Abstract
Aging leads to numerous transitions in brain physiology including synaptic dysfunction and disturbances in cognition and memory. With a few clinically relevant drugs, a substantial portion of aging population at risk for age-related neurodegenerative disorders require nutritional intervention. Dietary intake of polyphenols is known to attenuate oxidative stress and reduce the risk for related neurodegenerative diseases such as Alzheimer's disease (AD), stroke, multiple sclerosis (MS), Parkinson's disease (PD), and Huntington's disease (HD). Polyphenols exhibit strong potential to address the etiology of neurological disorders as they attenuate their complex physiology by modulating several therapeutic targets at once. Firstly, we review the advances in the therapeutic role of polyphenols in cell and animal models of AD, PD, MS, and HD and activation of drug targets for controlling pathological manifestations. Secondly, we present principle pathways in which polyphenol intake translates into therapeutic outcomes. In particular, signaling pathways like PPAR, Nrf2, STAT, HIF, and MAPK along with modulation of immune response by polyphenols are discussed. Although current polyphenol researches have limited impact on clinical practice, they have strong evidence and testable hypothesis to contribute clinical advances and drug discovery towards age-related neurological disorders.
1. Introduction
Neurodegenerative disorders such as Alzheimer's disease (AD), stroke, and Parkinson's disease (PD) represent a major clinical problem in the developed countries [1, 2] and are major economic burdens for health care systems [3]. Dietary [4], genetic, and molecular factors [5] are important determinants in progression and intervention of neurodegenerative diseases. AD is a common cause of dementia and mortality in the United States. Total numbers of reported deaths due to AD have increased in past years, and it is among 10 leading causes of deaths in the United States [6]. Amyloid-β (Aβ) peptides derived from amyloid precursor protein (APP) via γ-secretase and β-secretase cleavage are hallmarks of AD [7]. Cellular prion protein (PrP(C)) [8] and oxidative stress [9] mediate Aβ neurotoxicity, and the latter contributes to neuronal death by lowering intracellular glutathione. Along with Aβ, tau protein alteration in neuronal microtubules also contributes to the pathology of AD [10]. Abnormal phosphorylation and aggregation of tau protein leads to neural dysfunction and leads to pathological events which cause neuronal dysfunction in AD [11]. Failed clearance of Aβ aggregates resulting from impaired autophagy may also contribute to AD [12]. AD is also characterized by elevated peripheral blood cytokine concentrations for interleukin- (IL-) 6, tumor necrosis factor alpha (TNF-α), IL-1β, transforming growth factor beta (TGF-β), IL-12, and IL-18 suggestive of a pro-inflammatory response in AD pathology [13].
Multiple sclerosis (MS) is another neurodegenerative disease characterized by chronic inflammation accompanied by demyelination of neurons in brain [14]. MS is characterized by symptoms like mood disorder, fatigue, vision changes, muscle weakness, and motor changes [15]. Chemokines like IL-17, chemokine (C-C motif) ligand 17 (CCL17), and CCL20 are suggested as major mediators in MS neuroinflammation and pathology [16].
Stroke is the third leading cause of mortality, loss of cognitive functions, and heavy socioeconomic burden in the United States [17]. Similar to MS, stroke or cerebral ischemia is a pathological condition accompanied by inflammation and immune system disease [18]. One minute of cerebral ischemia is estimated to destroy approximately 2 million neurons and 14 million synapses [19]. Like AD and MS, inflammatory cytokines including TNF-α and IL-1 and IL-6 play modulatory role in stroke pathology [20]. Transcription factor, nuclear factor kappa B (NFκB), is an important regulator in pathology of inflammation and neuronal cell survival, as its activation leads to cell death in cerebral ischemia [21].
PD is a progressive neurodegenerative disease; its familial forms are characterized by mutations of six genes including clinically important ATP13A3 resulting in cognitive impairment and depression [22]. Some PD cases also involve changes in micro-RNA and α-synuclein level in patients [23]. Like other neurodegenerative diseases, PD also involves elevated levels of proinflammatory cytokines monocyte chemoattractant protein-1 (MCP-1), CCL-5, macrophage inflammatory protein-1α (MIP-1α), IL-8, interferon-gamma (IFNγ), IL-1β, and TNFα [24]. After examining cytokines in 52 PD patients, researchers [25] suggested involvement of TNF-α in production and maintenance of nonmotor symptoms. Mitochondrial dysfunction also plays an important role in pathogenesis of PD [26] similar to AD [27], MS [28], and stroke (CI) [29].
Huntington's disease (HD) is another neurological disorder causing cognitive impairment, accompanied by oxidative stress and mitochondrial dysfunction. It results from increased number of CAG triplet nucleotide repeats and expanded polyglutamine region of huntingtin protein [30]. HD pathology leads to elevated levels of chemokines like eotaxin-3, MIP-1β, eotaxin, MCP-1, and MCP-4 [31].
The pathophysiology of neurological disorders is also accompanied by alterations in electrical activity of neurons at cellular level. The voltage gated ion channels are required for action potential generation and its propagation in neurons, and their dysfunction contributes to pathology of neurodegenerative diseases. The brain electrical activity is significantly changed in AD and dementia leading to impaired verbal memory and cognitive skills [32]. The Kv3 subfamily of K+ channel subunits, which possess ability of fast repolarization of action potential [33], are compromised and slowed down in AD [34]. The upregulation of the K(v)1.3 potassium channel also plays important role in immunopathogenesis of multiple sclerosis and presents therapeutic option by blocking Kv channels [35]. As sodium channels (Nav1.8, Nav1.5) play an important role in electrical activity of neurons, their overloading is thus an important mediator in axonal degeneration in MS [36, 37]. Such electric disturbances are also found in PD [38] which impose energy burden by Ca2+ entry through L type voltage-dependent channels [39]. Similarly, sodium and potassium channel abnormalities are proposed to contribute to HD pathogenesis [40, 41].
There are a few clinically relevant medicines and therapies available for AD, HD, MS, PD, and stroke. A few clinically active, yet expensive, options such as acetylcholinesterase inhibitors, interferon β-1a, levodopa, tetrabenazine, and tissue type plasminogen activator (tPA) are available for AD [42], MS [43], PD [44], HD [45], and stroke [46], respectively. In wake of these pathologies and limited clinical treatments, alternative and preventive therapeutics are required which can control the occurrence and progression of neurodegenerative diseases. All the neurodegenerative diseases discussed above have the common features of pathogenesis which include cytokine changes, genetic alterations, immunomodulation, inflammation, mitochondrial dysfunction, oxidative stress, prions, and protein dysfunction. Recent research has shown that dietary polyphenols target the pathological manifestations of neurological disorders with their ability to cross blood-brain barrier [47] as they control neuronal disease pathogenesis at a molecular and symptomatic level by targeting these common features of neurodegeneration pathology. Polyphenols are naturally occurring phytochemicals found in fruits and vegetables, exhibiting strong neuroprotective properties [48]. Important dietary sources of polyphenols include apples, berries, cocoa, herbs, red wines, seeds, onions, and tea [49]. Dietary polyphenols have also been implicated in prevention of oxidative damage and LDL oxidation [50–52]. This review briefly outlines the pharmacological role of polyphenols in preventing neurodegenerative diseases based on the most recent scientific literature (Figure 1).
Figure 1.
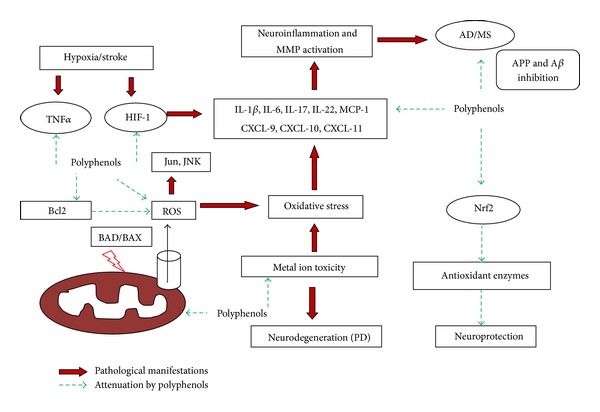
Neuroprotection by polyphenols against neurological disorders.
2. Polyphenols and Pharmacological Properties
2.1. Alzheimer's Disease and Dementia
Polyphenols exhibit neuroprotective properties including therapeutic action in AD and dementia. Green and white tea extracts have been shown to inhibit acetylcholinesterase which indicates their potential in treatment of age-related disorders such as AD [53]. Green tea polyphenols protect primary rat cortical neurons against Aβ-induced cytotoxicity [54]. In mouse model studies [55], polyphenols of grapes improved cognitive functions in mouse model of AD. As well, epicatechin metabolite 3′-O-methyl-epicatechin-5-O-β-glucuronide had improved synaptic transmission through cyclic adenosine monophosphate (cAMP) response element binding protein. In transgenic mice model studies, grape seed polymeric polyphenol extract has been shown to inhibit oligomerization of Aβ peptides and contributed to reduction in cognitive impairments in transgenic mice [56]. A similar study showed that polyphenols of grapes exhibited potential in neutralizing abnormal folding of tau proteins [57]. Earlier studies using animal models [58, 59] also confirm anti-Aβ action of grape seed polyphenols.
Resveratrol, a polyphenol abundant in grapes and red wines, inhibited Aβ 42 fibril formation [60] and protected from Aβ neurotoxicity by inhibiting inducible nitric oxide synthase inhibition [61]. Resveratrol, with possibly high bioavailability in lipid core nanocapsules, exhibited therapeutic action in AD [62]. Flavonoid fisetin and its analogues also inhibited Aβ fibril formation and have emerged as new drug candidates for AD treatment [63]. Morin (2′,3,4′,5,7-pentahydroxyflavone) has shown to prevent neuronal cell death by protecting neurons against tau hyperphosphorylation induced by Aβ [64]. Similarly, in transgenic mouse model studies [65], tannic acid has displayed the attenuation of Aβ deposition by decreasing cleavage of β-carboxyl terminal amyloid precursor protein (APP) fragment and controlled neural inflammation. A flavonoid, 7,8-dihydroxyflavone, has been shown to improve cognitive abilities in 5XFAD transgenic mouse model of AD by activation of tyrosine receptor kinase B leading to reduction in β-secretase enzyme levels and amyloid beta (Aβ) synthesis [66]. Similarly, liquiritigenin improved memory in Tg2576 mice model of AD, as it attenuated astrocytosis and decreased the Notch-2 expression as the latter can contribute to neuronal decay [67]. Unlike resveratrol, quercetin and rutin not only inhibited Aβ formation but also disaggregated Aβ fibrils in AD studies [68]. Both compounds also prevented scopolamine-induced amnesia in animal model systems [69]; however, resveratrol did not reverse scopolamine-induced deficit [70]. Rutin has been found to control oxidative stress, malondialdehyde, and glutathione disulfide formation in SH-SY5Y neuroblastoma cells. Rutin has also attenuated the inflammatory cascade by decreasing cytokines like TNF-α and IL-1β [71]. Ferulic acid, a phenolic acid, has also exhibited higher neuroprotection against Aβ toxicity than quercetin [72]. Recent research findings have shown that polyphenols have therapeutic relevance in both cell and animal model studies. The ability of polyphenols to improve synaptic transmission by elevating cAMP, target multiple signaling pathways, and reduce Aβ toxicity suggests their therapeutic utility for age-related disorders like AD and dementia.
2.2. Multiple Sclerosis
Multiple sclerosis is a neurodegenerative disease characterized by autoimmune-mediated demyelination in the CNS resulting in paralysis and cognitive deficits. MS therapies can reduce inflammation and downregulate immune function [73]. Resveratrol, a silent mating type information regulation 2 homolog1 (SIRT1) activator, has exhibited prevention of neural loss without immunosuppression in experimental autoimmune encephalomyelitis (EAE) model of MS [74]. Pharmaceutical grade formulation of resveratrol SRT501 was found to attenuate neural damage in EAE through SIRT1 activation [75]. Cell culture studies [76] have also shown SIRT1-mediated neuroprotection by resveratrol. Quercetin was found to control immune response via modulation of IL-1β and TNF-α and reduced the proliferation of peripheral blood mononuclear cells isolated from multiple sclerosis patients [77]. Epigallocatechin-3-gallate (EGCG) exhibited neuroprotective effects by modulating neuroinflammation and attenuating neural damage [78]. Quercetin [79], apple polyphenols [80], myricetin, and piceatannol [81] have also activated SIRT1, thus exhibiting potential in MS treatment. Earlier studies have also shown [82] that flavonoids limit demyelination in MS suggesting their potential against neuro-inflammation and related disorders. Preclinical data has shown that polyphenols exhibit potential to block neural inflammation and damage by activation of SIRT1 pathway along with modulation of inflammatory cytokines. The potential of polyphenols on limiting demyelination makes them prospective therapeutics in age-related MS and amyotrophic lateral sclerosis (ALS).
2.3. Ischemic Stroke
Various epidemiological studies suggest that diet rich in polyphenols can extend neuroprotection and lower the risk and severity of stroke, the third leading cause of mortality [83]. Experimental evidence using rodent and cellular models also indicates neuroprotective potential of dietary polyphenols in cerebral ischemia. Green tea polyphenol, EGCG, has exhibited neuroprotective action by downregulation of matrix metalloproteinases (MMP) in mice model of cerebral ischemia [84]. Green tea polyphenols have also been found to protect neurons against hypoxia-induced ischemic injury by controlling inflammation cascade and attenuating decline in transmembrane potential [85]. Quercetin has been found to attenuate ischemic injury by controlling acid-sensing ion channel led calcium dysregulation and lipid peroxidation in neurons [86]. Another study with similar experimentation has supported neuroprotective role of quercetin, based on its ability to block sodium channels [87]. Quercetin with similar antioxidant therapy to green tea polyphenols has reduced the level of MMP-9 and attenuated blood-brain barrier disruption in cerebral ischemia (CI) studies [88]. Researchers have also hypothesized neuroprotective action of quercetin in CI to be based on its inhibitory action against MMP [89]. Rutin has been found to control neural damage in CI through downregulation of p53, a protein which leads to necrosis in stroke [90]. It has also shown the attenuation of glutathione peroxidase, glutathione reductase, and inflammatory cytokines in rodent model of ischemic stroke [91]. In addition, resveratrol has been found to extend protection against ischemic injury by improving brain energy metabolism and controlling oxidative stress during ischemia injury in animal model studies [92], along with the modulation of release of multiple therapeutic neurotransmitters and neuromodulators during ischemic injury [93]. The flavonoid fisetin has shown neuroprotective action during cerebral ischemia as it stopped infiltration of macrophages and dendritic cells into ischemic hemisphere, thus controlling neural inflammation and damage [94]. Another flavonoid baicalin has been shown to reduce ischemic stroke damage by targeting multiple therapeutic targets like MMP-9 [95], caspase-3, oxidative stress [96], and p38 mitogen-activated protein kinase (MAPK) [97] and by downregulating toll-like receptor (TLR2/4) pathway [98]. The experimental data reveals that polyphenols may prevent, attenuate, or slow down, via multiple mechanisms, the course of stroke and age-related neural disorders. Since the risk for stroke increases with age, consumption of polyphenol rich diet seems to be an important preventive strategy.
2.4. Parkinson's Disease (PD)
PD is a neurodegenerative disease accompanied by inflammation and oxidative stress resulting in loss of dopaminergic neurons in the substantia nigra [137]. Polyphenols with their ability to attenuate oxidative stress and inflammation present therapeutic option in neurodegenerative disease. Resveratrol has been shown to inhibit the loss of dopaminergic neurons in rat model of PD [76]. Resveratrol has also been shown to reduce neural inflammation in PD by lowering mRNA levels of cyclooxygenase-2 (COX-2) and TNF-α mRNA in the substantia nigra [100] along with attenuation of oxidative stress, lipid peroxidation, and protein carbonyl (PC) in rat model of PD [138]. Oxyresveratrol has demonstrated attenuation of neural damage in SH-SY5Y cells by elevating levels of SIRT1 and downregulating expression of caspase-3, c-Jun N-terminal kinase (JNK), and c-Jun transcription factors [139]. Ferulic acid, like oxyresveratrol, has demonstrated neuroprotective effect via downregulation of JNK pathway [140]. Quercetin administration to neurons attenuated 1-methyl-4-phenylpyridinium (MMP) evoked microglia activation, which is a precursor for PD pathogenesis [124]. Studies have also shown that quercetin promises neuroprotection in PD mice model by stimulating glutathione peroxidase (GPx), superoxide dismutase (SOD), Na(+), and K(+)-ATPase [141]. Quercetin suppressed cell death in PD cell model while its metabolite quercetin-3-O-β-glucuronide, due to its low absorption, did not affect cell viability [142]. Another study showed that conversion of quercetin metabolites to its aglycone in neural cells is essential for neuroprotective activity [143]. These studies had shown consistent results as compared to a contradictory report [144] which showed that quercetin had no neuroprotective role in PD cells and rat models. Other polyphenols such as baicalein [145], kaempferol [146], caffeic acid [147], and EGCG [148] have been shown to extend neuroprotection in PD studies. Similarly, polyphenolic extracts from various plants have also exhibited pharmacological role in PD studies. For instance, polyphenols-rich mulberry fruit extracts have shown antioxidant and antiapoptotic effect in SH-SY5Y cells by modulating caspase-3, B-cell lymphoma (Bcl-2), and BCL2-associated X protein (Bax) [149].
2.5. Huntington's Disease
CAG triplet nucleotide repeats and expanded polyglutamine region of huntingtin protein form basis of HD [30]. Polyphenols hold pharmacological relevance, as they are associated with numerous benefits including antiaging, anti-inflammatory, and anticancer effects. Resveratrol has been found to exhibit positive effects in transgenic mouse model of HD via SIRT1 activation of peroxisome proliferative activated receptor, gamma, and coactivator 1 alpha (PGC-1α) signaling pathway [76]. Studies have further demonstrated the Ras-extracellular signal-regulated kinase activation by resveratrol and fisetin as the basis for neuroprotection in models of HD [150]. Likewise, hesperidin and naringenin, abundant in citrus fruits, induced neuroprotection in rats possibly via nitric oxide synthase (NOS) inhibition [151]. Curcumin has been shown to control Huntington aggregates and improve various transgene-dependent parameters, thereby promising therapeutic action in HD [152]. Grape and green tea polyphenols have also exhibited potential for treating/preventing HD disease pathogenesis [153, 154]. The overall preclinical data suggests that polyphenols extend strong neuroprotection through genetic and immunological modulation, thus promising clinical prevention or delay of neurological disorders like PD and HD.
3. Polyphenols and Oxidative Stress
A large body of literature supports the antioxidant potential of polyphenols against oxidative stress. Resveratrol is a potent antioxidant in vitro [155] and in vivo as it attenuates oxidative stress in both animal [156] and various cell model studies [157]. Resveratrol has been shown to extend antioxidant effect by reducing the production of reactive oxygen species (ROS) and superoxide ions [158]. Similarly, quercetin has also shown protection against oxidative stress and related disorders [159]. In a variety of cell and disease models, quercetin has been shown to engage in various signaling pathways to attenuate oxidative stress and exhibit pharmacological properties [51, 114]. Polyphenol-rich green tea and its principal constituent EGCG were found to ameliorate oxidative stress in various studies [160, 161]. Other polyphenols such as puerarin [162], baicalin [163], and phloridzin [136] also attenuated oxidative stress in various disease models. Apart from in vitro and in vivo evidence, sufficient clinical evidence also suggests the antioxidant potential of polyphenols. A clinical study [164] showed that polyphenol-rich diet reduced LDL oxidation and modulated cluster of differentiation 40-ligand (CD40L) gene expression, thus controlling atherogenesis and inflammation in humans. Polyphenol-rich fruit extracts have been shown to control free radicals and ROS. Polyphenol-rich bilberry juice was found to decrease oxidative stress and inflammatory markers in humans [165]. A 13-year long clinical study indicated that higher intake of antioxidant polyphenols including flavonoids and phenolic acids helps in improving memory and has potential for inhibiting brain aging [166]. Antioxidant-rich polyphenol supplementation as beverage has also been found to decrease plasma total homocysteine, thus contributing to attenuation of AD pathology [167]. It can be concluded that polyphenols are strong antioxidants in vitro and in vivo in both animal models and humans. Clinical translation of polyphenols as antioxidant therapy is a promising approach to attenuate oxidative damage due to aging and age-related disorders.
4. Polyphenols and Signal Transduction Pathways
4.1. Akt/P13K/mTOR Pathway
Resveratrol has exhibited neuroprotection against brain ischemia through P13K/Akt pathway by downregulating the expression of glycogen synthase kinase 3 (GSK-3β) and cAMP response element binding (CREB) proteins [99]. Resveratrol increased cAMP and modulated Akt pathway in cell model studies [144]. Baicalein also protects against ischemia through P13K/Akt pathway [126]. The scientific evidence suggests therapeutic intervention by polyphenols via P13K/Akt pathway (Table 1).
Table 1.
Neuroprotective signal transduction by polyphenols.
| Pathway | Polyphenol | References |
|---|---|---|
| P13K/AkT pathway | Resveratrol | [99, 100] |
| Baicalein | [97] | |
| NFκB pathway | Kaempferol, acacetin, apigenin, luteolin | [101] |
| Soybean isoflavones | [102] | |
| Fisetin | [94] | |
| Resveratrol | [103] | |
| Baicalein | [104] | |
| Silymarin | [105] | |
| Tetrahydroxystilbene | [58] | |
| Quercetin | [101, 106] | |
| Catechin hydrate | [94] | |
| STAT pathway | Silymarin | [105] |
| PPAR pathway | Baicalein | [104] |
| Resveratrol | [107] | |
| Pterostilbene | [108] | |
| Nrf2/HO1/ARE pathway | Epicatechin | [109] |
| Resveratrol | [110] | |
| HIF-1α | Xanthohumol | [111] |
| Resveratrol | [112] | |
| MAPK | Flavone glycoside | [113] |
| Quercetin | [114] |
4.2. NFκB Pathway
NFκB is an important mediator in inflammatory process and contributes to Aβ toxicity. Flavonoids and other dietary polyphenols have shown neuroprotective effects in neuronal ischemia through NFκB pathway. Flavonoids, including kaempferol, quercetin, acacetin, apigenin, and luteolin, inhibit Aβ1-40 and Aβ1-42 via NFκB pathway downregulation [101]. Similarly, soybean isoflavone had reversed memory impairment in rats through decrease in NFκB expression [102]. Other flavonoids such as resveratrol and baicalin inhibit Aβ-induced neural inflammation via a mechanism involving downregulation of NFκB signaling pathway [103, 168]. Activation of NFκB is an important event in ischemic injury and contributes to both inflammation and cell death [21]. Silymarin, a flavonolignan from milk thistle (Silybum marianum), has protected against cerebral ischemia by inhibiting signal transducer and activating transcription (STAT-1) pathway and NFκB [105]. Tetrahydroxystilbene glucoside from Polygonum multiforum has protected neurons from cerebral ischemia by activating SIRT1 and inhibiting NFκB signaling pathway in neurons [58]. Quercetin administration has also protected rat brain against oxidative stress and hypoxia-induced damage through NFκB inhibition [106]. Similarly, catechin hydrate and fisetin have been shown to protect rat brain against ischemic injury and oxidative damage by inhibiting expression of NFκB and proinflammatory cytokines such as IL-1β and TNF-α [83, 94]. It is observed that recent research advances confirm an immunomodulatory role of polyphenols as they control inflammatory response by inhibiting NFκB expression.
4.3. PPAR Pathway
Peroxisome proliferator activated receptor gamma (PPAR gamma) plays a role of a biomarker in cerebral ischemia as CI results in its upregulation and translocation to nucleus from the cytosol. Baicalein has reversed PPAR gamma expression and also suppresses its translocation to nucleus [104]. Resveratrol has attenuated the MMP-9 by modulating the peroxisome proliferator activated receptor (PPAR) alpha expression in hypoxia model of neurons [107]. Pterostilbene, a resveratrol derivative, has significant effect on the downregulation and normalization of PPAR-α expression in SAMP8 mouse model studies [108]. The scientific evidence shows that benefits associated with polyphenols in age-related and other disorders are associated with downregulation of PPAR pathway.
4.4. Nrf2/ARE/HO1 and HIF-1 Pathway
Nuclear factor (erythroid-derived 2-) like 2 (Nrf2) pathway is also involved in Sestrin2 (Sesn2), also known as Hi95, a p53 target gene expression which leads to encoding of antioxidant proteins [169]. Epicatechin has protected neurons against stroke and oxidative stress by upregulation of Nrf2 cascade and heme oxygenase-1 (HO1) enzyme [109]. Similarly, resveratrol has also been shown to protecte brain through increased expression of Nrf2, HO-1 expression, and downregulation of apoptotic enzymes like caspase-3 [110]. Xanthohumol, a prenylated chalcone, has demonstrated neuroprotective action by inhibiting HIF-1 pathway and it further stopped signal transduction pathway leading to apoptosis by caspases [111]. Resveratrol, apart from Nrf2 and HO-1 expression, has protected against ischemic injury in cells by downregulation of mRNA expression of hypoxia inducible factors-1α (HIF-1α) [112]. Upregulation of Nrf2 and HO-1 pathways by polyphenols in response to oxidative insult shows protective role of these compounds in brain health and oxidative damage.
5. Polyphenols and Immune Response
Proinflammatory cytokines and genes contribute to inflammation and neuronal death in various neurological disorders. Most of the therapeutics target cytokines and other immune responses for therapeutic intervention. Polyphenols are well known for their anti-inflammatory activities and thus control neuroinflammation and neural death. EGCG has been found to inhibit expression of monocyte chemotactic protein (MCP-1/CCL2) and IL-1β, thus protecting blood-brain barrier (BBB) integrity during pathological inflammation [170]. In another study, EGCG inhibited cytokine and chemokines including IL-1β, IL-6, and MCP-1 [116]. Resveratrol has also controlled hippocampal inflammation by reducing expression of MCP-1 mRNA levels [118]. Polyphenols, that is, catechin, caffeic acid, and transresveratrol, reduced production of inflammatory markers MCP-1, MIP-1α, MIP-1β, chemokine receptor-1 (CCR1), and CCR2 in vascular wall [119]. Polyphenol-rich olive oil controlled inflammation by inhibiting proinflammatory CD40, a costimulatory protein found on antigen presenting cells, gene expression [164]. EGCG reduced expression of inflammatory cytokines and chemokines such as chemokine (C-X-C motif) ligand (CXCL10), CCL22, CCL 17, and TGF-β, promising strong neuroprotection in AD and stroke [117]. Quercetin has been shown to inhibit proinflammatory cytokines like IL-1β, IL-6, COX-2, CD40, and TNFα receptor-associated factor-1 (TRAF1) [120]. A similar study [121] showed that quercetin exhibits neuroprotective effect in PC12 cells and zebrafish possibly by downregulating expression of proinflammatory genes like IL-1β and COX-2. Resveratrol also reduced neuroinflammation and improved memory along with IL-1β inhibition [115]. Resveratrol has exhibited restoration of BBB integrity and inhibited rising levels of IL-17A, T-helper 17 lymphocytes, and MMP [171]. Apple polyphenols have also reduced expression of a wide range of neuroinflammatory markers including IL-1β, IL-6, IL-17, 1L-22, CXCL9, CXCL10, CXCL11, and IFN-γ, thus providing immune-modulatory effects against inflammation [122]. Studies have also shown that blueberry and apple polyphenols can attenuate neuroinflammation and improve cognitive impairment, possibly by lowering the expression of IL-1β and TNFα in rat hippocampus [172, 173]. About 20 structurally related flavonoids have been shown to inhibit hypoxia-induced STAT3 tyrosine phosphorylation promoting cell survival [174]. Fisetin and quercetin protected neurons against LPS-induced inflammation by inhibiting TNFα production and JNK/Jun phosphorylation [120, 139]. Resveratrol administration during ischemic stroke inhibits neuronal damage along with reduced expression of IL-1β and TNFα [175]. Overall, polyphenols modulate immune response in neurodegenerative diseases as they induce expression of antiapoptotic factors, control neuroinflammation, and modulate cell signaling under stress (Table 2). These features of polyphenols make them strong neuroprotective candidates and support their translation from laboratory to clinical trials.
Table 2.
Modulation of cytokines and inflammatory targets by polyphenols.
| Polyphenol | Target | References |
|---|---|---|
| EGCG | IL-1β | [115] |
| IL-6 | [116] | |
| MCP-1 | [115] | |
| CXCL 10, | [116] | |
| CCL22, CCL 17 | [117] | |
| TGF β | [117] | |
| Resveratrol | MCP-1 | [118] |
| Catechin | MCP-1 (α and β) | [119] |
| Caffeic acid | CCR1, CCR2 | [119] |
| Quercetin | IL-1β, IL-6 | [120] |
| COX-2 | [120] | |
| COX-20, TRAF1 | [121] | |
| Apple polyphenols | IL-1β, IL-6, IL-17, IL-22 CXCL-9, CXCL-10, CXCL-11, |
[122, 123] |
6. Polyphenols and Metal Ion Chelation
Iron and copper play important roles in the generation of ROS through redox cycling and subsequent neurodegeneration. Metal accumulation in brain contributes to pathology of diseases like AD, MS, PD, and HD [176, 177]. EGCG exhibited iron chelating ability in SH-SY5Y neuroblastoma cells along with the inhibition of apoptotic factors like BCL2-associated agonist of cell death (Bad), Bax, and caspase-3 [178]. EGCG has exhibited stronger chelation of iron compared to desferrioxamine and increased transferrin receptor protein along with the elevation in mRNA levels in SH-SY5Y neuroblastoma cells [179]. Electron paramagnetic resonance studies [180] demonstrated the interaction of EGCG and gallic acid as ligands to copper coordination sphere, thus demonstrating Cu modulatory potential of these polyphenols. Also, iron modulation by curcumin in rat brain homogenate has been observed, thus warranting that curcumin-based therapy in AD and PD disease models [181]. Curcumin has extended neuroprotection in a rat model of PD against 6-hydroxydopamine treatment through its iron chelating activity and reduced degeneration of neurons [182]. Similarly, curcumin's ability to reverse neurodegeneration in hemi-Parkinson's mice model has been shown [183]. Apart from iron chelation, NFκB modulation by curcumin has also contributed to the reduction in 6-OHDA-induced neurodegeneration [184]. Rosmarinic acid, a phenolic acid found in Lamiaceae herbs, protected neurons against 6-OHDA treatment by lowering the expression of Bax/Bcl-2 at gene level and decreasing iron level in both MES23.5 dopaminergic cells and rat model of PD [185, 186]. It is evident that polyphenols are potent metal chelators and extend neuroprotection against iron- and copper-induced oxidative stress and neurotoxicity via metal chelation, modulation of signal transduction, oxidative stress, and inflammation.
7. Polyphenols and Prions
Prion proteins are involved in neurodegenerative diseases, and their conformational transition forms basis of prion diseases. The pathology of prion proteins has been inhibited by EGCG and ECG, thus exhibiting neuroprotective potential [187]. Studies have also confirmed the antiprion activity of resveratrol through autophagy activation in neuroblastoma cells [188]. Resveratrol also protected mouse neurons against PG14-PrP (mutant prion protein) expression [189]. Curcumin also downregulated the prion pathology in neuroblastoma cells [190]. Therefore, polyphenols seem to protect neurons against prion diseases by controlling prion mutation and pathology.
8. Polyphenol and Anti Acetylcholinesterase Activity
Pathology of neurodegenerative diseases including AD includes deficiency of neuromediator acetylcholine, thus making acetylcholinesterase (AChE) inhibitors as important clinically relevant drugs in AD and other dementia [191]. Black chokeberry extract, a rich source of polyphenols, in combination with lemon juice inhibited AChE [192]. The prenylated flavonols from paper mulberry (Broussonetia papyrifera) were potent inhibitors of AChE, thus exhibiting neuroprotective potential [193]. Studies have shown that a polyphenol-rich blueberry extract also inhibited AChE activity in vitro [194]. Polyphenols extracted from Paulownia tomentosa fruits exhibited inhibitory action against both AChE and butyrylcholinesterase (BChE) [195]. Quercetin was found to improve cognitive ability and exhibit neuroprotection against trimethyltin-induced neurotoxicity by inhibiting AChE [196]. A report has also shown that quercetin inhibited AChE activity and improved cognitive abilities in streptozotocin-treated mice [197]. Quercetin and macluraxanthone, from Maclura tinctoria and Dyer's mulberry, respectively, inhibited both AChE and BChE in vitro by competitive and noncompetitive inhibition, respectively [191]. Molecular docking studies [191] have indicated the hydrophobic interactions and strong hydrogen bonding of both flavonoids with enzymes as basis of their inhibitory activity. Polyphenols from Cistus laurifolius L. also exhibited cholinesterase inhibitory effects against AChE and BChE, supporting a neuroprotective role of polyphenols [198]. A herbal tea from Paulownia barbatus leaves reduced AChE activity by 40% and its principal constituent, natural polyphenol rosmarinic acid, reduces AChE activity by 25% [199]. Galangin, a flavonol isolated from rhizome of Alpiniae officinarum, has also exhibited strong AChE inhibition [200]. EGCG enhances huperzine A's (acetylcholinesterase inhibitor) effects against AChE as its supplementation leads to 88–91% inhibition [201]. Later reports have supported that EGCG supplementation with huperzine A improves cognitive abilities in AD [202]. Linarin, a flavonoid found in Linaria species, inhibited AChE activity in neuronal PC12 cells and extended potential for neuroprotection in AD and related disorders [203]. All these pieces of evidences suggest that polyphenols are potent AChE and BChE inhibitors, thus warranting neuroprotection and improved cognitive functions in AD and related dementia.
9. Polyphenols and Autophagy-Related Proteins
Flavonoids such as hesperetin and hesperidin inhibited Aβ-induced glucose metabolism impairment in neurons and downregulated Aβ stimulated autophagy, resulting in improved cognitive functions [204]. Kaempferol also protected SH-SY5Y and primary neurons from rotenone toxicity through induction of autophagy [154]. Resveratrol exhibited neuroprotective effect by activating AMPK-SIRT1 autophagy pathway in PD cell model studies [205]. Brain-related autophagy studies have a wide research gap, and polyphenols have strong potential for inducing neuroprotection via autophagy and its related pathways.
10. Polyphenols as Neuronal Mitochondria Medicine
Polyphenols from wine are known to reduce oxidative stress and increase the expression of antioxidant enzymes like catalase, superoxide dismutase, glutathione reductase, and glutathione peroxidase [131]. Resveratrol upregulates antiapoptotic Bcl-2 protein and downregulates Bax protein expression [206]. Resveratrol also acted as mitochondrial antioxidant by elevating the levels of antioxidants trioredoxin-2 (TRX2) and X-chromosome-linked inhibitor of apoptosis protein [207]. Another study has shown that resveratrol increased expression of Bcl-2, thus preventing neuronal apoptosis [100]. Similarly, resveratrol controlled oxidative stress in PC12 cells and inhibited mitochondria-mediated apoptosis by downregulating Bax and upregulating Bcl-2 [112]. Similarly, lutein has shown protection of mice against ischemic injury by enhancing the Bcl-2 levels and downregulated Cox-2 and pancreatic ER kinase (PERK) [125]. Baicalein also regulated Bcl-2 and antagonized cytochrome c release in cytosol [126]. Similarly, ferulic acid, a phenolic acid, attenuates mitochondria apoptosis by inhibiting Bax, tBid expression, and elevating Bcl-2-like proteins [124]. Important transcription factors of ERK/Nrf2 pathway like glutamate cysteine ligase catalytic (GCLC) and glutamate-cysteine ligase, modifier subunit (GCLM), are upregulated by flavones like chrysin, apigenin, and luteolin to combat oxidative stress [129]. Glutathione peroxidase (GPx) levels were modulated by red wine polyphenols resulting in combat of oxidative stress [132]. Similarly, phenolic antioxidant 3,3′,5,5′-tetra-t-butyl-biphenyl-4,4′-diol also controlled expression of GPx and HIF-1α in hypoxia studies [133]. Various polyphenols like butein, phloretin, chrysin, apigenin, and luteolin activated HO-1 (HMOX1), GCLC, and GCLM through expression of ERK/Nrf2 pathway [129, 134]. Quercetin has also downregulated inflammatory cascade by lowering the expression of JNK, c-Jun, and interferon-γ inducible protein [135]. Similarly, p-JNK and COX-2 were downregulated by polyphenols from Hibiscus sabdariffa L. providing relief from oxidative stress and pathological inflammation [128]. EGCG controlled mitochondria lead inflammation by lowering transcription of JNK and activator protein-1 (AP-1) [116]. Neuroprotection through the phosphatidylinositol 3-kinase (P13K) and MAPK has also been shown by flavone glycoside [113]. Hesperidin carsonic acid, a major rosemary polyphenol, exhibited strong anti-inflammatory action in neurons under hypoxia stress by inhibiting ROS, MAPKs, caspase-3, and COX-2 [127]. Lowering of JNK serves as mitochondrial therapy not only in stroke but also in AD as JNK activation in AD brain leads to tau hyperphosphorylation and Aβ pathogenesis [208]. Curcumin and resveratrol exhibited neuroprotection through increased activity of NAD(P)H quinone oxidoreductase (NQO1) via Nrf2 pathway in astrocytes [209]. Similarly, structurally modified isomers of resveratrol also elevated the NQO1 activity, thus promising antioxidant effects through NRf2 pathway [210]. ECG modulated endophilin-B1, also known as SH3GLB1, which is required for maintaining mitochondrial morphology and plays important role in apoptosis [211, 212]. EGCG has also increased expression of mitochondrial antioxidant enzymes including superoxide dismutase (SOD) and glutathione peroxidase (GPX1) [130]. Flavonoid-enriched fraction (AF4) isolated from the peel of “Northern Spy” apples has been shown to suppress the expression of IL-1β, TNF-α, and IL-6 in a mouse model of hypoxic-ischemic (HI) brain damage [123]. Phloridzin, also an apple polyphenol, has been shown to increase expression of SOD1 and SOD2 genes, thus protecting mitochondria against oxidative stress [80]. Polyphenols are important mitochondrial therapeutics as they play a role in mitochondrial biochemistry by modulating apoptosis, antioxidant action, signal transduction, and inflammation (Table 3).
Table 3.
Modulation of mitochondrial targets by polyphenols.
| Target | Polyphenol | Effect | References |
|---|---|---|---|
| AP-1 | EGCG | Downregulation | [116] |
| Bad/Bax | Resveratrol | Downregulation | [112] |
| Ferulic acid | Downregulation | [124] | |
| Bcl-2 | Lutein | Upregulation | [125] |
| Baicalein | Upregulation | [126] | |
| Cox-2 | Lutein | Downregulation | [125] |
| Hesperidin | Downregulation | [127] | |
| Hibiscus sabdariffa polyphenols | Downregulation | [128] | |
| GCLM | Chrysin, apigenin, luteolin | Upregulation | [129] |
| GCLC | Chrysin, apigenin, luteolin | Upregulation | [129] |
| GPX | EGCG | Upregulation | [130] |
| Red wine polyphenols | Upregulation | [131, 132] | |
| 3,3′,5,5′-tetra-t-butyl-biphenyl-4,4′-diol | Upregulation | [133] | |
| HO-1 | Butein, apigenin | Upregulation | [129] |
| Luteolin | Upregulation | [134] | |
| IFN-γ | Quercetin | Downregulation | [135] |
| JNK | Quercetin | Downregulation | [135] |
| EGCG | Downregulation | [116] | |
| Hibiscus sabdariffa polyphenols | Downregulation | [128] | |
| JUN | Quercetin | Downregulation | [135] |
| SOD | Phloridzin | Upregulation | [136] |
11. Polyphenols and Ion Channels
The neuroprotective benefits of polyphenols are often attributed to their antioxidant activity and their ability to modulate the cell signaling pathways [105, 124, 155]. Sodium channels (Nav 1.5) involved in pathology of MS were found to be blocked by red grape polyphenols like quercetin, catechin, and resveratrol in rodent and cell model studies [87, 213]. G protein-coupled inwardly rectifying potassium (KIR3) channels, involved in neuron signaling and membrane excitability [214], are activated by naringin (flavonoid glycoside), thus exhibiting potential for improving cognition in AD [215]. EGCG's neuroprotective effect was proposed to occur through inhibition of high-voltage-activated calcium currents (I HVA) and NMDA-induced inward currents (I NMDA) along with elevation of Ca2+ through PLC-IP3 pathway [216]. Similarly, curcumin exhibited modulation of a wide range of ionic channels including Ca2+-release-activated Ca2+ channels (I CRAC), voltage-gated K+ channel (I Kv), intermediate-conductance Ca2+-activated K+ channel (I SK4), and the cytoplasmic Ca2+ concentration Δ[Ca2+]C in Jurkat-T cells [217]. However, the current literature has a research gap of specific ion channel study (Kv3 subfamily of K+ channel subunits) in disease-specific conditions. Overall, the ability of polyphenols to modulate ion channels and action potential [218] complements their ability to protect neurons from disorders and thus supports the growing evidence that polyphenols may act as neuroprotectants in several neuropathological conditions.
12. Concluding Remarks
In conclusion, recent scientific evidence suggests that neurodegenerative diseases are accompanied by oxidative stress, inflammation, metal accumulation, and mitochondrial dysfunctions. Various physiological mechanisms are altered by these pathological changes which contribute to etiology of neurodegenerative diseases like stroke, MS, PD, AD, and HD. The prevention and treatment of these disorders with complex mechanisms need novel therapeutic strategies targeted for multiple genes and proteins. Polyphenols are natural plant secondary metabolites which exhibit remarkable multipotent ability to control and modulate ROS, metal toxicity, inflammation, apoptosis, signal transduction, ion channels, and neurotransmitters. Polyphenolic dietary antioxidants, particularly resveratrol, EGCG, quercetin, and other fruit polyphenols, are potent neuroprotectants (Figure 2). Their direct usage and dietary supplementation could act as antioxidant and neuroprotective therapy for treatment of these diseases. Most of experimental and epidemiological studies suggest that dietary polyphenols activate antioxidant pathways such as Nrf2/HO1 and downregulate NFκB, MMPs, PPAR, HIF-1, and STAT pathways. Polyphenols also modulate immune response by inhibiting proinflammatory biomarkers such as CCL17, CCL22, CCR1, CCR2, MIP1α, MIP 1β, CXCL (9, 10, 11), IFN-γ, TNF-α, and IL(1β, 6, 17A, 22). These salient properties of polyphenols help to reduce two hallmarks of neurodegeneration, that is, oxidative damage and inflammation.
Figure 2.
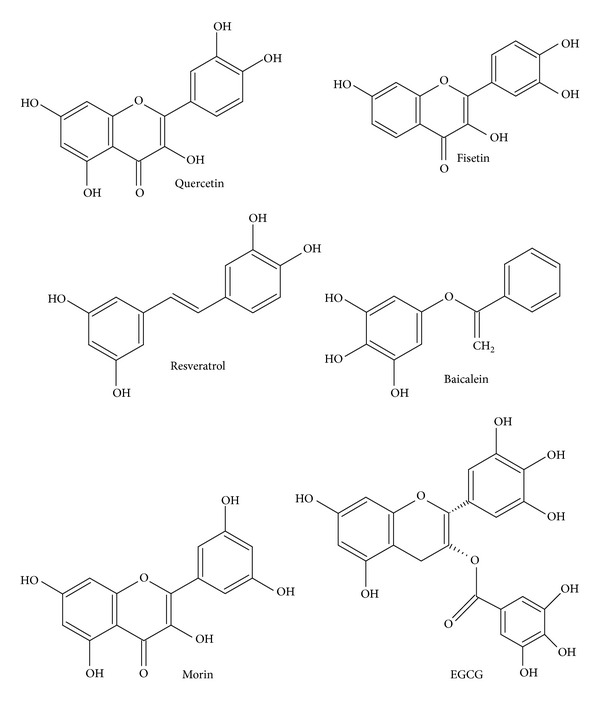
Chemical structure of polyphenols with therapeutic use in age-related neurological diseases.
Polyphenols also protect mitochondria from pathological events by triggering prosurvival cell signaling. Polyphenols increase antioxidant enzymes, that is, catalase, superoxide dismutase (SOD1, SOD2), and prosurvival Bcl-2 and PERK pathways. Downregulation of Bad/Bax, c-jun, JNK, COX-2, AP-1, and caspase-3 also contributes to the survival of neurons. Polyphenols also help in improving cognitive abilities by inhibiting AChE and BChE. The inhibition of these enzymes plays an important role in clinical medicine of AD. Apart from their anti-AChE activity, polyphenols also induce metal chelation and modulate autophagy and prion proteins. These features along with reduction of Aβ toxicity, reduction of neural lesions, and activation of cell survival genes are of particular relevance to neurodegenerative diseases. The activation of novel spectrum of these molecular targets forms underlying mechanism of neuroprotection by polyphenols. The lack of toxic effect and availability from natural sources makes polyphenols as clinically relevant therapeutics in neurodegeneration.
The future of polyphenol research needs to aim towards clinical acceptance of health claims from preclinical in vitro and animal model studies. Therefore, future studies focusing on human clinical trials of several potent polyphenols and their combinations should be carried out. Furthermore, polyphenols must be investigated for the risk assessment and safety evaluation to observe any undesirable effects. The success in clinical research of polyphenols will decide their pharmacological relevance for humans.
Abbreviations
- AAP:
Amyloid precursor protein
- AChE:
Acetylcholinesterase
- AD:
Alzheimer's disease
- AP-1:
Activator protein-1
- Aβ:
Amyloid beta
- Bad:
BCL2-associated agonist of cell death
- BAX:
BCL2-associated X protein
- BBB:
Blood-brain barrier
- BChE:
Butyrylcholinesterase
- Bcl:
B-cell lymphoma
- Bcl2:
B-cell lymphoma 2
- cAMP:
Cyclic adenosine monophosphate
- CCL:
Chemokine (C-C motif) ligand
- CCR:
Chemokine receptor
- CD:
Cluster of differentiation
- CI:
Cerebral ischemia
- COX:
Cyclooxygenase
- CREB:
cAMP response element binding protein
- CXCL:
Chemokine (C-X-C motif) ligand
- EAE:
Experimental autoimmune encephalomyelitis
- ECG:
Epicatechin gallate
- EGCG:
Epigallocatechin gallate
- GCLC:
Glutamate cysteine ligase catalytic
- GCLM:
Glutamate-cysteine ligase, modifier subunit
- GPx:
Glutathione peroxidase
- GSK:
Glycogen synthase kinase
- HD:
Huntington's disease
- HIF:
Hypoxia inducible factor
- HIF-1:
Hypoxia inducible factor-1
- HO:
Heme oxygenase
- IFNγ:
Interferon-gamma
- IL:
Interleukin
- JNK:
c-Jun N-terminal kinase
- MAPK:
Mitogen activated kinase-like protein
- MCP-1:
Monocyte chemoattractant protein-1
- MIP:
Macrophage inflammatory protein
- MMP:
Metalloproteinases
- MS:
Multiple sclerosis
- NFκB:
Nuclear factor-kappa B
- NOS:
Nitric oxide synthase
- NQO1:
NAD(P)H quinone oxidoreductase
- Nrf2:
Nuclear factor- (erythroid-derived 2-) like 2
- PC:
Protein carbonyl
- PD:
Parkinson's disease
- PERK:
Pancreatic ER kinase
- PGC-1α:
Peroxisome proliferative activated receptor, coactivator 1 alpha
- PI3K:
Phosphatidylinositol 3-kinase
- PPAR:
Peroxisome proliferator activated receptor
- PrP(c):
Cellular prion protein
- ROS:
Reactive oxygen species
- SIRT-1:
Silent mating type information regulation 2 homolog1
- SOD:
Superoxide dismutase
- STAT:
Signal transducer and activation of transcription
- TGFβ:
Transforming growth factors β
- TLR:
Toll-like receptor
- TNFα:
Tumor necrosis factor-alpha
- TRAF:
TNF receptor associated factor
- TRX:
Thioredoxin.
References
- 1.Hung CW, Chen YC, Hsieh WL, Chiou SH, Kao CL. Ageing and neurodegenerative diseases. Ageing Research Reviews. 2010;9(1):S36–S46. doi: 10.1016/j.arr.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Ross CA, Tabizi SJ. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancelet Neurology. 2011;10(1):83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- 3.Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jonsson B. The economic cost of brain disorders in Europe. European Journal of Neurology. 2012;19(1):155–162. doi: 10.1111/j.1468-1331.2011.03590.x. [DOI] [PubMed] [Google Scholar]
- 4.Albarracin SL, Stab B, Casas Z, et al. Effects of natural antioxidants in neurodegenerative diseases. Nutritional Neuroscience. 2012;15(1):1–9. doi: 10.1179/1476830511Y.0000000028. [DOI] [PubMed] [Google Scholar]
- 5.Lundkvist J, Näslund J. γ-secretase: a complex target for Alzheimer's disease. Current Opinion in Pharmacology. 2007;7(1):112–118. doi: 10.1016/j.coph.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Heron M. Deaths: leading causes for 2008. National Vital Statistics Reports. 2012;6(60):1–94. [PubMed] [Google Scholar]
- 7.Phiel CJ, Wilson CA, Lee VMY, Klein PS. GSK-3α regulates production of Alzheimer's disease amyloid-β peptides. Nature. 2003;423(6938):435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 8.Biasini E, Unterberger U, Solomon IH, et al. A mutant prion protein sensitizes neurons to glutamate-induced excitotoxicity. Journal of Neuroscience. 2013;33(6):2408–2418. doi: 10.1523/JNEUROSCI.3406-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zampagni M, Wright D, Cascella R, et al. Novel s-acyl glutathione derivatives prevent amyloid oxidative stress and cholinergic dysfunction in Alzheiner disease models. Free Radical Biology and Medicine. 2012;52(8):1362–1371. doi: 10.1016/j.freeradbiomed.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Kolarova M, Sierra FG, Bartos A, Rincy J. Structure and pathology of tau protein in Alzheimer’s disease. International Journal of Alzheimer’s Disease. 2012;2012:13 pages. doi: 10.1155/2012/731526.731526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mi K, Johnson GV. The role of tau phosphorylation in pathogenesis of Alzheimer’s disease. Current Alzheimer’s Research. 2006;3(5):449–463. doi: 10.2174/156720506779025279. [DOI] [PubMed] [Google Scholar]
- 12.Yang DS, Stavrides P, Mohan PS, et al. Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer's disease ameliorates amyloid pathologies and memory deficits. Brain. 2011;134(1):258–277. doi: 10.1093/brain/awq341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swardfager W, Lanctt K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer's disease. Biological Psychiatry. 2010;68(10):930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Dutta R, Trapp BD. Gene expression profiling in multiple sclerosis brain. Neurobiology of Disease. 2012;45(1):108–114. doi: 10.1016/j.nbd.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziemssen T. Symptom management in patients with multiple sclerosis. Journal of Neurological Sciences. 2011;311(1):S48–S52. doi: 10.1016/S0022-510X(11)70009-0. [DOI] [PubMed] [Google Scholar]
- 16.Łyszczarz AK, Szczuciński A, Pawlak MA, Losy J. Clinical study on CXCL13, CCL17, CCL20 and IL-17 as immune cell migration navigators in relapsing-remitting multiple sclerosis patients. Journal of the Neurological Sciences. 2011;300(1-2):81–85. doi: 10.1016/j.jns.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 17.Menezea AR, Lavie CJ, Milani RV, Okeefe J. The effects of statins on prevention of stoke and dementia: a review. Psychosomatics. 2012;32(5):240–249. doi: 10.1097/HCR.0b013e31825d2a03. [DOI] [PubMed] [Google Scholar]
- 18.Luheshi NM, Kovacs KJ, Lopez-Castejon G, Brough D, Denes A. Interleukin-1α expression precedes IL-1β after ischemic brain injury and is localized to area of focal neuronal loss and penumbral tissues. Journal of Neuroinflammation. 2011;29(8):p. 186. doi: 10.1186/1742-2094-8-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saver JL. Time is brain—quantified. Stroke. 2006;37(1):263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 20.Tuttolomondo A, Di Raimondo D, di Sciacca R, Pinto A, Licata G. Inflammatory cytokines in acute ischemic stroke. Current Pharmaceutical Design. 2008;14(33):3574–3589. doi: 10.2174/138161208786848739. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Potrovita I, Tarabin V, et al. Neuronal activation of NF-κB contributes to cell death in cerebral ischemia. Journal of Cerebral Blood Flow and Metabolism. 2005;25(1):30–40. doi: 10.1038/sj.jcbfm.9600004. [DOI] [PubMed] [Google Scholar]
- 22.Bekris LM, Mata IF, Zabetian CP. The genetics of Parkinson disease. Journal of Geriatric Psychiatry and Neurology. 2010;23(4):228–242. doi: 10.1177/0891988710383572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harraz MM, Dawson TM, Dawson VL. MicroRNAs in Parkinson's disease. Journal of Chemical Neuroanatomy. 2011;42(2):127–130. doi: 10.1016/j.jchemneu.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reale M, Iarlori C, Thomas A, et al. Peripheral cytokines profile in Parkinson's disease. Brain, Behavior, and Immunity. 2009;23(1):55–63. doi: 10.1016/j.bbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Menza M, Dobkin RD, Marin H, et al. The role of inflammatory cytokines in cognition and other non-motor symptoms of Parkinson's disease. Psychosomatics. 2010;51(6):474–479. doi: 10.1176/appi.psy.51.6.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Exner N, Lutz AK, Haass C, Winklhofer KF. Mitochondrial dysfunction in Parkinson’s disease: molecular mechanisms and pathophysiological consequences. The EMBO Journal. 2012;31(14):3038–3062. doi: 10.1038/emboj.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedskog L, Zhang S, Ankarcrona M. Strategic role for mitochondria in Alzheimer’s disease and cancer. Antioxidants and Redox Signaling. 2012;16(12):1476–1491. doi: 10.1089/ars.2011.4259. [DOI] [PubMed] [Google Scholar]
- 28.Van Horssen J, Witte M, Ciccarelli O. The role of mitochondria in axonal degeneration and tissue repair. Multiple Sclerosis. 2012;18(8):1058–1067. doi: 10.1177/1352458512452924. [DOI] [PubMed] [Google Scholar]
- 29.Pandya JD, Sullivan PG, Pettigrew LC. Focal cerebral ischemia and mitochondrial dysfunction in the TNFα-transgenic rat. Brain Research. 2011;1384:151–160. doi: 10.1016/j.brainres.2011.01.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanco S, Suarez A, Gandia-Pla S, et al. Use of capillary electrophoresis for accurate determination of CAG repeats causing Huntington disease. An oligonucleotide design avoiding shadow bands. Scandinavian Journal of Clinical and Laboratory Investigation. 2008;68(7):577–584. doi: 10.1080/00365510801915171. [DOI] [PubMed] [Google Scholar]
- 31.Wild E, Magnusson A, Lahiri N, et al. Abnormal peripheral chemokine profile in Huntington's disease. PLOS Currents. 2011;3:p. RRN1231. doi: 10.1371/currents.RRN1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duffy FH, Albert MS, McAnulty G. Brain electrical activity in patients with presenile and senile dementia of the Alzheimer type. Annals of Neurology. 1984;16(4):439–448. doi: 10.1002/ana.410160404. [DOI] [PubMed] [Google Scholar]
- 33.Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends in Neurosciences. 2001;24(9):517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- 34.Boda E, Hoxha E, Pinni A, Montarolo F, Tempia F. Brain expression of Kv3 subunits during development, adulthood and aging and in a murine model of Alzheimer’s disease. Journal of Molecular Neuroscience. 2012;46(6):606–615. doi: 10.1007/s12031-011-9648-6. [DOI] [PubMed] [Google Scholar]
- 35.Judge SIV, Lee JM, Bever CT, Hoffman PM. Voltage-gated potassium channels in multiple sclerosis: overview and new implications for treatment of central nervous system inflammation and degeneration. Journal of Rehabilitation Research and Development. 2006;43(1):111–122. doi: 10.1682/jrrd.2004.09.0116. [DOI] [PubMed] [Google Scholar]
- 36.Smith KJ. Sodium channels and multiple sclerosis: roles in symptom production, damage and therapy. Brain Pathology. 2007;17(2):230–242. doi: 10.1111/j.1750-3639.2007.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Black JA, Newcombe J, Waxman SG. Astrocytes within multiple sclerosis lesions upregulate sodium channel Nav1.5. Brain. 2010;133(3):835–846. doi: 10.1093/brain/awq003. [DOI] [PubMed] [Google Scholar]
- 38.Stiefelhagen P. Treatment of Parkinson's disease, epilepsy and pain with electrical impulses when drugs are inadequate. MMW-Fortschritte der Medizin. 2003;145(48):p. 14. [PubMed] [Google Scholar]
- 39.Surmeier DJ, Schumacker PT. Calcium, bioenergetics and neuronal vulnerability in Parkinson’s disease. The Journal of Biological Chemistry. 2013;288(15):10736–10741. doi: 10.1074/jbc.R112.410530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oyama F, Miyazaki H, Sakamoto N, et al. Sodium channel β4 subunit: down-regulation and possible involvement in neuritic degeneration in Huntington's disease transgenic mice. Journal of Neurochemistry. 2006;98(2):518–529. doi: 10.1111/j.1471-4159.2006.03893.x. [DOI] [PubMed] [Google Scholar]
- 41.Ariano MA, Cepeda C, Calvert CR, et al. Striatal potassium channel dysfunction in Huntington's disease transgenic mice. Journal of Neurophysiology. 2005;93(5):2565–2574. doi: 10.1152/jn.00791.2004. [DOI] [PubMed] [Google Scholar]
- 42.Small G, Bullock R. Defining optimal treatment with cholinesterase inhibitors in Alzheimer’s disease. Alzheimer’s and Dementia. 2011;7(2):177–184. doi: 10.1016/j.jalz.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Lampl C, You X, Limmroth V. Weekly IM interferon beta-1a in multiple sclerosis patients over 50 years of age. European Journal of Neurology. 2012;19(1):142–148. doi: 10.1111/j.1468-1331.2011.03460.x. [DOI] [PubMed] [Google Scholar]
- 44.Singer C. Managing the patient with newly diagnosed Parkinson's disease. Cleveland Clinical Journal of Medicine. 2012;79(2):S3–S7. doi: 10.3949/ccjm.79.s2a.01. [DOI] [PubMed] [Google Scholar]
- 45.Tommaso M, Serpino C, Sciruicchio V. Management of Huntington's disease: role of tetrabenazine. Therapeutics and Clinical Risk Management. 2011;7:123–129. doi: 10.2147/TCRM.S17152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haile WB, Wu J, Echeverry R, Wu F, An J, Yepes M. Tissue type plasminogen activator has a neuroprotective effect in the ischemic brain mediated by neuronal TNF-α . Journal of Cerebral Blood Flow and Metabolism. 2012;32(1):57–59. doi: 10.1038/jcbfm.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faria A, Pestana D, Teixeira D, et al. Flavonoid transport across RBE4 cells: a blood-brain barrier model. Cellular and Molecular Biology Letters. 2010;15(2):234–241. doi: 10.2478/s11658-010-0006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basli A, Soulet S, Chaher N, et al. Wine polyphenols: potential agents in neuroprotection. Oxidative Medicine and Cellular Longevity. 2012;2012:14 pages. doi: 10.1155/2012/805762.805762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pérez-Jiménez J, Neveu V, Vos F, Scalbert A. Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database. European Journal of Clinical Nutrition. 2010;64(3):S112–S120. doi: 10.1038/ejcn.2010.221. [DOI] [PubMed] [Google Scholar]
- 50.Cieslik E, Greda A, Adamus W. Contents of polyphenols in fruit and vegetables. Food Chemistry. 2006;94(1):135–142. [Google Scholar]
- 51.Jones QR, Warford J, Rupasinghe HPV, Robertson GS. Target-based selection of flavonoids for neurodegenerative disorders. Trends in Pharmacological Sciences. 2012;33(11):602–610. doi: 10.1016/j.tips.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Thilakarathna SH, Rupasinghe HPV, Needs PW. Apple peel bioactive rich extracts effectively inhibit in vitro human LDL oxidation. Food Chemistry. 2013;138(1):463–470. doi: 10.1016/j.foodchem.2012.09.121. [DOI] [PubMed] [Google Scholar]
- 53.Okello EJ, Leylabi R, McDougall J. Inhibition of acetylcholinesterase by green and white tea and their simulated intestinal metabolites. Food and Function. 2012;3(6):651–661. doi: 10.1039/c2fo10174b. [DOI] [PubMed] [Google Scholar]
- 54.Qin XY, Cheng Y, Yu LC. Potential protection of green tea polyphenols against intracellular amyloid beta induced toxicity on primary cultured prefrontal cortical neurons of rats. Neuroscience Letters. 2012;513(2):170–173. doi: 10.1016/j.neulet.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Ferruzzi MG, Ho L, et al. Brain-targeted proanthocyandinin metabolites for Alzheimer’s disease treatment. Journal of Neuroscience. 2012;32(15):5144–5150. doi: 10.1523/JNEUROSCI.6437-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu P, Kemper LJ, Wang J, Zahs KR, Ashe KH, Pasinetti GM. Grape seed polyphenolic extract specifically decreases Aβ ∗56 in the brains of Tg2576 mice. Journal of Alzheimer’s Disease. 2011;26(4):657–666. doi: 10.3233/JAD-2011-110383. [DOI] [PubMed] [Google Scholar]
- 57.Reding HK, Ho L, Santa-Maria I, Diaz-Ruiz C, Wang J, Pasinetti GM. Ultrastructural alterations of Alzheimer's disease paired helical filaments by grape seed-derived polyphenols. Neurobiology of Aging. 2012;33(7):1427–1439. doi: 10.1016/j.neurobiolaging.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 58.Wang T, Gu J, Wu PF, et al. Protection by tetrahydroxystilbene glucoside against cerebral ischemia: involvement of JNK, SIRT1, and NF-κB pathways and inhibition of intracellular ROS/RNS generation. Free Radical Biology and Medicine. 2009;47(3):229–240. doi: 10.1016/j.freeradbiomed.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 59.Thomas P, Wang YJ, Zhong JH, et al. Grape seed polyphenols and curcumin reduce genomic instability events in a transgenic mouse model for Alzheimer's disease. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis. 2009;661(1-2):25–34. doi: 10.1016/j.mrfmmm.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 60.Feng Y, Wang XP, Yang SG, et al. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. NeuroToxicology. 2009;30(6):986–995. doi: 10.1016/j.neuro.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 61.Huang TC, Lu KT, Wo YY, Wu YJ, Yang YL. Resveratrol protects rats from Aβ induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS ONE. 2011;6(12) doi: 10.1371/journal.pone.0029102.e29102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frozza RL, Bernardi A, Paese K, et al. Characterization of trans-resveratrol-loaded lipid-core nanocapsules and tissue distribution studies in rats. Journal of Biomedical Nanotechnology. 2010;6(6):694–703. doi: 10.1166/jbn.2010.1161. [DOI] [PubMed] [Google Scholar]
- 63.Ushikubo H, Watanabe S, Tanimoto Y, et al. 3, 3′, 4′, 5, 5′ Pentahyroxyflavone is a potent inhibitor of amyloid β fibril formation. Neuroscience Letters. 2012;513(1):51–56. doi: 10.1016/j.neulet.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 64.Gong EJ, Park HR, Kim ME, et al. Morin attenuates tau hyperphosphorylation by inhibiting GSK3β . Neurobiology of Disease. 2011;44(2):223–230. doi: 10.1016/j.nbd.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mori T, Rezai-Zedeh K, Koyama N, et al. Tannic acid is a natural β-secretase inhibitor that prevents cognitive impairment and mitigates Alzheimer-like pathology in transgenic mice. The Journal of Biological Chemistry. 2012;287(9):6912–6927. doi: 10.1074/jbc.M111.294025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Devi L, Ohno M. 7, 8-dihyroxyflavone, a small molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2012;37(2):434–444. doi: 10.1038/npp.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu RT, Tang JT, Zhou LB, Fu JY, Lu QJ. Liquiritigenin attenuates the learning and memory deficits in an amyloid protein precursor transgenic mouse model and the underlying mechanisms. European Journal of Pharmacology. 2011;669(1–3):76–83. doi: 10.1016/j.ejphar.2011.07.051. [DOI] [PubMed] [Google Scholar]
- 68.Jiménez-Aliaga K, Bermejo-Bescَs P, Benedí J, Martín-Aragón S. Quercetin and rutin exhibit antiamyloidogenic and fibril-diasggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sciences. 2011;89(25-26):939–945. doi: 10.1016/j.lfs.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 69.Richetti SK, Blank M, Capiotti KM, et al. Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish. Behavioural Brain Research. 2011;217(1):10–15. doi: 10.1016/j.bbr.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 70.Gupta R, Gupta LK, Mediratta PK, Bhattacharya SK. Effect of resveratrol on scopolamine induced cognitive impairment in mice. Pharmacological Reports. 2012;64(2):438–444. doi: 10.1016/s1734-1140(12)70785-5. [DOI] [PubMed] [Google Scholar]
- 71.Wang SW, Wang YJ, Su YJ, et al. Rutin inhibits β-amyloid aggregation and cytotoxicity, attenuates oxidative stress and decreases the production of nitric oxide and proinflammatory cytokines. Neurotoxicology. 2012;33(3):482–490. doi: 10.1016/j.neuro.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 72.Jagota S, Rajadas J. Effect of phenolic compounds against Aβ aggregation and Aβ-induced toxicity in transgenic C. elegans . Neurochemical Research. 2012;37(1):40–48. doi: 10.1007/s11064-011-0580-5. [DOI] [PubMed] [Google Scholar]
- 73.Sievers C, Meira M, Hoffmann F, Fontoura P, Kappos L, Lindberg RL. Altered microRNA expression in B lymphocytes in multiple sclerosis: towards a better understanding of treatment effects. Clinical Immunology. 2012;144(1):70–79. doi: 10.1016/j.clim.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 74.Fonseca-Kelly Z, Nassrallah M, Uribe J, et al. Resveratrol neuroprotection in a chronic mouse model of multiple sclerosis. Frontiers in Neurology. 2012;3:p. 84. doi: 10.3389/fneur.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shindler KS, Ventura E, Dutt M, Elliott P, Fitzgerald DC, Rostami A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. Journal of Neuro-Ophthalmology. 2010;30(4):328–339. doi: 10.1097/WNO.0b013e3181f7f833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ho DJ, Calingasan NY, Wille E, Dumont M, Beal MF. Resveratrol protects against peripheral deficits in a mouse model of Huntington's disease. Experimental Neurology. 2010;225(1):74–84. doi: 10.1016/j.expneurol.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 77.Sternberg Z, Chadha K, Lieberman A, et al. Quercetin and interferon-β modulate immune response(s) in peripheral blood mononuclear cells isolated from multiple sclerosis patients. Journal of Neuroimmunology. 2008;205(1-2):142–147. doi: 10.1016/j.jneuroim.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 78.Herges K, Millward JM, Hentschel N, Infante-Duarte C, Aktas O, Zipp F. Neuroprotective effect of combination therapy of glatiramer acetate and epigallocatechin-3-gallate in neuroinflammation. PLoS ONE. 2011;6(10) doi: 10.1371/journal.pone.0025456.e25456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davis JM, Murphy EA, Carmichael MD, Davis B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. American Journal of Physiology. 2009;296(4):R1071–R1077. doi: 10.1152/ajpregu.90925.2008. [DOI] [PubMed] [Google Scholar]
- 80.Xiang L, Sun K, Lu J, et al. Anti-aging effects of phloridzin, an apple polyphenol, on yeast via the SOD and Sir2 genes. Bioscience, Biotechnology and Biochemistry. 2011;75(5):854–858. doi: 10.1271/bbb.100774. [DOI] [PubMed] [Google Scholar]
- 81.Hong KS, Park JI, Kim MJ, et al. Involvement of SIRT1 in hypoxic down-regulation of c-Myc and β-catechin and hypoxic precondition effect of polyphenol. Toxicology and Applied Pharmacology. 2012;259(2):210–218. doi: 10.1016/j.taap.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 82.Hendriks JJA, De Vries HE, Van Der Pol SMA, Van Den Berg TK, Van Tol EAF, Dijkstra CD. Flavonoids inhibit myelin phagocytosis by macrophages; a structure-activity relationship study. Biochemical Pharmacology. 2003;65(5):877–885. doi: 10.1016/s0006-2952(02)01609-x. [DOI] [PubMed] [Google Scholar]
- 83.Ashafaq M, Raza SS, Khan MM, et al. Catechin hydrate ameliorates redox imbalance and limits inflammatory response in focal cerebral ischemia. Neurochemical Research. 2012;37(8):1747–1760. doi: 10.1007/s11064-012-0786-1. [DOI] [PubMed] [Google Scholar]
- 84.Park JW, Hong JS, Lee KS, Kim HY, Lee JJ, Lee SR. Green tea polyphenol (-)-epigallocatechin gallate reduces matrix metalloproteinase-9 activity following transient focal cerebral ischemia. Journal of Nutritional Biochemistry. 2010;21(11):1038–1044. doi: 10.1016/j.jnutbio.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 85.Panickar KS, Polansky MM, Anderson RA. Green tea polyphenols attenuate glial swelling and mitochondrial dysfunction following oxygen-glucose deprivation in cultures. Nutritional Neuroscience. 2009;12(3):105–113. doi: 10.1179/147683009X423300. [DOI] [PubMed] [Google Scholar]
- 86.Pandey AK, Hazari PP, Patnaik R, Mishra AK. The role of ASIC1a in neuroprotection elicited by quercetin in focal cerebral ischemia. Brain Research. 2011;1383:289–299. doi: 10.1016/j.brainres.2011.01.085. [DOI] [PubMed] [Google Scholar]
- 87.Yao Y, Han DD, Zhang T, Yang Z. Quercetin improves cognitive deficits in rats with chronic cerebral ischemia and inhibits voltage-dependent sodium channels in hippocampal CA1 pyramidal neurons. Phytotherapy Research. 2010;24(1):136–140. doi: 10.1002/ptr.2902. [DOI] [PubMed] [Google Scholar]
- 88.Lee JK, Kwak HJ, Piao MS, Jang JW, Kim SH, Kim HS. Quercetin reduces the elevated matrix metalloproteinases-9 level and improves functional outcome after cerebral focal ischemia in rats. Acta Neurochirurgica. 2010;112(6):1477–1487. doi: 10.1007/s00701-010-0889-x. [DOI] [PubMed] [Google Scholar]
- 89.Pandey AK, Verma S, Bhattacharya P, Paul S, Mishra A, Patnaik R. An in-silico strategy to explore neuroprotection by quercetin in cerebral ischemia: a novel hypothesis based on inhibition of matrix metalloproteinase (MMPs) and acid sensing ion channel 1a (ASIC1a) Medical Hypothesis. 2012;79(1):76–81. doi: 10.1016/j.mehy.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 90.Khan MM, Ahmad A, Ishrat T, et al. Rutin protects the neural damage induced by transient focal ischemia in rats. Brain Research. 2009;1292:123–135. doi: 10.1016/j.brainres.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 91.Abd-El-Fattah AA, El-Sawalhi MM, Rashed ER, El-Ghazaly MA. Possible role of vitamin E, coenzyme Q10 and rutin in protection against cerebral ischemia/reperfusion injury in irradiated rats. International Journal of Radiation Biology. 2010;86(12):1070–1078. doi: 10.3109/09553002.2010.501844. [DOI] [PubMed] [Google Scholar]
- 92.Li H, Yan Z, Zhu J, Yang J, He J. Neuroprotective effects of resveratrol on ischemic injury mediated by improving brain energy metabolism and alleviating oxidative stress in rats. Neuropharmacology. 2011;60(2-3):252–258. doi: 10.1016/j.neuropharm.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 93.Li C, Yan Z, Yang J, et al. Neuroprotective effects of resveratrol on ischemic injury mediated by modulating the release of neurotransmitter and neuromodulator in rats. Neurochemistry International. 2010;56(3):495–500. doi: 10.1016/j.neuint.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 94.Gelderblom M, Leypoldt F, Lewerenz J, et al. The flavonoid fisetin attenuates postischemic immune cell infiltration, activation and infract size after transient cerebral middle artery occlusion in mice. Journal of Cerebral Flow and Blood Metabolism. 2012;32(5):835–843. doi: 10.1038/jcbfm.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tu XK, Yang WZ, Liang RS, et al. Effect of baicalin on matrix metalloproteinase-9 expression and blood brain barrier permeability following focal cerebral ischemia in rats. Neurochemical Research. 2011;36(11):2022–2028. doi: 10.1007/s11064-011-0526-y. [DOI] [PubMed] [Google Scholar]
- 96.Cao Y, Mao X, Sun C, et al. Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Research Bulletin. 2011;85(6):396–402. doi: 10.1016/j.brainresbull.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 97.Cui L, Zhang X, Yang R, et al. Baicalein is neuroprotective in rat MCAO model: role of 12/15-lipoxygenase, mitogen-activated protein kinase and cytosolic phospholipase A2. Pharmacology Biochemistry and Behavior. 2010;96(4):469–475. doi: 10.1016/j.pbb.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 98.Tu XK, Yang WZ, Shi SS, et al. Baicalin inhibits TLR2/4 signaling pathway in rat brain following permanent cerebral ischemia. Inflammation. 2011;34(5):463–470. doi: 10.1007/s10753-010-9254-8. [DOI] [PubMed] [Google Scholar]
- 99.Simao F, Matte A, Pagnussant AS, Netto CA, Salbego CG. Resveratrol prevents CA1 neurons against ischemic injury by parallel modulation of both GSK-3β and CREB through P13-K/Akt pathways. European Journal of Neuroscience. 2012;36(7):2899–2905. doi: 10.1111/j.1460-9568.2012.08229.x. [DOI] [PubMed] [Google Scholar]
- 100.Jin F, Wu Q, Lu YF, Gong QH, Shi JS. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson's disease in rats. European Journal of Pharmacology. 2008;600(1–3):78–82. doi: 10.1016/j.ejphar.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 101.Paris D, Matura V, Ait-Ghezala G, et al. Flavonoids lower Alzheimer’s Aβ production via NFκB dependent mechanism. Bioinformation. 2011;6(6):229–236. doi: 10.6026/97320630006229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ding BJ, Ma WW, He LL, et al. Soybean isoflavone alleviates β-amyloid 1-42 induced inflammatory response to improve learning and memory ability by down regulation of Toll-like receptor 4 expression and nuclear factor-κB activity in rats. International Journal of Developmental Neuroscience. 2011;29(5):537–542. doi: 10.1016/j.ijdevneu.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 103.Capiralla H, Vingtdeux V, Zhao H, et al. Resveratrol mitigates lipopolysaccharide-and Aβ mediated microglial inflammation by inhibiting the TLR4/NFκB/STAT signaling cascade. Journal of Neurochemistry. 2012;120(3):461–472. doi: 10.1111/j.1471-4159.2011.07594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu YW, Sun L, Liang H, Sun GM, Cheng Y. 12/15-Lipoxygenase inhibitor baicalein suppresses PPARγ expression and nuclear translocation induced by cerebral ischemia/reperfusion. Brain Research. 2010;1307:149–157. doi: 10.1016/j.brainres.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 105.Hou YC, Liou KT, Chern CM, et al. Preventive effect of silymarin in cerebral ischemia-reperfusion-induced brain injury in rats possibly through impairing NF-κB and STAT-1 activation. Phytomedicine. 2010;17(12):963–973. doi: 10.1016/j.phymed.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 106.Patir H, Sarada SK, Singh S, Mathew T, Singh B, Bansal A. Quercetin as a prophylactic measure against high altitude cerebral edema. Free Radical Biology and Medicine. 2012;54(4):659–668. doi: 10.1016/j.freeradbiomed.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 107.Cheng G, Zhang X, Gao D, Jiang X, Dong W. Resveratrol inhibits MMP-9 expression by up-regulating PPAR α expression in an oxygen glucose deprivation-exposed neuron model. Neuroscience Letters. 2009;451(2):105–108. doi: 10.1016/j.neulet.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 108.Chang J, Rimando A, Pallas M, et al. Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. Neurobiology of Aging. 2012;33(9):2062–2071. doi: 10.1016/j.neurobiolaging.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 109.Shah ZA, Li RC, Ahmad AS, et al. The flavanol (-)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. Journal of Cerebral Blood Flow and Metabolism. 2010;30(12):1951–1961. doi: 10.1038/jcbfm.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ren J, Fan C, Chen N, Huang J, Yang Q. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf-2 and HO-1 in rats. Neurochemical Research. 2011;36(12):2352–2362. doi: 10.1007/s11064-011-0561-8. [DOI] [PubMed] [Google Scholar]
- 111.Yen TL, Hsu CK, Lu WJ, et al. Neuroprotective effects of Xanthohumol, a prenylated flavonoid from hops (Humulus lupus), in ischemic stroke of rats. Journal of Agricultural and Food Chemistry. 2012;60(8):1937–1944. doi: 10.1021/jf204909p. [DOI] [PubMed] [Google Scholar]
- 112.Agrawal M, Kumar V, Kashyap MP, Khanna VK, Randhawa GS, Pant AB. Ischemic insult induced apoptotic changes in PC12 cells: protection by trans resveratrol. European Journal of Pharmacology. 2011;666(1–3):5–11. doi: 10.1016/j.ejphar.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 113.Nones J, -Spohr TCE, Gomes FC. Hesperidin, a flavone glycoside, as mediator of neuronal survival. Neurochemical Research. 2011;36(10):1776–1784. doi: 10.1007/s11064-011-0493-3. [DOI] [PubMed] [Google Scholar]
- 114.Liu CM, Sun YZ, Sun JM, Ma JQ, Cheng C. Protective role of quercetin lead-induced inflammatory response in rat kidney through the ROS-mediated MAPKs and NFκB pathway. Biochemica et Biophysica Acta. 2012;1820(10):1693–1703. doi: 10.1016/j.bbagen.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 115.Abraham J, Johnson RW. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Research. 2009;12(6):445–453. doi: 10.1089/rej.2009.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cavet ME, Harrington KL, Vollmer TR, Ward KW, Zhang JZ. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Molecular Vision. 2011;17:533–542. [PMC free article] [PubMed] [Google Scholar]
- 117.Noh SU, Park YM. The effect of green tea polyphenols on macrophage migration inhibitory factor-associated steroid resistance. The British Journal of Dermatology. 2012;166(3):653–657. doi: 10.1111/j.1365-2133.2011.10720.x. [DOI] [PubMed] [Google Scholar]
- 118.Lee EO, Park HJ, Kang JL, Kim HS, Chong YH. Resveratrol reduces glutamate-mediated monocyte chemotactic protein-1 expression via inhibition of extracellular signal-regulated kinase 1/2 pathway in rat hippocampal slice cultures. Journal of Neurochemistry. 2010;112(6):1477–1487. doi: 10.1111/j.1471-4159.2009.06564.x. [DOI] [PubMed] [Google Scholar]
- 119.Norata GD, Marchesi P, Passamonti S, Pirillo A, Violi F, Catapano AL. Anti-inflammatory and anti-atherogenic effects of cathechin, caffeic acid and trans-resveratrol in apolipoprotein E deficient mice. Atherosclerosis. 2007;191(2):265–271. doi: 10.1016/j.atherosclerosis.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 120.Qureshi AA, Tan X, Reis JC, et al. Inhibition of nitric oxide in LPS-stimulated macrophages of young and senescent mice by d-tocotrienol and quercetin. Lipids in Health and Disease. 2011;10(239):1–22. doi: 10.1186/1476-511X-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang ZJ, Cheang LCV, Wang MW, Lee SMY. Quercetin exerts a neuroprotective effect through inhibition of the iNOS/NO system and pro-inflammation gene expression in PC12 cells and in zebrafish. International Journal of Molecular Medicine. 2011;27(2):195–203. doi: 10.3892/ijmm.2010.571. [DOI] [PubMed] [Google Scholar]
- 122.Skyberg JA, Robison A, Golden S, et al. Apple polyphenols require T cells to ameliorate dextran sulfate sodium-induced colitis and dampen proinflammatory cytokine expression. Journal of Leukocyte Biology. 2011;90(6):1043–1054. doi: 10.1189/jlb.0311168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Keddy PGW, Dunlop K, Warford J, et al. Neuroprotective and anti-inflammatory effects of the flavonoid-enriched fraction AF4 in a mouse model of hypoxic-ischemia brain injury. PLoS ONE. 2012;7(12) doi: 10.1371/journal.pone.0051324.e51324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bournival J, Plouffe M, Renaud J, Provencher C, Martinoli MG. Quercetin and sesamin protects dopaminergic cells from MMP(+)-Induced neuroinflammation in a microglial (N9)-neuronal (PC12) coculture system. Oxidative Stress and Cellular Longevity. 2012;2012 doi: 10.1155/2012/921941.921941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li SY, Yang D, Fu ZJ, Woo T, Wong D, Lo AC. Lutein enhances survival and reduces neuronal damage in a mouse model of ischemic stroke. Neurobiology of Disease. 2012;45(1):624–632. doi: 10.1016/j.nbd.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 126.Liu C, Wu J, Xu K, et al. Neuroprotection by baicalein in ischemic brain injury involves PTEN/AKT pathway. Journal of Neurochemistry. 2010;112(6):1500–1512. doi: 10.1111/j.1471-4159.2009.06561.x. [DOI] [PubMed] [Google Scholar]
- 127.Hou CW, Lin YT, Chen YL, et al. Neuroprotective effects of carnosic acid on neuronal cells under ischemic and hypoxic stress. Nutritional Neuroscience. 2012;15(6):257–263. doi: 10.1179/1476830512Y.0000000021. [DOI] [PubMed] [Google Scholar]
- 128.Kao ES, Hsu JD, Wang CJ, Yang SH, Cheng SY, Lee HJ. Polyphenols extracted from hibiscus sabdariffa L. inhibited lipopolysaccharide-induced inflammation by improving antioxidative conditions and regulating cyclooxygenase-2 expression. Bioscience, Biotechnology and Biochemistry. 2009;73(2):385–390. doi: 10.1271/bbb.80639. [DOI] [PubMed] [Google Scholar]
- 129.Huang CS, Lii CK, Lin AH, et al. Protection by chrysin, apigenin and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Archives of Toxicology. 2013;87(1):167–168. doi: 10.1007/s00204-012-0913-4. [DOI] [PubMed] [Google Scholar]
- 130.Ye Q, Ye L, Xu X, et al. Epigallocatechin-3-gallate suppresses 1-methyl-4-phenyl-pyridine-induced oxidative stress in PC12 cells via the SIRT1/PGC-1a signaling pathway. BMC Complementary and Alternative Medicine. 2012;28(12):p. 82. doi: 10.1186/1472-6882-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martín S, González-Burgos E, Carretero ME, Gómez-Serranillos MP. Neuroprotective properties of Spanish red wine and its isolated polyphenols on astrocytes. Food Chemistry. 2011;128(1):40–48. doi: 10.1016/j.foodchem.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 132.Fernández-Pachón MS, Berná G, Otaolaurruchi E, Troncoso AM, Martín F, García-Parrilla MC. Changes in antioxidant endogeneous enzymes (activity and gene expression levels) after repeated red wine intake. Journal of Agriculture and Food Chemistry. 2009;57(15):6578–6583. doi: 10.1021/jf901863w. [DOI] [PubMed] [Google Scholar]
- 133.Duong TTH, Antao S, Ellis NA, Myers SJ, Witting PK. Supplementation with a synthetic polyphenol limits oxidative stress and enhances neuronal cell viability in response to hypoxia-re-oxygenation injury. Brain Research. 2008;1219:8–18. doi: 10.1016/j.brainres.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 134.Yang YC, Lii CK, Lin AH, et al. Induction of glutathione synthesis and heme oxygenase 1 by flavonoids butein and phloretin is mediated through ERK/Nrf2 pathway and protects against oxidative stress. Free Radical Biology and Medicine. 2011;51(11):2073–2081. doi: 10.1016/j.freeradbiomed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 135.Overman A, Chuang CC, McIntosh M. Quercetin attenuates inflammation in human macrophages exposed to macrophage-conditioned media. International Journal of Obesity. 2011;35(9):1165–1172. doi: 10.1038/ijo.2010.272. [DOI] [PubMed] [Google Scholar]
- 136.Deng G, Wang J, Zhang Q, et al. Hepatoprotective effects of phloridzin on hepatoprotective fibrosis induced by carbon tetrachloride against stress triggered damage and fibrosis in rats. Biological and Pharmaceutical Bulletin. 2012;35(7):1118–1125. doi: 10.1248/bpb.b12-00057. [DOI] [PubMed] [Google Scholar]
- 137.Tufekci KU, Meuwissen R, Genc S, Genc K. Inflammation in Parkinson’s disease. Advances in Protein Chemistry and Structural Biology. 2012;88:69–132. doi: 10.1016/B978-0-12-398314-5.00004-0. [DOI] [PubMed] [Google Scholar]
- 138.Khan MM, Ahmad A, Ishrat T, et al. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson's disease. Brain Research. 2010;1328:139–151. doi: 10.1016/j.brainres.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 139.Chao J, Yu MS, Ho YS, Wang M, Chang RCC. Dietary oxyresveratrol prevents parkinsonian mimetic 6-hydroxydopamine neurotoxicity. Free Radical Biology and Medicine. 2008;45(7):1019–1026. doi: 10.1016/j.freeradbiomed.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 140.Kim HY, Lee SM. Ferulic acid attenuates ischemia/reperfusion-induced hepatocyte apoptosis via inhibition of JNK activation. European Journal of Pharmaceutical Sciences. 2012;45(5):708–705. doi: 10.1016/j.ejps.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 141.Lv C, Hong T, Yang Z, et al. Effect of quercetin in the 1-Methyl-4phenyl-1,2,3,6-tetrahyropyridine-induced mouse model of Parkinson’s disease. Evidence Based Complement and Alternative Medicine. 2012;2012 doi: 10.1155/2012/928643.928643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mukai R, Kawabata K, Otsuka S, et al. Effect of quercetin and its glucuronide metabolite upon 6-hyroxydopamine-induced oxidative damage in Neuro-2a cells. Free Radical Research. 2012;46(8):1019–1028. doi: 10.3109/10715762.2012.673720. [DOI] [PubMed] [Google Scholar]
- 143.Kääriäinen TM, Piltonen M, Ossola B, et al. Lack of robust protective effect of quercetin in two types of 6-hydroxydopamine-induced parkinsonian models in rats and dopaminergic cell cultures. Brain Research. 2008;1203:149–159. doi: 10.1016/j.brainres.2008.01.089. [DOI] [PubMed] [Google Scholar]
- 144.Mu X, He G, Cheng Y, Li X, Xu B, Du G. Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental parkinsonism in vivo and in vitro . Pharmacology Biochemistry and Behavior. 2009;92(4):642–648. doi: 10.1016/j.pbb.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 145.Filomeni G, Graziani I, Zio DD, et al. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: possible implications for Parkinson's disease. Neurobiology of Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 146.Vauzour D, Corona G, Spencer JPE. Caffeic acid, tyrosol and p-coumaric acid are potent inhibitors of 5-S-cysteinyl-dopamine induced neurotoxicity. Archives of Biochemistry and Biophysics. 2010;501(1):106–111. doi: 10.1016/j.abb.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 147.Tai KK, Truong DD. (-)-Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, reduces dichlorodiphenyl-trichloroethane (DDT)-induced cell death in dopaminergic SHSY-5Y cells. Neuroscience Letters. 2010;482(3):183–187. doi: 10.1016/j.neulet.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 148.Ho DJ, Calingasan NY, Wille E, Dumont M, Beal MF. Resveratrol protects against peripheral deficits in a mouse model of Huntington's disease. Experimental Neurology. 2010;225(1):74–84. doi: 10.1016/j.expneurol.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 149.Kim HG, Ju MS, Shim JS, et al. Mulberry fruit protects dopaminergic neurons in toxin-induced Parkinson's disease models. The British Journal of Nutrition. 2010;104(1):8–16. doi: 10.1017/S0007114510000218. [DOI] [PubMed] [Google Scholar]
- 150.Maher P, Dargusch R, Bodai L, Gerard PE, Purcell JM, Marsh JL. Erk activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington's disease. Human Molecular Genetics. 2011;20(2):261–270. doi: 10.1093/hmg/ddq460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kumar P, Kumar A. Protective effect of hesperidin and naringin against 3-nitropropionic acid induced Huntington's like symptoms in rats: possible role of nitric oxide. Behavioural Brain Research. 2010;206(1):38–46. doi: 10.1016/j.bbr.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 152.Hickey MA, Zhu C, Medvedeva V, et al. Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntington’s disease. Molecular Neurodegeneration. 2003;4(7):7–12. doi: 10.1186/1750-1326-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ehrnhoefer DE, Duennwald M, Markovic P, et al. Mitochondrial dysfunction and tau hyperphosphorylation in Ts1Cje, a mouse model for down syndrome. Human Molecular Genetics. 2006;15(18):2752–2762. doi: 10.1093/hmg/ddl211. [DOI] [PubMed] [Google Scholar]
- 154.Wang J, Pfleger CM, Friedman L, et al. Potential application of grape derived polyphenols in huntington's disease. Translational Neuroscience. 2010;1(2):95–100. doi: 10.2478/v10134-010-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sueishi Y, Ishikawa M, Yoshioka D, et al. Oxygen radical absorbance capacity (ORAC) of cyclodextrin-solubilized flavonoids, resveratrol and astaxanthin as measured with ORAC-EPR method. Journal of Clinical Biochemistry and Nutrition. 2012;50(2):127–132. doi: 10.3164/jcbn.11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chang CC, Chang CY, Huang JP, Hung LM. Effect of resveratrol on oxidative and inflammatory stress in liver and spleen of streptozotocin-induced type 1diabetic rats. The Chinese Journal of Physiology. 2012;55(3):192–201. doi: 10.4077/CJP.2012.BAA012. [DOI] [PubMed] [Google Scholar]
- 157.Mikula-Pietrasik J, Kuczmarska A, Rubis B, et al. Resveratrol delays replicative senescence of human mesothelial cells via mobilization of antioxidative and DNA repair mechanisms. Free Radical Biology and Medicine. 2012;52(11-12):2234–2245. doi: 10.1016/j.freeradbiomed.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 158.Gresele P, Pignatelli P, Guglielmini G, et al. Resveratrol, at concentrations attainable with moderate wine consumption, stimulates human platelet nitric oxide production. Journal of Nutrition. 2008;138(9):1602–1608. doi: 10.1093/jn/138.9.1602. [DOI] [PubMed] [Google Scholar]
- 159.Akhlaghi M, Bandy B. Preconditioning and acute effects of flavonoids in protecting cardiomyocytes from oxidative cell death. Oxidative Medicine and Cellular Longevity. 2012;2012:9 pages. doi: 10.1155/2012/782321.782321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brückner M, Westphal S, Domschke W, Kucharzik T, Lügering A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. Journal of Crohn’s and Colotis. 2012;6(2):226–235. doi: 10.1016/j.crohns.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 161.Xie T, Wang WP, Mao ZF, et al. Effects of epigallocatechin-3-gallate on pentylenetetrazole-inducing kindling, cognitive impairment and oxidative stress in rats. Neuroscience Letters. 2012;516(2):237–241. doi: 10.1016/j.neulet.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 162.Liu CM, Ma JQ, Sun YZ. Puerarin protects rat kidney from leas-induced apoptosis by modulating the P13K/Akt/Enos pathway. Toxicology and Applied Pharmacology. 2012;258(3):330–342. doi: 10.1016/j.taap.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 163.Ai ZL, Zhang WS, Yao SK, Xie BS, Gao C. Effect of baicalin on liver fatty acid binding protein in oxidative stress model in vitro . Zhonghua Gan Zang Bing Za Zhi. 2011;19(12):927–931. doi: 10.3760/cma.j.issn.1007-3418.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 164.Castañer O, Covas MI, Khymenets O, et al. Protection of LDL from oxidation by olive oil polyphenols is associated with a downregulation of CD40-ligand expression and its downstream products in vivo in humans. The American Journal of Clinical Nutrition. 2012;95(5):1238–1244. doi: 10.3945/ajcn.111.029207. [DOI] [PubMed] [Google Scholar]
- 165.Karlsen A, Paur I, Bøhn SK, et al. Bilberry juice modulates plasma concentration of NF-κB related inflammatory markers in subjects at increased risk of CVD. European Journal of Nutrition. 2010;49(6):345–355. doi: 10.1007/s00394-010-0092-0. [DOI] [PubMed] [Google Scholar]
- 166.Kesse-Guyot E, Fezeu L VA, Andreeva, et al. Total and specific polyphenol intake in midlife are associated with cognitive function measured 13 years later. The Journal of Nutrition. 2012;142(1):76–83. doi: 10.3945/jn.111.144428. [DOI] [PubMed] [Google Scholar]
- 167.Morillas-Ruiz JM, Rubio-Perez JM, Albaladejo MD, Zafrilla P, Parra S, Vidal-Guevara ML. Effect of an antioxidant drink on homocysteine levels in Alzheimer's patients. Journal of the Neurological Sciences. 2010;299(1-2):175–178. doi: 10.1016/j.jns.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 168.Xue X, Qu XJ, Yang Y, et al. Baicalin attenuates focal cerebral ischemic reperfusion injury through inhibition of nuclear factor κB p65 activation. Biochemical and Biophysical Research Communications. 2010;403(3-4):398–404. doi: 10.1016/j.bbrc.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 169.Shin BY, Jin SH, Chou IJ, Ki SH. Nrf2-ARE pathway regulates induction of Sestrin-2 expression. Free Radical Biology and Medicine. 2012;53(4):834–841. doi: 10.1016/j.freeradbiomed.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 170.Li J, Ye L, Wang X, et al. (-)-Epigallocatechin gallate inhibits endotoxin-induced expression of inflammatory cytokines in human cerebral microvascular endothelial cells. Journal of Neuroinflammation. 2012;9(161):1–13. doi: 10.1186/1742-2094-9-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Saha A, Sarkar C, Singh SP, et al. The blood brain barrier is disrupted in a mouse model of infantile neuronal ceroid lipofuscinosis: amelioration by resveratrol. Human Molecular Genetics. 2012;21(10):2233–2244. doi: 10.1093/hmg/dds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shukitt-Hale B, Lau FC, Carey AN, et al. Blueberry polyphenols attenuate kainic acid-induced decrements in cognition and alter inflammatory gene expression in rat hippocampus. Nutritional Neuroscience. 2008;11(4):172–182. doi: 10.1179/147683008X301487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jung M, Triebel S, Anke T, Richling E, Erkel G. Influence of apple polyphenols on inflammatory gene expression. Molecular Nutrition and Food Research. 2009;53(10):1263–1280. doi: 10.1002/mnfr.200800575. [DOI] [PubMed] [Google Scholar]
- 174.Ansó E, Zuazo A, Irigoyen M, Urdaci MC, Rouzaut A, Martínez-Irujo JJ. Flavonoids inhibit hypoxia-induced vascular endothelial growth factor expression by a HIF-1 independent mechanism. Biochemical Pharmacology. 2010;79(11):1600–1609. doi: 10.1016/j.bcp.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 175.Shin JA, Lee KE, Kim HS, Park EM. Acute resveratrol treatment modulates multiple signaling pathways in the ischemic brain. Neurochemical Research. 2012;37(12):2686–2696. doi: 10.1007/s11064-012-0858-2. [DOI] [PubMed] [Google Scholar]
- 176.Bonda DJ, Lee H, Blair JA, Zhu X, Perry G, Smith MA. Role of metal dyshomeostasis in Alzheimer’s disease. Metallomics. 2011;3:267–270. doi: 10.1039/c0mt00074d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schneider SA, Hardy J, Bhatia KP. Syndromes of neurodegeneration with brain iron accumulation (NBIA): an update on clinical presentations, histological and genetic underpinnings and treatment considerations. Movement Disorders. 2012;27(1):42–53. doi: 10.1002/mds.23971. [DOI] [PubMed] [Google Scholar]
- 178.Avramovich-Tirosh Y, Reznichenko L, Amit T, et al. Neurorescue activity, APP regulation and amyloid-β peptide reduction by novel multi-functional brain permeable iron-chelating-antioxidants, M-30 and green tea polyphenol, EGCG. Current Alzheimer Research. 2007;4(4):403–411. doi: 10.2174/156720507781788927. [DOI] [PubMed] [Google Scholar]
- 179.Reznichenko L, Amit T, Zheng H, et al. Reduction of iron-regulated amyloid precursor protein and β-amyloid peptide by (-)-epigallocatechin-3-gallate in cell cultures: implications for iron chelation in Alzheimer's disease. Journal of Neurochemistry. 2006;97(2):527–536. doi: 10.1111/j.1471-4159.2006.03770.x. [DOI] [PubMed] [Google Scholar]
- 180.Pirker KF, Baratto MC, Bososi R, Goodman BA. Influence of pH on the speciation of copper (II) in reactions with the green tea polyphenols, epigallocatechin gallate and gallic acid. Journal of Inorganic Biochemistry. 2012;112:10–16. doi: 10.1016/j.jinorgbio.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dairam A, Fogel R, Daya S, Limson JL. Antioxidant and iron-binding properties of curcumin, capsaicin, and S-allylcysteine reduce oxidative stress in rat brain homogenate. Journal of Agricultural and Food Chemistry. 2008;56(9):3350–3356. doi: 10.1021/jf0734931. [DOI] [PubMed] [Google Scholar]
- 182.Du XX, Xu HM, Jiang H, Song N, Wang J, Xie JX. Curcumin protects nigral dopaminergic neurons by iron-chelation in the 6-hyroxydopamine rat model of Parkinson’s disease. Neuroscience Bulletin. 2012;28(3):253–258. doi: 10.1007/s12264-012-1238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tripanichkul W, Jaroensuppaperch EO. Curcumin protects nigrostriatal dopaminergic neurons and reduces glial activation in 6-hyroxydopmanine hemiparkinsonian mice model. The International Journal of Neuroscience. 2012;122(5):263–270. doi: 10.3109/00207454.2011.648760. [DOI] [PubMed] [Google Scholar]
- 184.Wang J, Du XX, Jiang H, Xie JX. Curcumin attenuates 6-hydroxydopamine-induced cytotoxicity by anti-oxidation and nuclear factor-kappaB modulation in MES23.5 cells. Biochemical Pharmacology. 2009;78(2):178–183. doi: 10.1016/j.bcp.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 185.Ren J, Fan C, Chen N, Huang J, Yang Q. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf-2 and HO-1 in rats. Neurochemical Research. 2011;36(12):2352–2362. doi: 10.1007/s11064-011-0561-8. [DOI] [PubMed] [Google Scholar]
- 186.Wang J, Xu H, Jiang H, Du X, Su P, Xie J. Neurorescue effect of rosmaric acid on 6-hyroxydopamine-lesioned nigral dopamine neurons in rat model of Parkinson’s disease. Journal of Molecular Neuroscience. 2012;47(1):113–119. doi: 10.1007/s12031-011-9693-1. [DOI] [PubMed] [Google Scholar]
- 187.Rambold AS, Miesbauer M, Olschewski D, et al. Green tea extracts interfere with the stress-protective activity of PrPC and the formation of PrPSc. Journal of Neurochemistry. 2008;107(1):218–229. doi: 10.1111/j.1471-4159.2008.05611.x. [DOI] [PubMed] [Google Scholar]
- 188.Jeong JK, Moon MH, Bae BC, et al. Autophagy induced by resveratrol prevents human prion protein mediated neurotoxicity. Neurochemical Research. 2012;73(2):99–105. doi: 10.1016/j.neures.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 189.Bizat N, Peyrin JM, Haïk S, et al. Neuron dysfunction is induced by prion protein with an insertional mutation via a fyn kinase and reversed by sirtuin activation in Caenorhabditis elegans . Journal of Neuroscience. 2010;30(15):5394–5403. doi: 10.1523/JNEUROSCI.5831-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hafner-Bratkovic I, Gaspersic J, Smid LM, Bresjanac M, Jerala R. Neuron dysfunction is induced by prion protein with an insertional mutation via a Fyn kinase and reversed by sirtuin activation in Caenorhabiditis elegans . Journal of Neurochemistry. 2010;30(15):5394–5403. doi: 10.1523/JNEUROSCI.5831-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Khan MTH, Orhan I, Şenol FS, et al. Cholinesterase inhibitory activities of some flavonoid derivatives and chosen xanthone and their modecular docking studies. Chemical Biological Interactions. 2009;181(3):383–389. doi: 10.1016/j.cbi.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 192.Girones-Vilaplana A, Valentao P, Andrade PB, Ferreres F, Moreno DA, Garcia-Viguera C. Phytochemical profile of a blend of black chokeberry and lemon juice with cholinesterase inhibitory effect and antioxidant potential. Food Chemistry. 2012;134:2090–2096. doi: 10.1016/j.foodchem.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 193.Ryu HW, Curtis-Long MJ, Jung S, et al. Anticholinesterase potential of flavonols from paper mulberry (Broussonetia papyrifera) and their kinetic studies. Food Chemistry. 2012;132(3):1244–1250. doi: 10.1016/j.foodchem.2011.11.093. [DOI] [PubMed] [Google Scholar]
- 194.Papandreou MA, Dimakopoulou A, Linardaki ZI, et al. Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behavioural Brain Research. 2009;198(2):352–358. doi: 10.1016/j.bbr.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 195.Cho JK, Ryu YB, Curtis-Long MJ, et al. Cholinesterase inhibitory effects of geranylated flavonoids from Paulownia tomentosa fruits. Bioinorganic and Medicinal Chemistry. 2012;20(8):2595–2602. doi: 10.1016/j.bmc.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 196.Choi GN, Kim JH, Kwak JH, et al. Effect of quercetin on learning and memory performance in ICR mice under neurotoxic trimethyltin exposure. Food Chemistry. 2012;132(2):1019–1024. [Google Scholar]
- 197.Tota S, Awasthi H, Kamat PK, Nath C, Hanif K. Protective effect of quercetin against intracerebral streptozotocin induced reduction in cerebral blood flow and impairment of memory in mice. Behavioural Brain Research. 2010;209(1):73–79. doi: 10.1016/j.bbr.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 198.Akkol EK, Orhan IO, Yesilada E. Anticholinesterase and antioxidant effects of the ethanol extract, ethanol fractions and isolated flavonoids from Cistus laurifolius L. leaves. Food Chemistry. 2012;131(2):626–631. [Google Scholar]
- 199.Porfírio S, Falé PLV, Madeira PJA, Florêncio MH, Ascensão L, Serralheiro MLM. Antiacetylcholinesterase and antioxidant activities of Plectranthus barbatus tea, after in vitro gastrointestinal metabolism. Food Chemistry. 2010;122(1):179–187. [Google Scholar]
- 200.Guo AJY, Xie HQ, Choi RCY, et al. Galangin, a flavonol derived from Rhizoma Alpiniae Officinarum, inhibits acetylcholinesterase activity in vitro . Chemico-Biological Interactions. 2010;187(1–3):246–248. doi: 10.1016/j.cbi.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 201.Xiao J, Chen X, Zhang L, Talbot SG, Li GC, Xu M. Investigation of the Mechanism of enhances effect of EGCG on huperzine A’s inhibition of acetylcholinesterase activity in rats by a multispectroscopic method. Journal of Agricultural and Food Chemistry. 2008;56:910–915. doi: 10.1021/jf073036k. [DOI] [PubMed] [Google Scholar]
- 202.Zhang L, Cao H, Wen J, Xu M. Green tea polyphenol (-)-epigallocatechin-3-gallate enhances the inhibitory effect of huperzine A on acetylcholinesterase by increasing the affinity with serum albumin. Nutritional Neuroscience. 2009;12(4):142–148. doi: 10.1179/147683009X423283. [DOI] [PubMed] [Google Scholar]
- 203.Lou H, Fan P, Perez RG, Lou H. Neuroprotective effects of linarin through activation of the PI3K/Akt pathway in amyloid-β-induced neuronal cell death. Bioorganic and Medicinal Chemistry. 2011;19(13):4021–4027. doi: 10.1016/j.bmc.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 204.Huang SM, Tsai SY, Lin JA, Wu CH, Yen GC. Cytoprotective effects of hesperetin and hesperidin against amyloid β-induced impairment of glucose transport through downregulation of neuronal autophagy. Molecular Nutrition and Food Research. 2012;56(4):601–609. doi: 10.1002/mnfr.201100682. [DOI] [PubMed] [Google Scholar]
- 205.Wu Y, Li X, Zhu JX, et al. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals. 2011;19(3):163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li Z, Pang L, Fang F, et al. Resveratrol attenuates brain damage in a rat model of focal ischemia via up-regulation of hippocampal Bcl-2. Brain Research. 2012;1450:116–124. doi: 10.1016/j.brainres.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 207.Kairisalo M, Bonomo A, Hyrskyluoto A, et al. Resveratrol reduces oxidative stress and cell death and increases mitochondrial antioxidants and XIAP in PC6.3-cells. Neuroscience Letters. 2011;488(3):263–266. doi: 10.1016/j.neulet.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 208.Ploai C, Antoniou X, Sclip A, et al. JNK plays a key role in tau hyperphosphorylation in Alzheimet’s disease models. Journal of Alzheimer’s Research. 2011;26(2):315–329. doi: 10.3233/JAD-2011-110320. [DOI] [PubMed] [Google Scholar]
- 209.Erlank H, Elmann A, Kohen R, Kanner J. Polyphenols activate Nrf2 in astrocytes via H2O2, semiquinones and quinones. Free Radical Biology and Medicine. 2011;51(12):2319–2327. doi: 10.1016/j.freeradbiomed.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 210.Zhang W, Go ML. Methoxylation of resveratrol: effects on induction of NAD(P)H Quinone-oxidoreductase 1 (NQO1) activity and growth inhibitory properties. Bioorganic and Medicinal Chemistry Letters. 2011;21(3):1032–1035. doi: 10.1016/j.bmcl.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 211.Karbowski M, Jeong SY, Youle RJ. Endophilin B1 is required for the maintenance of mitochondrial morphology. Journal of Cell Biology. 2004;166(7):1027–1039. doi: 10.1083/jcb.200407046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ahn JI, Jeong KJ, Ko MJ, et al. Changes of miRNA and mRNA expression in HepG2 cells treated by epigallocatechin gallate. Molecular and Cellular Toxicology. 2010;6(2):169–177. [Google Scholar]
- 213.Wallace CHR, Baczkó I, Jones L, Fercho M, Light PE. Inhibition of cardiac voltage-gated sodium channels by grape polyphenols. British Journal of Pharmacology. 2006;149(6):657–665. doi: 10.1038/sj.bjp.0706897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ma D, Zerangue N, Raab-Graham K, Fried SR, Jan YN, Jan LY. Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and heart. Neuron. 2002;33(5):715–729. doi: 10.1016/s0896-6273(02)00614-1. [DOI] [PubMed] [Google Scholar]
- 215.Yow TT, Pera E, Absalom N, et al. Naringin directly activates inwardly rectifying potassium channels at an overlapping binding site to tertiapin-Q. British Journal of Pharmacology. 2011;163(5):1017–1033. doi: 10.1111/j.1476-5381.2011.01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang JH, Cheng J, Li CR, Ye M, Ma Z, Cai F. Modulation of Ca2+ signals by epigallocatechin-3-gallate(EGCG) in cultured rat hippocampal neurons. International Journal of Molecular Sciences. 2011;12(1):742–754. doi: 10.3390/ijms12010742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shin DH, Seo EY, Pang B, et al. Inhibition of Ca2+-release-activated Ca2+ channel (CRAC) and K+ channels by curcumin in Jurkat-T cells. Journal of Pharmacological Sciences. 2011;115(2):144–154. doi: 10.1254/jphs.10209FP. [DOI] [PubMed] [Google Scholar]
- 218.Wu YL, Ohsaga A, Oshiro T, et al. Suppressive effects of red wine polyphenols on voltage-gated ion channels in dorsal root ganglionic neuronal cells. Tohoku Journal of Experimental Medicine. 2005;206(2):141–150. doi: 10.1620/tjem.206.141. [DOI] [PubMed] [Google Scholar]


