Abstract
Purpose
To report a case of a macular vitelliform lesion associated with desferrioxamine treatment.
Methods
Ocular, electrophysiological, psychophysical, perimetric, fluorescein angiographic, fundus autofluorescence, and spectral-domain OCT examinations were obtained on a 45-year old Caucasian woman with thalassemia major treated with blood transfusions and desferrioxamine.
Results
The patient was observed to have a vitelliform macular lesion in the right eye with a hypopigmented macular lesion and retinal pigment mottling in the left. At the most recent follow-up visit, best-corrected visual acuity was 20/70 in the right eye and 20/25 in left. Full-field electroretinogram (ERG) testing showed normal cone and rod responses. Mild localized elevations of rod psychophysical thresholds were found.
Conclusion
A vitelliform macular lesion can develop in patients treated with desferrioxamine. Some such patients may not show diffuse photoreceptor cell functional loss as determined by electrophysiological testing.
Keywords: Desferrioxamine toxicity, Macular vitelliform lesion, Electroretinogram measurement
Introduction
Desferrioxamine mesylate is an iron chelating agent used in the treatment of chronic iron overload in patients with thalassemia major and other hematologic conditions requiring regular blood transfusions [1]. Ocular toxicity secondary to prolonged treatment with desferrioxamine may result in night blindness, a centrocaecal scotoma, constriction of the peripheral visual field, pigmentary retinopathy, or optic neuropathy [2–8].
Macular and/or peripheral pigmentary degeneration are the most common changes described. The earliest fundus changes described are subtle opacification or loss in transparency of the outer retina and retinal pigment epithelium (RPE). These changes precede the development of RPE mottling. Peripapillary, papillomacular, and paramacular patterns of RPE degeneration have been previously demonstrated [9, 10].
Case report
A 45-year old Caucasian woman of Italian ancestry had a history of progressive decrease in central vision of each eye (more in the right eye than the left). There was a 10 years history for the sudden onset of decreased visual acuity in each eye. The patient's only other visual complaints were intermittent photoaversion and difficulty seeing in dim light. There was no history of impairment for peripheral vision or color vision. The patient had a known history for thalassemia major with regular blood transfusions and had been treated with intramuscular or subcutaneous desferrioxamine for the past 20 years. A general review of systems indicated a history for mild hearing impairment. The patient previously had a splenectomy, appendectomy, tonsillectomy as well as gall bladder surgery for cholelithiasis. There was a history for smoking one pack of cigarettes a week for 20 years.
At the time of her initial presentation, the fundus exam showed a stippled appearance to the right macula including both hypopigmentation and pigment clumping. The stippling extended just anterior to the vascular arcades (most apparent inferiorly). The left eye showed similar changes with an isolated white spot inferior to the vascular arcades and a choroidal nevus just inferior to fovea. Best-corrected visual acuity was 20/20 in each eye. Three years after the initial visit, the fundus exam of the right eye showed a small yellowish lesion at the inferior margin of the fovea with hypopigmentary changes superiorly. The left eye showed a similar small yellowish lesion and atrophy within the macula in addition to the choroidal nevus inferior to the fovea. Best-corrected visual acuity was 20/20–2 in each eye. The optic discs and the retinal vessels were normal in both eyes at all of the follow-up visits.
On her most recent visit, distance visual acuity was correctable to 20/70+2 OD with a +1.75+0.50 × 65° and 20/25–2 OS with a +1.00 sphere. External examination showed that both eyes were orthophoric and there was a full range of ocular motion in all directions of gaze. Pupils were round and reacted normally without an afferent pupillary defect. Slit-lamp examination showed no evidence of conjunctival injection. The corneas, lenses, and anterior chambers were clear. Ocular pressures measured by applanation tonometry were 14 mmHg OD and 12 mmHg OS.
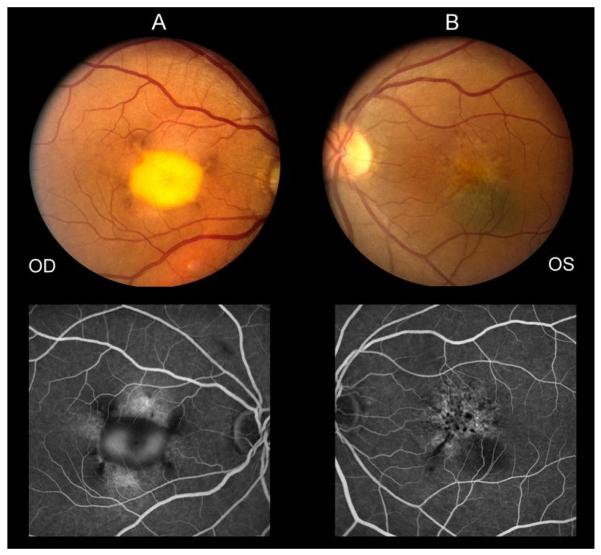
Dilated fundoscopic examination at the most recent visit showed that the retina was flat and the retinal vasculature was normal in each eye. The cup to disc ratio was 0.2 in each eye. The central foveal region of the right eye showed an elevated vitelliform-appearing lesion with hyperpigmented changes at the margins (Fig.1A). The macula of the left eye showed a hypopigmentary lesion with mild RPE pigment mottling and clumping. The previously observed small yellowish macular lesion was no longer apparent. The flat choroidal nevus was noted inferior to the fovea. The nevus was approximately 1 by 1 ½ disc diameters in size. There was an isolated druse temporal to the macula and just anterior to the vascular arcades (Fig.1B).
Fig. 1.
Fundus photograph obtained at the most recent follow-up visit of the right eye (A) demonstrating a vitelliform-appearing macular lesion and left eye (B) demonstrating macular hypopigmentary changes, RPE mottling, and a flat choroidal nevus inferior to the fovea. Fluorescein angiogram (late frames) of both eyes shows window defects and late staining in the macula of each eye with pooling of fluorescein dye in the macula of the right eye as well as hypofluorescent loci in each eye.
Fluorescein angiography at the most recent visit of the right eye showed a normal retinal vasculature. In the foveal region there were regions of hypofluorescence, which correlated with the hyperpigmentary changes seen fundoscopically, and late pooling of fluorescein which corresponded to the vitelliform lesion. Window defects of hyperfluorescence were noted at superior and inferior margins of the vitelliform lesion. There was no leakage or choroidal neovascularization (CNVM) in late frames of the angiogram. The left eye showed multiple punctate areas of hyperfluorescence corresponding to RPE window defects as well as areas of punctate blocked fluorescence corresponding to RPE hyperpigmentation. Hypofluorescence was seen to correspond to the choroidal nevus. There was no evidence of CNVM (Fig.1A and B).
Visual field examination was performed by Goldmann kinetic perimetry 940 (Haag-Streit AG, Switzerland), using I-2-e, II-2-e and II-4-e test targets. The testing showed a normal peripheral boundary to all test targets in each eye. The right eye showed a small relative central scotoma while the left eye was normal.
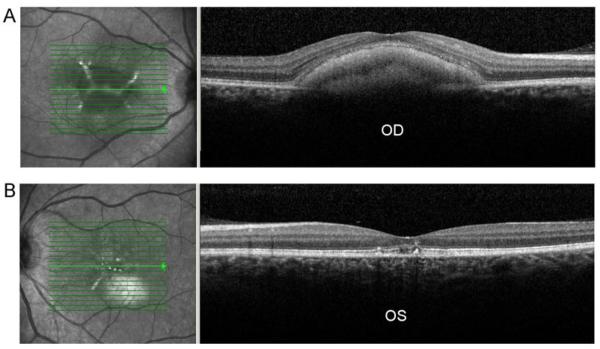
A spectral domain-OCT (SD-OCT) examination was performed at the most recent visit by using a Spectralis HRA+OCT instrument (Heidelberg Retina Angiograph (HRA), Heidelberg Engineering, Heidelberg, Germany). OCT testing of the right eye showed an elevation of the RPE and accumulation of material in the sub-RPE space associated with the vitelliform macular lesion. The left eye showed disruption of the normal anatomy within the macula which involved the inner/outer segment (IS/OS) junction of the photoreceptors with focal foveal areas of RPE loss and disruption (Fig.2A and B).
Fig. 2.
SD-OCT of the right eye (A) shows an elevation of the RPE associated with the vitelliform macular lesion and left eye (B) showing foveal disruption of the inner/outer segment junction of the photoreceptors with focal RPE disruption and loss. Infrared image (IR) of right eye (A) shows a well-demarcated vitelliform-appearing macular lesion with an enhanced reflectivity within the lesion. Left eye IR image (B) shows several foci of enhanced reflectively in the fovea and a hyper-reflective lesion inferior to fovea corresponding to the choroidal nevus seen ophthalmoscopically.
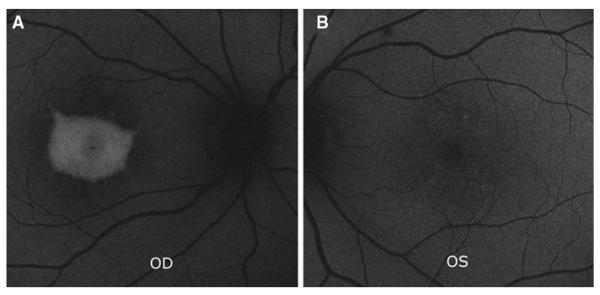
Fundus autofluorescence (FAF) was performed with a confocal laser scanning ophthalmoscope (cSLO) (Spectralis; Heidelberg Engineering, Heidelberg, Germany). Infrared (IR) reflectance images were obtained as well by the Spectralis system. FAF image of right eye showed an area of intense enhanced autofluorescence corresponding to the vitelliform macular lesion while the left eye showed a few scattered foci of faint enhanced autofluorescence corresponding to the RPE changes seen fundoscopically (Fig.3).
Fig. 3.
Autofluorescence (AF) image of the right eye (A) shows an area of enhanced autofluorescence corresponding to the vitelliform macular lesion. Left eye image (B) shows scattered foci of faint enhanced autofluorescence corresponding to the RPE changes observed fundoscopically.
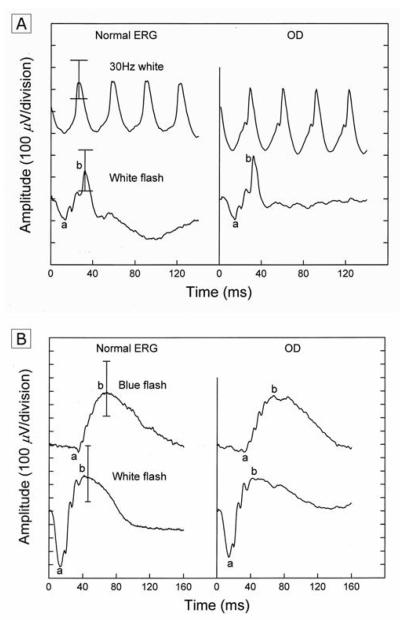
A full-field electroretinogram (ERG) (Nicolet Viking IV system, Nicolet Biomedical Inc) was performed in the right eye by using a unipolar Burian-Allen contact lens electrode. The ERG responses were obtained according to the International Society for Clinical Electrophysiological of Vision (ISCEV) standards. Parameters included amplitudes and implicit times for each of the major waveform components [11]. The full-field ERG did not show any evidence of diffuse cone or rod impairment as the ERG b-wave amplitudes and implicit times were normal to all stimulus parameters (Fig.4). Psychophysical threshold screening with a Tübingen perimeter showed several loci of elevated rod final thresholds, no greater than 0.3 log units, within 45 degrees of the fovea.
Fig. 4.
Full-field electroretinogram (ERG) responses from the right eye show normal cone and rod amplitudes and implicit times under light-adapted (right panel, A) and dark-adapted (right panel, B) conditions.
Discussion
Ocular toxicity secondary to desferrioxamine has been reported in several studies [2–8]. Survival of patients with thalassemia necessitates long-term blood transfusions with consequent iron overload of vital organs, for which chelation therapy is mandatory. Desferrioxamine toxicity is thought to arise secondary to chelation of metals such as iron, copper, and zinc, which are essential for normal retinal function [12, 13].
We report a case with a vitelliform-appearing macular lesion after prolonged desferrioxamine therapy on clinical fundus examination and SD-OCT testing. Findings on the SD-OCT showed that the vitelliform lesion was located in the sub-RPE space. An explanation for the enhanced autofluorescence of this lesion remains conjectural, although a fluorophore of lipofuscin as a component of the vitelliform lesion seems plausible. The patient's history and clinical exam, including a late-onset decrease in her central acuity (fourth decade of life), prolonged history of desferrioxamine use (20 years), and negative family history of any ocular disorders, favor an acquired cause for her maculopathy. While the vitelliform-like macular lesion seen in our patient has a phenotype simulating the vitelliform lesion seen in Best's macular dystrophy, and some forms of pattern dystrophy, the vitelliform lesion in our patient is highly likely related to the use of desferrioxamine as noted in a previous case report [14].
The longitudinal changes observed in the evolution of our patient's macular lesions were not reminiscent of those seen in the dominantly transmitted bestrophin gene. Our patient initially demonstrated a stippled appearance to the posterior pole that included both hypopigmentation and pigment clumping extending anterior to the vascular arcade. This phenotypic appearance is characteristic of fundus changes observed in patients with desferrioxamine retinal toxicity. What was unusual in our patient was the vitelliform-appearing lesion that developed beyond the fourth decade of life, a finding previously reported in a single similar case [14].
Patients with peripherin/RDS gene mutation associated with pattern dystrophy generally have an autosomal dominant transmission and do not begin with atrophic macular lesions or diffuse retinal pigmentary stippling but most often pigmentary changes in the macula with a pattern that has been likened to a butterfly in many instances. It is true that some such patients may present with a vitelliform lesion but do not show diffuse granular stippling as noted in our patient. Patients with bestrophinopathy tend to have diffuse changes throughout the posterior pole including, on occasion, a vitelliform lesion of the macula, and multiple yellow-white smaller flecks-like lesions. Diffuse granular stippling is not part of the phenotype.
Several previous reports demonstrated a different retinopathy secondary to desferrioxamine therapy such as early subtle RPE opacification, RPE pigment mottling, and a bull's eye maculopathy [2, 3, 9, 10]. To our knowledge, only a single report demonstrated the presence of a similar vitelliform-appearing macular lesion in a patient with desferrioxamine toxicity [14]. Although electrophysiologic testing can be useful in demonstrating photoreceptor and retinal pigment epithelial cell dysfunction in patients with desferrioxamine toxicity, these tests are not specific and variable results have been reported [9,15,16]. Our patient's full-field ERG showed normal cone and rod responses. This finding differs from some previous reports which demonstrated ERG abnormalities, including reduced cone and rod amplitudes and increased implicit times [9,15,16]. However, a normal ERG has also been previously described in patients with desferrioxamine retinopathy [9].
Previous studies evaluated the correlation between the dosage of desferrioxamine used and ocular toxicity without a definitive conclusion [2, 14, 17, 18]. Roulez showed that ocular changes with the use of desferrioxamine could be reversed if treatment was discontinued at initial stages of toxicity [17]. However, Bene et al., showed an irreversible retinopathy even after a small dose of desferrioxamine [18].
Our case report highlights the association of an adult-onset vitelliform macular lesion and a reduction in visual acuity with RPE degenerative changes associated with prolonged treatment with desferrioxamine. In our case, electrophysiological testing did not find a reduction in cone or rod function. Psychophysically determined retinal sensitivity found only mild elevations of rod absolute thresholds.
Acknowledgements
Supported by funds from the Foundation Fighting Blindness, Owings Mills, Maryland; Grant Healthcare Foundation, Lake Forest, Illinois; NIH core grant EYO1792; and an unrestricted departmental grant from Research to Prevent Blindness.
Footnotes
The authors have no proprietary interests in this report.
References
- 1.Porter JB. A risk-benefit assessment of iron-chelation therapy. Drug Saf. 1997;17:407–421. doi: 10.2165/00002018-199717060-00006. [DOI] [PubMed] [Google Scholar]
- 2.Lakhanpal V, Schocket SS, Jiji R. Deferoxamine (Desferal)-induced toxic retinal pigmentary degeneration and presumed optic neuropathy. Ophthalmology. 1984;91:443–451. doi: 10.1016/s0161-6420(84)34267-1. [DOI] [PubMed] [Google Scholar]
- 3.Davies SC, Marcus RE, Hungerford JL, Miller MH, Arden GB, Huehns ER. Ocular toxicity of high-dose intravenous desferrioxamine. Lancet. 1983;2:181–184. doi: 10.1016/s0140-6736(83)90170-8. [DOI] [PubMed] [Google Scholar]
- 4.Orton RB, de Veber LL, Sulh HM. Ocular and auditory toxicity of long-term, high-dose subcutaneous Deferoxamine therapy. Can J Ophthalmol. 1985;20:153–156. [PubMed] [Google Scholar]
- 5.Cases A, Kelly J, Sabater F, Torras A, Griño MC, Lopez-Pedret J, Revert L. Ocular and auditory toxicity in hemodialyzed patients receiving desferrioxamine. Nephron. 1990;56:19–23. doi: 10.1159/000186094. [DOI] [PubMed] [Google Scholar]
- 6.Mehta AM, Engstrom RE, Jr, Kreiger AE. Deferoxamine associated retinopathy after subcutaneous injection [letter] Am J Ophthalmol. 1994;118:260–262. doi: 10.1016/s0002-9394(14)72914-9. [DOI] [PubMed] [Google Scholar]
- 7.De Virgiliis S, Congia M, Turco MP, Frau F, Dessi C, Argiolu F, Sorcinelli R, Sitzia A, Cao A. Depletion of trace elements and acute ocular toxicity induced by desferrioxamine in patients with thalassaemia. Arch Dis Child. 1988;63:250–255. doi: 10.1136/adc.63.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen A, Martin M, Mizanin J, Konkle DF, Schwartz E. Vision and hearing during deferoxamine therapy. J Pediatr. 1990;117:326–330. doi: 10.1016/s0022-3476(05)80556-6. [DOI] [PubMed] [Google Scholar]
- 9.Haimovici R, D'Amico DJ, Gragoudas ES, Sokol S. The expanded clinical spectrum of deferoxamine retinopathy. Ophthalmology. 2002;109:164–171. doi: 10.1016/s0161-6420(01)00947-2. [DOI] [PubMed] [Google Scholar]
- 10.Bansal V, Elgarbly I, Ghanchi FD, Atkinson PL. Bull's eye maculopathy with deferoxamine. Eur J Haematol. 2003;70(6):420–421. doi: 10.1034/j.1600-0609.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 11.Marmor MF, Holder GE, Seeliger MW, Yamamoto S. Standard for clinical electroretinography (2004 update) Doc Ophthalmol. 2004;108:107–114. doi: 10.1023/b:doop.0000036793.44912.45. [DOI] [PubMed] [Google Scholar]
- 12.Pall H, Blake DR, Winyard P, Lunec J, Williams A, Good PA, Kritzinger EE, Cornish A, Hider RC. Ocular toxicity of desferrioxamine- an example of copper promoted auto-oxidative damage? Br J Ophthalmol. 1989;73:42–47. doi: 10.1136/bjo.73.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaston TB, Richardson DR. Iron chelators for the treatment of iron overload disease: relationship between structure, redox activity, and toxicity. Am J Hematol. 2003;73:200–210. doi: 10.1002/ajh.10348. [DOI] [PubMed] [Google Scholar]
- 14.Gonzales CR, Lin AP, Engstrom RE, Kreiger AE. Bilateral vitelliform maculopathy and deferoxamine toxicity. Retina. 2004;24(3):464–467. doi: 10.1097/00006982-200406000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Arden GB, Wonke B, Kennedy C, Huehns ER. Ocular changes in patients undergoing long-term desferrioxamine treatment. Br J Ophthalmol. 1984;68(12):873–877. doi: 10.1136/bjo.68.12.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang C, Hansen RM, Gee BE, Kurth SS, Fulton AB. Rod and rod mediated function in patients with beta-thalassemia major. Doc Ophthalmol. 1999;96(4):333–345. doi: 10.1023/a:1001728512515. [DOI] [PubMed] [Google Scholar]
- 17.Roulez F. Retinal pigment epithelium-desferal. Bull Soc Belge Ophtalmol. 2007;(304):59–66. [PubMed] [Google Scholar]
- 18.Bene C, Manzler A, Bene D, Kranias G. Irreversible ocular toxicity from single “challenge” dose of deferoxamine. Clin Nephrol. 1989;31(1):45–8. [PubMed] [Google Scholar]