Abstract
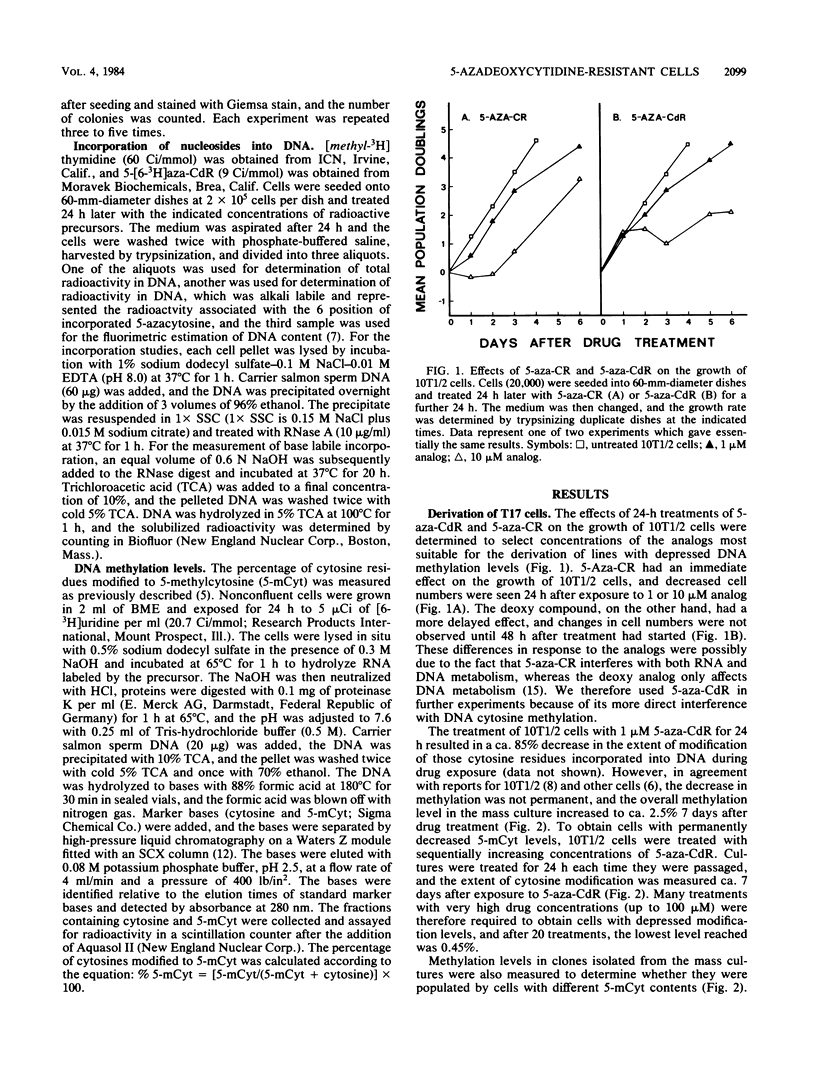
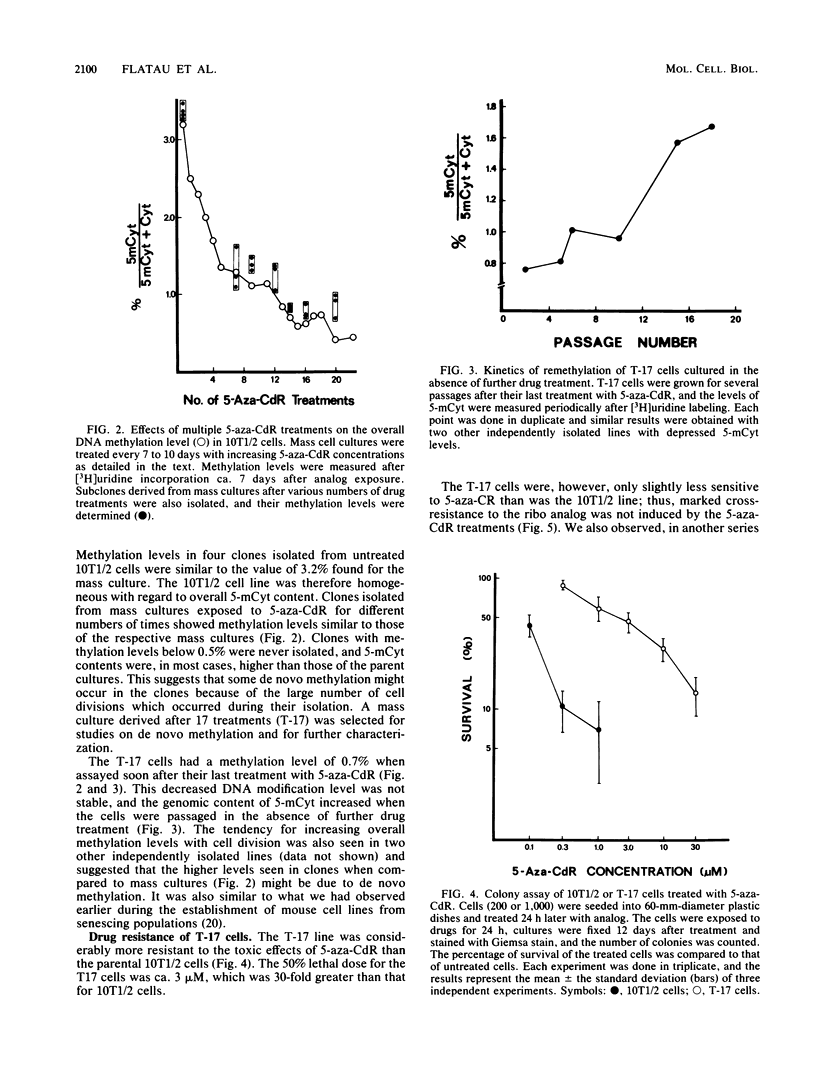
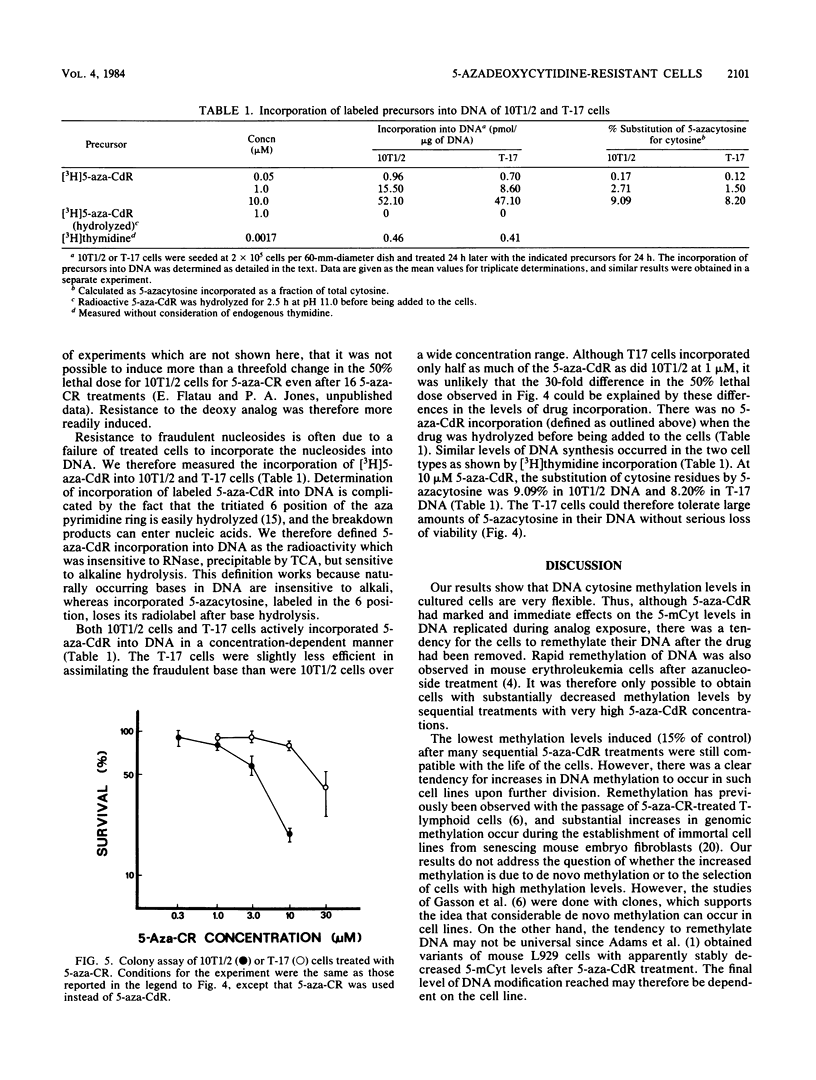
A cell line (T17) was derived from C3H 10T1/2 C18 cells after 17 treatments with increasing concentrations of 5-aza-2'-deoxycytidine. The T17 cell line was very resistant to the cytotoxic effects of 5-aza-2'-deoxycytidine, and the 50% lethal dose for 5-aza-2'-deoxycytidine was ca. 3 microM, which was 30-fold greater than that of the parental C3H 10T1/2 C18 cells. Increased drug resistance was not due to a failure of the T17 cell line to incorporate 5-aza-2'-deoxycytidine into DNA. The cells were also slightly cross-resistant to 5-azacytidine. The percentage of cytosines modified to 5-methylcytosine in T17 cells was 0.7%, a 78% decrease from the level of 3.22% in C3H 10T1/2 C18 cells. The DNA cytosine methylation levels in several clones isolated from the treated lines were on the order of 0.7%, and clones with methylation levels lower than 0.45% were not obtained even after further drug treatments. These highly decreased methylation levels appeared to be unstable, and DNA modification increased as the cells divided in the absence of further drug treatment. The results suggest that it may not be possible to derive mouse cells with vanishingly low levels of 5-methylcytosine and that considerable de novo methylation can occur in cultured lines.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L., Fulton J., Kirk D. The effect of 5-azadeoxycytidine on cell growth and DNA methylation. Biochim Biophys Acta. 1982 Jun 30;697(3):286–294. doi: 10.1016/0167-4781(82)90091-4. [DOI] [PubMed] [Google Scholar]
- Constantinides P. G., Jones P. A., Gevers W. Functional striated muscle cells from non-myoblast precursors following 5-azacytidine treatment. Nature. 1977 May 26;267(5609):364–366. doi: 10.1038/267364a0. [DOI] [PubMed] [Google Scholar]
- Constantinides P. G., Taylor S. M., Jones P. A. Phenotypic conversion of cultured mouse embryo cells by aza pyrimidine nucleosides. Dev Biol. 1978 Sep;66(1):57–71. doi: 10.1016/0012-1606(78)90273-7. [DOI] [PubMed] [Google Scholar]
- Creusot F., Acs G., Christman J. K. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2'-deoxycytidine. J Biol Chem. 1982 Feb 25;257(4):2041–2048. [PubMed] [Google Scholar]
- Flatau E., Bogenmann E., Jones P. A. Variable 5-methylcytosine levels in human tumor cell lines and fresh pediatric tumor explants. Cancer Res. 1983 Oct;43(10):4901–4905. [PubMed] [Google Scholar]
- Gasson J. C., Ryden T., Bourgeois S. Role of de novo DNA methylation in the glucocorticoid resistance of a T-lymphoid cell line. Nature. 1983 Apr 14;302(5909):621–623. doi: 10.1038/302621a0. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Momparier R. L., Gonzales F. A. Effect of intravenous infusion of 5-aza-2'-deoxycytidine on survival time of mice with L1210 leukemia. Cancer Res. 1978 Sep;38(9):2673–2678. [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Riggs A. D., Jones P. A. 5-methylcytosine, gene regulation, and cancer. Adv Cancer Res. 1983;40:1–30. doi: 10.1016/s0065-230x(08)60678-8. [DOI] [PubMed] [Google Scholar]
- Singer J., Stellwagen R. H., Roberts-Ems J., Riggs A. D. 5-Methylcytosine content of rat hepatoma DNA substituted with bromodeoxyuridine. J Biol Chem. 1977 Aug 10;252(15):5509–5513. [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Mechanism of action of eukaryotic DNA methyltransferase. Use of 5-azacytosine-containing DNA. J Mol Biol. 1982 Dec 15;162(3):679–692. doi: 10.1016/0022-2836(82)90395-3. [DOI] [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979 Aug;17(4):771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- Veselý J., Cihák A., Sorm F. Association of decreased uridine and deoxycytidine kinase with enhanced RNA and DNA polymerase in mouse leukemic cells resistant to 5-azacytidine and 5-aza-2'-deoxycytidine. Cancer Res. 1970 Aug;30(8):2180–2186. [PubMed] [Google Scholar]
- Veselý J., Cihák A., Sorm F. Biochemical mechanisms of drug resistance. IV. Development of resistance to 5-azacytidine and simultaneous depression of pyrimidine metabolism in leukemic mice. Int J Cancer. 1967 Nov 15;2(6):639–646. doi: 10.1002/ijc.2910020625. [DOI] [PubMed] [Google Scholar]
- Veselý J., Cihák A., Sorm F. Characteristics of mouse leukemic cells resistant to 5-azacytidine and 5-aza-2'-deoxycytidine. Cancer Res. 1968 Oct;28(10):1995–2000. [PubMed] [Google Scholar]
- Von Hoff D. D., Slavik M., Muggia F. M. 5-Azacytidine. A new anticancer drug with effectiveness in acute myelogenous leukemia. Ann Intern Med. 1976 Aug;85(2):237–245. doi: 10.7326/0003-4819-85-2-237. [DOI] [PubMed] [Google Scholar]
- Wilson V. L., Jones P. A. DNA methylation decreases in aging but not in immortal cells. Science. 1983 Jun 3;220(4601):1055–1057. doi: 10.1126/science.6844925. [DOI] [PubMed] [Google Scholar]


