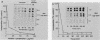Abstract
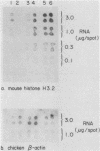
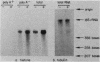
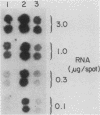
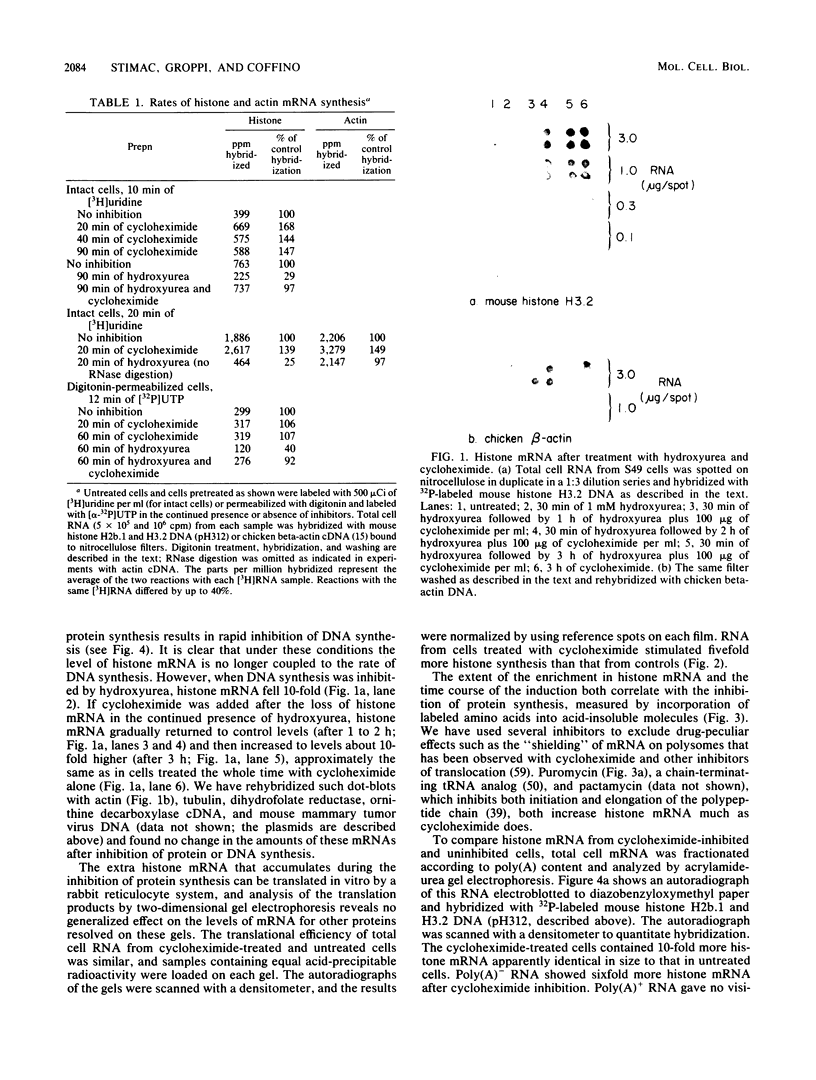
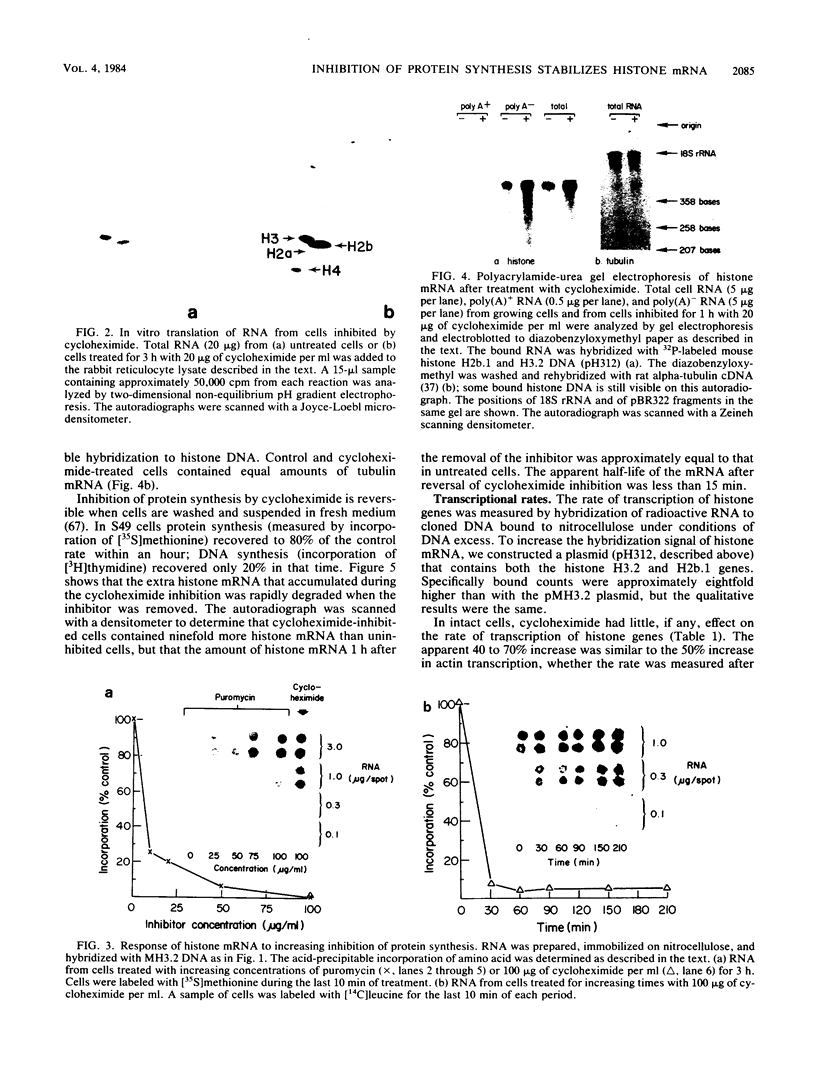
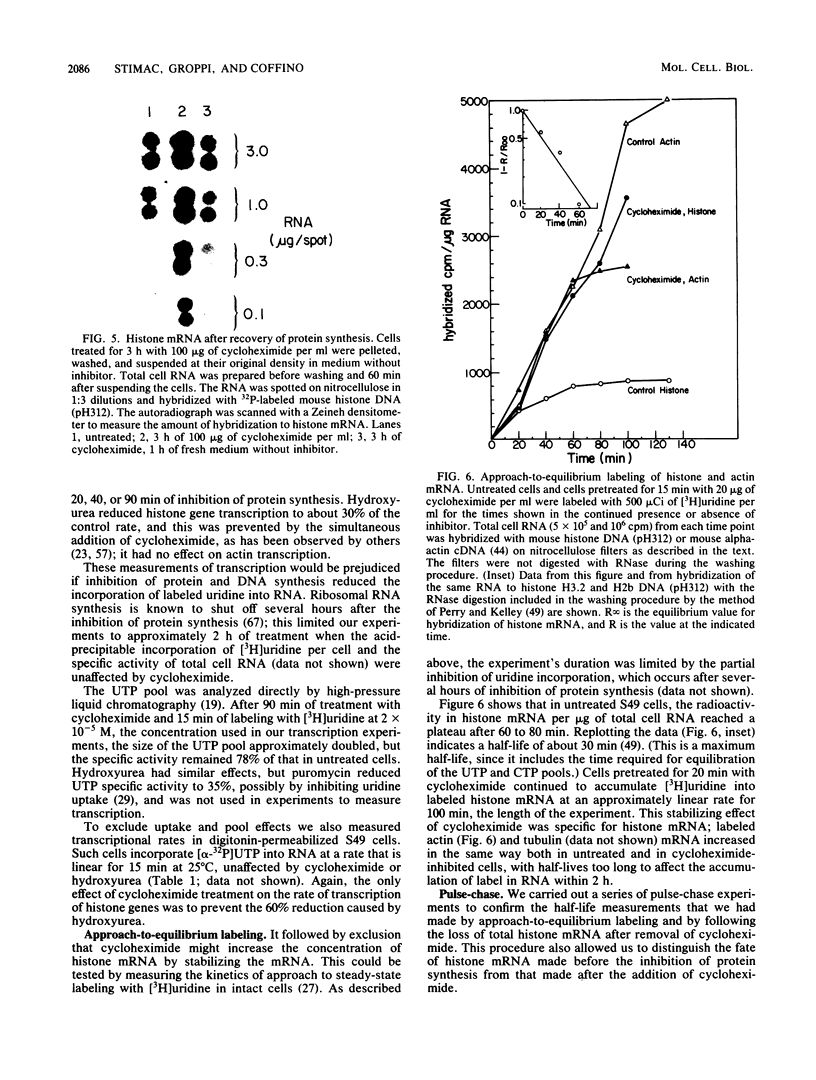
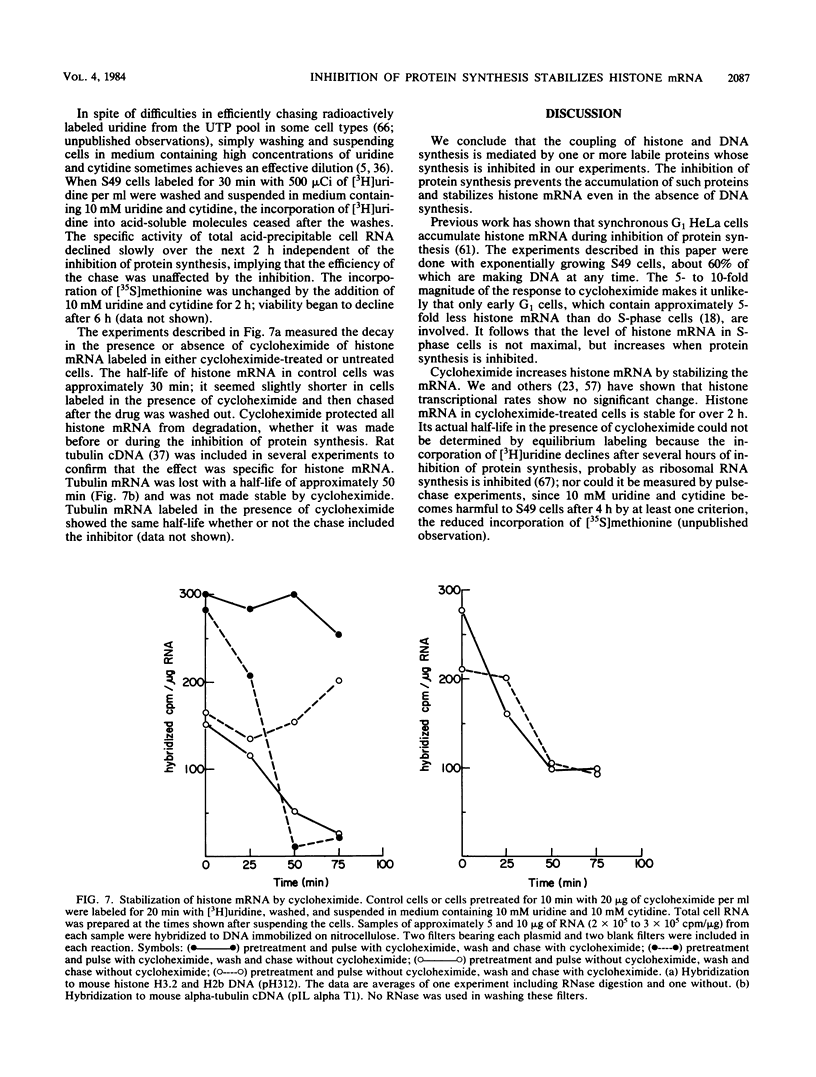
The inhibition of protein synthesis in exponentially growing S49 cells leads to a specific fivefold increase in histone mRNA in 30 min. The rate of transcription of histone mRNA, measured in intact or digitonin-permeabilized cells, is increased slightly, if at all, by cycloheximide inhibition of protein synthesis. Both approach-to-equilibrium labeling and pulse-chase experiments show that cycloheximide prolongs histone mRNA half-life from approximately 30 min to greater than 2 h. Histone mRNA made before the addition of cycloheximide becomes stable after the inhibition of protein synthesis, whereas removal of the inhibitor is followed by rapid degradation of histone mRNA. This suggests that the increased stability of histone mRNA during inhibition of protein synthesis results not from alteration of the structure of the mRNA, but from the loss of an activity in the cell which regulates histone mRNA turnover.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Alterman R. B., Ganguly S., Schulze D. H., Marzluff W. F., Schildkraut C. L., Skoultchi A. I. Cell cycle regulation of mouse H3 histone mRNA metabolism. Mol Cell Biol. 1984 Jan;4(1):123–132. doi: 10.1128/mcb.4.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. V., Lengyel J. A. Changing rates of histone mRNA synthesis and turnover in Drosophila embryos. Cell. 1980 Oct;21(3):717–727. doi: 10.1016/0092-8674(80)90435-3. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos R. N., Volloch Z., Aviv H. Messenger RNA population analysis during erythroid differentiation: a kinetical approach. J Mol Biol. 1977 Feb 25;110(2):191–203. doi: 10.1016/s0022-2836(77)80068-5. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R., Penman S. Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell. 1979 Jun;17(2):319–325. doi: 10.1016/0092-8674(79)90157-0. [DOI] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Rapidly labeled, polyribosome-associated RNA having the properties of histone messenger. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1977–1983. doi: 10.1073/pnas.58.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breindl M., Gallwitz D. On the translational control of histone synthesis. Quantitation of biologically active histone mRNA from synchronized HeLa cells and its translation in different cell-free systems. Eur J Biochem. 1974 Jun 1;45(1):91–97. doi: 10.1111/j.1432-1033.1974.tb03533.x. [DOI] [PubMed] [Google Scholar]
- Brock M. L., Shapiro D. J. Estrogen stabilizes vitellogenin mRNA against cytoplasmic degradation. Cell. 1983 Aug;34(1):207–214. doi: 10.1016/0092-8674(83)90151-4. [DOI] [PubMed] [Google Scholar]
- Butler W. B., Mueller G. C. Control of histone synthesis in HeLa cells. Biochim Biophys Acta. 1973 Feb 4;294(1):481–496. doi: 10.1016/0005-2787(73)90104-4. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Havercroft J. C. Is apparent autoregulatory control of tubulin synthesis nontranscriptionally regulated? J Cell Biol. 1983 Sep;97(3):919–924. doi: 10.1083/jcb.97.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., Sherline P., Kirschner M. W. Unpolymerized tubulin modulates the level of tubulin mRNAs. Cell. 1981 Aug;25(2):537–546. doi: 10.1016/0092-8674(81)90072-6. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Cohen M. B., Maybaum J., Sadee W. Guanine nucleotide depletion and toxicity in mouse T lymphoma (S-49) cells. J Biol Chem. 1981 Aug 25;256(16):8713–8717. [PubMed] [Google Scholar]
- Cozzarelli N. R. The mechanism of action of inhibitors of DNA synthesis. Annu Rev Biochem. 1977;46:641–668. doi: 10.1146/annurev.bi.46.070177.003233. [DOI] [PubMed] [Google Scholar]
- Craig E. A., McCarthy B. J., Wadsworth S. C. Sequence organization of two recombinant plasmids containing genes for the major heat shock-induced protein of D. melanogaster. Cell. 1979 Mar;16(3):575–588. doi: 10.1016/0092-8674(79)90031-x. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- DeLisle A. J., Graves R. A., Marzluff W. F., Johnson L. F. Regulation of histone mRNA production and stability in serum-stimulated mouse 3T6 fibroblasts. Mol Cell Biol. 1983 Nov;3(11):1920–1929. doi: 10.1128/mcb.3.11.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke S., Lichtler A., Phillips I., Stein J., Stein G. Reassessment of histone gene expression during cell cycle in human cells by using homologous H4 histone cDNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4995–4999. doi: 10.1073/pnas.76.10.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallwitz D., Mueller G. C. Histone synthesis in vitro on HeLa cell microsomes. The nature of the coupling to deoxyribonucleic acid synthesis. J Biol Chem. 1969 Nov 10;244(21):5947–5952. [PubMed] [Google Scholar]
- Gray J. W., Coffino P. Cell cycle analysis by flow cytometry. Methods Enzymol. 1979;58:233–248. doi: 10.1016/s0076-6879(79)58140-3. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- Groppi V. E., Jr, Coffino P. G1 and S phase mammalian cells synthesize histones at equivalent rates. Cell. 1980 Aug;21(1):195–204. doi: 10.1016/0092-8674(80)90127-0. [DOI] [PubMed] [Google Scholar]
- Hauschka P. V. Analysis of nucleotide pools in animal cells. Methods Cell Biol. 1973;7:361–462. doi: 10.1016/s0091-679x(08)61787-2. [DOI] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereford L. M., Osley M. A., Ludwig T. R., 2nd, McLaughlin C. S. Cell-cycle regulation of yeast histone mRNA. Cell. 1981 May;24(2):367–375. doi: 10.1016/0092-8674(81)90326-3. [DOI] [PubMed] [Google Scholar]
- Hereford L., Bromley S., Osley M. A. Periodic transcription of yeast histone genes. Cell. 1982 Aug;30(1):305–310. doi: 10.1016/0092-8674(82)90036-8. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Karin M., Andersen R. D., Slater E., Smith K., Herschman H. R. Metallothionein mRNA induction in HeLa cells in response to zinc or dexamethasone is a primary induction response. Nature. 1980 Jul 17;286(5770):295–297. doi: 10.1038/286295a0. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Khym J. X., Jones M. H., Lee W. H., Regan J. D., Volkin E. On the question of compartmentalization of the nucleotide pool. J Biol Chem. 1978 Dec 25;253(24):8741–8746. [PubMed] [Google Scholar]
- Lemischka I., Sharp P. A. The sequences of an expressed rat alpha-tubulin gene and a pseudogene with an inserted repetitive element. Nature. 1982 Nov 25;300(5890):330–335. doi: 10.1038/300330a0. [DOI] [PubMed] [Google Scholar]
- Leys E. J., Kellems R. E. Control of dihydrofolate reductase messenger ribonucleic acid production. Mol Cell Biol. 1981 Nov;1(11):961–971. doi: 10.1128/mcb.1.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. G., Housman D., Jacobsen M. Initiation of hemoglobin synthesis. Specific inhibition by antibiotics and bacteriophage ribonucleic acid. Biochemistry. 1971 Jun 8;10(12):2348–2356. doi: 10.1021/bi00788a027. [DOI] [PubMed] [Google Scholar]
- Marashi F., Baumbach L., Rickles R., Sierra F., Stein J. L., Stein G. S. Histone proteins in HeLa S3 cells are synthesized in a cell cycle stage specific manner. Science. 1982 Feb 5;215(4533):683–685. doi: 10.1126/science.7058333. [DOI] [PubMed] [Google Scholar]
- Mayo K. E., Palmiter R. D. Glucocorticoid regulation of metallothionein-I mRNA synthesis in cultured mouse cells. J Biol Chem. 1981 Mar 25;256(6):2621–2624. [PubMed] [Google Scholar]
- McConlogue L., Gupta M., Wu L., Coffino P. Molecular cloning and expression of the mouse ornithine decarboxylase gene. Proc Natl Acad Sci U S A. 1984 Jan;81(2):540–544. doi: 10.1073/pnas.81.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Oliver D., Granner D., Chalkley R. Identification of a distinction between cytoplasmic histone synthesis and subsequent histone deposition within the nucleus. Biochemistry. 1974 Feb 12;13(4):746–749. doi: 10.1021/bi00701a017. [DOI] [PubMed] [Google Scholar]
- Osley M. A., Hereford L. M. Yeast histone genes show dosage compensation. Cell. 1981 May;24(2):377–384. doi: 10.1016/0092-8674(81)90327-5. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Chen H. Y., Brinster R. L. Differential regulation of metallothionein-thymidine kinase fusion genes in transgenic mice and their offspring. Cell. 1982 Jun;29(2):701–710. doi: 10.1016/0092-8674(82)90186-6. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA turnover in mouse L cells. J Mol Biol. 1973 Oct 5;79(4):681–696. doi: 10.1016/0022-2836(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Plumb M., Stein J., Stein G. Coordinate regulation of multiple histone mRNAs during the cell cycle in HeLa cells. Nucleic Acids Res. 1983 Apr 25;11(8):2391–2410. doi: 10.1093/nar/11.8.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. The end of the message and beyond. Nature. 1984 Feb 2;307(5950):412–413. doi: 10.1038/307412a0. [DOI] [PubMed] [Google Scholar]
- Rickles R., Marashi F., Sierra F., Clark S., Wells J., Stein J., Stein G. Analysis of histone gene expression during the cell cycle in HeLa cells by using cloned human histone genes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):749–753. doi: 10.1073/pnas.79.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robbins E., Borun T. W. The cytoplasmic synthesis of histones in hela cells and its temporal relationship to DNA replication. Proc Natl Acad Sci U S A. 1967 Feb;57(2):409–416. doi: 10.1073/pnas.57.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1849–1853. doi: 10.1073/pnas.80.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Structure of a cluster of mouse histone genes. Nucleic Acids Res. 1983 Oct 11;11(19):6679–6697. doi: 10.1093/nar/11.19.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman B. M., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983 Aug 25;258(16):10083–10089. [PubMed] [Google Scholar]
- Stiles C. D., Lee K. L., Kenney F. T. Differential degradation of messenger RNAs in mammalian cells. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2634–2638. doi: 10.1073/pnas.73.8.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimac E., Groppi V. E., Coffino P. Increased histone mRNA levels during inhibition of protein synthesis. Biochem Biophys Res Commun. 1983 Jul 18;114(1):131–137. doi: 10.1016/0006-291x(83)91604-2. [DOI] [PubMed] [Google Scholar]
- Stimac E., Housman D., Huberman J. A. Effects of inhibition of protein synthesis on DNA replication in cultured mammalian cells. J Mol Biol. 1977 Sep 25;115(3):485–511. doi: 10.1016/0022-2836(77)90167-x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucker D. S., Ross S. R., Yamamoto K. R. Mammary tumor virus DNA contains sequences required for its hormone-regulated transcription. Cell. 1981 Dec;27(2 Pt 1):257–266. doi: 10.1016/0092-8674(81)90409-8. [DOI] [PubMed] [Google Scholar]
- Ucker D. S., Yamamoto K. R. Early events in the stimulation of mammary tumor virus RNA synthesis by glucocorticoids. Novel assays of transcription rates. J Biol Chem. 1984 Jun 25;259(12):7416–7420. [PubMed] [Google Scholar]
- Volloch V., Housman D. Stability of globin mRNA in terminally differentiating murine erythroleukemia cells. Cell. 1981 Feb;23(2):509–514. doi: 10.1016/0092-8674(81)90146-x. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Soeiro R., Birnboim H. C., Girard M., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. I. Identification by zone sedimentation of a heterogeneous fraction separate from ribosomal precursor RNA. J Mol Biol. 1966 Aug;19(2):349–361. doi: 10.1016/s0022-2836(66)80009-8. [DOI] [PubMed] [Google Scholar]
- Willems M., Penman M., Penman S. The regulation of RNA synthesis and processing in the nucleolus during inhibition of protein synthesis. J Cell Biol. 1969 Apr;41(1):177–187. doi: 10.1083/jcb.41.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. C., Darnell J. E., Jr Control of messenger RNA concentration by differential cytoplasmic half-life. Adenovirus messenger RNAs from transcription units 1A and 1B. J Mol Biol. 1981 May 25;148(3):231–251. doi: 10.1016/0022-2836(81)90537-4. [DOI] [PubMed] [Google Scholar]
- Wiskocil R., Bensky P., Dower W., Goldberger R. F., Gordon J. I., Deeley R. G. Coordinate regulation of two estrogen-dependent genes in avian liver. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4474–4478. doi: 10.1073/pnas.77.8.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]