093/genetics/105.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque A., Mizusawa H., Seidman M. M. Rearrangement and mutagenesis of a shuttle vector plasmid after passage in mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3010–3014. doi: 10.1073/pnas.80.10.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sakaguchi J., Yamamoto M. Cloned ural locus of Schizosaccharomyces pombe propagates autonomously in this yeast assuming a polymeric form. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7819–7823. doi: 10.1073/pnas.79.24.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechtman M. G., Yanofsky C. Structure of the trifunctional trp-1 gene from Neurospora crassa and its aberrant expression in Escherichia coli. J Mol Appl Genet. 1983;2(1):83–99. [PubMed] [Google Scholar]
- Schweizer M., Case M. E., Dykstra C. C., Giles N. H., Kushner S. R. Identification and characterization of recombinant plasmids carrying the complete qa gene cluster from Neurospora crassa including the qa-1+ regulatory gene. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5086–5090. doi: 10.1073/pnas.78.8.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stohl L. L., Collins R. A., Cole M. D., Lambowitz A. M. Characterization of two new plasmid DNAs found in mitochondria of wild-type Neurospora intermedia strains. Nucleic Acids Res. 1982 Mar 11;10(5):1439–1458. doi: 10.1093/nar/10.5.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl L. L., Lambowitz A. M. A colony filter-hybridization procedure for the filamentous fungus Neurospora crassa. Anal Biochem. 1983 Oct 1;134(1):82–85. doi: 10.1016/0003-2697(83)90266-x. [DOI] [PubMed] [Google Scholar]
- Stohl L. L., Lambowitz A. M. Construction of a shuttle vector for the filamentous fungus Neurospora crassa. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1058–1062. doi: 10.1073/pnas.80.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland W N, Perkins D D, Veatch C C. Linkage Data for Group V Markers in Neurospora. Genetics. 1959 Nov;44(6):1221–1226. doi: 10.1093/genetics/44.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó G., Schablik M., Fekete Z., Zsindely A. A comparative study of DNA-induced transformants and spontaneous revertants of inositolless Neurospora crassa. Acta Biol Acad Sci Hung. 1978;29(4):375–384. [PubMed] [Google Scholar]
- Szostak J. W., Wu R. Insertion of a genetic marker into the ribosomal DNA of yeast. Plasmid. 1979 Oct;2(4):536–554. doi: 10.1016/0147-619x(79)90053-2. [DOI] [PubMed] [Google Scholar]
- Tilburn J., Scazzocchio C., Taylor G. G., Zabicky-Zissman J. H., Lockington R. A., Davies R. W. Transformation by integration in Aspergillus nidulans. Gene. 1983 Dec;26(2-3):205–221. doi: 10.1016/0378-1119(83)90191-9. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Hautala J. A., Jacobson J. W., Giles N. H., Kushner S. R. Expression in Escherichia coli K-12 of the structural gene for catabolic dehydroquinase of Neurospora crassa. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3508–3512. doi: 10.1073/pnas.74.8.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]