Abstract
Stem cells are essential for the regeneration and homeostasis of many organs, such as tooth, hair, skin, and intestine. Although human tooth regeneration is limited, a number of animals have evolved continuously growing teeth that provide models of stem cell-based organ renewal. A well-studied model is the mouse incisor, which contains dental epithelial stem cells in structures known as cervical loops. These stem cells produce progeny that proliferate and migrate along the proximo-distal axis of the incisor and differentiate into enamel-forming ameloblasts. Here, we studied the role of E-cadherin in behavior of the stem cells and their progeny. Levels of E-cadherin are highly dynamic in the incisor, such that E-cadherin is expressed in the stem cells, downregulated in the transit-amplifying cells, re-expressed in the pre-ameloblasts and then downregulated again in the ameloblasts. Conditional inactivation of E-cadherin in the cervical loop led to decreased numbers of label-retaining stem cells, increased proliferation, and decreased cell migration in the mouse incisor. Using both genetic and pharmacological approaches, we showed that Fibroblast Growth Factors regulate E-cadherin expression, cell proliferation and migration in the incisor. Together, our data indicate that E-cadherin is an important regulator of stem cells and their progeny during growth of the mouse incisor.
Keywords: E-cadherin, Epithelial stem cells, Cell migration, Cell proliferation, Incisor, Ameloblasts, Fibroblast Growth factors (FGFs), Sprouty genes, Mouse
Introduction
Adult stem cells enable the regeneration and renewal of many vertebrate organs, including the nervous system, gut, gonads, skin and hematopoietic system (Alvarez-Buylla et al., 2001; Barker et al., 2007; de Rooij and Grootegoed, 1998; Fuchs and Segre, 2000; Morrison et al., 1997). A striking example of another regenerative organ is the ever-growing rodent incisor, in which stem cells fuel continuous growth by producing progeny at a remarkably rapid rate. This lifelong growth is counterbalanced by abrasion from material in the animal's diet. Enamel, the hardest dental tissue, is asymmetrically deposited on the labial (lip) but not the lingual (tongue) surface of the incisor (Fig. 1A), because enamel-secreting ameloblasts are present exclusively on the labial side (Fig. 1B). This arrangement leads to preferential abrasion on the lingual, enamel-free side.
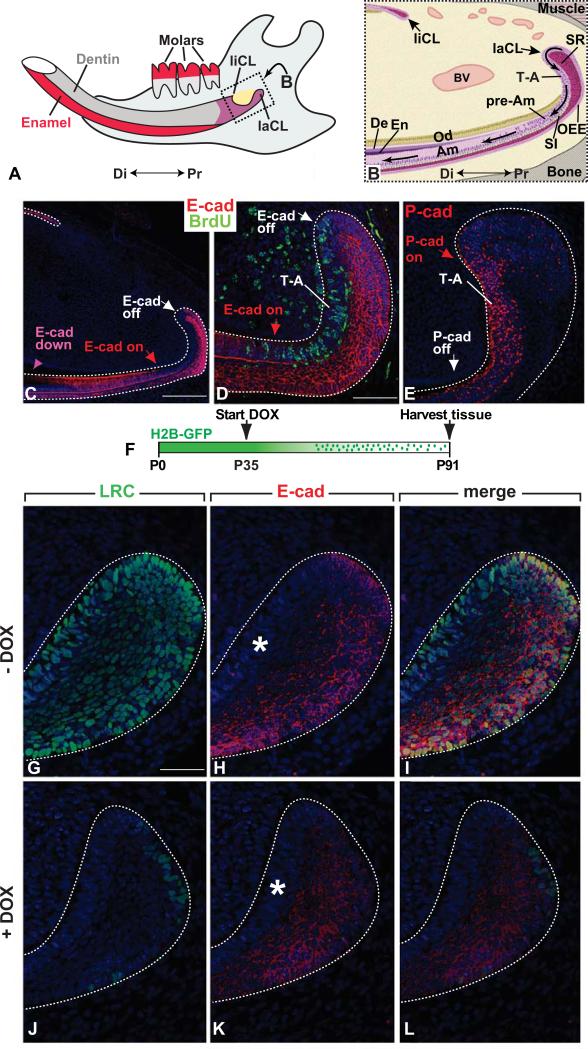
Fig. 1.
E-cadherin expression in the cervical loop of the mouse incisor. (A) Schematic diagram of an adult incisor. Enamel is produced by ameloblasts, which are present only on the labial surface. Dentin, produced by odontoblasts, is deposited on both of the labial and lingual surface. (B) Schematic diagram of the proximal region of an adult mouse incisor (sagittal section). The dental epithelial stem cells reside in the stellate reticulum (SR) or the outer enamel epithelium (OEE) regions of the labial cervical loop (laCL). These cells differentiate to form proliferating progenitors called transit-amplifying (T-A) cells. T-A cells further give rise to the preameloblasts (pAm) that differentiate into ameloblasts (Am). The stratum intermedium (SI), which comprises a single layer that subtends the ameloblasts, also arises from stem cells. BV, blood vessel; Di, distal; De, dentin; En, enamel; liCL, lingual cervical loop; Od, odontoblasts; Pr, proximal. (C-D) E-cadherin is highly expressed in the SR, SI and OEE of the laCL. In the highly proliferative T-A cells (D), the expression of E-cadherin is downregulated. In pAm, E-cadherin is re-expressed, and when the cells differentiate further into mature ameloblasts, E-cadherin expression is again downregulated. (E) P-cadherin is only expressed in the T-A region, where E-cadherin is not expressed. (F) Time-course for treatment of K5tTA;H2B-GFP mice with doxycycline (DOX). (G-I) The laCL of K5tTA;H2B-GFP mice prior to DOX treatment; the entire laCL epithelium was labeled with H2B-GFP. (J-L) H2B-GFP marked LRCs in laCL of K5tTA; H2B-GFP mice treated with DOX for 8 weeks stained with anti-GFP and anti-E-cadherin antibodies. Dotted lines outline the laCL and liCL epithelium. White asterisks in H and K mark region of absent E-cadherin expression. Scale bars: 250 μm in C; 100 μm in D, E; 50 μm in G-L.
Several studies have shown that incisor epithelial stem cells reside in niches, known as cervical loops (CLs), at the proximal end of the incisor (Fig. 1B) (Harada et al., 1999; Mitsiadis et al., 2007). Early experiments showed that epithelial progenitors in the labial cervical loop (laCL) give rise to transit-amplifying (T-A) cells that differentiate into ameloblasts as they migrate distally (Fig. 1B) (Harada et al., 1999; Smith and Warshawsky, 1975). After the ameloblasts mature and secrete enamel, they undergo apoptosis, and fresh ameloblasts derived from the laCL stem cells replace them.
The niche, which is the microenvironment where stem cells reside, influences stem cell maintenance in various tissue types. Signals emanating either from the niche or outside the niche are thought to determine stem cell behaviors (Kai and Spradling, 2003). Adherens junction molecules, such as E-cadherin, have been shown to influence the proliferation of stem/progenitor cells in different tissues (Karpowicz et al., 2009). Growth factors secreted from tissues surrounding the niche are also important regulators of the fate of stem/progenitor cells. For example, members of the Fibroblast Growth Factor (FGF) family influence cellular behavior such as cell proliferation, migration or differentiation in many tissues and organs (Ciruna and Rossant, 2001; Franzdottir et al., 2009; Ngan et al., 2002; Robinson, 2006; Zhao et al., 2008).
Recent studies have identified two members of the FGF family, Fgf3 and Fgf10, as key signaling molecules for the maintenance of dental epithelial stem cell populations within the incisor niche. Fgf10 is expressed in the mesenchyme that surrounds the posterior laCL epithelium, as well as in the mesenchyme adjacent to the T-A cells, whereas Fgf3 expression is restricted to the mesenchyme adjacent to the T-A cells (Harada et al., 1999; Harada et al., 2002; Wang et al., 2007). The receptors for these signaling molecules are highly expressed in the T-A cells as well as in the stratum intermedium (SI) cells that subtend T-A cells (Harada et al., 1999; Parsa et al., 2010). Genetic analysis has shown that Fgf3 and Fgf10 play a role in regulating ameloblast formation and function, as Fgf3–/– mice have teeth with defective enamel and incisors of Fgf3–/–;Fgf10+/– mice show hypoplasia of the laCL and failure of ameloblast formation (Wang et al., 2007). Sprouty genes, which antagonize FGF and other receptor-tyrosine kinase (RTK) signaling pathways, also have been shown to regulate tooth development (Klein et al., 2008; Klein et al., 2006). Loss of Sprouty function causes development of ectopic stem cell progeny in the liCL of the incisor.
Currently, it is not clear how FGFs regulate incisor stem cells and their progeny. A number of reports have demonstrated that, in other tissues, FGF signaling affects cell behavior by controlling expression of E-cadherin (gene symbol Cdh1) (Ciruna and Rossant, 2001; Ngan et al., 2002). In addition, E-cadherin has been shown to be essential for maintenance of stem cells and proliferation of their progeny in Drosophila and mouse (Karpowicz et al., 2009; Song et al., 2002) and is required for generation of induced pluripotent stem cells (Chen et al., 2010). These studies demonstrate that E-cadherin can exert a critical influence over both the decision between stem cell self-renewal and differentiation as well as the behavior of stem cell progeny. E-cadherin also plays an important role in regulation of tissue morphogenesis and polarity (Larue et al., 1994). Studies in C. elegans, Drosophila, and mammals have indicated that E-cadherin is crucial for proper cell migration and tissue shape (Larue et al., 1994). During tooth development, E-cadherin is expressed at high levels both during early morphogenesis and by more differentiated cells as the tooth matures (Kieffer-Combeau et al., 2001). At the onset of enamel secretion, E-cadherin is downregulated and is later absent from the post-secretory ameloblasts (Terling et al., 1998).
Here, we explored the role of E-cadherin in regulating proliferation and migration of stem cells and their progeny in the mouse incisor. We found high levels of E-cadherin expression in the CLs where dental epithelial stem cells reside. Expression is downregulated in the T-A cells that are the direct progeny of the stem cells and then upregulated in pre-ameloblasts. Loss of E-cadherin caused abnormal morphology of the laCL, which was accompanied by a reduced number of label-retaining cells (LRCs) and increased cell proliferation rates in the laCL. Furthermore, E-cadherin deletion slowed migration of the stem cell progeny along the proximo-distal axis of the incisor. Genetic and in vitro analysis indicated that E-cadherin expression was controlled by FGF signaling. Together, these results demonstrate that E-cadherin functions downstream of FGF signaling and is an important regulator of dental epithelial stem cell maintenance and behavior in the laCL of the mouse incisor.
Material and Methods
Animals and drug administration
Mouse lines carrying Gli1lacZ (Bai et al., 2002), Gli1CreERT2 (Ahn and Joyner, 2004), K5tTA (Diamond et al., 2000), H2B-GFP (Tumbar et al., 2004), a conditional allele for E-cadherin (Cdh1) (Boussadia et al., 2002), and null alleles of Fgf10 (Min et al., 1998), Spry2 (Shim et al., 2005), and Spry4 (Klein et al., 2006) were maintained and genotyped as previously reported. 5-bromo-2′-deoxyuridine (BrdU) was injected intraperitoneally as a single dose (20 μg/g), and mice were euthanized at indicated time points. For conditional inactivation of Cdh1, the Gli-CreERT2;Cdh1fl/– mice were injected intraperitoneally with 2.5 mg tamoxifen twice daily for three days, followed by a chase period to ensure maximal recombination. For MEK antagonism, the MEK1 inhibitor PD325901 (Solit et al., 2006) (12 mg/kg) was administered by oral gavage daily for two weeks. Adult mice were used for all experiments. For LRC production, mice were treated with doxycycline for 8 weeks.
Tissue preparation and histological analysis
Mice were perfused with 15 ml PBS followed by 15 ml 4% paraformaldehyde (PFA) in PBS. After fixation, the jaws were dissected, postfixed with 4% PFA overnight at 4°C, and decalcified with 0.5M ethylenediaminetetraacetic acid (EDTA). After 16 days of decalcification, tissues were embedded in paraffin. Sections were cut at 6 μm and stained with hematoxylin and eosin (H&E) using standard methods. For ultrastructural analysis, mice were perfused with 0.2 M Na-cacidylate buffer (pH 7.2) followed by 2% PFA with 2.5% glutaraldehyde in 0.2 M Na-cacodylate buffer (pH 7.2). After fixation, the mandibles were removed from the heads, freed from adherent soft tissues, and divided in two halves along the mandibular symphysis. Hemimandibles were decalcified in 10% EDTA containing 0.2% glutaraldehyde for 4-5 weeks at room temperature and rinsed in 0.185 M Na-cacodylate buffer (pH 7.2). Thereafter, the incisors were divided into blocks suitable for embedding and postfixed in 1.33% Os-tetroxide in 0.067 M s-collidine buffer for 3 hours at room temperature. Following rinsing in several changes of 0.185 M Na-cacodylate buffer, tissue blocks were dehydrated in ascending grades of ethanol, transferred to propylene oxide and embedded in Epon. Sections of 1-2 μm in thickness were cut using Histodiamond knives (Diatome AG, Biel, Switzerland) and a Reichert Ultracut ultramicrotome (Leica Microsystems, Heerbrugg, Switzerland). They were stained with toluidine blue O and microphotographed at a resolution of 2592 × 1944 px using a DM 6000B light microscope equipped with a DFC 420C camera (Leica Microsystems). Ultrathin sections of 60-80 nm in thickness were cut using the same ultramicrotome, collected on copper grids and contrasted with U-acetate and Pb-citrate. They were examined in an EM 400T transmission electron microscope (FEI, Eindhoven, The Netherlands) at an accelerating voltage of 60 kV and micrographed at a resolution of 2272 × 2272 px using a Hamamatsu ORCA-HR camera (Hamamatsu Photonics, Hamamatsu, Japan). Continuous series of images obtained at magnifications of 1200 × and 3300 × were assembled into composite micrographs using Photoshop V10 (Adobe, San Jose, CA).
Immunofluorescence staining
Paraffin sections were rehydrated, and antigen retrieval was performed by 6 cycles of boiling in 1 mM EDTA for 20 seconds followed by a 5 minute cool down. Antibodies and dilutions used were as follows: E- and P-cadherin (rat, 1:1000; Zymed), β-catenin (rabbit, 1:1000; Abcam), BrdU (rat, 1:500; Abcam), PCNA (Proliferating Cell Nuclear Antigen) (mouse, 1:500; Thermo), (phospho-Histone 3) (rabbit, 1:1000; Upstate), GFP (chicken, 1:1000; Abcam), β-galactosidase (rabbit, 1:1000; Abcam). Secondary antibodies were coupled to Alexa 488, Alexa 555, Alexa 633 (Invitrogen), or Biotin (Vector). A TSA kit (PerkinElmer) was used for signal amplification. Nuclear counterstaining was performed with DAPI (Invitrogen), and all images were acquired using a Leica-TCS SP5 confocal microscope.
TUNEL assay
Paraffin sections were rehydrated and treated with Trypsin before TUNEL staining. The processed samples were incubated with the TUNEL reaction mixture containing terminal transferase (TdT) and TMR-dUTP. TdT catalyzes the attachment of TMR-dUTP to free 3′OH ends in the DNA. The incorporated TMR-dUTP was visualized with a Leica DM 5000B fluorescence microscope.
Colony forming assay
Primary dental epithelial cells were isolated from the laCLs of wild type CD-1 mice and cultured in vitro. Briefly, the lower incisors were dissected from the mandible and treated with 2% collagenase type I for 3 hours on ice. After the laCL epithelium, which consists largely of stem cells, was mechanically separated from the mesenchyme, cells were isolated, treated with Accumax dissociation media (Sigma) and dispersed by pipetting to generate a single cell suspension. A total of 6000 cells were seeded to 6 well tissue culture plates with or without anti-E-cadherin antibody (Invitrogen). For FGF10 treatment, the cells were treated with different doses of FGF10 (R&D Systems) as described in the figure legend. The cells were cultured for 3 weeks in the presence of antibody or FGF10. After 3 weeks, the cells were fixed with 4% paraformaldehyde for 15 minutes, colony numbers were counted and colony size was measured (n=3).
In vivo cell migration assay
The animals were intraperitoneally injected with a single dose of BrdU and chased for 1.5, 24 and 48 hrs. At each time point, the jaws were harvested and immunofluorescence staining was performed to detect BrdU positive cells in the laCL. The distance the cells travelled along the axis of the surface of the mouse incisor was measured, with the start point set in the T-A region. At least 3 animals were used at each time point.
Quantification of fluorescent punctae in incisor sections
E-cadherin-positive punctae were identified with a Leica-TCS SP5 confocal microscope (63x); TSA amplification was not used for these studies. All images were taken with the same laser settings. The E-cadherin punctae in the laCL were counted in fifty cells per section from vehicle and MEK antagonist treated mice (n=3).
Statistical analysis
Data were reported as means ± standard error of mean (SEM). Unpaired Student's t tests were performed where significance was indicated by two-tailed p values; * indicates p<0.05 and ** indicates p<0.001.
Results
Expression of cadherins in subdomains of the adult labial cervical loop
We first examined the expression of E-cadherin in the incisor laCLs of adult mice. Immunofluorescence staining demonstrated that the distinct cell types in the laCL had varying levels of E-cadherin expression (Fig. 1 C, D). E-cadherin was highly expressed in the cells of the outer enamel epithelial (OEE) and stellate reticulum (SR) regions where the cell proliferation rate is relatively low. In the T-A cells, which are descended from the stem cells and are highly proliferative, the expression of E-cadherin was not detectable (Fig. 1 D). However, cells that had left this region and started to differentiate into preameloblasts reinitiated the expression of E-cadherin (Fig. 1 C, D). By contrast, P-cadherin, another important epithelial Cadherin, was exclusively expressed in cells in the T-A region (Fig. 1 E). Previous studies have shown that dental epithelial stem cells and their progeny are present in the laCLs of the mouse incisor (Harada et al., 1999; Seidel et al.; Smith and Warshawsky, 1975). We have previously identified slowly cycling stem cells in the incisor using mice carrying a doxycycline-repressible H2B-GFP transgene whose expression is controlled by the Keratin 5 (K5) promoter (Seidel et al., 2010; Tumbar et al., 2004) (Fig. 1 F). In these mice, GFP is uniformly expressed in the nucleus of all incisor epithelial cells in the absence of doxycycline (Fig. 1 G-I). After 8 weeks of exposure to doxycycline, the expression of H2B-GFP is repressed, and existing H2B-GFP is diluted out in rapidly proliferating cells, resulting in a population of label retaining cells (LRCs) in the proximal part of the laCL (Fig. 1 J). These cells are restricted to a Gli1/E-cadherin positive region (Fig.1 K, L ; Fig. S1). Previous lineage tracing work has shown that Gli1-positive cells gave rise to mature ameloblasts (Seidel et al., 2010). These data suggest that E-cadherin regulates dental epithelial stem cell function during generation of mature dental epithelial cells.
Conditional ablation of E-cadherin in the mouse incisor results in morphological defects of the cervical loop
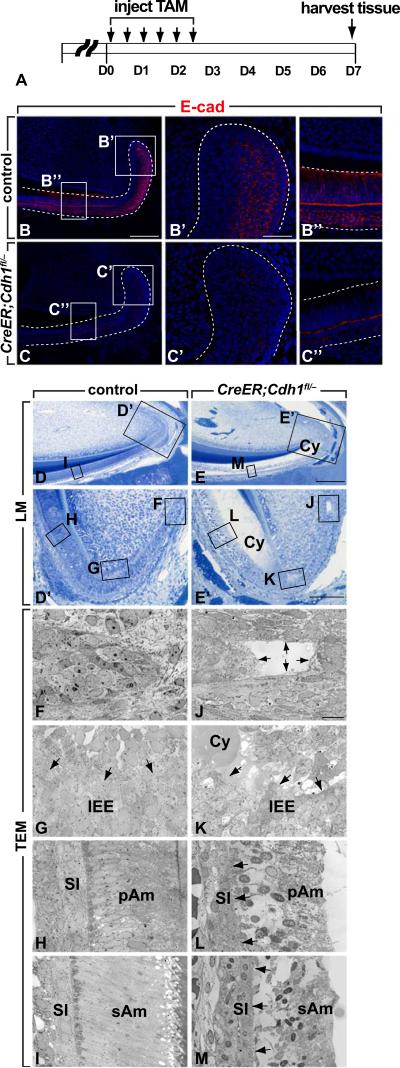
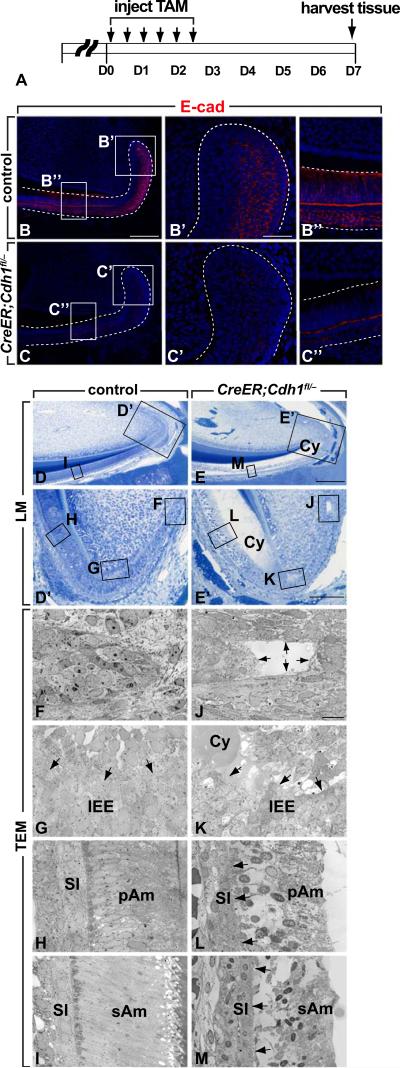
To determine the function of E-cadherin in the proximal incisor, we set out to conditionally inactivate E-cadherin in the laCL (Fig. 2 A). To delete E-cadherin in the dental epithelial stem cells, we produced mice carrying one null and one flox allele of Cdh1 as well as an inducible Cre recombinase under the control of the Gli1 promoter (Gli1CreERT2) (Ahn and Joyner, 2004); we chose this approach because Gli1 is expressed in the OEE and SR regions of the laCL, where epithelial stem cells reside (Fig. S1). Uninduced GliCreERT2;Cdh1fl/– mice showed no obvious phenotype, and E-cadherin expression was identical to control mice (Fig. 2 B-B”). When GliCreERT2;Cdh1fl/– mice were treated with tamoxifen for three days and chased for five days, E-cadherin expression was dramatically downregulated in most of the epithelial cells in the laCL (Fig. 2 C-C”). Histologically, the overall structure of the mutant laCL at low magnification appeared relatively normal (Fig. S2 A, B), but closer examination revealed several changes in the mutant. In the control, the outer and inner enamel epithelial regions were composed of well-organized columnar cells. The cells between these two layers contained two distinct cell populations. One population, known as the stellate reticulum, had a relatively clear cytoplasm, and another population was composed of larger cells that were more tightly clustered and had a more eosinophilic cytoplasm; a clear boundary was present between these two cell types (green arrows in Fig. S2 A’; Fig. S2 A”). In contrast, in the absence of E-cadherin expression, the outer and inner epithelial cells appeared less organized, lost their columnar shape, and the boundaries between the different cell types disappeared (green arrows in Fig. S2 B’; Fig. S2 B”). Ultrastructural analysis through slightly more lateral sections revealed that in the mutant laCL, there was progressive disintegration of the epithelial cells, beginning at the tip of the laCL (Fig. 2 E, E’, J, K, compare to D, D’, F, G). Cells in the region of differentiating ameloblasts disintegrated but still remained attached to the cells of the SI (Fig. 2 L, compare to H). However, in the area of the fully differentiated secretory ameloblasts, cells detached from the SI (Fig. 2 M, compare to I). These data showed that E-cadherin is essential for maintaining the integrity of cells in the laCLs.
Fig. 2.
Deletion of E-cadherin causes disorganized structure of the cervical loop (CL). (A) Time-course for conditional deletion of E-cadherin in the CL. Tamoxifen was administered twice a day for 3 days to induce Cre activity and mice were sacrificed 5 days later. (B-C”) E-cadherin expression in the CL before and after induction of Cre activity. (D-E’) Light microscope structure of the CLs from control and mutant; note cyst (Cy) in mutant. (F-M) Ultrustructural analysis of incisors from controls and mutants. Arrows in J indicates the disintegration of the CL (compare to F). Arrows in K indicate separation of cells in the T-A region (compare to G). Arrows in L indicate separation between SI and pAm (compare to H). Arrows in M indicate separation between SI and sAm (compare to I). IEE, inner enamel epithelium; pAm, preameloblasts; sAm, secretory ameloblasts; SI, stellate reticulum; TAM, tamoxifen. Scale bars: 250 μm in D, E; 100 μm in B, C, D’ and E’; 50 μm in B’, C’, B”, C”; 10 μm in F, G, H, I, J, K, L and M.
Cell proliferation is increased and cell migration is decreased markedly when E-cadherin is inactivated in the cervical loop
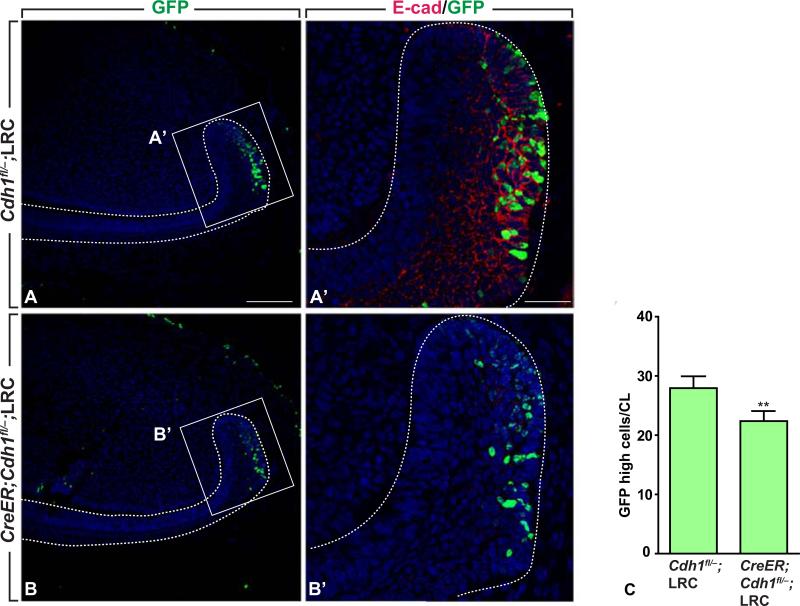
Next, we investigated whether E-cadherin affects cell proliferation and migration in the mouse incisor. We first analyzed whether loss of E-cadherin affected stem cell maintenance by counting LRC number. GliCreERT2;Cdh1fl/–;K5tTA;H2BGFP mice were chased with doxycycline to produce LRCs and injected with tamoxifen to delete Cdh1, and cells that were brightly GFP positive were counted (dimly positive cells were considered to be undergoing division). Deletion of E-cadherin significantly decreased the number of LRCs in the laCLs (Fig. 3 A-C, p<0.05), indicating that E-cadherin functions to maintain dental epithelial stem cells in the laCLs.
Fig. 3.
Decreased numbers of GFP-high LRCs after deletion of E-cadherin in the cervical loops. (A, A’) Incisor section of control and E-cadherin mutant stained with GFP antibody. (B, B’) Incisor section of control and E-cadherin mutant stained with GFP and E-cadherin antibodies. (C) Quantification of GFP-high LRCs in controls and E-cadherin mutants; mean ± SEM (n=3; p<0.05). Dotted lines outline the labial cervical loops. Scale bars: 100 μm in A, C; 50 μm in B, D.
We examined cell proliferation in the laCL after E-cadherin deletion by staining for proliferating cell nuclear antigen (PCNA) and found that, in the mutants, PCNA positive cells were significantly increased compared to controls (Fig. 4 A-C, p<0.05). To directly test whether proliferation was negatively regulated by E-cadherin, we used an in vitro approach in which we cultured primary cells from the CL epithelium. In this assay, single cells of primary dental epithelium formed colonies, similar to keratinocyte stem cells (Barrandon and Green, 1987), and these colonies express E-cadherin (Fig. S3). Blockade of E-cadherin function with an antibody resulted in an increase of the number and size of colonies, further supporting the notion that E-cadherin regulates cell proliferation in the laCLs (Fig. 4 D- H, p<0.05). To test whether the loss of E-cadherin function also affects cell death, a TUNEL assay was performed. This experiment showed that apoptosis was not increased with the deletion of E-cadherin in the laCLs (Fig. S4). No obvious changes in cervical loop size were detected.
Fig. 4.
Increased cell proliferation after inhibition of E-cadherin function in incisor epithelium. (A, B) Incisor sections of control (A) and E-cadherin mutant (B) stained with anti-PCNA antibody. (C) Quantification of cell proliferation in the labial cervical loop of control and mutants; mean ± SEM (n=3; p<0.05). (D-F) In vitro colony forming assay with increasing doses of anti-E-cadherin antibody. (G) Quantification of the number of colonies after anti-E-cadherin antibody treatment; mean ± SEM (n=3, p<0.05). (H) Quantification of the size of colonies after anti-E-cadherin antibody treatment; mean ± SEM (n=3, p<0.05). Dotted lines outline the labial and lingual cervical loops. Scale bars: 100 μm in A-C; 4 mm in D, E and F.
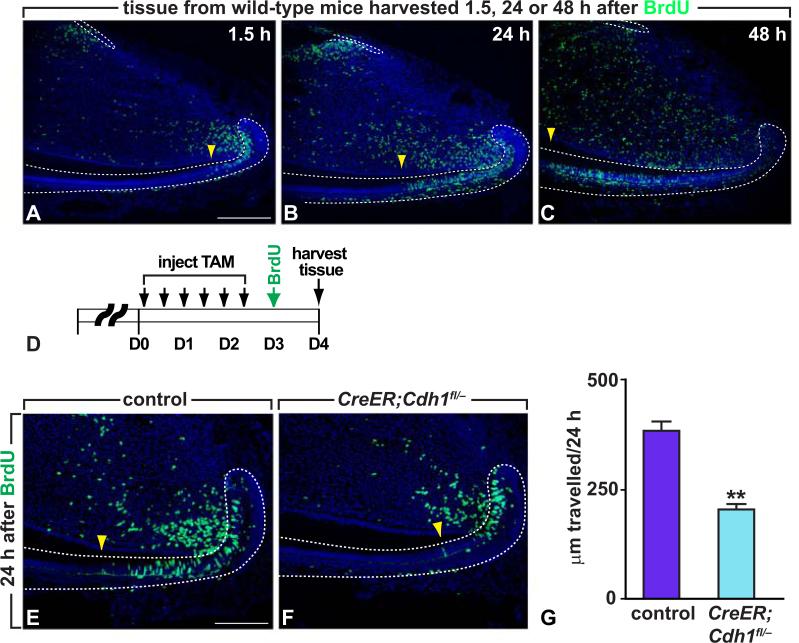
Next, the effects of E-cadherin on cell migration were assayed by measuring the distance that cells migrated along the proximo-distal axis of the tooth. Adult mice were given a single intraperitoneal injection of BrdU to label dental epithelial cells during S-phase (n=3 mice per time point). The mice were then sacrificed 1.5, 24 or 48 hours after injecting BrdU to visualize the distance that labeled cells had migrated from the laCL at these different time points. In controls, cells migrated out of the laCL towards the ameloblast region at a rate of approximately 470 μm per 24 hours (Fig. 5 A-C). This rate was slightly slower than that in rat and was similar to what was previously found in mouse with tritiated thymidine labeling, in which the migration rate was 408 μm per day (Hwang and Tonna, 1965; Smith and Warshawsky, 1975). Loss of E-cadherin expression in the laCL caused a significant slowing of cell migration, such that cells in the mutant moved at approximately half the speed of cells in the control (Fig. 5 E, F and G, p<0.001).
Fig. 5.
Decreased cell migration after deletion of E-cadherin in incisor epithelium. (A-C) Tissue from wild-type mice harvested 1.5, 24 or 48 h after BrdU injection. Yellow arrowheads indicate the migrating front. (D) Protocol used to label proliferating cells with BrdU after E-cadherin deletion. (E, F) Incisor sections of controls (E) and mutants (F) stained with anti-BrdU antibody. Yellow arrowheads indicate the migrating front. (G) Quantification of cell migration in controls and mutants; mean ± SEM (n=3; p<0.05). Dotted lines outline the labial and lingual cervical loops. Scale bars: 250 μm in A-C; 100 μm in E, F.
E-cadherin expression is regulated by FGF signaling in CLs of mouse incisor
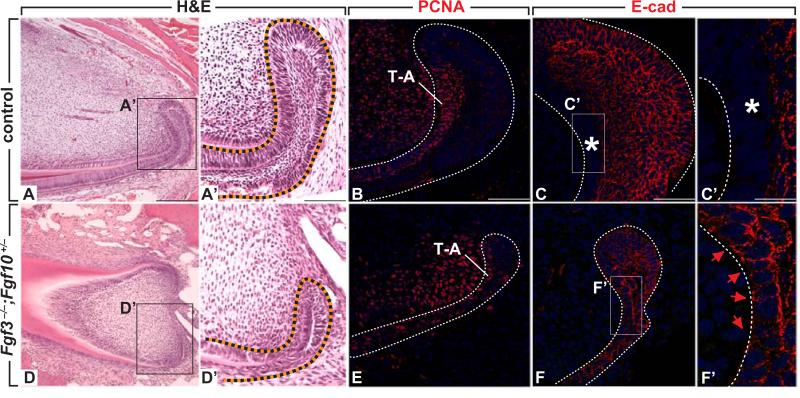
To determine if FGF signaling regulates E-cadherin expression in the adult incisors, as it does in other systems (Ciruna and Rossant, 2001), we first examined Fgf3–/–;Fgf10+/– adult mice, which have been previously reported to have small, enamel-free incisors (Wang et al., 2007). Hematoxylin & eosin (H&E) staining of incisor sections from these mutants confirmed the presence of hypoplastic laCLs (Wang et al., 2007), and further showed that no ameloblasts formed (compare Fig. 6 A, A’, D and D’). Next, we assayed the number of PCNA positive cells. We found noticeably less cell proliferation in the laCL of Fgf3–/–;Fgf10+/– mutants compared to controls (Fig. 6 B, E). In the mutants, E-cadherin was expressed broadly throughout the laCL, and the cells in the T-A region maintained high levels of E-cadherin (Fig. 6 F, F’). These results suggest that mesenchymal FGFs may affect the proliferation of the T-A cells through regulation of E-cadherin expression. To test this possibility, we used the in vitro colony formation assay described above. Wild-type colonies were treated with increasing doses of FGF10, and colony numbers as well as E-cadherin expression were analyzed. In controls, two types of colony were present, both of which were E-cadherin positive (Fig. S5 A-D). However, after FGF10 treatment, a third type of colony which was E-cadherin negative was present as well (Fig. S5 E). Additionally, FGF10 treatment led to dose-dependent increases in colony numbers (Fig. S5 F, p<0.05). These data indicate that FGF10 inhibits E-cadherin expression and induces cell proliferation in vitro.
Fig. 6.
FGF signaling regulates cell proliferation and E-cadherin expression in the labial cervical loop (laCL). (A, D) H&E staining of the incisor in controls (A, A’) and Fgf3–/–;Fgf10+/– mutants (D, D’). (B, E) Cell proliferation as assayed by PCNA staining in the laCL of controls (B) and Fgf3–/–;Fgf10+/– mutants (E). (C, C’, F, F’) E-cadherin expression in laCL of controls (C, C’) and Fgf3–/–; Fgf10+/– mutant (F, F’). Asterisk in C, C’ indicates downregulation of E-cadherin and red arrows in F, F’ indicate upregulation of E-cadherin. Scale bars: 250 μm in A, D, G; 100 μm in H; 80 μm in A’, D’ B, E; 50 μm in C, F; 17 μm in C’, F’.
We also used genetic approaches to increase FGF signaling and analyzed E-cadherin expression and cell proliferation in CLs. For these studies, we took advantage of mice carrying loss of function alleles of members of the Sprouty gene family. Sprouty proteins antagonize FGFs and other RTK signaling pathways (Mason et al., 2006), and thus loss of Sprouty function renders cells hypersensitive to FGF signaling and increases the effective level of FGF signaling. The normal downregulation of E-cadherin expression in the T-A region makes it problematic to analyze whether increased FGF signaling in Sprouty mutants inhibits E-cadherin expression in the laCL. Because essentially no E-cadherin is expressed in the T-A region of the wild-type, it is not possible to further decrease E-cadherin levels in this region. However, we have previously reported that inactivation of three Sprouty alleles in Spry4–/–;Spry2+/– mice causes generation of mature ameloblasts in the liCL as a result of upregulated FGF signaling (Klein et al., 2008). Therefore, we turned to the liCL as a system in which E-cadherin is normally widely expressed and in which the cells are relatively less proliferative. We first analyzed whether loss of Sprouty genes affects E-cadherin expression and cell proliferation in this structure. E-cadherin was expressed throughout the epithelium in the wild-type liCL (Fig. S6 A, A’). Incisors from Spry4–/– mice showed similar E-cadherin expression patterns as the control (Fig. S6 B, B’). By contrast, in Spry4–/–;Spry2+/– mice, E-cadherin expression was inhibited in the T-A region of the liCL (Fig. S6 C, C’), as is typically seen in the wild-type laCL. Thus, the liCL in the Spry4–/–;Spry2+/– mice mimics the arrangement of cells found in the wild-type laCL. We assayed proliferation in the liCL using both PCNA and phospho-histone (pH3) staining, and found that in the Spry4–/–;Spry2+/– mice, proliferation was increased dramatically in the liCL (Fig. S6 D, E, F, J and K).
We next investigated whether increased FGF signaling in the Sprouty mutants affects migration of dental epithelial cells. In Spry4 null incisors, cell migration was similar to controls in the laCL (Fig. S6 G, H, and L). In contrast, the Spry4–/–;Spry2+/– mice differed from the control in both the liCL and laCLs. In the laCL, migration was slowed relative to the control (Fig. S6 G, H, I and L, p<0.001). On the lingual side, the control CL is very small, and it is not possible to detect the low levels of cell migration that may occur there using the BrdU labeling technique. However, in Spry4–/–;Spry2+/– mice, the liCL enlarges significantly and becomes similar to the laCL in wild-type mice (Klein et al., 2008). Therefore, we compared cell migration in the liCL of Spry4–/–;Spry2+/– mice to that in the laCL of control mice. We found that cell migration was slower in the liCL of Spry4–/–;Spry2+/– mice relative to the laCL of the control (Fig. S6 L, P<0.001).
Finally, we analyzed whether cell migration was affected when the FGF signaling pathway is downregulated. In Fgf3–/–;Fgf10+/– mice, FGF signaling is low from the earliest stages of organogenesis, and these mice do not form proper ameloblasts. Thus, it was difficult to analyze how FGFs regulate cell migration in the Fgf3–/–;Fgf10+/– adult incisor. Therefore, we utilized PD325901, a well-characterized chemical inhibitor of signaling that blocks the MAPK pathway by antagonizing MEK (Solit et al., 2006). By administering this drug to wild-type adult mice (Fig. S7 A), we were able to examine the effects of blocking signaling in an organ that had developed in the context of normal signaling. We first analyzed E-cadherin expression after MEK antagonism and then examined cell migration using the BrdU chase method described above. Compared to the controls, an increased density of E-cadherin punctae was detected in the ameloblasts of the treated mice (Fig. S7 B-B”, D-D”, and G, p<0.001) and cell migration was significantly increased compared to the vehicle-treated control (Fig. S7 C, E, F, p<0.001). These results indicate that in the adult incisor the MAPK pathway regulates E-cadherin expression in more differentiated cells and suggest that the pathway controls cell migration through E-cadherin.
Discussion
Many adult stem cell populations are relatively slow-cycling, and when these stem cells begin to differentiate, their progeny often first enter a transient state of rapid proliferation (Oakberg, 1971; Potten, 1975). During this stage, the transit amplifying (T-A) cells are highly mitotic and produce a large number of progeny that will contribute to various lineages of differentiated cells. Upon exhaustion of their proliferative potential, the T-A cells exit the cell cycle and progress to terminal differentiation (Potten et al., 1979). In the mouse incisor, a wide range of cell types, from undifferentiated stem cells to highly specialized differentiated cells, is arrayed in a linear fashion and is visible in a single section. Thus, this tooth provides an excellent model system for the study of the interplay between proliferation, migration and differentiation in an organ in which continuous growth is fueled by adult stem cells.
Here, we found that the cell adhesion protein E-cadherin, which is expressed in the incisor epithelium, is required for proper proliferation and migration of dental stem cell progeny as well as the normal morphology of the laCL. Conditional deletion of E-cadherin in the stem cell-containing laCL caused disorganization of this structure, decreased LRC number and increased cell proliferation in the laCL, and decreased cell migration along the proximo-distal axis of the incisor.
Role of E-cadherin in proliferation and migration
Previous work with mouse lens and corneal epithelia demonstrated that, whereas cell division is suppressed in the migrating cell front, proliferation behind the migrating front is required to expand the size of the epithelial cell sheet (Zelenka and Arpitha, 2008). In mouse incisors, the differentiating preameloblasts do not proliferate as they migrate towards the distal tip of the tooth. However, behind the differentiated cells, there is a proliferating zone that drives the growth of the epithelial cell sheet. We found that E-cadherin may influence the balance between proliferation and migration. Our observation that deletion of E-cadherin in epithelial stem cells causes reduced LRC number and increased cell proliferation in vivo and in vitro supports the notion that E-cadherin maintains stem cells in the niche and restricts their proliferation. Without E-cadherin, the stem cells produced more proliferative progenitors. This is consistent with observations in other systems showing that E-cadherin is required for housing stem cells in their niches (Di Carlo and De Felici, 2000; Karpowicz et al., 2009; Niewiadomska et al., 1999; Song et al., 2002).
Deletion of E-cadherin decreased cell migration towards the tip of the incisor as well, showing that the presence of E-cadherin is necessary for the proper migration of differentiated cells. Epithelial cell migration is tightly regulated because the structural integrity of the epithelial cell sheet must be maintained as it migrates (Zelenka and Arpitha, 2008), and a number of studies have shown that E-cadherin is important for this process. In Drosophila, border cell migration is highly dependent on E-cadherin (called DE-cadherin) (Bai et al., 2000; Montell, 2001), and in mouse primordial germ cell development, the migration of germ cells to gonadal ridges also requires E-cadherin expression for collective migration (Di Carlo and De Felici, 2000; Okamura et al., 2003). Our results also indicate that E-cadherin is required for collective cell migration.
Together, our results indicate that E-cadherin has two functions in the mouse incisor; one is to maintain epithelial stem cells in the laCL niche and another is to enable proper cell migration. Inactivation of E-cadherin leads stem cells to prematurely differentiate into T-A cells and increases their proliferation rates, which subsequently will delay the start of their migration. Because we used a short term chase after tamoxifen injection to analyze the function of E-cadherin, we were not able to determine whether deletion of E-cadherin affects enamel deposition, as this process takes many weeks to complete. Future long term studies will be needed to understand the effect of E-cadherin during enamel formation.
Regulation of E-cadherin expression by growth factors
Growth factors function to coordinate cell proliferation, migration and differentiation (Hashimoto, 2000; Robinson, 2006; Thompson et al., 2007; Vaudry et al., 2002). In mice, FGFs are known to have essential functions during tooth morphogenesis and homeostasis, but the cellular mechanisms by which these functions are carried out are incompletely understood. Here, we found that FGFs regulate E-cadherin expression in the CL of the incisor. Fgf3 and Fgf10 are expressed in the mesenchyme adjacent to the laCL (Harada et al., 1999; Harada et al., 2002) and they signal to the epithelium, where the receptors that transduce their signals are expressed. In Fgf3–/–;Fgf10+/– incisors, decreased FGF levels in the laCL were associated with upregulation of E-cadherin expression in the transit-amplifying region.
An important function of FGFs is to regulate epithelial cell proliferation, migration and differentiation (Franzdottir et al., 2009; Zhao et al., 2008). We propose that E-cadherin function during cell proliferation in the laCL depends on FGF signaling. The in vivo and in vitro data presented here showed that inhibition of E-cadherin increased cell proliferation. FGFs are well-known to have a mitogenic role in many contexts (Dailey et al., 2005), and our studies suggest that these two functions of FGF signaling – downregulation of E-cadherin expression and increased cell proliferation – may be linked in the laCL of the incisor. In FGF mutants, E-cadherin was not downregulated and cell proliferation was decreased in the laCL. In contrast, increased FGF signaling as a result of mutations in Sprouty genes and exogenous FGF10 treatment in vitro caused downregulation of E-cadherin in the T-A region and increased cell proliferation in vivo, increased formation of E-cadherin negative colonies and increased total colony numbers in vitro. These results suggest that FGF signaling is necessary for downregulation of E-cadherin expression, which allows stem cells to become proliferating progenitors.
Our findings also suggested that FGF signaling affects cell migration through regulation of E-cadherin expression. We used a MEK antagonist to block the MAPK pathway, which is downstream of FGF signaling, in wild-type adults. Inhibition of the MAPK pathway increased and extended E-cadherin expression in the ameloblasts, and cell migration was accelerated, supporting the notion that FGF signaling is upstream of E-cadherin expression and regulates cell migration. It is interesting that inhibition of the MAPK pathway in adults caused increased E-cadherin expression in the ameloblast region but not in the T-A region. In contrast, decreased FGF signaling from development through adulthood in the Fgf3–/–;Fgf10+/– animals led to increased E-cadherin expression in the T-A region. One explanation for this may be that FGFs regulate E-cadherin expression in both the T-A and ameloblast regions, but by different mechanisms. For example, it is possible that in the T-A region, FGFs transcriptionally regulate E-cadherin expression, whereas in the ameloblast region, FGFs may regulate E-cadherin post-transcriptionally. While further investigations will be needed to address this issue, it is worth noting that, in addition to the transcriptional effects described above, inhibition of the MAPK pathway has been shown to stabilize the E-cadherin protein. For example, in intestinal epithelium, activation of p38 MAPK caused downregulation of E-cadherin protein (Zohn et al., 2006). Thus, the FGF pathway may regulate E-cadherin expression in stem cell progeny and control the balance between their proliferation and migration. It may also regulate the expression of E-cadherin in differentiated cells and control epithelial sheet migration via effects by the MAPK pathway more directly on E-cadherin protein levels.
It is well established that FGF signaling is negatively regulated by Sprouty proteins (Mason et al., 2006). Incisors from Spry4–/–;Spry2+/– mice displayed upregulation of Fgf3 and Fgf10 in the mesenchyme adjacent to the liCL, which in turn contributed to formation of mature ameloblasts in the liCL (Klein et al., 2008). In the laCL, deletion of Sprouty genes also caused increased FGF signaling to the epithelium (Klein et al., 2008), but we could not readily analyze the effect of FGF signaling on E-cadherin expression on the labial side of the Spry4–/–;Spry2+/– mutant. However, in the liCL, E-cadherin was expressed in all of the cells, and in the Spry4–/–;Spry2+/– mutant, E-cadherin expression in the liCL was absent in the T-A region. Thus, increased levels of FGF signaling in this model were associated with decreased E-cadherin. Interestingly, cell migration in the laCL of the Spry4–/–;Spry2+/– mutant was slowed compared to the wild-type indicating that increased FGF signaling is correlated with decreased migration in this tissue. The mechanisms underlying migration of epithelial cells in the incisor have not been previously studied, and our data suggest that FGF-dependent regulation of E-cadherin expression is necessary for correct cell migration in this organ.
E-cadherin is necessary for the normal structure of the mouse incisor
The epithelium of the incisor possesses different cell types. Deletion of E-cadherin disrupted the structure of the laCL and caused separation of mature ameloblasts from the SI, which has some similarities to the phenotype of p120ctn mutant mice (Bartlett et al., 2010). However, although deletion of p120ctn affected ameloblasts, the laCL appeared normal. In contrast, E-cadherin deletion affected cells in the laCL and also caused disintegration of the ameloblast-SI boundary. Thus, these results indicate that E-cadherin and p120ctn have different functions in regulation of the growth of mouse incisors. Interestingly, inactivation of both Nectin and PERP (Barron et al., 2008; Jheon et al., 2011; Yoshida et al., 2010), two other cell adhesion molecules, caused disruption of intercellular contacts between epithelial cells of the enamel organ (including the ameloblasts and stratum intermedium) and presented ultrastructural details which were milder, but otherwise comparable, to those obtained in this study. However, the stem cells per se were not studied in either of these mutants.
Concluding remarks
The studies presented here demonstrated an essential role for E-cadherin in regulating cell proliferation and migration in the continuously growing mouse incisor. This work indicates that E-cadherin contributes to incisor homeostasis in two ways. First, E-cadherin expression in the stem cell niche maintains the integrity of the laCL which controls proliferation of stem cells and their progeny. Second, E-cadherin expressed in preameloblasts is necessary for their proper migration. Finally, FGFs, which are important signaling molecules during odontogenesis, regulate the expression of E-cadherin in the laCL of the incisor.
Supplementary Material
Fig. S1. E-cadherin is expressed in Gli1-expressing cells in the cervical loop (CL). CL from Gli1-lacZ incisor co-stained with anti-E-cadherin (red) and anti-beta-galactosidase (green) antibodies. Scale bar: 100 μm.
Fig. S2. H&E staining of the cervical loop (CL) after conditional deletion of E-cadherin. (A-B) Low magnification of CLs in controls and mutants. (A’-B’) High magnification of CLs in controls and mutants. Green arrows indicate disorganized region in B” compared with A”. Dotted lines outline the cervical loop epithelium. Scale bars: 250 μm in A, B; 50 μm in A’, B’; 20 μm in A”, B”.
Fig. S3. Expression of E-cadherin in in vitro formed colonies of wild-type cervical loops (CLs). (A) Phase contrast view of colony formed from primary cultured CLs. (B-D) E-cadherin expression in a colony formed from primary cultured CLs. Scale bars: 400 μm in A-D.
Fig. S4. TUNEL staining after deletion of E-cadherin in CLs. TUNEL staining in control and E-cadherin mutant CLs (A, B). Scale bars: 100 μm in A, B.
Fig. S5. Colony formation after FGF10 treatment. (A-E) E-cadherin expression in colonies formed in vitro from wild-type labial cervical loops (laCLs) after FGF10 treatment. In the absence of FGF10 treatment, two types of colonies formed. The first type (A) consisted of larger colonies with small, tightly-clustered cells. The second type (B) consisted of smaller colonies with larger cells. Both types of colonies were E-cadherin positive. After FGF10 treatment, in addition to the two types of colonies observed in the untreated control (C, D), a third type of colony that did not express E-Cadherin was present (E). (F) Quantification of colonies after FGF10 treatment in vitro. Dotted lines outline the laCLs. Scale bars: 250 μm A, C and E; 100 μm in B, D.
Fig. S6. E-cadherin expression and cell proliferation are increased and cell migration is decreased in Spry4–/–;Spry2+/– mice. (A-C’) E-cadherin expression in the lingual cervical loop in control (A, A’), Spry4–/– (B, B’) and Spry4–/–;Spry2+/– (C, C’) mice. (D-F) Cell proliferation as measured by PCNA and phospho-Histone 3 (pH3) staining in control (D), Spry4–/– (E) and Spry4–/–;Spry2+/– (F) mice. . (J-K) Quantification of cell proliferation detected with antibodies against PCNA (K) and pH3 (L); mean ±SEM (n=3, p<0.001). (G-I) Cell migration in control (G), Spry4–/– (H) and Spry4–/–;Spry2+/– (I) mice. Yellow arrowhead indicates the migrating front. (L) Quantification of the distance travelled by cells in the cervical loop in control, Spry4–/– and Spry4–/–;Spry2+/– mice; mean ±SEM (n=3; p<0.001). For the Spry4–/–;Spry2+/– mice, distance travelled by both labial and lingual cervical loop cells is shownDotted lines outline the cervical loop epithelium. Scale bars: 100 μm in A’F; 250 μm in A-C, G-I.
Fig. S7. Inhibition of the MAPK pathway with MEK1 inhibitor PD325901 (MEK antag) leads to expanded E-cadherin expression in the ameloblast region and increased migration of stem cell progeny. (A) Protocol used to label the cells with BrdU after treatment of animals with MEK1 inhibitor. (B-B”, D-D”) E-cadherin protein in control (B) and MEK 1 inhibitor treated incisor (D). (C, E) Cell migration in incisors from control (C) or animals treated with MEK1 inhibitor (E). Yellow arrowheads indicate the migrating front. (F) Quantification of the distance that cells traveled in control and MEK1 inhibitor treated cervical loop; mean ± SEM (n=3; p<0.001). (G) Quantification of E-cadherin punctae in ameloblasts in control and MEK1 inhibitor treated incisors; mean ± SEM (n=3; p<0.001). Dotted lines outline the cervical loop epithelium. Scale bars: 250 μm in B, D, C, F; 50 μm in B’, D’; 20 μm in B”, D”.
Highlights.
E-cadherin regulates dental epithelial stem cell progeny proliferation and migration in the mouse incisor.
FGF3 and FGF10, and their antagonists encoded by the Sprouty genes, control E-cadherin expression in the incisor.
Acute blockade of the MAP kinase pathway in adult incisors affects migration of the stem cell progeny.
Acknowledgments
We are grateful to D.B. Lyons for initial observations that led us to pursue these studies and for insightful discussions over the years. We thank B. Biehs, S. Ching, A. Jheon, K. Seidel, W. Yu and other members of the Klein laboratory for helpful advice, and N. Strauli, D.-K. Tran and P. Mostowfi for technical assistance. We also thank X.-P. Wang for help with the in vitro primary culture system. This work was supported in part by Basil O'Connor Starter Scholar Research Award Grant No. 5-FY09-140 from the March of Dimes Foundation and a CIRM New Faculty Award II to O. D. Klein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: C.-Y. Li and O.D. Klein designed experiments, analyzed data and wrote the manuscript; C.-Y. Li, W. Cha, H.U. Luder and R.-P. Charles performed experiments; all authors reviewed the manuscript and discussed the data.
References
- Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. doi: 10.1016/j.cell.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- Bai J, Uehara Y, Montell DJ. Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell. 2000;103:1047–1058. doi: 10.1016/s0092-8674(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci U S A. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron MJ, Brookes SJ, Draper CE, Garrod D, Kirkham J, Shore RC, Dixon MJ. The cell adhesion molecule nectin-1 is critical for normal enamel formation in mice. Hum Mol Genet. 2008;17:3509–3520. doi: 10.1093/hmg/ddn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JD, Dobeck JM, Tye CE, Perez-Moreno M, Stokes N, Reynolds AB, Fuchs E, Skobe Z. Targeted p120-catenin ablation disrupts dental enamel development. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev. 2002;115:53–62. doi: 10.1016/s0925-4773(02)00090-4. [DOI] [PubMed] [Google Scholar]
- Chen T, Yuan D, Wei B, Jiang J, Kang J, Ling K, Gu Y, Li J, Xiao L, Pei G. E-cadherin-mediated cell-cell contact is critical for induced pluripotent stem cell generation. Stem Cells. 2010;28:1315–1325. doi: 10.1002/stem.456. [DOI] [PubMed] [Google Scholar]
- Ciruna B, Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell. 2001;1:37–49. doi: 10.1016/s1534-5807(01)00017-x. [DOI] [PubMed] [Google Scholar]
- Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–247. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, Grootegoed JA. Spermatogonial stem cells. Curr Opin Cell Biol. 1998;10:694–701. doi: 10.1016/s0955-0674(98)80109-9. [DOI] [PubMed] [Google Scholar]
- Di Carlo A, De Felici M. A role for E-cadherin in mouse primordial germ cell development. Dev Biol. 2000;226:209–219. doi: 10.1006/dbio.2000.9861. [DOI] [PubMed] [Google Scholar]
- Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–794. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- Franzdottir SR, Engelen D, Yuva-Aydemir Y, Schmidt I, Aho A, Klambt C. Switch in FGF signalling initiates glial differentiation in the Drosophila eye. Nature. 2009;460:758–761. doi: 10.1038/nature08167. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Segre JA. Stem cells: a new lease on life. Cell. 2000;100:143–155. doi: 10.1016/s0092-8674(00)81691-8. [DOI] [PubMed] [Google Scholar]
- Harada H, Kettunen P, Jung HS, Mustonen T, Wang YA, Thesleff I. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J Cell Biol. 1999;147:105–120. doi: 10.1083/jcb.147.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Toyono T, Toyoshima K, Yamasaki M, Itoh N, Kato S, Sekine K, Ohuchi H. FGF10 maintains stem cell compartment in developing mouse incisors. Development. 2002;129:1533–1541. doi: 10.1242/dev.129.6.1533. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Regulation of keratinocyte function by growth factors. J Dermatol Sci. 2000;24(Suppl 1):S46–50. doi: 10.1016/s0923-1811(00)00141-9. [DOI] [PubMed] [Google Scholar]
- Hwang WS, Tonna EA. Autoradiographic Analysis of Labeling Indices and Migration Rates of Cellular Component of Mouse Incisors Using Tritiated Thymidine (H3tdr). J Dent Res. 1965;44:42–53. doi: 10.1177/00220345650440012901. [DOI] [PubMed] [Google Scholar]
- Jheon AH, Mostowfi P, Snead ML, Ihrie RA, Sone E, Pramparo T, Attardi LD, Klein OD. PERP regulates enamel formation via effects on cell-cell adhesion and gene expression. J Cell Sci. 2011;124:745–754. doi: 10.1242/jcs.078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai T, Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci U S A. 2003;100:4633–4638. doi: 10.1073/pnas.0830856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpowicz P, Willaime-Morawek S, Balenci L, DeVeale B, Inoue T, van der Kooy D. E-Cadherin regulates neural stem cell self-renewal. J Neurosci. 2009;29:3885–3896. doi: 10.1523/JNEUROSCI.0037-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer-Combeau S, Meyer JM, Lesot H. Cell-matrix interactions and cell-cell junctions during epithelial histo-morphogenesis in the developing mouse incisor. Int J Dev Biol. 2001;45:733–742. [PubMed] [Google Scholar]
- Klein OD, Lyons DB, Balooch G, Marshall GW, Basson MA, Peterka M, Boran T, Peterkova R, Martin GR. An FGF signaling loop sustains the generation of differentiated progeny from stem cells in mouse incisors. Development. 2008;135:377–385. doi: 10.1242/dev.015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein OD, Minowada G, Peterkova R, Kangas A, Yu BD, Lesot H, Peterka M, Jernvall J, Martin GR. Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev Cell. 2006;11:181–190. doi: 10.1016/j.devcel.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci U S A. 1994;91:8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, DeRose M, Simonet WS. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–3161. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiadis TA, Barrandon O, Rochat A, Barrandon Y, De Bari C. Stem cell niches in mammals. Exp Cell Res. 2007;313:3377–3385. doi: 10.1016/j.yexcr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Montell DJ. Command and control: regulatory pathways controlling invasive behavior of the border cells. Mech Dev. 2001;105:19–25. doi: 10.1016/s0925-4773(01)00393-8. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- Ngan ES, Ma ZQ, Chua SS, DeMayo FJ, Tsai SY. Inducible expression of FGF-3 in mouse mammary gland. Proc Natl Acad Sci U S A. 2002;99:11187–11192. doi: 10.1073/pnas.172366199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiadomska P, Godt D, Tepass U. DE-Cadherin is required for intercellular motility during Drosophila oogenesis. J Cell Biol. 1999;144:533–547. doi: 10.1083/jcb.144.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakberg EF. A new concept of spermatogonial stem-cell renewal in the mouse and its relationship to genetic effects. Mutat Res. 1971;11:1–7. doi: 10.1016/0027-5107(71)90027-3. [DOI] [PubMed] [Google Scholar]
- Okamura D, Kimura T, Nakano T, Matsui Y. Cadherin-mediated cell interaction regulates germ cell determination in mice. Development. 2003;130:6423–6430. doi: 10.1242/dev.00870. [DOI] [PubMed] [Google Scholar]
- Parsa S, Kuremoto K, Seidel K, Tabatabai R, Mackenzie B, Yamaza T, Akiyama K, Branch J, Koh CJ, Al Alam D, Klein OD, Bellusci S. Signaling by FGFR2b controls the regenerative capacity of adult mouse incisors. Development. 2010;137:3743–3752. doi: 10.1242/dev.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS. Epidermal cell production rates. J Invest Dermatol. 1975;65:488–500. doi: 10.1111/1523-1747.ep12610194. [DOI] [PubMed] [Google Scholar]
- Potten CS, Schofield R, Lajtha LG. A comparison of cell replacement in bone marrow, testis and three regions of surface epithelium. Biochim Biophys Acta. 1979;560:281–299. doi: 10.1016/0304-419x(79)90022-2. [DOI] [PubMed] [Google Scholar]
- Robinson ML. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 2006;17:726–740. doi: 10.1016/j.semcdb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel K, Ahn CP, Lyons D, Nee A, Ting K, Brownell I, Cao T, Carano RA, Curran T, Schober M, Fuchs E, Joyner A, Martin GR, de Sauvage FJ, Klein OD. Hedgehog signaling regulates the generation of ameloblast progenitors in the continuously growing mouse incisor. Development. 2010;137:3753–3761. doi: 10.1242/dev.056358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim K, Minowada G, Coling DE, Martin GR. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev Cell. 2005;8:553–564. doi: 10.1016/j.devcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Smith CE, Warshawsky H. Cellular renewal in the enamel organ and the odontoblast layer of the rat incisor as followed by radioautography using 3H-thymidine. Anat Rec. 1975;183:523–561. doi: 10.1002/ar.1091830405. [DOI] [PubMed] [Google Scholar]
- Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, Golub TR, Sebolt-Leopold J, Sellers WR, Rosen N. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Zhu CH, Doan C, Xie T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296:1855–1857. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]
- Terling C, Heymann R, Rozell B, Obrink B, Wroblewski J. Dynamic expression of E-cadherin in ameloblasts and cementoblasts in mice. Eur J Oral Sci. 1998;106(Suppl 1):137–142. doi: 10.1111/j.1600-0722.1998.tb02166.x. [DOI] [PubMed] [Google Scholar]
- Thompson RC, Deo M, Turner DL. Analysis of microRNA expression by in situ hybridization with RNA oligonucleotide probes. Methods. 2007;43:153–161. doi: 10.1016/j.ymeth.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Stork PJ, Lazarovici P, Eiden LE. Signaling pathways for PC12 cell differentiation: making the right connections. Science. 2002;296:1648–1649. doi: 10.1126/science.1071552. [DOI] [PubMed] [Google Scholar]
- Wang XP, Suomalainen M, Felszeghy S, Zelarayan LC, Alonso MT, Plikus MV, Maas RL, Chuong CM, Schimmang T, Thesleff I. An integrated gene regulatory network controls stem cell proliferation in teeth. PLoS Biol. 2007;5:e159. doi: 10.1371/journal.pbio.0050159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Miyoshi J, Takai Y, Thesleff I. Cooperation of nectin-1 and nectin-3 is required for normal ameloblast function and crown shape development in mouse teeth. Dev Dyn. 2010;239:2558–2569. doi: 10.1002/dvdy.22395. [DOI] [PubMed] [Google Scholar]
- Zelenka PS, Arpitha P. Coordinating cell proliferation and migration in the lens and cornea. Semin Cell Dev Biol. 2008;19:113–124. doi: 10.1016/j.semcdb.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yang T, Madakashira BP, Thiels CA, Bechtle CA, Garcia CM, Zhang H, Yu K, Ornitz DM, Beebe DC, Robinson ML. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008;318:276–288. doi: 10.1016/j.ydbio.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohn IE, Li Y, Skolnik EY, Anderson KV, Han J, Niswander L. p38 and a p38-interacting protein are critical for downregulation of E-cadherin during mouse gastrulation. Cell. 2006;125:957–969. doi: 10.1016/j.cell.2006.03.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. E-cadherin is expressed in Gli1-expressing cells in the cervical loop (CL). CL from Gli1-lacZ incisor co-stained with anti-E-cadherin (red) and anti-beta-galactosidase (green) antibodies. Scale bar: 100 μm.
Fig. S2. H&E staining of the cervical loop (CL) after conditional deletion of E-cadherin. (A-B) Low magnification of CLs in controls and mutants. (A’-B’) High magnification of CLs in controls and mutants. Green arrows indicate disorganized region in B” compared with A”. Dotted lines outline the cervical loop epithelium. Scale bars: 250 μm in A, B; 50 μm in A’, B’; 20 μm in A”, B”.
Fig. S3. Expression of E-cadherin in in vitro formed colonies of wild-type cervical loops (CLs). (A) Phase contrast view of colony formed from primary cultured CLs. (B-D) E-cadherin expression in a colony formed from primary cultured CLs. Scale bars: 400 μm in A-D.
Fig. S4. TUNEL staining after deletion of E-cadherin in CLs. TUNEL staining in control and E-cadherin mutant CLs (A, B). Scale bars: 100 μm in A, B.
Fig. S5. Colony formation after FGF10 treatment. (A-E) E-cadherin expression in colonies formed in vitro from wild-type labial cervical loops (laCLs) after FGF10 treatment. In the absence of FGF10 treatment, two types of colonies formed. The first type (A) consisted of larger colonies with small, tightly-clustered cells. The second type (B) consisted of smaller colonies with larger cells. Both types of colonies were E-cadherin positive. After FGF10 treatment, in addition to the two types of colonies observed in the untreated control (C, D), a third type of colony that did not express E-Cadherin was present (E). (F) Quantification of colonies after FGF10 treatment in vitro. Dotted lines outline the laCLs. Scale bars: 250 μm A, C and E; 100 μm in B, D.
Fig. S6. E-cadherin expression and cell proliferation are increased and cell migration is decreased in Spry4–/–;Spry2+/– mice. (A-C’) E-cadherin expression in the lingual cervical loop in control (A, A’), Spry4–/– (B, B’) and Spry4–/–;Spry2+/– (C, C’) mice. (D-F) Cell proliferation as measured by PCNA and phospho-Histone 3 (pH3) staining in control (D), Spry4–/– (E) and Spry4–/–;Spry2+/– (F) mice. . (J-K) Quantification of cell proliferation detected with antibodies against PCNA (K) and pH3 (L); mean ±SEM (n=3, p<0.001). (G-I) Cell migration in control (G), Spry4–/– (H) and Spry4–/–;Spry2+/– (I) mice. Yellow arrowhead indicates the migrating front. (L) Quantification of the distance travelled by cells in the cervical loop in control, Spry4–/– and Spry4–/–;Spry2+/– mice; mean ±SEM (n=3; p<0.001). For the Spry4–/–;Spry2+/– mice, distance travelled by both labial and lingual cervical loop cells is shownDotted lines outline the cervical loop epithelium. Scale bars: 100 μm in A’F; 250 μm in A-C, G-I.
Fig. S7. Inhibition of the MAPK pathway with MEK1 inhibitor PD325901 (MEK antag) leads to expanded E-cadherin expression in the ameloblast region and increased migration of stem cell progeny. (A) Protocol used to label the cells with BrdU after treatment of animals with MEK1 inhibitor. (B-B”, D-D”) E-cadherin protein in control (B) and MEK 1 inhibitor treated incisor (D). (C, E) Cell migration in incisors from control (C) or animals treated with MEK1 inhibitor (E). Yellow arrowheads indicate the migrating front. (F) Quantification of the distance that cells traveled in control and MEK1 inhibitor treated cervical loop; mean ± SEM (n=3; p<0.001). (G) Quantification of E-cadherin punctae in ameloblasts in control and MEK1 inhibitor treated incisors; mean ± SEM (n=3; p<0.001). Dotted lines outline the cervical loop epithelium. Scale bars: 250 μm in B, D, C, F; 50 μm in B’, D’; 20 μm in B”, D”.