Abstract
The authors present the case of an 8-year-old boy with a long-term diagnosis of unilateral optic nerve hypoplasia (ONH) of unknown cause in the right eye. Spectral- domain optical coherence tomography (SD-OCT) of the central macula was consistent with hypoplasia greatest in the inner retinal layers, but also involving the outer retinal layers when compared with the unaffected contralateral eye. Although ONH is commonly associated with hypoplasia of the nerve fiber and ganglion cell layers, it can also be associated with hypoplasia of other layers in the inner and outer retina, including the outer nuclear and photoreceptor inner/outer segment layers, as evidenced by SD-OCT.
INTRODUCTION
Optic nerve hypoplasia (ONH) is one of the most common congenital optic nerve abnormalities and is a cause of vision loss in young children.1 ONH is accompanied by congenital deficiency of retinal ganglion cells and axons that compose the retinal nerve fiber layer (RNFL) and optic nerve head.2 Therefore, most reports of patients with ONH who underwent spectral-domain optical coherence tomography (SD-OCT) focus on a decrease in RNFL thickness and a small optic nerve. We describe a patient with a long-term diagnosis of ONH of unknown cause in the right eye who was found to have less central retinal thickness of both the inner and outer retinal layers compared with the unaffected left eye. Although ONH is commonly associated with lack of development in RNFL and retinal ganglion cell layers, it can also involve hypoplasia of the inner and outer retinal layers.
CASE REPORT
An 8-year-old boy presented with a long-term diagnosis of ONH. He had undergone a genetics consult/work-up and magnetic resonance imaging of the brain and orbits 6 years prior, which were unremarkable except for a small right optic nerve. He was born full-term via a normal, spontaneous vaginal delivery with a birth weight of 9 pounds 8 ounces. He had a history of amblyopia in the right eye (status post-occlusion therapy with a historical best-corrected visual acuity of 20/125), as well as exotropia and an afferent pupillary defect. His medical and developmental history were otherwise unremarkable.
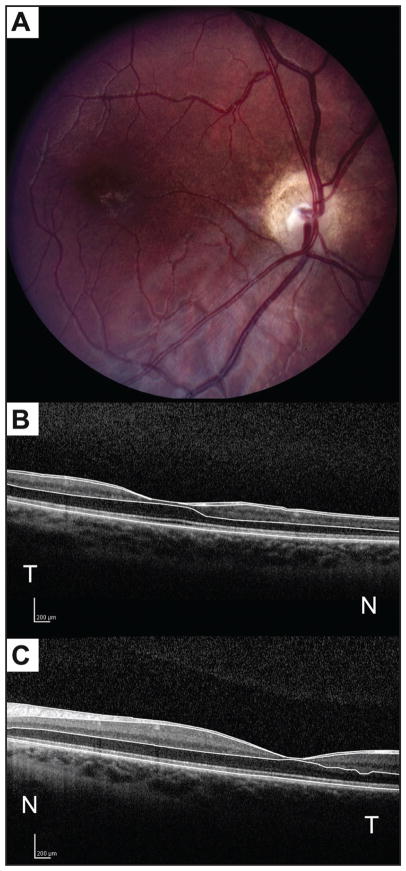
On presentation, best-corrected visual acuity was 20/200 in the right eye (with a manifest refraction of −1.25 +1.25 × 095), and 20/25 in the left eye (−2.00 +0.50 × 090). A right exotropia of approximately 15° was noted. Anterior segment examination was un-remarkable in both eyes. Ophthalmoscopy showed a small, pale nerve in the right eye with overlying pigment, a double-ring sign, and a hypoplastic rim that was most prominent nasally. The optic nerve head in the left eye appeared normal with a healthy neuroretinal rim. Macular examination using a 78-diopter lens revealed a subtle pigment change in the central macula in the right eye (Fig. 1A). The macula in the left eye was unremarkable.
Figure 1.
Fundus photograph of the right eye (A) and horizontal optical coherence tomography (OCT) scan through the fovea of the right eye (B) and the left eye (C). The photograph depicts optic nerve head hypoplasia and pallor, a double-ring sign, and pigmentary changes in the central macula. Examination of the right eye with spectral-domain OCT (SD-OCT) (B) shows a difference in retinal nerve fiber layer and a difference in all retinal layers in the central macula, most noticeably in the inner layers, but also affecting the outer layers when compared to SD-OCT of the left eye (C). Manual segmentation lines (B and C) delineate the inner limiting membrane, posterior border of the nerve fiber layer, posterior border of the outer plexiform layer, and the neural retina–retinal pigment epithelium interface. N = nasal; and T = temporal to the fovea.
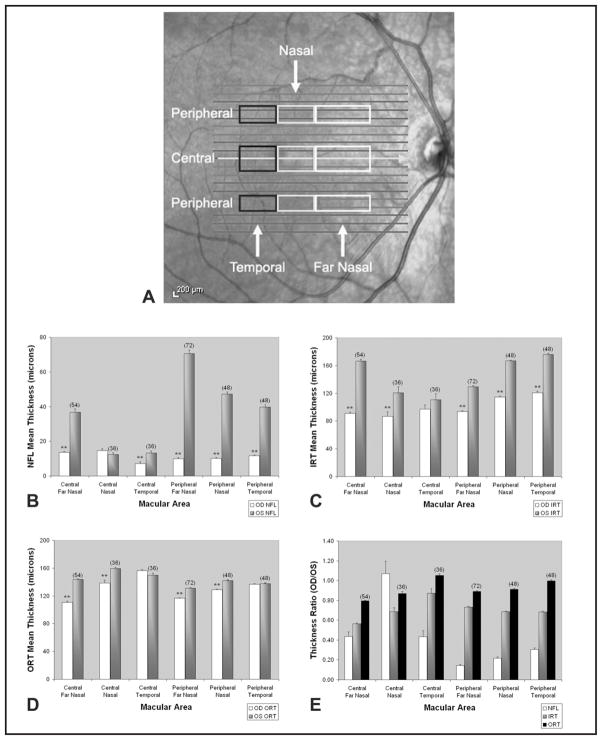
SD-OCT imaging was performed for both eyes using a commercially available instrument (Spectralis; Heidelberg Engineering, Heidelberg, Germany). SD-OCT of the RNFL in the right eye showed diffuse hypoplasia with relatively intact NFL between the 5- and 8-o’clock positions. Nineteen 6-mm horizontal OCT scans, each separated by 250 μm, were generated. The central foveal OCT scan in the right eye (Fig. 1B) compared with the left eye (Fig. 1C) demonstrated hypoplasia of all retinal layers in the central macula with the greatest difference in the inner retina, but also involving the outer retinal layers. Four peripheral macular scans (scans 4, 5, 14, and 15, respectively located at 1,500 and 1,250 μm inferior and 1,250 and 1,500 μm superior to the foveal center) and three central macular scans (scans 9, 10, and 11, respectively located 250 μm inferior to the fovea, at the foveal center, and 250 μm superior to the fovea) were selected for analysis (Fig. 2A).
Figure 2.
Map of macular regions selected for analysis (denoted by rectangles; (A). “Central” represents data points averaged from three horizontal scans located at 250 μm inferior, 250 μm superior, and through the foveal center. “Peripheral” represents data points averaged from four horizontal scans located at 1,250 and 1,500 μm superior, and 1,250 and 1,500 μm inferior to the fovea center. Measurements from 1,200 μm nasal to the foveal center extending up to the vertical midline of the fovea were defined as “nasal.” Measurements from the vertical midline of the fovea to 1,200 μm temporally were defined as “temporal.” Measurements from 3,000 to 1,200 μm nasal to the vertical mid-line of the fovea were defined as “far nasal.” Mean nerve fiber layer (NFL) thickness (B), inner retinal thickness (IRT; C), and outer retinal thickness (ORT; D) in central and peripheral areas, nasal and temporal to the fovea, comparing the right eye (OD) to the left eye (OS). Thickness measurements from the eye with optic nerve hypoplasia (OD) were divided by thickness measurements in the control eye (OS) at corresponding eccentricities and OD/OS thickness ratios were calculated (E). Sample size of data points was equal for both eyes in the respective areas, indicated within brackets. Error bars represent standard error of the means. A two-tailed t test was performed for data found in B, C, and D. ** = P < .005.
RESUlTS
Currently, Spectralis SD-OCT software provides total thickness maps but not thickness segmented into the layers of interest in this study. Therefore, for quantitative analysis of retinal hypoplasia, segmentation lines were manually drawn by one observer (QVH) using an image processing software (ImageJ, Bethesda, MD). Segmentation lines were drawn at the internal limiting membrane, posterior boundary of the NFL, posterior boundary of the outer plexiform layer, and at the neural retina–retinal pigment epithelium interface (Figs. 1B and 1C). A dedicated software program developed in Matlab (The Mathworks Inc., Natick, MA) was used to measure RNFL thickness (internal limiting membrane to posterior NFL), inner retinal thickness (posterior NFL to posterior outer plexiform layer), and outer retinal thickness (posterior outer plexiform layer to retinal pigment epithelium interface) based on the distance between segmentation lines.
Thickness profiles were generated from measurements obtained at 100-μm intervals laterally along OCT scans. Thickness profiles generated in the right and left eye were first aligned based on the minimum inner retinal thickness at the fovea center. As shown in Figure 2, the macula was divided into regions for more detailed analysis between the eyes. Measurements were made in “far nasal,” “nasal,” and “temporal” regions and averaged among “central” or “peripheral” macular scans (Fig. 2A). Mean NFL thickness, inner retinal thickness, and outer retinal thickness measurements in the central and peripheral macular areas of the two eyes are shown in Figures 2B, 2C, and 2D, respectively. Thickness ratio was calculated by dividing thickness measurements in the eye with ONH (the right eye) by thickness measurements in the control eye (the left eye) at corresponding locations (Fig. 2E).
The data revealed significant difference between the NFL in the right eye compared with the left eye in all regions except central nasal (Fig. 2B), with the greatest difference in peripheral areas and specifically in the far nasal area (a region between the optic nerve and fovea from 3,000 to 1,200 μm nasal to the fovea). In the peripheral far nasal area, NFL thickness of the right eye (10 ± 0.65 μm; mean ± standard error mean; n = 72 data points) was 14% ± 1% of NFL thickness of the left eye and significantly less thick by 61 μm (P < .005).
Similarly, in all regions there was difference in inner retinal thickness of the right eye compared to the left eye (Fig. 2C). This difference was statistically significant in all regions except the central temporal region. The greatest difference was in the central far nasal area, where mean inner retinal thickness of the right eye (91.2 ± 1.7 μm; n = 54) was 56% ± 1% of inner retinal thickness of the left eye. Additionally, in the peripheral temporal area, mean inner retinal thickness of the right eye (120.6 ± 2.5 μm; n = 48) was 68% ± 1% of inner retinal thickness of the left eye.
Furthermore, outer retinal thickness of the right eye demonstrated significant difference in nasal and far nasal areas, greater in central compared with peripheral areas (Figs. 1B, 1C, and 2D). Specifically, in the central far nasal area, outer retinal thickness of the right eye (110.6 ± 1.7 μm; n = 54) was 80% ± 1% of outer retinal thickness of the left eye, and in the peripheral nasal area, outer retinal thickness of the right eye (116.7 ± 0.7 μm; n = 72) was 89% ± 0.5% of outer retinal thickness of the left eye. In both areas, differences were found to be statistically significant (P < .005). In temporal regions, outer retinal thickness was not significantly different between eyes.
DISCUSSION
ONH results from congenital deficiency of retinal ganglion cells and retinal ganglion cell axons that lead to a disorganization of the retinal ganglion cell layer, NFL thinning, and a small optic nerve head.1,2 Srinivasan et al. demonstrated that OCT is a valid technique for quantitative assessment of optic disc dimensions, NFL features, and macular thickness measurement in a patient with unilateral ONH.3 Specifically, they reported comparable foveal thickness in both eyes, but with loss of foveal depression in the eye with ONH due to less than normal total retinal thickness, but inner and outer retinal layers were not evaluated separately.3 In a naturally occurring animal model of canine ONH, histologic studies suggested that although NFL and retinal ganglion cell layer thickness were hypoplastic, the outer retinal layers were preserved.4 Macular hypoplasia has also been reported in other optic neuropathies such as the morning glory disc anomaly,5 as well as in cases of acquired optic neuropathy associated with non-arteritic ischemic optic neuropathy, optic nerve head drusen, systemic lupus erythematosus, and pseudotumor cerebri.6 In these optic neuropathies, cone photoreceptors displayed structural changes in the presence of permanent damage to the overlying inner retina layers (retinal ganglion cell-NFL-inner plexiform).6
Through the use of SD-OCT and manual segmentation, our report demonstrates thinning of not only inner retinal layers but also outer retinal layers (outer nuclear and photoreceptor inner/outer segment layers) in a patient with congenital, unilateral ONH. We speculate that this subtle but significant hypoplasia of the outer retina may be due to a relative lack of neurotrophic factors during development. This lack of outer retinal development may also contribute to decreased vision in patients with ONH.
Although ONH is commonly associated with hypoplasia of the nerve fiber and ganglion cell layers, it can also be associated with hypoplasia of other layers in the inner retina and outer retina, including the outer nuclear and photoreceptor inner/outer segment layers, as evidenced by SD-OCT.
Acknowledgments
Supported by NIH grants EY014275 and EY01792, Department of VA, and a senior scientific investigator award and an unrestricted departmental grant from Research to Prevent Blindness.
Footnotes
The authors have no financial or proprietary interest in the materials presented herein.
References
- 1.Kim MR, Park SE, Oh SY. Clinical feature analysis of congenital optic nerve abnormalities. Jpn J Ophthalmol. 2006;50:250–255. doi: 10.1007/s10384-006-0310-8. [DOI] [PubMed] [Google Scholar]
- 2.Patel L, McNally RJ, Harrison E, Lloyd IC, Clayton PE. Geographical distribution of optic nerve hypoplasia and septo-optic dysplasia in Northwest England. J Pediatr. 2006;148:85–88. doi: 10.1016/j.jpeds.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan G, Venkatesh P, Garg S. Optic nerve head, retinal nerve fiber layer, and macular thickness characteristics on optical coherence tomography in optic disc hypoplasia. J Pediatr Ophthalmol Strabismus. 2007;44:140–141. doi: 10.3928/0191-3913-20070301-01. [DOI] [PubMed] [Google Scholar]
- 4.Negishi H, Hoshiya T, Tsuda Y, Doi K, Kanemaki N. Unilateral optical nerve hypoplasia in a Beagle dog. Lab Anim. 2008;42:383–388. doi: 10.1258/la.2007.007033. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan G, Venkatesh P, Garg S. Optical coherence tomographic characteristics in glory disc anomaly. Can J Ophthalmol. 2007;42:307–309. [PubMed] [Google Scholar]
- 6.Choi SS, Zawadzki RJ, Keltner JL, Werner JS. Changes in cellular structures revealed by ultra-high resolution retinal imaging in optic neuropathies. Invest Ophthalmol Vis Sci. 2008;49:2103–2119. doi: 10.1167/iovs.07-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]