Abstract
The visual cortex has been traditionally considered as a stimulus-driven, unimodal system with a hierarchical organization. However, recent animal and human studies have shown that the visual cortex responds to non-visual stimuli, especially in individuals with visual deprivation congenitally, indicating the supramodal nature of the functional representation in the visual cortex. To understand the neural substrates of the cross-modal processing of the non-visual signals in the visual cortex, we firstly showed the supramodal nature of the visual cortex. We then reviewed how the nonvisual signals reach the visual cortex. Moreover, we discussed if these non-visual pathways are reshaped by early visual deprivation. Finally, the open question about the nature (stimulus-driven or top-down) of non-visual signals is also discussed.
1. Introduction
The visual cortex has been traditionally considered as a stimulus-driven, unimodal system with a hierarchical organization, in which the early visual areas (V1, V2) tune to general features while the higher-tier ones (V3A, V4v, V7, hMT+, and V8) respond selectively to the specific features of a visual stimulus [1–5]. Two parallel visual streams have been proposed to generalize the hierarchical organization of the visual processing [6–8]. The dorsal stream or “where” pathway serves to analyze visual spatial information about object location, motion, and visuomotor planning. In this pathway, visual signals are conveyed to the posterior parietal cortex through the dorsal part of the visual cortex (such as the V3d, V3A, V7, and hMT+) and finally reach the prefrontal cortex. The ventral stream or “what” pathway has been associated with the processing of form, object identity, and color. This pathway conveys visual signals along the ventral part of visual cortex (such as VP, V4, and V8), the inferior temporal (IT) areas, and finally to the prefrontal cortex.
The structural and functional organization of the visual areas is supposed to develop through a combination of genetic instruction [9–11] and experience-dependent refinement [12, 13]. The role of visual experience in the development of the visual areas is supported by a large number of neuroimaging studies revealing that the visual areas of congenitally blind (CB) and early blind (EB) subjects have increased cortical thickness [14–17], local brain spontaneous activity [18], metabolism, and blood flow [19–22] and decreased regional volume [23–25], white matter integrity [26, 27], anatomical network efficiency [28, 29], and altered resting-state functional connectivity (rsFC) [30, 31]. Moreover, converging evidence suggests that both the early and higher-tier visual areas in CB subjects are recruited during performing a variety of tasks given through nonvisual sensory modalities, as detailed in previous reviews [32–36].
However, the notion of the visual cortex as a unimodal system molded only by visual experience has recently been challenged because the visual cortex of both the sighted controls (SC) and the CB responded to a variety of nonvisual perceptive stimuli, including tactile, auditory, and olfactory. Furthermore, the visual cortex of the CB was also involved in cognitive processes, such as linguistic processing, working memory, and attention. Although the extent and magnitude of the activation in the visual areas depend on the tasks and subjects' characteristics [37, 38], the coactivation of several visual areas by nonvisual tasks in SC and CB highly indicates that the development of the functional organization of these visual areas does not require visual experience.
The main topic of this review is to elucidate how the nonvisual signals recruit the visual cortex. We firstly provide evidence if the visual cortex is supramodal in nature, and then we reviewed how the nonvisual signals reach the visual cortex. Next, we discussed if these nonvisual pathways are reshaped by early visual deprivation. Finally, we also discussed about the nature (stimulus-driven or top-down) of nonvisual signals.
2. The Supramodal Nature of Visual Cortex
2.1. Cross-Modal Processing of Nonvisual Signals in the Visual Cortex in Sighted Subjects
2.1.1. Tactile Stimuli Activate the Visual Cortex in Sighted Subjects
Unimodal theory supposes that the visual cortex is specifically allocated to process visual stimuli in sighted people; however, this hypothesis has recently been challenged. Using the positron emission tomography (PET), Sathian et al. first reported that the extrastriate area close to the parietooccipital fissure (V6) was activated during discrimination of grating orientation compared with discrimination of grating groove width, suggesting this visual area is recruited in the processing spatial information of tactile signals [39]. To further confirm that the occipital area is functionally involved in nonvisual processing, the transcranial magnetic stimulation (TMS) technique was used to transiently disrupt the functioning of this area. The authors found impaired tactile discrimination of grating orientation after exerting TMS on this occipital area and concluded that this occipital area is really functionally involved in tactile spatial perception [40]. Since then, many studies have reported the involvement of visual areas in a series of tactile processing, including the hMT+ complex for tactile motion perception [37, 41] and the ventral visual pathway for tactile object discrimination [42–49]. It is interesting to note that the hMT+ is capable of processing motion-related information even when the stimulus is delivered to the tongue [50]. Furthermore, several studies using both visual and tactile stimuli showed that these two modalities drive the same visual areas for motion and object processing, supporting the cross-modal involvement of visual areas in the abstract representation of the concepts of objects, space, and motion [38, 42].
2.1.2. Auditory Stimuli Activate the Visual Cortex in Sighted Subjects
The cross-modal recruitment of the visual cortex has also been reported in auditory domain. As early as 1972, Morrell found that up to 41% of recorded neurons in extrastriate cortex of adult cats were modulated by both visual and auditory stimuli and that the receptive fields for both responses typically overlapped in space [51]. Recent studies in humans have also provided evidence of occipital activation in auditory processing [52, 53]. For example, the hMT+ complex was activated in sighted subjects while listening to auditory motion stimuli [52, 54, 55]. In accordance with the neuroimaging findings, several TMS studies showed that transient disruption of the specific occipital regions can impair the auditory perception in sighted subjects, such as inhibition of the extrastriate cortex induced a systematic error in auditory spatial perception [56], and disruption of the dorsal extrastriate cortex impaired the sound localization [57, 58]. The TMS evidence supports that the visual cortex is involved in spatial hearing in sighted subjects. It is also interesting to note that the visual cortex in sighted subjects cannot only percept the sound itself but also response to abstract auditory information such as action sounds [59].
2.2. Cross-Modal Processing of Nonvisual Signals in the Visual Cortex in Early Blind Subjects
Relative to the sighted subjects, cross-modal processing of nonvisual signals in the visual cortex has been more extensively reported in the CB and EB subjects when they perform nonvisual perception and high-order cognitive tasks.
2.2.1. Tactile Perception
Numerous studies reported that the visual cortex was recruited during diverse tactile tasks, such as the early and higher visual areas were activated in vibrotactile frequency discrimination [60], the hMT+ in tactile motion perception [37, 50, 61], and the ventral visual pathway in tactile object perception [38]. The visual cortex is also involved in tactile perception of the tongue [42, 62, 63]. Using the tongue display device (TDU), a tactile-to-vision sensory substitution device that translates a visual image into electrotactile stimulation, several studies have shown that the specific visual areas were recruited to process different types of tongue tactile stimuli, such as the ventral stream for tactile-form recognition [42], the hMT+ complex for tactile motion discrimination [50], and the ventral lateral occipitotemporal cortex for virtual route recognition [62]. Moreover, rTMS inhibition of the human hMT+ impaired the tactile speed discrimination, indicating that the recruitment of hMT+ is necessary for tactile motion processing [41]. Interestingly, TMS stimulation of the visual cortex can induce subjective tongue-tactile sensations in the CB who is proficient at the use of the TDU, which indicates that the perceptual correlate of activity in the visual cortex reflects the characteristics of its novel sensory input source [64].
2.2.2. Auditory Perception
Similar with the tactile perception, the activation of the visual cortex by auditory perception was also frequently reported in CB subjects [54, 65, 66]. In an early PET study, Weeks et al. [67] reported that the right dorsal visual cortex was activated by auditory perception task in the CB but not in the SC. This region was also activated in EB subjects by an auditory spatial processing task using a sensory substitution prosthesis translating visual information into sounds [65]. The activation pattern in EB was also confirmed by recent studies [66, 68, 69]. Accordingly, the visual areas previously considered to be involved in visual motion processing (such as the hMT+) were specifically recruited in the EB by motion stimuli presented through the auditory modality [50, 54, 55, 70, 71]. The ventral pathway can be recruited to process auditory object recognition [72, 73] in the CB. In a recent study, Striem-Amit et al. [74] showed that the dorsal stream processed the location information, whereas the ventral stream responded to shape information via the vision-to-sound substitutes in CB subjects. The dorsal and ventral pathway recruited by auditory perception was further confirmed by electrophysiological and TMS evidence. Using ERP, several groups showed greater amplitude of the N1 component at the visual region in sound localization, suggesting the involvement of the visual cortex in early auditory processing in the EB [75–77]. TMS inhibition of the visual area can impair specific auditory performance in the EB [57, 78, 79]. For example, rTMS delivered to right dorsal extrastriate cortex disrupted the spatial processing of sounds in the EB [57, 78], and rTMS over the LOC can impair a EB subject's ability to identify objects [79].
2.2.3. Olfactory Sensation
In the CB, besides auditory and tactile perception, the visual cortex was also recruited in olfactory processing [80]. In this study, a simple odor detection task, the authors found that CB subjects not only showed strong activation in the olfactory cortex, but also showed widespread activation in the visual cortex [80]. Combining earlier studies reported that superior olfactory perception in the CB [81, 82]; the recruitment of the visual cortex during odor detection suggests a preferential access of olfactory stimuli to this area in the CB.
2.2.4. Cognitive Processing
The visual cortex in EB subjects is not only involved in nonvisual perception, but also takes part in the process of higher-level cognitive tasks, such as language, attention, and working memory. Converging evidence supports the involvement of the visual cortex in language processing in the CB or EB subjects. The medial visual cortex was recruited during Braille reading [83–85] and the occipitotemporal visual areas were activated during covert verb generation in the EB [86, 87]. Further studies reveal that the visual cortex is preferentially recruited by semantic relative to phonological processing [88], and the magnitude of fMRI activation is associated with both semantic and syntactic complexity [89]. A recent study showed that the visual word form area (VWFA), a component of the ventral stream that develops expertise for visual reading, can also process Braille reading in the EB [90]. Further evidence for the involvement of the visual cortex in language processing in the EB has been provided by a combination of task activation and functional connectivity analyses [91]. The authors found that (1) the responses of the visual regions and classic language regions across conditions were similar; (2) language sensitivity was restricted to the left visual cortex; (3) the left visual regions that responded to language had increased functional connectivity with classic language regions [91]. Besides the neuroimaging findings, in the CB, disruption of the visual cortex by TMS or lesions impairs the performance of Braille reading and verb generation [84, 92–95].
Besides the language processing, the visual cortex that normally subserves vision is activated in the CB subjects when performing nonvisual attention-demanding tasks, such as spatial attention discrimination [32, 35, 36]. More importantly, the amplitude of the occipital activation in the CB was correlated with the spatial attention performance [66, 96, 97]. These findings suggest that the occipital activation is associated with the enhanced nonvisual attention abilities in the CB.
The visual cortex can also be activated by memory task with nonvisual stimuli or without any sensory input [98–101]. The posterior occipital region (including V1) was recruited during a verbal memory task even without real sensory stimulation in the CB, and the activation magnitude of this region was correlated with verbal memory performance [101]. Bonino et al. reported that tactile spatial working memory task activated the dorsal extrastriate areas in the CB individuals [100]. Moreover, using three different kinds of working memory tasks (verbal, tactile, and auditory), a recent study showed that the visual cortex of the EB subjects responded to all types of stimuli [99].
2.3. Supramodal versus Plastic Mechanisms of the Occipital Activation
Two neural mechanisms have been proposed to explain the involvement of the visual cortex in the processing of nonvisual stimuli. One hypothesis holds that the visual cortex is supramodal in nature, which means that an occipital area relies on a common, abstract representation of the perceived stimuli irrespective of the sensory modality. Another hypothesis is the cross-modal plasticity. In the CB or EB, the visual cortex that normally serves to process visual input shifts to cross-modal process nonvisual information via plastic reorganization of the inner structure and function. However, the two mechanisms are not mutually exclusive, and they might coexist in the EB.
As discussed above, many pieces of evidence that showed the involvement of the visual areas in processing nonvisual inputs in both the SC and EB may support the supramodal hypothesis (see Sections 2.1 and 2.2). The cross-modal involvement of the visual cortex in processing nonvisual stimuli means that the functional specialization of the visual areas is task-dependent rather than sensory modality-dependent. Furthermore, this pattern cannot be fully explained by visual imagery [102–108] because the CB subjects who never have visual experience also show occipital response to the nonvisual stimuli [69, 80]. The supramodal hypothesis can explain that visual experience is not necessary to develop the normal functional organization of the visual areas, which has been confirmed in a variety of previous studies on the CB [74, 90, 109, 110], because the development of the functional organization may be driven by inputs from other sensory modalities.
The cross-modal plasticity is supported by the following evidence. The CB subjects commonly showed superior performance during auditory or tactile perception than normal sighted subjects [97, 101, 111–115]. The superior performance has also been associated with the occipital activation in the CB [96, 97, 101], suggesting the hypothesis of the cross-modal plasticity. This mechanism can also be applied to explain the involvement of the visual cortex in the higher-level cognitive tasks in the CB but not in the SC [84, 92–95]. Furthermore, the plasticity mechanism may also partly explain the increased cortical thickness [14–17], local brain spontaneous activity [18], metabolism and blood flow [19–22], and rsFC [30, 31] in the EB.
Studies on the functional characteristics of the hMT+ have well described the coexistence of the two mechanisms in the EB. In sighted subjects, the hMT+ complex is segregated into an anterior part (supramodal region), that is, involved in processing both visual and tactile motion, and a posterior part (unimodal region), which is only involved in processing visual information. In the EB, however, the entire hMT+ is involved in the representation of tactile motion, suggesting the coexistence of the supramodal (anterior part) and plastic (posterior part) mechanisms in this region. These results represent competitive interactions between visual and nonvisual inputs in the reshape of hMT+ complex [37, 116].
It should be noted that the improved nonvisual perception performance in the early blind subjects can also be the consequences of the experience-dependent plasticity of their auditory or somatosensory related cortices. For example, the mice that are binocularly enucleated from birth demonstrate remarkable expansion of their barrel cortex, which may be interpreted by increased usage of the whiskers after visual deprivation [117]. Cats that were deprived of vision from birth also show expanded primary somatosensory and auditory areas; furthermore, the neurons in the anterior ectosylvian visual area (AEV) that normally respond to visual stimuli are replaced by neurons of neighboring auditory ectosylvian area (AEA), which is accompanied by the improvement of auditory spatial tuning of this region than sighted controls [118, 119]. As a result, the improved auditory/tactile perceptive performance might both be caused by the experience-dependent plasticity of the classic auditory/tactile regions with expanded cortical area, and by classic visual regions that turn to subserve the nonvisual information. Caution should be paid that some cortical regions such as the “AEV” in the congenitally blind subjects are actually replaced by expanded auditory cortices and their activation by auditory stimuli cannot be interpreted as cross-modal involvement of visual areas any more.
3. The Candidate Pathways That Nonvisual Signals Reach the Visual Cortex
As discussed above, much evidence supports the cross-modal processing of nonvisual signals in the visual cortex. It is then important to understand how the nonvisual sensory information reaches the occipital areas, especially in the CB subjects who have no visual experience about the external world. The candidate pathways include the thalamooccipital and corticooccipital pathway, which is categorized in Figures 1 and 2.
Figure 1.
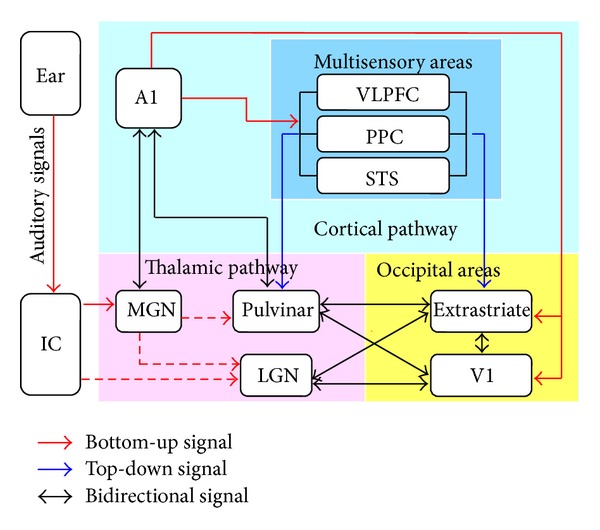
Schematic of neural pathways that convey auditory signals into visual areas. The solid line represents the existing connections in the normal sighted animals or human; dash line represents the rewired connections after early visual deprivation; arrows with red, blue, and black color represent the bottom-up, top-down, and bidirectional auditory signals, respectively. All these connections are confirmed by previous animal or human studies (for details see Section 3). A1: primary auditory cortex; IC: inferior colliculus; MGN: medial geniculate nucleus; LGN: lateral geniculate nucleus; PPC: post parietal cortex; STS: superior temporal cortex; VLPFC: ventral lateral prefrontal cortex; V1: primary visual cortex.
Figure 2.
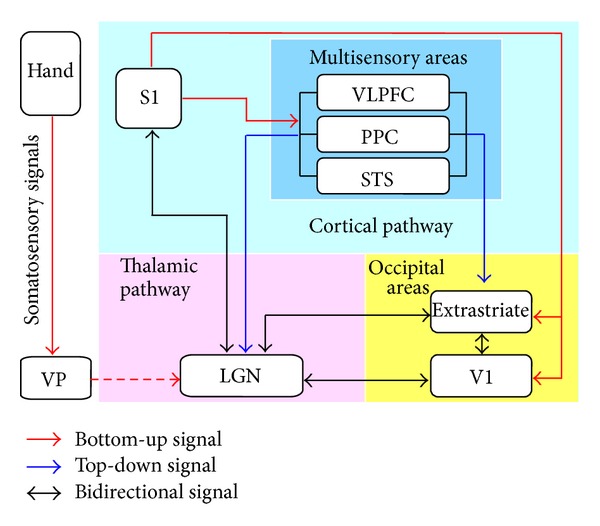
Schematic of neural pathways that convey somatosensory signals into visual areas. The solid line represents the existing connections in the normal sighted animals or human; dash line represents the rewired connections after early visual deprivation; arrows with red, blue, and black color represent the bottom-up, top-down, and bidirectional somatosensory signals, respectively. LGN: lateral geniculate nucleus; PPC: post parietal cortex; S1: primary somatosensory cortex; STS: superior temporal cortex; VLPFC: ventral lateral prefrontal cortex; V1: primary visual cortex; VP: ventral posterior nuclei.
3.1. Thalamooccipital Pathway
The thalamus is an important relay that receives afferents from different sensory organs and sends efferents to the primary sensory cortex. Under normal condition, the thalamic nuclei are relay stations via which sensory information from the peripheral sensory receptors can reach the primary sensory cortex. For example, the lateral geniculate nucleus (LGN), the “visual” thalamic relay, mainly transfers visual signals to the primary visual cortex (V1); the medial geniculate nucleus (MGN) is the “auditory” thalamic relay that connects the inferior colliculus (IC) and the primary auditory cortex (A1); and the ventral posterior nuclei (VP) is the “somatosensory” thalamic relay that receives tactile signals and transmits them to the primary somatosensory cortex (S1) [120, 121]. Furthermore, the thalamus can also receive feedback signals from the sensory cortex [122–125] and the associate cortex [126–132] to modulate the input signals. It is hypothesized that nonvisual signals may bypass the traditional sensory pathway and “rewire” into the “visual” thalamus and project to the V1. This hypothesis is supported by the findings that the LGN receives rewired auditory projections from the inferior colliculus [133–139] and the MGN [140], receives rewired somatosensory projections from the VP [140, 141], and then projects efferent fibers to the visual areas in enucleated animals since birth [142].
Recent findings have shown that the LGN and its output projections to the V1 are atrophied in early blind subjects [24, 26, 27], which seems to contradict with this hypothesis. One putative explanation is that the atrophy of the LGN and the retinofugal pathway is the consequence of the interaction between disused neurodegeneration of the “visual” part of the pathway and cross-modal plasticity of the “rewired” nonvisual part of the pathway. In fact, pervious animal experiments have shown that the visual projections to the dorsal lateral geniculate nucleus are dramatically reduced in blind animals, while the auditory projections to the same region are strengthened [133, 142]. Another possibility is that the nonvisual signals bypass traditional retinofugal pathway (from the LGN to the V1) and pass through the pulvinar-occipital pathway [143–146], or from the LGN to the higher visual areas (such as the hMT+ and V4) [147–150]. In agreement with this interpretation, a recent fMRI study in monkeys demonstrates that direct LGN projections to the extrastriate cortex have a critical functional contribution to blindsight with V1 lesions [151]; a human study shows a direct anatomical connection between the thalamus and the hMT+ complex, that would directly convey motion information to the hMT+, thereby bypassing the V1 [147].
3.2. Corticooccipital Pathway
An alternative pathway is that the visual areas receive nonvisual sensory information through corticooccipital connections between these sensory modalities [140, 152, 153]. These corticooccipital connections can be further subdivided into direct corticooccipital connections and indirect polysynaptic corticooccipital connections. The former has been found between the A1 and visual cortex in adult Mongolian gerbils [154], cats [155], primates [153, 156, 157], humans [158], and congenitally blind opossums [140], and between S1 and V1 in enucleated opossums [140]. The latter has been indicated by studies showing multisensory processing in the association cortices [159], such as the posterior parietal area (PPA) [160, 161], superior temporal sulcus (STS) [162–164], ventral lateral prefrontal cortex (VLPFC) [165–167], and extrastriate areas [155, 168, 169]. The dense anatomical connections between multisensory areas and both the visual and nonvisual sensory cortices have been identified in both animal and human studies [155, 159, 163, 170, 171].
The corticooccipital pathway hypothesis is also supported by task-based fMRI studies [165, 172] and a resting-state fMRI study [173] in sighted subjects, an effective connectivity study [174], and TMS studies in sighted and EB subjects [23, 78, 175, 176]. In a recent study by our group, we found the rsFC between the early visual areas and S1 was dramatically decreased, while those between the higher-tier visual areas and S1, and between the early and higher-tier visual areas, were relatively preserved or even strengthened in the CB. Our findings support the hypothesis of the indirect corticooccipital pathway mediating nonvisual sensory information to the early visual areas via the relay of higher-tier ones [17].
It should be noted that these two pathways cannot be absolutely segregated in the brain network. They are interacted with each other by feed forward and feed back projections. For example, the auditory signals can first feed forward to the A1 for initial processing, and then feed back to the MGN and multimodal thalamic nuclei (e.g., the pulvinar) [177], and finally project to the V1 (cortico-thalamooccipital pathway). The multisensory areas such as the PPA and VLPFC also project efferents to the multimodal thalamus (the pulvinar and medial dorsal nucleus) [127–131], so they can convey the modulated nonvisual signals to the occipital area via the thalamus (Figure 1).
The existing thalamo-cortical and corticooccipital pathways found in the normal adult animals and humans provide anatomical evidence of the supramodal nature of the visual cortex. Auditory and tactile information can be conveyed to the occipital areas through the direct and indirect connections. This anatomical connections pattern can explain the cross-modal involvement of visual cortex by nonvisual sensory tasks; furthermore, it can explain the development of a portion of the visual areas does not depend on the visual experience because inputs from other sensory modalities are sufficient to support the development of these functional patterns.
4. Rewiring versus Unmasking of the Nonvisual Pathway after Visual Deprivation
Two competing hypotheses have been proposed to explain the neural mechanisms of cross-modal plasticity after early visual deprivation. According to the rewiring hypothesis, cross-modal brain responses are mediated by the formation of new pathways in the sensory deprived brain. For example, after experimental destruction of the superior colliculi and the visual cortex in neonatal hamsters, the authors observed a strong projection from the retina to the A1, which can “perceive” the visual information [178, 179]. Studies in animals have also shown that when the brain is deprived of peripheral visual input at an early age, auditory inputs are re-routed to the visual cortex via the thalamooccipital pathway [133, 139–141]. However, this subcortical rewired pathway is questioned because of the lack of in vivo evidence. In contrast, there are considerable studies showed that the whole segments of retinofugal pathway, including the optic tract, the LGN, and optic radiation, suffered atrophy and loss of integrity in humans after early visual deprivation [16, 23, 27].
The unmasking hypothesis proposed that the loss of a sensory input induces unmasking and/or strengthening of the existing neural pathways. As discussed in Sections 3.1 and 3.2, the tactile and auditory inputs can be conveyed to the visual cortex via the existing thalamooccipital pathway or corticooccipital pathway that have been confirmed in normal adult animals and humans. Generally, these nonvisual signals can modulate the processing of visual information in sighted subjects [180]; however, they cannot induce subjective nonvisual sensations and occipital activation due to being masked by the dominant visual input [40, 64, 176]. The occasional findings of occipital processing nonvisual signals might be task-dependent that dramatically reduced the masking effects of the visual input [40, 52, 55]. However, after early visual deprivation, nonvisual processing in the visual cortex is strengthened or unmasked because of the lack of visual input. The unmasking hypothesis is also supported by the cross-modal responses after short-term visual deprivation (blindfolding). Several hours to days blindfolding resulted in rapid, reversible improvement in task performance and recruitment of the visual cortex in nonvisual processing, such as tactile discrimination [181, 182], Braille reading [183], and sound localization [184, 185]. Rapid cross-modal responses exclude the possibility that these are mediated by the establishment of new anatomical connections. This claim was also supported by sensory substitution devices (SSD) studies that the visual cortex was also involved in processing nonvisual tasks after a short period of SSD training in sighted subject [50, 72, 186]. It is possible that a short period of blindfolding and SSD training unmasks and strengthens pre-existing connections between the nonvisual and the occipital cortices.
5. Stimulus-Driven or Top-Down Control of the Nonvisual Processing in the Visual Cortex
As shown in Figure 1, nonvisual signals can reach the visual cortex via thalamooccipital pathway, corticooccipital pathway, or combinations of these two pathways. An important but unsolved question is the nature of these nonvisual signals: stimulus-driven or top-down. The stimulus-driven signals refer to those from sensory organs or early sensory cortex, whereas the top-down signals are refer to those who came from the higher-level cortical regions. Clarifying this question can help us to understand the neural mechanisms underlying cross-modal recruitment of the visual cortex and to design appropriate interventions to improve the adaptive capacity of blind subjects to the external environments.
5.1. Stimulus-Driven Hypothesis
The following evidence supports stimulus-driven nature of the nonvisual signals that reach the visual cortex. These nonvisual signals can be conveyed from sensory organs to the visual cortex via rewired thalamic-cortical pathway [133–139] and those from the nonvisual primary sensory cortices via the direct corticooccipital connections (such as from A1 to V1) [153, 156, 157], which bypass the higher-tier “cognitive” cortex, so the inputted nonvisual signals may not be modulated and reflect the pure stimulus-driven information. The involvement of the visual cortex in nonvisual perception in both sighted [45, 46, 72] and EB [37, 42, 50, 61–63, 66, 68, 69, 80] subjects also suggests the stimulus-driven nature of these nonvisual signals because the top-down effects such as visual imagery and attention were well controlled in these studies. The event-related potentials (ERPs) studies demonstrated that the N1 component of occipital response following nonvisual stimulation was as early as the typical component of the visual perception in EB subjects [76, 77, 187]. For example, a recent report showed that the shape-selective activity in the LOC was present as early as 150 ms following the onset of tactile stimulation, which support the stimulus-driven somatosensory input to the LOC [187].
5.2. Top-Down Hypothesis
According to the top-down mechanism, the peripheral auditory/tactile signals are firstly modulated and refined by the higher-level cortical regions, such as the multisensory associate areas (VLPFC, PPA, and STS), and then feed back to the visual cortex through the indirect corticooccipital pathway and cortico-thalamooccipital pathway (Figure 1). The existing anatomical feedback projections from higher-level cortical regions to the thalamus and visual cortex support this top-down mechanism [132, 155, 159, 163, 170, 171]. Furthermore, many task-evoked and lesion studies have demonstrated the modulation effects of the fronto-parietal network on the thalamus and visual cortex [165, 188–191]. It seems that in CB subjects, the top-down attention modulation of the occipital activity was strengthened [75, 96, 192–195]. Visual imagery, a complex mental process, can also activate the specific visual areas that are usually recruited by certain visual properties (shape, space, and color), which supports the top-down controlling the visual activities [102–108]. It should be noted that the earlier ERP response cannot exclude the effects of top-down modulation, because top-down attention can also module the early components of ERP, for example, the N1 negativity or even earlier peak [196, 197]. Furthermore, a ERP study demonstrated that the top-down attention modulation of the occipital activity was significantly strengthened in EB subjects [75]. The following paragraphs state the two popular cognitive processes (visual imagery and attention) that might contribute to the top-down recruitment of the visual cortex.
5.2.1. Visual Imagery
Visual imagery mechanism supports the hypothesis of top-down recruitment of the visual cortex because the mental progress can recruit the visual cortex [102–108]. For example, Kosslyn et al. showed that the visual areas 17 and 18/19 were activated by a stripe imagery task, and rTMS delivered to area 17 did disrupt both visual perception and imagery performance [104]. This study indicates that the early visual areas are involved in at least some forms of visual imagery as well as in visual perception [104]. It is interesting to noted that not only the early visual area, but also the higher-tier ones can specifically respond to the visual imagery task, including the ventral stream for shape imagery [47, 198–200], and the dorsal stream for motion and spatial imagery [107, 108, 201–203], which highly corresponded with the hierarchy representation of the visual perception. De Volder showed that in both sighted and EB subjects, auditory triggered mental imagery of shape can also activate the ventral occipitotemporal and visual association areas [200].
5.2.2. Attention Controlling
Top-down attention may be the most extensively studied cognitive process that can modulate the activation of the visual cortex [204–208]. It is proposed that visual attention modulates visual processing even at an early stage; it not only modulates the gain on incoming visual information, but also adds a pure top-down signal that increases baseline activity in the visual cortex; moreover, attentional modulation can exert on different aspects of visual perception, such as locations, features, objects, or a combination [208]. Furthermore, not only the visual attention, but also the nonvisual attention can also activate the visual cortex [207, 209–211].
In the CB subjects, electrophysiological or neuroimaging studies have revealed that the top-down attention modulation was strengthened when they performing tactile/auditory attention-demanding tasks [75, 111, 112, 212–215]. Additionally, the occipital cortical areas were activated in the CB subjects by attention tasks through nonvisual modalities [60, 96, 194, 216, 217]. In combination with evidence of attention modulation on visual perception in the SC [218–220], these findings indicate increased top-down attention modulation of occipital activity in the CB.
In summary, the nature of the nonvisual signals to the visual cortex may be either stimulus-driven or top-down, or both. Indeed, effective connectivity analysis offers evidence for the coexistence of both bottom-up and top-down information flows during nonvisual perception [221–223].
6. Conclusions
The cross-modal processing of nonvisual signals in the occipital areas in both sighted and EB subjects suggests that the functional organization of the visual cortex is supramodal in nature. The cross-modal plasticity can also account for parts of the findings in the EB subjects. The normally existing thalamo-cortical and corticooccipital pathways provide anatomical evidence for the supramodal nature of the visual cortex. The cross-modal plasticity in the EB might be driven by two neural mechanisms: rewiring and unmasking, although there is lack of in vivo evidence for the former. Further studies with more advanced in vivo imaging techniques should be implemented to clarify this issue. Finally, the nature of the nonvisual signals to the visual cortex may be either stimulus-driven or top-down, or both. Further understanding the issue may help us to design appropriate interventions to improve the adaptive capacity of blind subjects to the external environments.
Acknowledgments
This study was supported by the National Basic Research Program of China (973 Program, 2011CB707801) and the National Natural Science Foundation of China (81271564).
References
- 1.Grill-Spector K, Malach R. The human visual cortex. Annual Review of Neuroscience. 2004;27:649–677. doi: 10.1146/annurev.neuro.27.070203.144220. [DOI] [PubMed] [Google Scholar]
- 2.Wandell BA, Dumoulin SO, Brewer AA. Visual field maps in human cortex. Neuron. 2007;56(2):366–383. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex. 1991;1(1):1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 4.Golarai G, Ghahremani DG, Whitfield-Gabrieli S, et al. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nature Neuroscience. 2007;10(4):512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seymour K, Clifford CWG, Logothetis NK, Bartels A. Coding and binding of color and form in visual cortex. Cerebral Cortex. 2010;20(8):1946–1954. doi: 10.1093/cercor/bhp265. [DOI] [PubMed] [Google Scholar]
- 6.Haxby JV, Grady CL, Horwitz B, et al. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(5):1621–1625. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodale MA, David Milner A. Separate visual pathways for perception and action. Trends in Neurosciences. 1992;15(1):20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 8.Konen CS, Kastner S. Two hierarchically organized neural systems for object information in human visual cortex. Nature Neuroscience. 2008;11(2):224–231. doi: 10.1038/nn2036. [DOI] [PubMed] [Google Scholar]
- 9.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SH. Neuroscience: experience-driven plasticity of visual cortex limited by myelin and nogo receptor. Science. 2005;309(5744):2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putignano E, Lonetti G, Cancedda L, et al. Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron. 2007;53(5):747–759. doi: 10.1016/j.neuron.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Triplett JW, Owens MT, Yamada J, et al. Retinal input instructs alignment of visual topographic maps. Cell. 2009;139(1):175–185. doi: 10.1016/j.cell.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Van Hooser SD, Mazurek M, White LE, Fitzpatrick D. Experience with moving visual stimuli drives the early development of cortical direction selectivity. Nature. 2008;456(7224):952–956. doi: 10.1038/nature07417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yazaki-Sugiyama Y, Kang S, Cteau H, Fukai T, Hensch TK. Bidirectional plasticity in fast-spiking GABA circuits by visual experience. Nature. 2009;462(7270):218–221. doi: 10.1038/nature08485. [DOI] [PubMed] [Google Scholar]
- 14.Jiang J, Zhu W, Shi F, et al. Thick visual cortex in the early blind. Journal of Neuroscience. 2009;29(7):2205–2211. doi: 10.1523/JNEUROSCI.5451-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park H, Lee JD, Kim EY, et al. Morphological alterations in the congenital blind based on the analysis of cortical thickness and surface area. NeuroImage. 2009;47(1):98–106. doi: 10.1016/j.neuroimage.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 16.Bridge H, Cowey A, Ragge N, Watkins K. Imaging studies in congenital anophthalmia reveal preservation of brain architecture in “visual“ cortex. Brain. 2009;132(12):3467–3480. doi: 10.1093/brain/awp279. [DOI] [PubMed] [Google Scholar]
- 17.Qin W, Liu Y, Jiang T, Yu C. The development of visual areas depends differently on visual experience. PLoS ONE. 2013;8(1) doi: 10.1371/journal.pone.0053784.e53784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Liu Y, Li W, et al. Increased regional homogeneity of blood oxygen level-dependent signals in occipital cortex of early blind individuals. NeuroReport. 2011;22(4):190–194. doi: 10.1097/WNR.0b013e3283447c09. [DOI] [PubMed] [Google Scholar]
- 19.De Volder AG, Bol A, Blin J, et al. Brain energy metabolism in early blind subjects: neural activity in the visual cortex. Brain Research. 1997;750(1-2):235–244. doi: 10.1016/s0006-8993(96)01352-2. [DOI] [PubMed] [Google Scholar]
- 20.Mishina M, Senda M, Kiyosawa M, et al. Increased regional cerebral blood flow but normal distribution of GABAA receptor in the visual cortex of subjects with early-onset blindness. NeuroImage. 2003;19(1):125–131. doi: 10.1016/s1053-8119(03)00051-x. [DOI] [PubMed] [Google Scholar]
- 21.Veraart C, De Volder AG, Wanet-Defalque MC, Bol A, Michel C, Goffinet AM. Glucose utilization in human visual cortex is abnormally elevated in blindness of early onset but decreased in blindness of late onset. Brain Research. 1990;510(1):115–121. doi: 10.1016/0006-8993(90)90735-t. [DOI] [PubMed] [Google Scholar]
- 22.Uhl F, Franzen P, Podreka I, Steiner M, Deecke L. Increased regional cerebral blood flow in inferior occipital cortex and cerebellum of early blind humans. Neuroscience Letters. 1993;150(2):162–164. doi: 10.1016/0304-3940(93)90526-q. [DOI] [PubMed] [Google Scholar]
- 23.Ptito M, Schneider FCG, Paulson OB, Kupers R. Alterations of the visual pathways in congenital blindness. Experimental Brain Research. 2008;187(1):41–49. doi: 10.1007/s00221-008-1273-4. [DOI] [PubMed] [Google Scholar]
- 24.Noppeney U, Friston KJ, Ashburner J, Frackowiak R, Price CJ. Early visual deprivation induces structural plasticity in gray and white matter. Current Biology. 2005;15(13):R488–R490. doi: 10.1016/j.cub.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 25.Pan W, Wu G, Li C, Lin F, Sun J, Lei H. Progressive atrophy in the optic pathway and visual cortex of early blind Chinese adults: a voxel-based morphometry magnetic resonance imaging study. NeuroImage. 2007;37(1):212–220. doi: 10.1016/j.neuroimage.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Shimony JS, Burton H, Epstein AA, McLaren DG, Sun SW, Snyder AZ. Diffusion tensor imaging reveals white matter reorganization in early blind humans. Cerebral Cortex. 2006;16(11):1653–1661. doi: 10.1093/cercor/bhj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shu N, Li J, Li K, Yu C, Jiang T. Abnormal diffusion of cerebral white matter in early blindness. Human Brain Mapping. 2009;30(1):220–227. doi: 10.1002/hbm.20507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shu N, Liu Y, Li J, Li Y, Yu C, Jiang T. Altered anatomical network in early blindness revealed by diffusion tensor tractography. PLoS ONE. 2009;4(9) doi: 10.1371/journal.pone.0007228.e7228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Liu Y, Qin W, et al. Age of onset of blindness affects brain anatomical networks constructed using diffusion tensor tractography. Cereb Cortex. 2013;23(3):542–551. doi: 10.1093/cercor/bhs034. [DOI] [PubMed] [Google Scholar]
- 30.Yu C, Liu Y, Li J, et al. Altered functional connectivity of primary visual cortex in early blindness. Human Brain Mapping. 2008;29(5):533–543. doi: 10.1002/hbm.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Yu C, Liang M, et al. Whole brain functional connectivity in the early blind. Brain. 2007;130(8):2085–2096. doi: 10.1093/brain/awm121. [DOI] [PubMed] [Google Scholar]
- 32.Bavelier D, Neville HJ. Cross-modal plasticity: where and how? Nature Reviews Neuroscience. 2002;3(6):443–452. doi: 10.1038/nrn848. [DOI] [PubMed] [Google Scholar]
- 33.Fiehler K, Rösler F. Plasticity of multisensory dorsal stream functions: evidence from congenitally blind and sighted adults. Restorative Neurology and Neuroscience. 2010;28(2):193–205. doi: 10.3233/RNN-2010-0500. [DOI] [PubMed] [Google Scholar]
- 34.Sadato N. Chapter 11 Cross-modal plasticity in the blind revealed by functional neuroimaging. Supplements to Clinical Neurophysiology. 2006;59:75–79. doi: 10.1016/s1567-424x(09)70015-7. [DOI] [PubMed] [Google Scholar]
- 35.Sathian K, Stilla R. Cross-modal plasticity of tactile perception in blindness. Restorative Neurology and Neuroscience. 2010;28(2):271–281. doi: 10.3233/RNN-2010-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collignon O, Voss P, Lassonde M, Lepore F. Cross-modal plasticity for the spatial processing of sounds in visually deprived subjects. Experimental Brain Research. 2009;192(3):343–358. doi: 10.1007/s00221-008-1553-z. [DOI] [PubMed] [Google Scholar]
- 37.Ricciardi E, Vanello N, Sani L, et al. The effect of visual experience on the development of functional architecture in hMT+ Cerebral Cortex. 2007;17(12):2933–2939. doi: 10.1093/cercor/bhm018. [DOI] [PubMed] [Google Scholar]
- 38.Pietrini P, Furey ML, Ricciardi E, et al. Beyond sensory images: object-based representation in the human ventral pathway. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(15):5658–5663. doi: 10.1073/pnas.0400707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sathian K, Zangaladze A, Hoffman JM, Grafton ST. Feeling with the mind’s eye. NeuroReport. 1997;8(18):3877–3881. doi: 10.1097/00001756-199712220-00008. [DOI] [PubMed] [Google Scholar]
- 40.Zangaladze A, Epstein CM, Grafton ST, Sathian K. Involvement of visual cortex in tactile discrimination orientation. Nature. 1999;401(6753):587–590. doi: 10.1038/44139. [DOI] [PubMed] [Google Scholar]
- 41.Ricciardi E, Basso D, Sani L, et al. Functional inhibition of the human middle temporal cortex affects non-visual motion perception: a repetitive transcranial magnetic stimulation study during tactile speed discrimination. Experimental Biology and Medicine. 2011;236(2):138–144. doi: 10.1258/ebm.2010.010230. [DOI] [PubMed] [Google Scholar]
- 42.Ptito M, Matteau I, Zhi Wang A, Paulson OB, Siebner HR, Kupers R. Crossmodal recruitment of the ventral visual stream in congenital blindness. Neural Plasticity. 2012;2012:9 pages. doi: 10.1155/2012/304045.304045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.James TW, Humphrey GK, Gati JS, Menon RS, Goodale MA. Differential effects of viewpoint on object-driven activation in dorsal and ventral streams. Neuron. 2002;35(4):793–801. doi: 10.1016/s0896-6273(02)00803-6. [DOI] [PubMed] [Google Scholar]
- 44.James TW, Humphrey GK, Gati JS, Servos P, Menon RS, Goodale MA. Haptic study of three-dimensional objects activates extrastriate visual areas. Neuropsychologia. 2002;40(10):1706–1714. doi: 10.1016/s0028-3932(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 45.Amedi A, Jacobson G, Hendler T, Malach R, Zohary E. Convergence of visual and tactile shape processing in the human lateral occipital complex zohary. Cerebral Cortex. 2002;12(11):1202–1212. doi: 10.1093/cercor/12.11.1202. [DOI] [PubMed] [Google Scholar]
- 46.Amedi A, Malach R, Hendler T, Peled S, Zohary E. Visuo-haptic object-related activation in the ventral visual pathway. Nature Neuroscience. 2001;4(3):324–330. doi: 10.1038/85201. [DOI] [PubMed] [Google Scholar]
- 47.Zhang M, Weisser VD, Stilla R, Prather SC, Sathian K. Multisensory cortical processing of object shape and its relation to mental imagery. Cognitive, Affective and Behavioral Neuroscience. 2004;4(2):251–259. doi: 10.3758/cabn.4.2.251. [DOI] [PubMed] [Google Scholar]
- 48.Stoesz MR, Zhang M, Weisser VD, Prather SC, Mao H, Sathian K. Neural networks active during tactile form perception: common and differential activity during macrospatial and microspatial tasks. International Journal of Psychophysiology. 2003;50(1-2):41–49. doi: 10.1016/s0167-8760(03)00123-5. [DOI] [PubMed] [Google Scholar]
- 49.Prather SC, Votaw JR, Sathian K. Task-specific recruitment of dorsal and ventral visual areas during tactile perception. Neuropsychologia. 2004;42(8):1079–1087. doi: 10.1016/j.neuropsychologia.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 50.Matteau I, Kupers R, Ricciardi E, Pietrini P, Ptito M. Beyond visual, aural and haptic movement perception: hMT+ is activated by electrotactile motion stimulation of the tongue in sighted and in congenitally blind individuals. Brain Research Bulletin. 2010;82(5-6):264–270. doi: 10.1016/j.brainresbull.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Morrell F. Visual system’s view of acoustic space. Nature. 1972;238(5358):44–46. doi: 10.1038/238044a0. [DOI] [PubMed] [Google Scholar]
- 52.Poirier C, Collignon O, DeVolder AG, et al. Specific activation of the V5 brain area by auditory motion processing: an fMRI study. Cognitive Brain Research. 2005;25(3):650–658. doi: 10.1016/j.cogbrainres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 53.Zimmer U, Lewald J, Erb M, Grodd W, Karnath H. Is there a role of visual cortex in spatial hearing? European Journal of Neuroscience. 2004;20(11):3148–3156. doi: 10.1111/j.1460-9568.2004.03766.x. [DOI] [PubMed] [Google Scholar]
- 54.Poirier C, Collignon O, Scheiber C, et al. Auditory motion perception activates visual motion areas in early blind subjects. NeuroImage. 2006;31(1):279–285. doi: 10.1016/j.neuroimage.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 55.Ricciardi E. Brain response to visual, tactile and auditory flow in sighted and blind individuals supports a supramodal functional organization in hMT+ complex. Neuroimage. 2006;31(1, supplement):p. 512. [Google Scholar]
- 56.Lewald J, Meister IG, Weidemann J, Töpper R. Involvement of the superior temporal cortex and the occipital cortex in spatial hearing: evidence from repetitive transcranial magnetic stimulation. Journal of Cognitive Neuroscience. 2004;16(5):828–838. doi: 10.1162/089892904970834. [DOI] [PubMed] [Google Scholar]
- 57.Collignon O, Davare M, Olivier E, De Volder AG. Reorganisation of the right occipito-parietal stream for auditory spatial processing in early blind humans. a transcranial magnetic stimulation study. Brain Topography. 2009;21(3-4):232–240. doi: 10.1007/s10548-009-0075-8. [DOI] [PubMed] [Google Scholar]
- 58.Collignon O, Davare M, De Volder AG, Poirier C, Olivier E, Veraart C. Time-course of posterior parietal and occipital cortex contribution to sound localization. Journal of Cognitive Neuroscience. 2008;20(8):1454–1463. doi: 10.1162/jocn.2008.20102. [DOI] [PubMed] [Google Scholar]
- 59.Ricciardi E, Bonino D, Sani L, et al. Do we really need vision? How blind people “see” the actions of others. Journal of Neuroscience. 2009;29(31):9719–9724. doi: 10.1523/JNEUROSCI.0274-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burton H, Sinclair RJ, McLaren DG. Cortical activity to vibrotactile stimulation: an fMRI study in blind and sighted individuals. Human Brain Mapping. 2004;23(4):210–228. doi: 10.1002/hbm.20064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ptito M, Matteau I, Gjedde A, Kupers R. Recruitment of the middle temporal area by tactile motion in congenital blindness. NeuroReport. 2009;20(6):543–547. doi: 10.1097/WNR.0b013e3283279909. [DOI] [PubMed] [Google Scholar]
- 62.Kupers R, Chebat DR, Madsen KH, Paulson OB, Ptito M. Neural correlates of virtual route recognition in congenital blindness. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(28):12716–12721. doi: 10.1073/pnas.1006199107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ptito M, Moesgaard SM, Gjedde A, Kupers R. Cross-modal plasticity revealed by electrotactile stimulation of the tongue in the congenitally blind. Brain. 2005;128(3):606–614. doi: 10.1093/brain/awh380. [DOI] [PubMed] [Google Scholar]
- 64.Kupers R, Fumal A, De Noordhout AM, Gjedde A, Schoenen J, Ptito M. Transcranial magnetic stimulation of the visual cortex induces somatotopically organized qualia in blind subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(35):13256–13260. doi: 10.1073/pnas.0602925103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arno P, De Volder AG, Vanlierde A, et al. Occipital activation by pattern recognition in the early blind using auditory substitution for vision. NeuroImage. 2001;13(4):632–645. doi: 10.1006/nimg.2000.0731. [DOI] [PubMed] [Google Scholar]
- 66.Renier LA, Anurova I, De Volder AG, Carlson S, VanMeter J, Rauschecker JP. Preserved functional specialization for spatial processing in the middle occipital gyrus of the early blind. Neuron. 2010;68(1):138–148. doi: 10.1016/j.neuron.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weeks R, Horwitz B, Aziz-Sultan A, et al. A positron emission tomographic study of auditory localization in the congenitally blind. Journal of Neuroscience. 2000;20(7):2664–2672. doi: 10.1523/JNEUROSCI.20-07-02664.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dormal G, Lepore F, Collignon O. Plasticity of the dorsal “spatial” stream in visually deprived individuals. Neural Plasticity. 2012;2012:12 pages. doi: 10.1155/2012/687659.687659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Collignon O, Vandewalle G, Voss P, et al. Functional specialization for auditory-spatial processing in the occipital cortex of congenitally blind humans. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(11):4435–4440. doi: 10.1073/pnas.1013928108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolbers T, Zahorik P, Giudice NA. Decoding the direction of auditory motion in blind humans. NeuroImage. 2011;56(2):681–687. doi: 10.1016/j.neuroimage.2010.04.266. [DOI] [PubMed] [Google Scholar]
- 71.Bedny M, Konkle T, Pelphrey K, Saxe R, Pascual-Leone A. Sensitive period for a multimodal response in human visual motion area MT/MST. Current Biology. 2010;20(21):1900–1906. doi: 10.1016/j.cub.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amedi A, Stern WM, Camprodon JA, et al. Shape conveyed by visual-to-auditory sensory substitution activates the lateral occipital complex. Nature Neuroscience. 2007;10(6):687–689. doi: 10.1038/nn1912. [DOI] [PubMed] [Google Scholar]
- 73.Kim J, Zatorre RJ. Tactile-auditory shape learning engages the lateral occipital complex. Journal of Neuroscience. 2011;31(21):7848–7856. doi: 10.1523/JNEUROSCI.3399-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Striem-Amit E, Dakwar O, Reich L, Amedi A. The large-scale organization of “visual” streams emerges without visual experience. Cereb Cortex. 2012;22(7):1698–1709. doi: 10.1093/cercor/bhr253. [DOI] [PubMed] [Google Scholar]
- 75.Röder B, Teder-Sälejärvi W, Sterr A, Rösler F, Hillyard SA, Neville HJ. Improved auditory spatial tuning in blind humans. Nature. 1999;400(6740):162–166. doi: 10.1038/22106. [DOI] [PubMed] [Google Scholar]
- 76.Leclerc C, Segalowitz SJ, Desjardins J, Lassonde M, Lepore F. EEG coherence in early-blind humans during sound localization. Neuroscience Letters. 2005;376(3):154–159. doi: 10.1016/j.neulet.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 77.Leclerc C, Saint-Amour D, Lavoie ME, Lassonde M, Lepore F. Brain functional reorganization in early blind humans revealed by auditory event-related potentials. NeuroReport. 2000;11(3):545–550. doi: 10.1097/00001756-200002280-00024. [DOI] [PubMed] [Google Scholar]
- 78.Collignon O, Lassonde M, Lepore F, Bastien D, Veraart C. Functional cerebral reorganization for auditory spatial processing and auditory substitution of vision in early blind subjects. Cerebral Cortex. 2007;17(2):457–465. doi: 10.1093/cercor/bhj162. [DOI] [PubMed] [Google Scholar]
- 79.Merabet LB, Battelli L, Obretenova S, Maguire S, Meijer P, Pascual-Leone A. Functional recruitment of visual cortex for sound encoded object identification in the blind. NeuroReport. 2009;20(2):132–138. doi: 10.1097/WNR.0b013e32832104dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kupers R, Beaulieu-Lefebvre M, Schneider FC, et al. Neural correlates of olfactory processing in congenital blindness. Neuropsychologia. 2011;49(7):2037–2044. doi: 10.1016/j.neuropsychologia.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 81.Beaulieu-Lefebvre M, Schneider FC, Kupers R, Ptito M. Odor perception and odor awareness in congenital blindness. Brain Research Bulletin. 2011;84(3):206–209. doi: 10.1016/j.brainresbull.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 82.Cuevas I, Plaza P, Rombaux P, De Volder AG, Renier L. Odour discrimination and identification are improved in early blindness. Neuropsychologia. 2009;47(14):3079–3083. doi: 10.1016/j.neuropsychologia.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 83.Sadato N, Pascual-Leone A, Grafman J, et al. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380(6574):526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- 84.Cohen LG, Weeks RA, Sadato N, Celnik P, Ishii K, Hallett M. Period of susceptibility for cross-modal plasticity in the blind. Annals of Neurology. 1999;45(4):451–460. doi: 10.1002/1531-8249(199904)45:4<451::aid-ana6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 85.Sadato N, Okada T, Honda M, Yonekura Y. Critical period for cross-modal plasticity in blind humans: a functional MRI study. NeuroImage. 2002;16(2):389–400. doi: 10.1006/nimg.2002.1111. [DOI] [PubMed] [Google Scholar]
- 86.Burton H, Snyder AZ, Conturo TE, Akbudak E, Ollinger JM, Raichle ME. Adaptive changes in early and late blind: a fMRI study of Braille reading. Journal of Neurophysiology. 2002;87(1):589–607. doi: 10.1152/jn.00285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burton H, Snyder AZ, Diamond JB, Raichle ME. Adaptive changes in early and late blind: a fMRI study of verb generation to heard nouns. Journal of Neurophysiology. 2002;88(6):3359–3371. doi: 10.1152/jn.00129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burton H, Diamond JB, McDermott KB. Dissociating cortical regions activated by semantic and phonological tasks: a fMRI study in blind and sighted people. Journal of Neurophysiology. 2003;90(3):1965–1982. doi: 10.1152/jn.00279.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Röder B, Stock O, Bien S, Neville H, Rösler F. Speech processing activates visual cortex in congenitally blind humans. European Journal of Neuroscience. 2002;16(5):930–936. doi: 10.1046/j.1460-9568.2002.02147.x. [DOI] [PubMed] [Google Scholar]
- 90.Reich L, Szwed M, Cohen L, Amedi A. A ventral visual stream reading center independent of visual experience. Current Biology. 2011;21(5):363–368. doi: 10.1016/j.cub.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 91.Bedny M, Pascual-Leone A, Dodell-Feder D, Fedorenko E, Saxe R. Language processing in the occipital cortex of congenitally blind adults. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(11):4429–4434. doi: 10.1073/pnas.1014818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cohen LG, Celnik P, Pascual-Leone A, et al. Functional relevance of cross-modal plasticity in blind humans. Nature. 1997;389(6647):180–183. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- 93.Amedi A, Floel A, Knecht S, Zohary E, Cohen LG. Transcranial magnetic stimulation of the occipital pole interferes with verbal processing in blind subjects. Nature Neuroscience. 2004;7(11):1266–1270. doi: 10.1038/nn1328. [DOI] [PubMed] [Google Scholar]
- 94.Hamilton R, Keenan JP, Catala M, Pascual-Leone A. Alexia for Braille following bilateral occipital stroke in an early blind woman. NeuroReport. 2000;11(2):237–240. doi: 10.1097/00001756-200002070-00003. [DOI] [PubMed] [Google Scholar]
- 95.Maeda K, Yasuda H, Haneda M, Kashiwagi A. Braille alexia during visual hallucination in a blind man with selective calcarine atrophy. Psychiatry and Clinical Neurosciences. 2003;57(2):227–229. doi: 10.1046/j.1440-1819.2003.01105.x. [DOI] [PubMed] [Google Scholar]
- 96.Stevens AA, Snodgrass M, Schwartz D, Weaver K. Preparatory activity in occipital cortex in early blind humans predicts auditory perceptual performance. Journal of Neuroscience. 2007;27(40):10734–10741. doi: 10.1523/JNEUROSCI.1669-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gougoux F, Zatorre RJ, Lassonde M, Voss P, Lepore F. A functional neuroimaging study of sound localization: visual cortex activity predicts performance in early-blind individuals. PLoS Biology. 2005;3(2, article e27) doi: 10.1371/journal.pbio.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burton H, Sinclair RJ, Agato A. Recognition memory for Braille or spoken words: an fMRI study in early blind. Brain Research. 2012;1438:22–34. doi: 10.1016/j.brainres.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park HJ. Activation of the occipital cortex and deactivation of the default mode network during working memory in the early blind. Journal of the International Neuropsychological Society. 2011;17: 407–422. doi: 10.1017/S1355617711000051. [DOI] [PubMed] [Google Scholar]
- 100.Bonino D, Ricciardi E, Sani L, et al. Tactile spatial working memory activates the dorsal extrastriate cortical pathway in congenitally blind individuals. Archives Italiennes de Biologie. 2008;146(3-4):133–146. [PubMed] [Google Scholar]
- 101.Amedi A, Raz N, Pianka P, Malach R, Zohary E. Early “visual” cortex activation correlates with superior verbal memory performance in the blind. Nature Neuroscience. 2003;6(7):758–766. doi: 10.1038/nn1072. [DOI] [PubMed] [Google Scholar]
- 102.Sathian K, Zangaladze A. Feeling with the mind’s eye: the role of visual imagery in tactile perception. Optometry and Vision Science. 2001;78(5):276–281. doi: 10.1097/00006324-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 103.Kosslyn SM, Thompson WL, Kim IJ, Alpert NM. Topographical representations of mental images in primary visual cortex. Nature. 1995;378(6556):496–498. doi: 10.1038/378496a0. [DOI] [PubMed] [Google Scholar]
- 104.Kosslyn SM, Pascual-Leone A, Felician O, et al. The role of area 17 in visual imagery: convergent evidence from PET and rTMS. Science. 1999;284(5411):167–170. doi: 10.1126/science.284.5411.167. [DOI] [PubMed] [Google Scholar]
- 105.Lee S, Kravitz DJ, Baker CI. Disentangling visual imagery and perception of real-world objects. NeuroImage. 2012;59(4):4064–4073. doi: 10.1016/j.neuroimage.2011.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cattaneo Z, Bona S, Silvanto J. Cross-adaptation combined with TMS reveals a functional overlap between vision and imagery in the early visual cortex. NeuroImage. 2012;59(3):3015–3020. doi: 10.1016/j.neuroimage.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 107.Seurinck R, de Lange FP, Achten E, Vingerhoets G. Mental rotation meets the motion aftereffect: the role of hV5/MT+ in visual mental imagery. Journal of Cognitive Neuroscience. 2011;23(6):1395–1404. doi: 10.1162/jocn.2010.21525. [DOI] [PubMed] [Google Scholar]
- 108.Kaas A, Weigelt S, Roebroeck A, Kohler A, Muckli L. Imagery of a moving object: the role of occipital cortex and human MT/V5+ NeuroImage. 2010;49(1):794–804. doi: 10.1016/j.neuroimage.2009.07.055. [DOI] [PubMed] [Google Scholar]
- 109.Mahon BZ, Schwarzbach J, Caramazza A. The representation of tools in left parietal cortex is independent of visual experience. Psychological Science. 2010;21(6):764–771. doi: 10.1177/0956797610370754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mahon BZ, Anzellotti S, Schwarzbach J, Zampini M, Caramazza A. Category-specific organization in the human brain does not require visual experience. Neuron. 2009;63(3):397–405. doi: 10.1016/j.neuron.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Collignon O, De Voider AG. Further evidence that congenitally blind participants react faster to auditory and tactile spatial targets. Canadian Journal of Experimental Psychology. 2009;63(4):287–293. doi: 10.1037/a0015415. [DOI] [PubMed] [Google Scholar]
- 112.Collignon O, Renier L, Bruyer R, Tranduy D, Veraart C. Improved selective and divided spatial attention in early blind subjects. Brain Research. 2006;1075(1):175–182. doi: 10.1016/j.brainres.2005.12.079. [DOI] [PubMed] [Google Scholar]
- 113.Lessard N, Paré M, Lepore F, Lassonde M. Early-blind human subjects localize sound sources better than sighted subjects. Nature. 1998;395(6699):278–280. doi: 10.1038/26228. [DOI] [PubMed] [Google Scholar]
- 114.Goldreich D, Kanics IM. Tactile acuity is enhanced in blindness. Journal of Neuroscience. 2003;23(8):3439–3445. doi: 10.1523/JNEUROSCI.23-08-03439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Van Boven RW, Hamilton RH, Kauffman T, Keenan JP, Pascual-Leone A. Tactile spatial resolution in blind Braille readers. Neurology. 2000;54(12):2230–2236. doi: 10.1212/wnl.54.12.2230. [DOI] [PubMed] [Google Scholar]
- 116.Sani L, Ricciardi E, Gentili C, Vanello N, Haxby JV, Pietrini P. Effects of visual experience on the human MT+ functional connectivity networks: an fMRI study of motion perception in sighted and congenitally blind individuals. Frontiers in Systems Neuroscience. 2010;4, article 159 doi: 10.3389/fnsys.2010.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rauschecker JP, Tian B, Korte M, Egert U. Crossmodal changes in the somatosensory vibrissa/barrel system of visually deprived animals. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(11):5063–5067. doi: 10.1073/pnas.89.11.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rauschecker JP, Korte M. Auditory compensation for early blindness in cat cerebral cortex. Journal of Neuroscience. 1993;13(10):4538–4548. doi: 10.1523/JNEUROSCI.13-10-04538.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rauschecker JP. Compensatory plasticity and sensory substitution in the cerebral cortex. Trends in Neurosciences. 1995;18(1):36–43. doi: 10.1016/0166-2236(95)93948-w. [DOI] [PubMed] [Google Scholar]
- 120.Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and stereotactic atlas of the human thalamus. Journal of Comparative Neurology. 1997;387(4):588–630. doi: 10.1002/(sici)1096-9861(19971103)387:4<588::aid-cne8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 121.Percheron G, François C, Talbi B, Yelnik J, Fénelon G. The primate motor thalamus. Brain Research Reviews. 1996;22(2):93–181. [PubMed] [Google Scholar]
- 122.Sillito AM, Cudeiro J, Jones HE. Always returning: feedback and sensory processing in visual cortex and thalamus. Trends in Neurosciences. 2006;29(6):307–316. doi: 10.1016/j.tins.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 123.Winer JA, Chernock ML, Larue DT, Cheung SW. Descending projections to the inferior colliculus from the posterior thalamus and the auditory cortex in rat, cat, and monkey. Hearing Research. 2002;168(1-2):181–195. doi: 10.1016/s0378-5955(02)00489-6. [DOI] [PubMed] [Google Scholar]
- 124.Li L, Ebner FF. Cortical modulation of spatial and angular tuning maps in the rat thalamus. Journal of Neuroscience. 2007;27(1):167–179. doi: 10.1523/JNEUROSCI.4165-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lam Y, Sherman SM. Functional organization of the somatosensory cortical layer 6 feedback to the thalamus. Cerebral Cortex. 2010;20(1):13–24. doi: 10.1093/cercor/bhp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Iacoboni M. Adjusting reaches: feedback in the posterior parietal cortex. Nature Neuroscience. 1999;2(6):492–494. doi: 10.1038/9136. [DOI] [PubMed] [Google Scholar]
- 127.Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex—I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. Journal of Neurophysiology. 2004;91(3):1381–1402. doi: 10.1152/jn.00738.2003. [DOI] [PubMed] [Google Scholar]
- 128.Romanski LM, Giguere M, Bates JF, Goldman-Rakic PS. Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology. 1997;379(3):313–332. [PubMed] [Google Scholar]
- 129.Salzmann E. Attention and memory trials during neuronal recording from the primate pulvinar and posterior parietal cortex (area PG) Behavioural Brain Research. 1995;67(2):241–253. doi: 10.1016/0166-4328(94)00153-7. [DOI] [PubMed] [Google Scholar]
- 130.Lin C-S, Kaas JH. Projections from the medial nucleus of the inferior pulvinar complex to the middle temporal area of the visual cortex. Neuroscience. 1980;5(12):2219–2228. doi: 10.1016/0306-4522(80)90138-4. [DOI] [PubMed] [Google Scholar]
- 131.Simpson DA. The projection of the pulvinar to the temporal lobe. Journal of Anatomy. 1952;86(1):20–28. [PMC free article] [PubMed] [Google Scholar]
- 132.Shipp S. The functional logic of cortico-pulvinar connections. Philosophical Transactions of the Royal Society B. 2003;358(1438):1605–1624. doi: 10.1098/rstb.2002.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chabot N, Charbonneau V, Laramée M, Tremblay R, Boire D, Bronchti G. Subcortical auditory input to the primary visual cortex in anophthalmic mice. Neuroscience Letters. 2008;433(2):129–134. doi: 10.1016/j.neulet.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 134.Izraeli R, Koay G, Lamish M, et al. Cross-modal neuroplasticity in neonatally enucleated hamsters: structure, electrophysiology and behaviour. European Journal of Neuroscience. 2002;15(4):693–712. doi: 10.1046/j.1460-9568.2002.01902.x. [DOI] [PubMed] [Google Scholar]
- 135.Doron N, Wollberg Z. Cross-modal neuroplasticity in the blind mole rat Spalax Ehrenbergi: a WGA-HRP tracing study. NeuroReport. 1994;5(18):2697–2701. doi: 10.1097/00001756-199412000-00072. [DOI] [PubMed] [Google Scholar]
- 136.Piché M, Chabot N, Bronchti G, Miceli D, Lepore F, Guillemot J-P. Auditory responses in the visual cortex of neonatally enucleated rats. Neuroscience. 2007;145(3):1144–1156. doi: 10.1016/j.neuroscience.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 137.Piché M, Robert S, Miceli D, Bronchti G. Environmental enrichment enhances auditory takeover of the occipital cortex in anophthalmic mice. European Journal of Neuroscience. 2004;20(12):3463–3472. doi: 10.1111/j.1460-9568.2004.03823.x. [DOI] [PubMed] [Google Scholar]
- 138.Bronchti G, Heil P, Sadka R, Hess A, Scheich H, Wollberg Z. Auditory activation of “visual” cortical areas in the blind mole rat (Spalax ehrenbergi) European Journal of Neuroscience. 2002;16(2):311–329. doi: 10.1046/j.1460-9568.2002.02063.x. [DOI] [PubMed] [Google Scholar]
- 139.Chabot N, Robert S, Tremblay R, Miceli D, Boire D, Bronchti G. Audition differently activates the visual system in neonatally enucleated mice compared with anophthalmic mutants. European Journal of Neuroscience. 2007;26(8):2334–2348. doi: 10.1111/j.1460-9568.2007.05854.x. [DOI] [PubMed] [Google Scholar]
- 140.Karlen SJ, Kahn DM, Krubitzer L. Early blindness results in abnormal corticocortical and thalamocortical connections. Neuroscience. 2006;142(3):843–858. doi: 10.1016/j.neuroscience.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 141.Rehkamper G, Necker R, Nevo E. Functional anatomy of the thalamus in the blind mole rat Spalax ehrenbergi: an architectonic and electrophysiologically controlled tracing study. Journal of Comparative Neurology. 1994;347(4):570–584. doi: 10.1002/cne.903470408. [DOI] [PubMed] [Google Scholar]
- 142.Bronchti G, Rado R, Terkel J, Wollberg Z. Retinal projections in the blind mole rat: a WGA-HRP tracing study of a natural degenertion. Developmental Brain Research. 1991;58(2):159–170. doi: 10.1016/0165-3806(91)90002-z. [DOI] [PubMed] [Google Scholar]
- 143.Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337(6095):753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kaas JH, Lyon DC. Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Research Reviews. 2007;55(2):285–296. doi: 10.1016/j.brainresrev.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Casanova C, Merabet L, Desautels A, Minville K. Higher-order motion processing in the pulvinar. Progress in Brain Research. 2001;134:71–82. doi: 10.1016/s0079-6123(01)34006-2. [DOI] [PubMed] [Google Scholar]
- 146.Grieve KL, Acuña C, Cudeiro J. The primate pulvinar nuclei: vision and action. Trends in Neurosciences. 2000;23(1):35–39. doi: 10.1016/s0166-2236(99)01482-4. [DOI] [PubMed] [Google Scholar]
- 147.Gaglianese A, Costagli M, Bernardi G, Ricciardi E, Pietrini P. Evidence of a direct influence between the thalamus and hMT+ independent of V1 in the human brain as measured by fMRI. NeuroImage. 2012;60(2):1440–1447. doi: 10.1016/j.neuroimage.2012.01.093. [DOI] [PubMed] [Google Scholar]
- 148.Schoenfeld MA, Heinze H-J, Woldorff MG. Unmasking motion-processing activity in human brain area V5/MT+ mediated by pathways that bypass primary visual cortex. NeuroImage. 2002;17(2):769–779. [PubMed] [Google Scholar]
- 149.Ungerleider LG, Desimone R, Galkin TW, Mishkin M. Subcortical projections of area MT in the Macaque. Journal of Comparative Neurology. 1984;223(3):368–386. doi: 10.1002/cne.902230304. [DOI] [PubMed] [Google Scholar]
- 150.Lysakowski A, Standage GP, Benevento LA. An investigation of collateral projections of the dorsal lateral geniculate nucleus and other subcortical structures to cortical areas V1 and V4 in the macaque monkey: a double label retrograde tracer study. Experimental Brain Research. 1988;69(3):651–661. doi: 10.1007/BF00247317. [DOI] [PubMed] [Google Scholar]
- 151.Schmid MC, Mrowka SW, Turchi J, et al. Blindsight depends on the lateral geniculate nucleus. Nature. 2010;466(7304):373–377. doi: 10.1038/nature09179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bock AS, Kroenke CD, Taber EN, Olavarria JF. Retinal input influences the size and corticocortical connectivity of visual cortex during postnatal development in the ferret. Journal of Comparative Neurology. 2012;520(5):914–932. doi: 10.1002/cne.22738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Falchier A, Clavagnier S, Barone P, Kennedy H. Anatomical evidence of multimodal integration in primate striate cortex. Journal of Neuroscience. 2002;22(13):5749–5759. doi: 10.1523/JNEUROSCI.22-13-05749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Budinger E, Heil P, Hess A, Scheich H. Multisensory processing via early cortical stages: connections of the primary auditory cortical field with other sensory systems. Neuroscience. 2006;143(4):1065–1083. doi: 10.1016/j.neuroscience.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 155.Ruth Clemo H, Sharma GK, Allman BL, Alex Meredith M. Auditory projections to extrastriate visual cortex: connectional basis for multisensory processing in ’unimodal’ visual neurons. Experimental Brain Research. 2008;191(1):37–47. doi: 10.1007/s00221-008-1493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Falchier A, Schroeder CE, Hackett TA, et al. Projection from visual areas V2 and prostriata to caudal auditory cortex in the monkey. Cerebral Cortex. 2010;20(7):1529–1538. doi: 10.1093/cercor/bhp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Clavagnier S, Falchier A, Kennedy H. Long-distance feedback projections to area V1: implications for multisensory integration, spatial awareness, and visual consciousness. Cognitive, Affective and Behavioral Neuroscience. 2004;4(2):117–126. doi: 10.3758/cabn.4.2.117. [DOI] [PubMed] [Google Scholar]
- 158.Beer AL, Plank T, Greenlee MW. Diffusion tensor imaging shows white matter tracts between human auditory and visual cortex. Experimental Brain Research. 2011;213(2-3):299–308. doi: 10.1007/s00221-011-2715-y. [DOI] [PubMed] [Google Scholar]
- 159.Driver J, Noesselt T. Multisensory interplay reveals crossmodal influences on “sensory-specific” brain regions, neural responses, and judgments. Neuron. 2008;57(1):11–23. doi: 10.1016/j.neuron.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. Journal of Comparative Neurology. 2000;428(1):112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 161.Beauchamp MS, Pasalar S, Ro T. Neural substrates of reliability-weighted visual-tactile multisensory integration. Frontiers in Systems Neuroscience. 2010;4, article 25 doi: 10.3389/fnsys.2010.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Calvert GA, Hansen PC, Iversen SD, Brammer MJ. Detection of audio-visual integration sites in humans by application of electrophysiological criteria to the BOLD effect. NeuroImage. 2001;14(2):427–438. doi: 10.1006/nimg.2001.0812. [DOI] [PubMed] [Google Scholar]
- 163.Smiley JF, Falchier A. Multisensory connections of monkey auditory cerebral cortex. Hearing Research. 2009;258(1-2):37–46. doi: 10.1016/j.heares.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Noesselt T, Rieger JW, Schoenfeld MA, et al. Audiovisual temporal correspondence modulates human multisensory superior temporal sulcus plus primary sensory cortices. Journal of Neuroscience. 2007;27(42):11431–11441. doi: 10.1523/JNEUROSCI.2252-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Werner S, Noppeney U. Distinct functional contributions of primary sensory and association areas to audiovisual integration in object categorization. Journal of Neuroscience. 2010;30(7):2662–2675. doi: 10.1523/JNEUROSCI.5091-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sugihara T, Diltz MD, Averbeck BB, Romanski LM. Integration of auditory and visual communication information in the primate ventrolateral prefrontal cortex. Journal of Neuroscience. 2006;26(43):11138–11147. doi: 10.1523/JNEUROSCI.3550-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Romanski LM. Representation and integration of auditory and visual stimuli in the primate ventral lateral prefrontal cortex. Cerebral Cortex. 2007;17, supplement:i61–69. doi: 10.1093/cercor/bhm099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rockland KS, Ojima H. Multisensory convergence in calcarine visual areas in macaque monkey. International Journal of Psychophysiology. 2003;50(1-2):19–26. doi: 10.1016/s0167-8760(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 169.Laramée ME, Kurotani T, Rockland KS, Bronchti G, Boire D. Indirect pathway between the primary auditory and visual cortices through layer V pyramidal neurons in V2L in mouse and the effects of bilateral enucleation. European Journal of Neuroscience. 2011;34(1):65–78. doi: 10.1111/j.1460-9568.2011.07732.x. [DOI] [PubMed] [Google Scholar]
- 170.Schmahmann JD, Pandya DN. The complex history of the fronto-occipital fasciculus. Journal of the History of the Neurosciences. 2007;16(4):362–377. doi: 10.1080/09647040600620468. [DOI] [PubMed] [Google Scholar]
- 171.Schmahmann JD, Pandya DN, Wang R, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130(3):630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 172.Den Ouden HEM, Friston KJ, Daw ND, McIntosh AR, Stephan KE. A dual role for prediction error in associative learning. Cerebral Cortex. 2009;19(5):1175–1185. doi: 10.1093/cercor/bhn161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Eckert MA, Kamdar NV, Chang CE, Beckmann CF, Greicius MD, Menon V. A cross-modal system linking primary auditory and visual cortices: evidence from intrinsic fMRI connectivity analysis. Human Brain Mapping. 2008;29(7):848–857. doi: 10.1002/hbm.20560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Klinge C, Eippert F, Röder B, Büchel C. Corticocortical connections mediate primary visual cortex responses to auditory stimulation in the blind. Journal of Neuroscience. 2010;30(38):12798–12805. doi: 10.1523/JNEUROSCI.2384-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wittenberg GF, Werhahn KJ, Wassermann EM, Herscovitch P, Cohen LG. Functional connectivity between somatosensory and visual cortex in early blind humans. European Journal of Neuroscience. 2004;20(7):1923–1927. doi: 10.1111/j.1460-9568.2004.03630.x. [DOI] [PubMed] [Google Scholar]
- 176.Ptito M, Fumal A, De Noordhout AM, Schoenen J, Gjedde A, Kupers R. TMS of the occipital cortex induces tactile sensations in the fingers of blind Braille readers. Experimental Brain Research. 2008;184(2):193–200. doi: 10.1007/s00221-007-1091-0. [DOI] [PubMed] [Google Scholar]
- 177.de la Mothe LA, Blumell S, Kajikawa Y, Hackett TA. Thalamic connections of auditory cortex in marmoset monkeys: lateral belt and parabelt regions. Anatomical Record. 2012;295(5):822–836. doi: 10.1002/ar.22454. [DOI] [PubMed] [Google Scholar]
- 178.Ptito M, Giguère J-F, Boire D, Frost DO, Casanova C. When the auditory cortex turns visual. Progress in Brain Research. 2001;134:447–458. doi: 10.1016/s0079-6123(01)34029-3. [DOI] [PubMed] [Google Scholar]
- 179.Ptito M, Kupers R. Cross-modal plasticity in early blindness. Journal of Integrative Neuroscience. 2005;4(4):479–488. doi: 10.1142/s0219635205000951. [DOI] [PubMed] [Google Scholar]
- 180.Macaluso E, Frith CD, Driver J. Modulation of human visual cortex by crossmodal spatial attention. Science. 2000;289(5482):1206–1208. doi: 10.1126/science.289.5482.1206. [DOI] [PubMed] [Google Scholar]
- 181.Facchini S, Aglioti SM. Short term light deprivation increases tactile spatial acuity in humans. Neurology. 2003;60(12):1998–1999. doi: 10.1212/01.wnl.0000068026.15208.d0. [DOI] [PubMed] [Google Scholar]
- 182.Boroojerdi B, Bushara KO, Corwell B, et al. Enhanced excitability of the human visual cortex induced by short-term light deprivation. Cerebral Cortex. 2000;10(5):529–534. doi: 10.1093/cercor/10.5.529. [DOI] [PubMed] [Google Scholar]
- 183.Merabet LB, Hamilton R, Schlaug G, et al. Rapid and reversible recruitment of early visual cortex for touch. PLoS ONE. 2008;3(8) doi: 10.1371/journal.pone.0003046.e3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pascual-Leone A, Hamilton R. The metamodal organization of the brain. Progress in Brain Research. 2001;134:427–445. doi: 10.1016/s0079-6123(01)34028-1. [DOI] [PubMed] [Google Scholar]
- 185.Lewald J. More accurate sound localization induced by short-term light deprivation. Neuropsychologia. 2007;45(6):1215–1222. doi: 10.1016/j.neuropsychologia.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 186.Amedi A, Raz N, Azulay H, Malach R, Zohary E. Cortical activity during tactile exploration of objects in blind and sighted humans. Restorative Neurology and Neuroscience. 2010;28(2):143–156. doi: 10.3233/RNN-2010-0503. [DOI] [PubMed] [Google Scholar]
- 187.Lucan JN, Foxe JJ, Gomez-Ramirez M, Sathian K, Molholm S. Tactile shape discrimination recruits human lateral occipital complex during early perceptual processing. Human Brain Mapping. 2010;31(11):1813–1821. doi: 10.1002/hbm.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rossi AF, Bichot NP, Desimone R, Ungerleider LG. Top-down attentional deficits in Macaques with lesions of lateral prefrontal cortex. Journal of Neuroscience. 2007;27(42):11306–11314. doi: 10.1523/JNEUROSCI.2939-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Friedman-Hill SR, Robertson LC, Desimone R, Ungerleider LG. Posterior parietal cortex and the filtering of distractors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(7):4263–4268. doi: 10.1073/pnas.0730772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. Natural frequencies of human corticothalamic circuits. Journal of Neuroscience. 2009;29(24):7679–7685. doi: 10.1523/JNEUROSCI.0445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008;456(7220):391–394. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Melzer I, Damry E, Landau A, Yagev R. The influence of an auditory-memory attention-demanding task on postural control in blind persons. Clinical Biomechanics. 2011;26(4):358–362. doi: 10.1016/j.clinbiomech.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 193.Burton H, Sinclair RJ, Dixit S. Working memory for vibrotactile frequencies: comparison of cortical activity in blind and sighted individuals. Human Brain Mapping. 2010;31(11):1686–1701. doi: 10.1002/hbm.20966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Garg A, Schwartz D, Stevens AA. Orienting auditory spatial attention engages frontal eye fields and medial occipital cortex in congenitally blind humans. Neuropsychologia. 2007;45(10):2307–2321. doi: 10.1016/j.neuropsychologia.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Després O, Candas V, Dufour A. Spatial auditory compensation in early-blind humans: involvement of eye movements and/or attention orienting? Neuropsychologia. 2005;43(13):1955–1962. doi: 10.1016/j.neuropsychologia.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 196.Neuhaus AH, Urbanek C, Opgen-Rhein C, et al. Event-related potentials associated with Attention Network Test. International Hournal of Psychophysiology. 2010;76(2):72–79. doi: 10.1016/j.ijpsycho.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 197.Zani A, Proverbio AM. Is that a belt or a snake? Object attentional selection affects the early stages of visual sensory processing. Behavioral and Brain Functions. 2012;8, article 6 doi: 10.1186/1744-9081-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Stokes M, Thompson R, Cusack R, Duncan J. Top-down activation of shape-specific population codes in visual cortex during mental imagery. Journal of Neuroscience. 2009;29(5):1565–1572. doi: 10.1523/JNEUROSCI.4657-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Stokes M, Saraiva A, Rohenkohl G, Nobre AC. Imagery for shapes activates position-invariant representations in human visual cortex. NeuroImage. 2011;56(3):1540–1545. doi: 10.1016/j.neuroimage.2011.02.071. [DOI] [PubMed] [Google Scholar]
- 200.De Volder AG, Toyama H, Kimura Y, et al. Auditory triggered mental imagery of shape involves visual association areas in early blind humans. NeuroImage. 2001;14(1 I):129–139. doi: 10.1006/nimg.2001.0782. [DOI] [PubMed] [Google Scholar]
- 201.Slotnick SD, Thompson WL, Kosslyn SM. Visual mental imagery induces retinotopically organized activation of early visual areas. Cerebral Cortex. 2005;15(10):1570–1583. doi: 10.1093/cercor/bhi035. [DOI] [PubMed] [Google Scholar]
- 202.Vanlierde A, De Volder AG, Wanet-Defalque M, Veraart C. Occipito-parietal cortex activation during visuo-spatial imagery in early blind humans. NeuroImage. 2003;19(3):698–709. doi: 10.1016/s1053-8119(03)00153-8. [DOI] [PubMed] [Google Scholar]
- 203.Mellet E, Tzourio N, Crivello F, Joliot M, Denis M, Mazoyer B. Functional anatomy of spatial mental imagery generated from verbal instructions. Journal of Neuroscience. 1996;16(20):6504–6512. doi: 10.1523/JNEUROSCI.16-20-06504.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tootell RBH, Hadjikhani N, Hall EK, et al. The retinotopy of visual spatial attention. Neuron. 1998;21(6):1409–1422. doi: 10.1016/s0896-6273(00)80659-5. [DOI] [PubMed] [Google Scholar]
- 205.Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3(3):284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- 206.Zhou H, Desimone R. Feature-based attention in the frontal eye field and area V4 during visual search. Neuron. 2011;70(6):1205–1217. doi: 10.1016/j.neuron.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cate AD, Herron TJ, Yund EW, et al. Auditory attention activates peripheral visual cortex. PLoS ONE. 2009;4(2, article e4645) doi: 10.1371/journal.pone.0004645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kanwisher N, Wojciulik E. Visual attention: insights from brain imaging. Nature Reviews Neuroscience. 2000;1(2):91–100. doi: 10.1038/35039043. [DOI] [PubMed] [Google Scholar]
- 209.Wu C-T, Weissman DH, Roberts KC, Woldorff MG. The neural circuitry underlying the executive control of auditory spatial attention. Brain Research. 2007;1134(1):187–198. doi: 10.1016/j.brainres.2006.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Saito DN, Yoshimura K, Kochiyama T, Okada T, Honda M, Sadato N. Cross-modal binding and activated attentional networks during audio-visual speech integration: a functional MRI study. Cerebral Cortex. 2005;15(11):1750–1760. doi: 10.1093/cercor/bhi052. [DOI] [PubMed] [Google Scholar]
- 211.Degerman A, Rinne T, Salmi J, Salonen O, Alho K. Selective attention to sound location or pitch studied with fMRI. Brain Research. 2006;1077(1):123–134. doi: 10.1016/j.brainres.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 212.Wakefield CE, Homewood J, Taylor AJ. Cognitive compensations for blindness in children: an investigation using odour naming. Perception. 2004;33(4):429–442. doi: 10.1068/p5001. [DOI] [PubMed] [Google Scholar]
- 213.Chen Q, Zhang M, Zhou X. Spatial and nonspatial peripheral auditory processing in congenitally blind people. NeuroReport. 2006;17(13):1449–1452. doi: 10.1097/01.wnr.0000233103.51149.52. [DOI] [PubMed] [Google Scholar]
- 214.Forster B, Eardley AF, Eimer M. Altered tactile spatial attention in the early blind. Brain Research. 2007;1131(1):149–154. doi: 10.1016/j.brainres.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 215.Röder B, Krämer UM, Lange K. Congenitally blind humans use different stimulus selection strategies in hearing: an ERP study of spatial and temporal attention. Restorative Neurology and Neuroscience. 2007;25(3-4):311–322. [PubMed] [Google Scholar]
- 216.Weaver KE, Stevens AA. Attention and sensory interactions within the occipital cortex in the early blind: an fMRI study. Journal of Cognitive Neuroscience. 2007;19(2):315–330. doi: 10.1162/jocn.2007.19.2.315. [DOI] [PubMed] [Google Scholar]
- 217.Burton H. Visual cortex activity in early and late blind people. Journal of Neuroscience. 2003;23(10):4005–4011. doi: 10.1523/JNEUROSCI.23-10-04005.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Saygin AP, Sereno MI. Retinotopy and attention in human occipital, temporal, parietal, and frontal cortex. Cerebral Cortex. 2008;18(9):2158–2168. doi: 10.1093/cercor/bhm242. [DOI] [PubMed] [Google Scholar]
- 219.Moradi F, Buračas GT, Buxton RB. Attention strongly increases oxygen metabolic response to stimulus in primary visual cortex. NeuroImage. 2012;59(1):601–607. doi: 10.1016/j.neuroimage.2011.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Brefczynski JA, DeYoe EA. A physiological correlate of the “spotlight” of visual attention. Nature Neuroscience. 1999;2(4):370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- 221.Deshpande G, LaConte S, James GA, Peltier S, Hu X. Multivariate granger causality analysis of fMRI data. Human Brain Mapping. 2009;30(4):1361–1373. doi: 10.1002/hbm.20606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Peltier S, Stilla R, Mariola E, LaConte S, Hu X, Sathian K. Activity and effective connectivity of parietal and occipital cortical regions during haptic shape perception. Neuropsychologia. 2007;45(3):476–483. doi: 10.1016/j.neuropsychologia.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 223.Deshpande G, Hu X, Lacey S, Stilla R, Sathian K. Object familiarity modulates effective connectivity during haptic shape perception. NeuroImage. 2010;49(3):1991–2000. doi: 10.1016/j.neuroimage.2009.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]


