Abstract
Purpose
To correlate macular structural changes by spectral domain optical coherence tomography (SD-OCT) with functional changes by scanning laser ophthalmoscope (SLO) microperimetry testing in patients with sickle cell hemoglobinopathies.
Design
Prospective, investigational study
Methods
Patients with electrophoretic confirmation of sickle cell hemoglobinopathies and normal subjects underwent SD-OCT and microperimetry testing with the OPKO Spectral OCT/SLO instrument. Based on SD-OCT findings, patients were grouped into those with focal macular thinning (Group A) and those without (Group B). Main outcome measure were mean retinal sensitivities measured by microperimetry and mean macular thicknesses in the 9 Early Treatment Diabetic Retinopathy Study (ETDRS)-like subfields.
Results
Thirty-seven eyes of 19 patients with sickle cell hemoglobinopathies (SS, SC, and S-Thalassemia) and 34 eyes of 34 age similar normal controls were included. Mean age and mean logMAR best corrected visual acuity between Group A and B were not statistically different (39.7 years vs. 36.5 years, p = 0.64 and 0.015 vs. 0.016, p = 0.93, respectively). Group A had significantly thinner retinas compared to Group B in the parafoveal superior (p = 0.019), parafoveal temporal (p < 0.004), parafoveal inferior (p = 0.003), perifoveal superior (p = 0.04), perifoveal temporal (p = 0.0005), and perifoveal inferior (p = 0.045) subfields. The overall mean microperimetry retinal sensitivities of Group A were significantly less than those of Group B (14.2 dB vs. 16.5 dB, p = 0.00005). However, there was no statistical difference between Group B and controls (16.5 dB vs. 16.7 dB, p = 0.63).
Conclusion
Sickle cell patients with focal macular thinning present on SD-OCT have significantly decreased retinal sensitivities compared to those without focal thinning or normal controls based on mean microperimetry sensitivities, despite similar age and visual acuity. Microperimetry is a sensitive measurement of macular function in patients with sickle cell hemoglobinopathies.
Introduction
Patients with sickle cell hemoglobinopathies inherit an abnormal globin protein chain that results in occlusions in various vascular systems. Vascular occlusions occur from sickling of the erythrocytes, increased viscosity, and veno-stasis especially in the setting of hypoxia, acidosis, and inflammation.1 In the retina, common findings include salmon-patch hemorrhages, iridescent spots, and black sunbursts. Non-proliferative sickle retinopathy with arteriolar occlusions and arteriovenous anastomoses can progress to proliferative sickle retinopathy with sea-fan neovascularization, vitreous hemorrhage, and retinal detachment.2 Profound visual loss resulting from vitreous hemorrhage and tractional retinal detachment has been reported to be as high as 10–20% in symptomatic patients in a referral setting3 but rare in a recent observational cohort study.4
Although sickle retinopathy primarily occurs in the retinal periphery, structural macular changes have been well documented by clinical examination,5 fluorescein angiography and histopathology.6–7 These changes include enlargement of the foveal avascular zone with perifoveal capillary dropout, nerve fiber layer infarcts, and vascular abnormalities including microaneurysm-like dots and hairpin-shaped venular loops.8 The temporal horizontal raphe represents a watershed area and is subject to terminal arteriolar occlusions. Previous studies from our group using spectral-domain optical coherence tomography (SD-OCT) showed focal macular thinning in about 50% of eyes with sickle cell hemoglobinopathies (Chau FY, et al. IOVS 2010;51:ARVO E-Abstract 3554). In those sickle cell patients without obvious focal macular thinning based on SDOCT, we found subclinical foveal thinning and splaying as well as thinning in the outer retina in the central foveal subfield and the temporal parafoveal regions, when compared to age-matched controls.9 However, the functional impairment caused by these structural changes is unknown, since a majority of these patients retain excellent visual acuity.
The introduction of microperimetry testing with a scanning laser ophthalmoscope (SLO) in 198210–11 added a useful tool for clinicians to evaluate macular function. In 2006, OPKO/OTI (OPKO Instrumentations, Miami, FL) developed a SLO microperimeter in conjunction with SD-OCT. The SLO provides high-resolution images of the posterior ocular structures and incorporates ocular tracking capabilities allowing excellent test reliability and reproducibility during SD-OCT and microperimetry testing. After data acquisition, this system superimposes perimetric sensitivity values on an SLO infrared image with associated retinal thickness values, thus allowing direct correlations of structural and functional abnormalities. In the current study, we used this device to examine the relationship between the retinal thickness and macular sensitivity in patients with sickle cell hemoglobinopathies.
Patients and Methods
All patients who participated in this prospective, unmasked, comparative investigational study were diagnosed at the University of Illinois at Chicago.
Patient Selection
All patients had electrophoretic confirmation of sickle cell disease (SS), sickle cell -hemoglobin C disease (SC), or sickle-cell thalassemia (S-thal). Medical histories and sickle hemoglobinopathy type were recorded from the medical record. Initial evaluation included best corrected visual acuity (BCVA) measurment by using the Snellen chart, Goldmann applanation tonometry, slit-lamp biomicroscopy, and dilated fundus examination. Staging of sickle retinopathy was based on the classification developed by Goldberg.2 Stage I: peripheral arteriolar occlusion; stage II: arteriovenous anastomoses; stage III: peripheral neovascularization; stage IV: presence of vitreous hemorrhage; and stage V: tractional and/or rhegmatogenous retinal detachment. All patients were staged by the same observer (JIL). Patients with lens or other ocular media opacities, extensive laser or cryotherapy treatment, diabetes mellitus, uncontrolled systemic hypertension, or clinical evidence of any other maculopathies were excluded from the current study.
SD-OCT Retinal Thickness Mapping
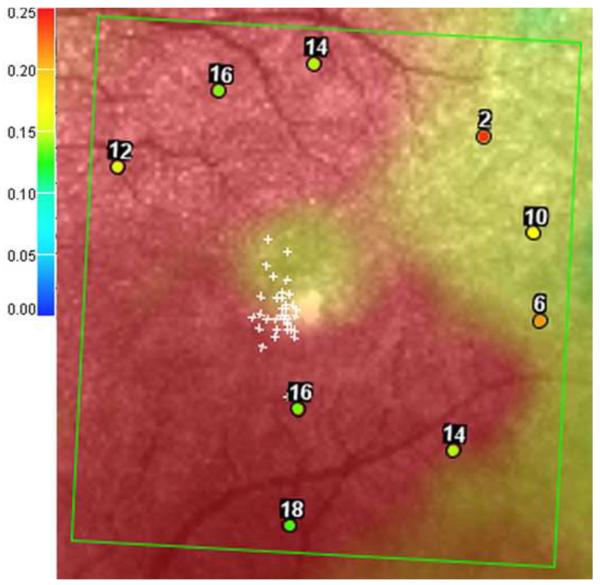
SD-OCT was performed using the Spectral OCT/SLO system (OPKO instrumentations, Miami, FL) to obtain both OCT and SLO images with an axial resolution of < 10 microns, and a transverse resolution of 20 microns (in tissue). The system uses an SLO fundus image for alignment, orientation, and registration of the OCT image topographic maps. Both the Line scan (B-scan) and the 3-Dimension Retinal Topography scan protocols were used for image acquisition. The Line scan mode allows the capture of cross-sectional B-scan OCT images of the vitreoretinal, retinal, and chorioretinal structures. A red scanning line on the SLO image represents the exact location of the cross sectional OCT image. We used the “Max Frame Count” of 32 frames. The “Max Frame Count” is the maximum sequentially captured frames of OCT and SLO images, which are captured and displayed as individual frames. The 3D Retinal Topography mode covers an area of 9.0 × 9.0 mm with a 2.0 mm depth. The retinal thickness map is displayed as 9 Early Treatment Diabetic Retinopathy Study (ETDRS)-like subfields that correspond to the microperimetry testing area, including central, parafoveal and perifoveal superior, temporal, inferior, and nasal subfields (Figure 1). The central subfield included the circle centered on the fovea with a radius of 0.5 mm. The parafoveal subfields included the concentric ring of retina around the central subfield with an inner radius of 0.5 mm and an outer radius of 1.5 mm from the fovea. The perifoveal subfields included the outer ring of retina beyond the parafoveal subfields concentric with the fovea and with an inner radius of 1.5 mm and an outer radius of 3 mm. Focal macular thinning was defined as abrupt asymmetric decrease in retinal thickness confirmed on thickness map and B-scan.
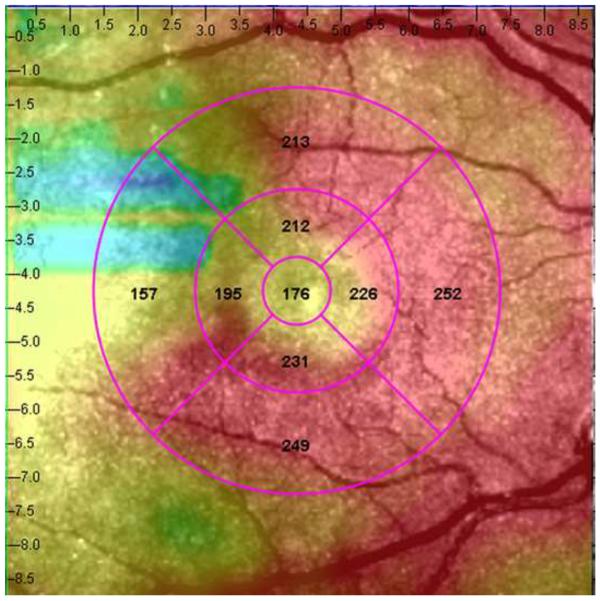
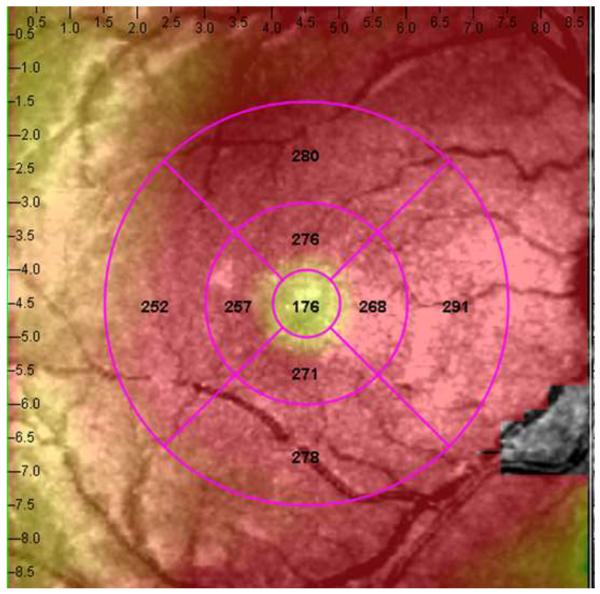
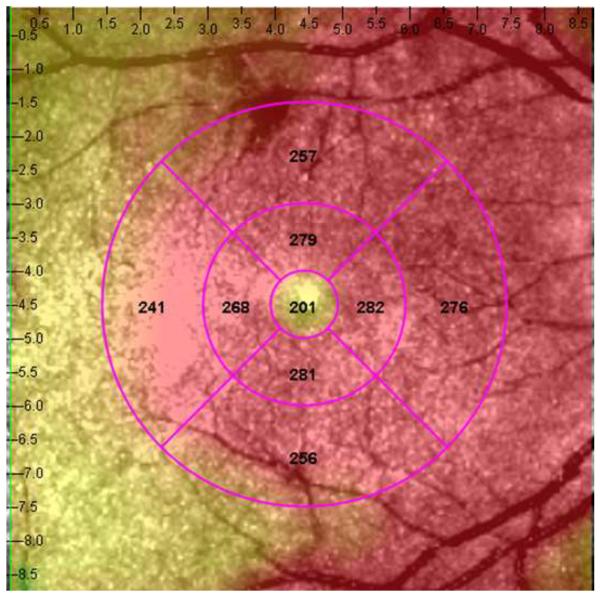
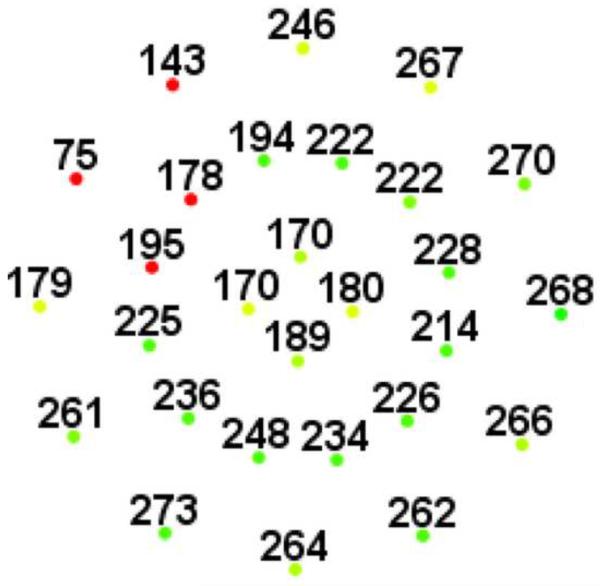
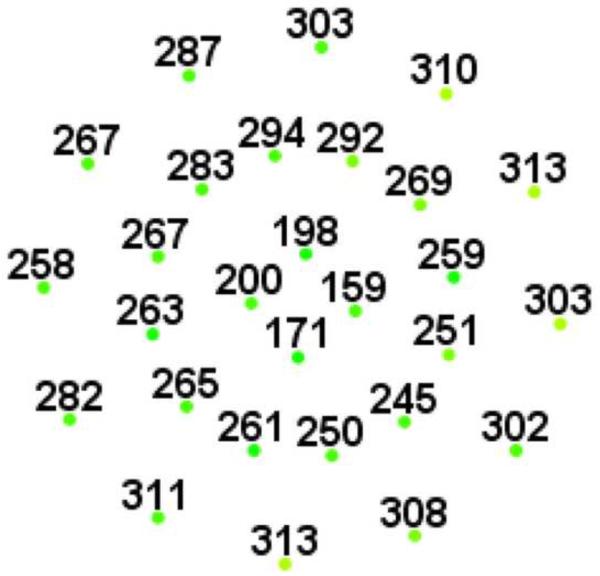
Figure 1.
Representative macular SD-OCT maps showing mean thicknesses of 9 ETDRS-like subfields within the polar 3 microperimetry testing grid. (Left) SD-OCT map of right eye of patient 3 (SS sickle cell patient, best corrected visual acuity 20/25), demonstrating significant perifoveal and parafoveal temporal thinning and therefore grouped to Group A (“with macular thinning”). (Middle) SD-OCT map of right eye of patient 14 (SS sickle cell patient, best corrected visual acuity 20/20) showing no macular focal thinning and therefore grouped to Group B (“without macular thinning”). (Right) SD-OCT map of right eye of a age similar normal control showing normal retinal thicknesses.
Macular Microperimetry Testing
Microperimetry was performed using the OPKO Spectral OCT/SLO system. All tests were performed after dilation of the pupil with 1% tropicamide and 2.5% phenylephrine. The non-tested eye was patched during testing. The subjects underwent a practice test and were instructed to fixate on a red square and to depress a button as soon as they saw the stimulus light. The Polar 3 testing grid was used for all subjects. Polar 3 is a standardized grid composed of 28 points arranged in three concentric circles (2.3° for inner, 6.6°for middle, and 11° for outer circle in diameter) within the central macula. The inner circle is composed of four points, whereas the middle and outer circles are each composed of 12 points. Parameters included a Goldmann III size stimulus (area of 4 mm2, diameter of 26 minute of arc or 0.4 degrees), a 200 millisecond duration of stimulus presentation, and a 4–2 test strategy. The 4–2 test strategy is the standard method in automated static perimetry for estimating threshold. It uses a staircase with an initial crossing of threshold in 4 dB increments and a final crossing in 2 dB increments.12
The system automatically tracks fundus localization according to retinal vessel alignment to ensure accurate stimuli placement. If focal macular thinning was found on SD-OCT, a customized testing pattern was created and projected to the area of focal thinning in addition to the standard Polar 3 pattern. After retinal thickness and microperimetry maps were generated, the images were aligned using native software so that the retinal thickness at each microperimetry test location could be obtained.
Normative sensitivity values and intrasession repeatability were measured in 32 normal subjects with the same instrument for comparison with our study cohort.13 In summary, the sensitivity values averaged across two tests for inner, middle, and outer circles ranged from 14.3 to 18.8 dB (median value of 16.9 dB), 13.8 to 18.3 dB (median value of 17.2 dB), and 11.3 to 18.3 dB (median value of 16.6 dB), respectively. The mean intrasession sensitivity variability between two tests was small (0.13 dB). Based on these results, retinal sensitivity of 12 dB or lower was considered abnormal for our particular instrument.
Data Analysis
For data analysis purposes, Snellen chart visual acuity was converted to logMAR visual acuity.14 Eyes with focal macular thinning were grouped into Group A and those without thinning were grouped into Group B. Two tail, unpaired t-test was performed between Group A, Group B, and controls using Microsoft Excel software. A p-value less than 0.05 generated by the program was considered statistically significant.
Results
Thirty-seven eyes of 19 African American patients with sickle cell hemoglobinopathies (SS, SC, S-thal) and 34 eyes of 34 age-similar, visually normal control subjects were included in this study. Fifteen patients had SS, two had SC, and two had S-thal disease. The sickle cell eyes were divided into 2 groups - those with focal macular thinning (Group A, 17 eyes of 10 patients) and those without (Group B, 20 eye of 11 patients) based on SD-OCT exam. Two patients had one eye in each group. Mean ages of Groups A and B were 39.7 years (range 20–66) and 36.5 years (range 22–66) respectively which was not statistically different (p = 0.64). Mean logMAR BCVA was not different between the two groups (0.0167 for Group A vs. 0.015 for Group B, p = 0.93). The median stage of sickle cell retinopathy for both groups was II (range I to IV). Demographics of patients are summarized in Table 1.
Table 1.
SD-OCT/SLO Microperimetry in Sickle Cell Hemoglobinopathies: Demographics of Enrolled Patients
| With Focal Thinning (Group A) | Without Focal Thinning (Group B) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Patient | Age | Eye | BCVA (Snellen) | BCVA (log MAR) | Sickle type | Stagea | Patient | Age | Eye | BCVA (Snellen) | BCVA (log MAR) | Sickle type | Stagea |
| 1 | 20 | OD | 20/20 | 0 | SS | II | 11 | 22 | OD | 20/20 | 0 | SS | II |
| 2 | 21 | OD | 20/15 | −0.1 | Sthal | III | OS | 20/25 | 0.1 | SS | I | ||
| OS | 20/15 | −0.1 | Sthal | II | 12 | 24 | OD | 20/20 | 0 | SS | II | ||
| 3 | 22 | OD | 20/25 | 0.1 | SS | II | OS | 20/20 | 0 | SS | II | ||
| OS | 20/20 | 0 | SS | II | 13 | 26 | OD | 20/20 | 0 | SS | II | ||
| 4 | 26 | OD | 20/20 | 0 | SS | I | OS | 20/20 | 0 | SS | II | ||
| 5 | 39 | OD | 20/20 | 0 | SS | II | 4 | 26 | OS | 20/20 | 0 | SS | I |
| OS | 20/25 | 0.1 | SS | II | 14 | 28 | OD | 20/20 | 0 | SS | I | ||
| 6 | 41 | OD | 20/20 | 0 | SS | II | OS | 20/20 | 0 | SS | I | ||
| OS | 20/25 | 0.1 | SS | II | 15 | 28 | OD | 20/25 | 0.1 | SS | I | ||
| 7 | 50 | OD | 20/25 | 0.1 | SS | IV | OS | 20/25 | 0.1 | SS | II | ||
| OS | 20/20 | 0 | SS | II | 16 | 39 | OD | 20/20 | 0 | SS | II | ||
| 8 | 54 | OD | 20/20 | 0 | SS | II | OS | 20/20 | 0 | SS | II | ||
| OS | 20/20 | 0 | SS | III | 17 | 40 | OD | 20/20 | 0 | SC | II | ||
| 9 | 58 | OD | 20/20 | 0 | SS | III | OS | 20/15 | −0.1 | SC | II | ||
| OS | 20/25 | 0.1 | SS | III | 18 | 43 | OD | 20/20 | 0 | SS | II | ||
| 10 | 66 | OD | 20/20 | 0 | SC | III | OS | 20/20 | 0 | SS | I | ||
| 19 | 59 | OD | 20/25 | 0.1 | Sthal | III | |||||||
| OS | 20/20 | 0 | Sthal | II | |||||||||
| 10 | 66 | OS | 20/20 | 0 | SC | IV | |||||||
Staging based on Goldberg classification.2
BCVA = Best corrected visual acuity; SS = sickle cell disease; SC = sickle cell-hemoglobin C disease; Sthal = sickle cell thalassemia
SD-OCT Retinal Thickness Map
In the standard ETDRS-like retinal thickness subfields that correspond to the Polar 3 microperimetry testing area, Group A had significantly thinner retina compared to Group B in the parafoveal superior (256 μm vs. 276 μm, p = 0.019), parafoveal temporal (234 μm vs. 266 μm, p < 0.004), parafoveal inferior (255 μm vs. 279 μm, p = 0.003), perifoveal superior (266 μm vs. 282 μm, p = 0.04), perifoveal temporal (222 μm vs. 262 μm, p = 0.0005), and perifoveal inferior (256 μm vs. 271 μm, p = 0.045) subfields. There was no significant difference in central macular subfield thickness between the two groups (190 μm vs. 189 μm, p = 0.89). Figure 1 shows the ETDRS-like retinal thickness subfields for a representative patient from Group A, Group B, and the control group.
Microperimetry Data
Our group has previously determined the normative values for the Polar 3 microperimetry on 34 visually healthy subjects (mean age 41.2 years, range 27–66) on the same SD-OCT/SLO system used in this study.13 Mean sensitivities of African American subjects was not statistically different from non-African American subjects (16.7 dB vs. 16.9 dB, p = 0.57), which was consistent with a previous report.15 The overall mean retinal sensitivity of Group A was significantly less than that of Group B (14.2 dB vs. 16.5 dB, p = 0.00005). However, there was no statistical difference between Group B and controls (16.5 dB vs. 16.7 dB, p = 0.63). The mean number of points out of the 28 tested points at or below 12 dB (which was defined as the lower limit of normal sensitivity threshold as described in material and methods section) was significantly more in Group A than in Group B (3.4 vs. 0.7, p = 0.00001). Figures 2 and 3 show representative cases from Groups A and B.
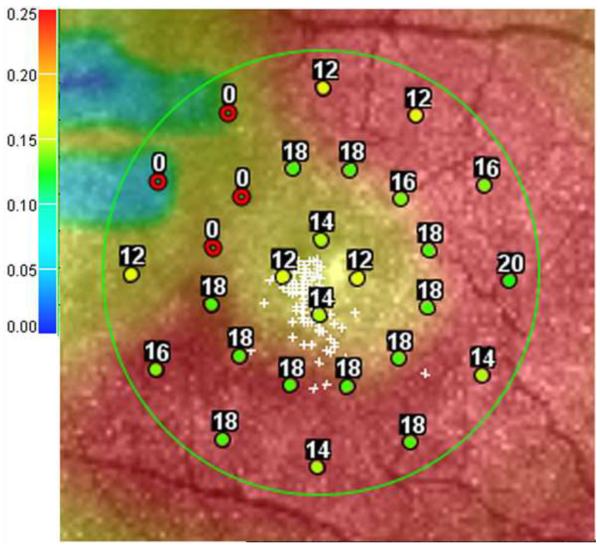
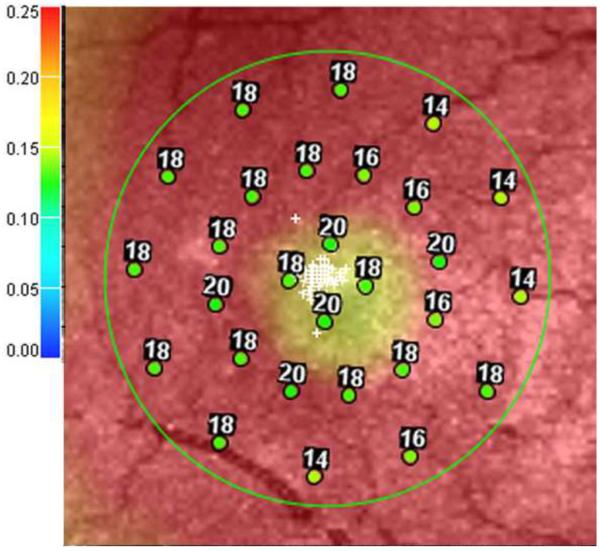
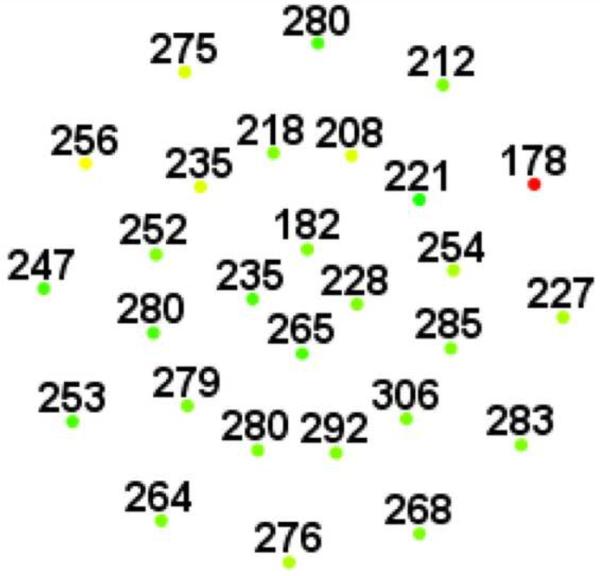
Figure 2.
Microperimetry and SD-OCT data of right eye of patient 3 with SS sickle cell disease and macular focal thinning (Group A, BCVA 20/25). (Top Left) Microperimetry Polar 3 test grid superimposed on the SLO infrared image. Small white crosses indicate fixation. Each value represents the light sensitivity for the corresponding retinal area. Based on normative data for this particular instrument, a sensitivity of > 12 dB was considered normal. Note markedly reduced retinal sensitivities temporally (4 data points of 0 dB temporally). (Top Right) Corresponding SD-OCT thickness (μm) at each of the 28 Polar 3 tested points showing thinning temporally. (Bottom) SD-OCT B-scan image showing focal macular thinning temporally seen as abrupt asymmetric decrease in retinal thickness as indicated by arrow.
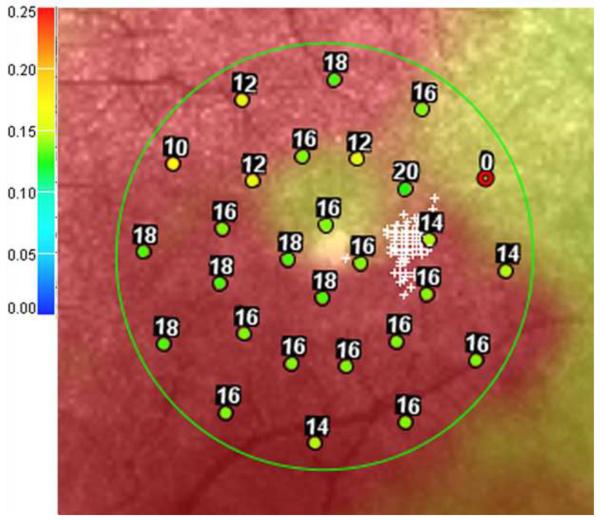
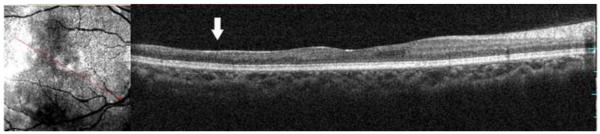
Figure 3.
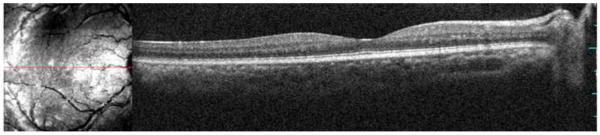
Microperimetry and SD-OCT data of right eye patient 14 with SS sickle cell disease and no focal thinning (Group B, BCVA 20/20). (Top Left) Microperimetry Polar 3 test grid superimposed on the SLO infrared image. Small white crosses indicate fixation. Each value represents the light sensitivity for the corresponding retinal area. Based on normative data for this particular instrument, a sensitivity of > 12 dB was considered normal. Note that all data points are > 12 dB in this patient. (Top Right) Corresponding SD-OCT thickness (μm) at each of the 28 Polar 3 tested points showing no macular thinning. (Bottom) SD-OCT B-scan image also showing no focal macular thinning.
In those patients with focal thinning, a customized pattern of sensitivity testing was performed in the area of thinning. These areas showed moderate to severely depressed sensitivity levels in the area of thinning. Figure 4 shows a representative case of this finding in patient 3 with focal thinning. However, these data points were not analyzed statistically since no normative data has been established for this customized pattern.
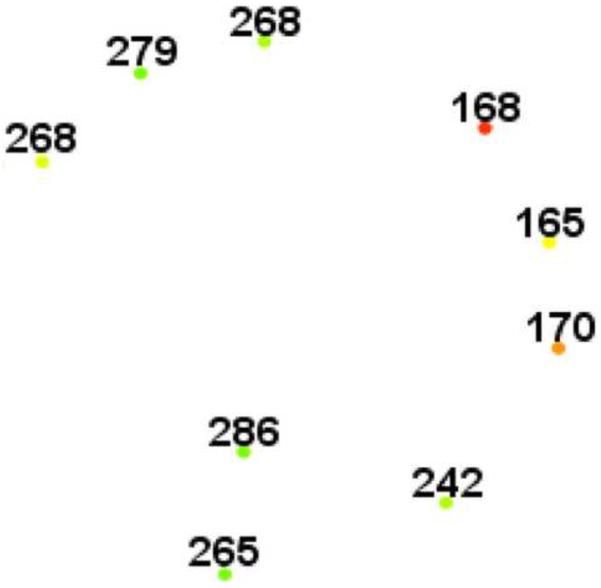
Figure 4.
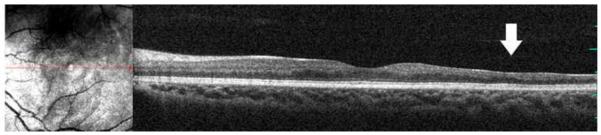
Microperimetry and SD-OCT of left eye of patient 3 with SS sickle cell disease and macular focal thinning (Group A, BCVA 20/20). (Top Left) Microperimetry Polar 3 test grid superimposed on the SLO infrared image. Small white crosses indicate fixation. Each value represents the light sensitivity for the corresponding retinal area. Based on normative data for this particular instrument, a sensitivity of > 12 dB was considered normal. Note markedly reduced retinal sensitivities at one test point. (Top Right) Corresponding SD-OCT thickness (μm) at each of the 28 Polar 3 tested points showing thinning temporally at that location. (Middle Left) Microperimetry data of a customized testing area temporal to the standard Polar 3 grid showing more reduced retinal sensitivities in the area of thinning. Note that these data points were not analyzed statistically since no normative data has been established for this customized pattern. (Middle Right) Corresponding SD-OCT thicknesses in the customized testing area showing reduced retinal thickness temporally. (Bottom) SD-OCT B-scan image showing focal macular thinning temporally seen as abrupt asymmetric decrease in retinal thickness as indicated by arrow.
Discussion
In our current study, we used a combined SD-OCT/SLO microperimeter to correlate structural changes and psychophysical function in patients with sickle cell hemoglobinopathies. This device enables simultaneous evaluation of macular sensitivity along with high-resolution SLO infrared images during testing to observe responses from the macula in vivo. Following testing, the instrument superimposes perimetric sensitivity values on an SLO infrared image, thus offering the opportunity to view and correlate functional responses with anatomical structures. Landa et al.16 used the same instrument to correlate the structure and function in various retinal diseases. The authors found that retinal thickening due to cystoid changes in the inner retinal layers (such as diabetes, retinal vein occlusion, cystoid macular edema) or thinning of the neurosensory retina (such as myopic degeneration, atrophic changes from age-related macular degeneration, and laser scars) on SD-OCT exam correlated most significantly with decreased retinal sensitivities on microperimetry testing. However, the direct correlation of structural and functional changes using this technique has not been reported in patients with sickle cell hemoglobinopathies. Visual acuity is not as useful for detection of small areas of decreased macular function in these sickle cell eyes as it is often normal.
Although findings of sickle retinopathy are mostly found in the periphery, structural changes from macular infarctions in sickle cell hemoglobinopathies have been documented using fluorescein angiography, electroretinography and histopathology.6,7,17 In fluorescein angiograhy studies, the foveal avascular zone was shown to be enlarged in sickle cell patients, with no significant difference in foveal avascular zone diameter with respect to stage of retinopathy, type of hemoglobinopathy or visual acuity.18 The macular function in sickle cell patients has also been reported. While kinetic perimetry failed to correlate the degree of macular ischemia to scotoma size,19 static perimetry demonstrated scotoma size correlated with the size of the foveal avascular zone20 and the area of retina depression.5 These studies19,20 suggest that static perimetry, rather than visual acuity, provides a more sensitive measurement of macular function in patients with sickle cell hemoglobinopathies.
In our current study, we found a strong correlation between the retinal sensitivities and presence of focal macular thinning. Sickle cell patients with focal macular thinning present on SD-OCT have significantly decreased retinal sensitivities compared to those without focal thinning or normal controls based on mean Polar 3 sensitivities (p = 0.00005) and number of test points at or below 12 dB (p = 0.00001), despite similar age and visual acuity. Thinning is found most notably in the temporal subfields. This is likely because macular ischemia tends to occur in watershed areas along the temporal horizontal raphe, thus sparing the fovea and central visual acuity. Therefore, microperimetry testing maybe a more sensitive tool to detect macular ischemia than visual acuity testing alone.
Of note, the retinal sensitivities remain normal at some test points where there is thinning on SD-OCT. This could certainly be due to false negative errors, but there are two other plausible explanations. Similar to glaucoma damage, there is likely a structural (thickness) threshold at which the retinal sensitivities drop off and above which the function remains relatively normal. A larger study will likely be necessary to determine such a threshold. Secondly, the test target (4 mm2) maybe large enough to cover an area that includes a focally thinned area as well as the adjacent normal retina so that the normal retina is in effect being tested. Such areas would be at risk for decreased function if the adjacent normal retina becomes damaged. It would be interesting to see how these data points behave over time to monitor progression of macular ischemia.
In this study, we found no statistically significant difference in microperimetry sensitivities between sickle cell patients without focal thinning on SD-OCT and controls (p = 0.63). Our group previously demonstrated that in those patients without focal thinning, there was subclinical foveal splaying and thinning as well as outer retinal thinning.9 The results from this study showed that these subclinical structural changes are not visually significant based on microperimetry testing. However, longitudinal follow-up with microperimetry testing could be useful for monitoring the natural history of any progressive loss of macular function in these patients. Furthermore, based on the findings of this and previous studies, SD-OCT maybe a more sensitive test than microperimetry to detect subclinical macular ischemic thinning, which may prompt a more thorough examination of the peripheral retina to look for evidence of peripheral ischemia and the more advanced stages of sickle retinopathy. Future study is underway to determine the longitudinal changes over time and to determine correlation with severity of systemic disease. Macular SD-OCT scans and/or microperimetry maybe of value some day as a clinical test (in addition to echocardiograms, creatinine levels, and complete blood count) to help hematologists who care for sickle patients in deciding on systemic therapy.
Acknowledgement
This study was supported by the Foundation Fighting Blindness, Owing Mills, Maryland, and Grant Healthcare Foundation, Chicago, Illinois (GF); NIH core grant EY01792; Gerhard Cless Retina Research Fund (JL); University of Illinois Core Grant EY495707, and an unrestricted grant from Research to Prevent Blindness, New York, New York. Contributions of authors: Design of the study (CC, MG, AA, FC, GF, JL), collection (CC, MG, AA, FC, JL), management (CC, MG, AA, FC, GF, JL), analysis (CC, MG, AA, FC, GF, JL), and interpretation (CC, MG, AA, FC, GF, JL); and preparation (CC, MG, AA, FC, GF, JL), review (CC, MG, AA, FC, GF, JL), approval of the manuscript (CC, MG, AA, FC, GF, JL). The University of Illinois – Chicago Institutional Review Board approved the research. Informed consent was obtained from all subjects before their participation in this study.
Biographies

Dr. Clement Chow received his medical degree at the University of Wisconsin School of Medicine and Public Health and is currently completing ophthalmology residency at the University of Illinois at Chicago Illinois Eye are Ear Infirmary, where he will start his surgical vitreoretinal fellowship in 2011.

Dr. Jennifer Lim is Professor of Ophthalmology and Director of the Retina Service at University of Illinois at Chicago. She is the Marion H. Schenk Esq., Chair in Ophthalmology for Research in the Aging Eye. Her research involves clinical trials and translational research in retinal degenerative and vascular diseases. She collaborates with basic scientists on retinal imaging and angiogenesis. She has been funded for these studies by the National Eye Institute, American Cancer Society and industry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial or proprietary interest in any of the products or techniques mentioned in this article.
Financial Disclosure(s): None of the authors have any financial interests to disclose.
References
- 1.Emerson GG, Harlan JB, Fekrat S, et al. Hemoglobinopathies. In: Ryan SJ, editor. Retina. 4th ed. Elsevier; Philadelphia, PA: 2006. pp. 1429–1445. [Google Scholar]
- 2.Goldberg MF. Classification and pathogenesis of proliferative sickle retinopathy. Am J Ophthalmol. 1971;71(3):649–65. doi: 10.1016/0002-9394(71)90429-6. [DOI] [PubMed] [Google Scholar]
- 3.Moriarty BJ, Acheson RW, Condon PI, Serjeant GR. Patterns of visual loss in untreated sickle cell retinopathy. Eye. 1988;2(Pt3):330–335. doi: 10.1038/eye.1988.62. [DOI] [PubMed] [Google Scholar]
- 4.Downes SM, Hambleton IR, Chuang EL, et al. Incidence and natural history of proliferative sickle cell retinopathy: observations from a cohort study. Ophthalmology. 2005;112(11):1869–1875. doi: 10.1016/j.ophtha.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 5.Goldbaum MH. Retinal depression sign indicating a small retinal infarct. Am J Ophthalmol. 1978;86(1):45–55. doi: 10.1016/0002-9394(78)90013-2. [DOI] [PubMed] [Google Scholar]
- 6.Acacio I, Goldberg MF. Peripapillary and macular vessel occlusions in sickle cell anemia. Am J Ophthalmol. 1973;75(5):861–866. doi: 10.1016/0002-9394(73)90892-1. [DOI] [PubMed] [Google Scholar]
- 7.Romayanada N, Goldberg MF, Green WR. Histopathology of sickle cell retinopathy. Trans Am Acad Ophthalmol Otolaryngol. 1973;77(5):OP642–76. [PubMed] [Google Scholar]
- 8.Asdourian GK, Nagpal KC, Busse B, et al. Macular and perimacular vascular remodelling sickling haemoglobinopathies. Br J Ophthalmol. 1976;60(6):431–453. doi: 10.1136/bjo.60.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoang QV, Chau FY, Shahidi M, Lim JI. Central Macular Splaying and Outer Retinal Thinning in Asymptomatic Sickle Cell Patients by Spectral Domain Optical Coherence Tomography. Am J Ophthalmol. 2011 doi: 10.1016/j.ajo.2010.12.010. forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mainster MA, Timberlake GT, Webb RH, Hughes GW. Scanning laser Ophthalmoscopy. Clinical applications. Ophthalmology. 1982;89(7):852–857. doi: 10.1016/s0161-6420(82)34714-4. [DOI] [PubMed] [Google Scholar]
- 11.Timberlake GT, Mainster MA, Webb RH, et al. Retinal localisation of scotomata by scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 1982;22(1):91–97. [PubMed] [Google Scholar]
- 12.Johnson CA, Chauhan BC, Shapiro LR. Properties of staircase procedures for estimating thresholds in automated perimetry. Invest Ophthalmol Vis Sci. 1992;33(10):2966–2974. [PubMed] [Google Scholar]
- 13.Anastasakis A, McAnany JJ, Fishman GA, Seiple WH. Clinical value, normative retinal sensitivity values, and intrasession repeatability using a combined spectral domain optical coherence tomography/scanning laser ophthalmoscope microperimeter. Eye (Lond) 2010 doi: 10.1038/eye.2010.158. forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holladay JT. Visual acuity measurements. J Cataract Refract Surg. 2004;30(2):287–290. doi: 10.1016/j.jcrs.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Shah VA, Chalam KV. Values for macular perimetry using the MP-1 microperimeter in normal subjects. Ophthalmic Res. 2009;41(1):9–13. doi: 10.1159/000162111. [DOI] [PubMed] [Google Scholar]
- 16.Landa G, Rosen RB, Garcia PM, Seiple WH. Combined three-dimensional spectral OCT/SLO topography and Microperimetry: steps toward achieving functional spectral OCT/SLO. Ophthalmic Res. 2010;43(2):92–98. doi: 10.1159/000247593. [DOI] [PubMed] [Google Scholar]
- 17.Knapp JW. Isolated macular infarction in sickle cell (SS) disease. Am J Ophthalmol. 1972;73(6):857–859. doi: 10.1016/0002-9394(72)90452-7. [DOI] [PubMed] [Google Scholar]
- 18.Sanders RJ, Brown GC, Rosenstein RB, Magargal L. Foveal avascular zone diameter and sickle cell disease. Arch Ophthalmol. 1991;109(6):812–815. doi: 10.1001/archopht.1991.01080060076029. [DOI] [PubMed] [Google Scholar]
- 19.Asdourian GK, Nagpal KC, Busse B, et al. Macular and perimacular vascular remodelling sickling haemoglobinopathies. Br J Ophthalmol. 1976;60(6):431–453. doi: 10.1136/bjo.60.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CM, Charles HC, Smith RT, et al. Quantification of macular ischaemia in sickle cell retinopathy. Br J Ophthalmol. 1987;71(7):540–545. doi: 10.1136/bjo.71.7.540. [DOI] [PMC free article] [PubMed] [Google Scholar]