Abstract
Diabetic kidney disease is the leading cause of end-stage renal disease worldwide. Podocytes are highly differentiated, pericyte-like cells that are essential for normal function of the kidney filter. Loss of podocytes is a hallmark of progressive kidney diseases including diabetic nephropathy. Podocytes are a direct target for angiotensin II –mediated injury by altered expression and distribution of podocyte proteins. Additionally, angiotensin II promotes podocyte injury indirectly by increasing calcium influx and production of reactive oxygen species. Notwithstanding the convincing rationale for angiotensin II blockade as a treatment modality, the incidence of diabetes-related end stage renal disease has increased steadily despite widespread use of angiotensin converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs). Recently published clinical trials have rekindled a debate on the safety and efficacy of dual blockade of the renin-angiotensin system (RAS).
This review summarizes the rationale for blockade of angiotensin II as a therapeutic target in treating diabetic kidney disease, including the critical role played by podocytes. Recent relevant clinical trials on the role of RAS blockade in the treatment of diabetic kidney disease are discussed.
Keywords: Angiotensin II, Podocyte, Diabetes mellitus, Proteinuria, Reactive oxygen species
INTRODUCTION
Blockade of the renin-angiotensin system is a widely established and utilized antiproteinuric and renoprotective modality. Many beneficial effects from these agents can be attributed to their blood-pressure lowering effects that lead to lowered glomerular filtration pressure. However, recent studies have provided valuable insight into the non-hemodynamic actions of Ang II and other components of the RAS in the progression of kidney disease.
The renin-angiotensin system plays a key role in maintaining the balance between extracellular matrix (ECM) synthesis and degradation with abnormal ECM remodeling associated with the progression of glomerular disease [1]. Consequently the ACEI enalaprilat normalizes the abnormal, high glucose-induced concentration of laminin, while it decreases fibronectin synthesis [2]. Ang II is known to induce mesangial TGF-β1 expression leading to matrix expansion as well as podocyte apoptosis [3]. Increased podocyte-derived VEGF via Ang II is thought to play a role more directly on proteinuria in diabetic kidney disease rather than matrix expansion [4]. Ang II also plays an active role in the inflammatory response in renal diseases [5, 6]. Ang II in vivo increases TNF-α production in the kidney and up regulates other inflammatory mediators including IL-6, MCP-1 and NF-kB [7].
Due to space limitations we have not discussed in detail the relative contributions of glomerular endothelial or mesangial cells to the progression of diabetic kidney disease. Excellent reviews incorporating the role of Ang II in these cells types have been previously published [8, 9]. Here we seek to review the contribution of Ang II to the progression of diabetic kidney disease with particular focus on the podocyte as a target in pathogenesis.
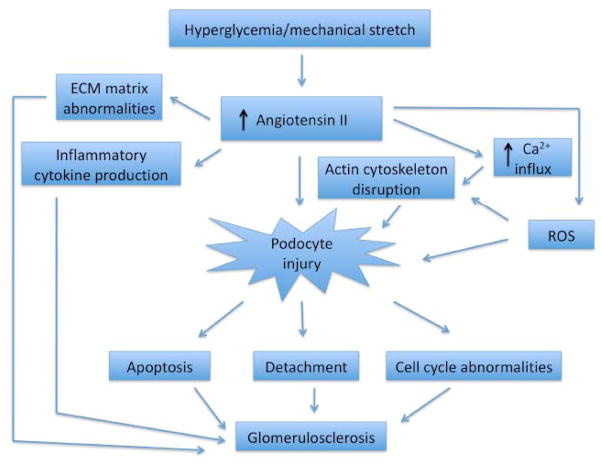
PODOCYTE INJURY IS A HALLMARK OF DIABETIC AND NON-DIABETIC KIDNEY DISEASE (FIG. 1)
Fig. 1.
Schematic of the role of Ang II in diabetic kidney disease. Podocyte injury is a hallmark of progressive diabetic kidney disease where increased calcium influx, generation of ROS and disruption of the actin cytoskeleton are processes mediated by Ang II. Progressive podocyte injury leads to apoptosis, detachment and cell cycle abnormalities that culminates in glomerulosclerosis. Ang II has actions outside the podocyte including abnormal ECM expansion and inflammatory cytokine production that also leads to the progression of diabetic kidney disease.
Podocytes are pericyte-like cells with an actin-based contractile apparatus [7, 10]. They consist of three morphologically distinct segments: a cell body, major processes and foot processes (FPs) [11]. FPs contain an actin-based cytoskeleton that is linked to the glomerular basement membrane (GBM) [10, 12, 13]. Podocyte FPs form an interdigitating network with FPs of neighboring podocytes connected by the slit diaphragm (SD). The SD is a modified adherens junction that covers the 30–50 nm wide filtration slits and serves as the final barrier to urinary protein loss [10, 13]. At the SD, multiple membrane proteins including nephrin, CD2AP, neph1 and podocin are present that are connected to the actin cytoskeleton via a variety of adaptor and effector molecules [12]. Podocyte injury is a hallmark of human and experimental glomerular disease including focal segmental glomerulosclerosis (FSGS), membranous glomerulopathy, minimal change disease and diabetic nephropathy [10, 13]. Human and experimental models of diabetic nephropathy have demonstrated that the onset of albuminuria correlates with markers of podocyte injury such as foot process effacement, podocyte hypertrophy, detachment and apoptosis [14–16].
Podocyte loss contributes to the progression of diabetic nephropathy in patients with type II DM [17]. Podocyte cell number is also decreased in patients of all ages with type I DM where the reduction in podocyte number correlates with an increased albumin excretion rate [18, 19]. Glucose-induced oxidative stress has been implicated as an important contributor to podocyte apoptosis [20]. Additionally, podocytes may detach from the GBM due to decreased expression of α3β1 integrin [21]. Viable urinary podocytes have indeed been detected in human as well as experimental diabetic nephropathy [22–24]. The lack of an appropriate compensatory increase in proliferation to replace lost podocytes likely occurs through alterations in cell cycle regulatory proteins, where an increase in expression and action of the cyclin-dependent kinase inhibitors p21 and p27 prevent cell-cycle progression [25].
ANGIOTENSIN II PROMOTES PODOCYTE INJURY
Podocytes contain a contractile apparatus and can adapt to mechanical strain [26]. Cultured podocytes exposed to mechanical stress exhibit a reorganization of the actin cytoskeleton, increase local expression of Ang II as well as its receptor AT1R, thereby perpetuating podocyte injury [27, 28]. Increased AT1R expression has specifically been demonstrated in vivo in podocytes of the experimental remnant kidney model of glomerular capillary hypertension [28]. The same authors have recently demonstrated increased local expression of Ang II via increased renin transcription in podocytes exposed to hyperglycemia [29]. There is evidence that local production of Ang II contributes to the progression of kidney disease by promoting apoptosis, matrix accumulation and aberrant proliferation [30, 31]. The finding that blockade of the RAS with the ACEI captopril did not abrogate podocyte stretch-induced or hyperglycemia-induced increase in local Ang II suggests that primarily non-ACE pathways are involved in cleavage of angiotensin I into Ang II in podocytes [28, 29].
Inhibition of the RAS restores the expression of nephrin in both experimental and human diabetic nephropathy [15, 32]. Nephrin is also retained at the slit diaphragm when rats with experimental membranous nephropathy were treated with an ACEI or ARB [33]. Additionally, the ACEI lisinopril prevents proteinuria as well as the redistribution of slit diaphragm associated ZO-1 in the MWF rat model [34]. Taken together, these findings present a correlation between the renoprotective effects of ACE inhibition and the preservation of slit diaphragm integrity.
Ang II increases free cytosolic calcium in podocytes via a release of calcium from intracellular stores as well as thru an influx from the extracellular space [35]. An increase of cytosolic calcium activates chloride channels in podocytes resulting in depolarization [36]. In vivo it has been demonstrated that Ang II depolarizes podocytes in the isolated rat glomerulus and Ang II increases the inward current of podocytes [37]. The ion channel TRPC6 is activated in podocytes directly by Ang II and interestingly, similar to patients with TRPC6 mutations, a podocyte-specific transgenic rat model for the human Ang II type 1 receptor (AT1R) develops progressive proteinuria culminating in FSGS [38–40].
Angiotensin converting enzyme 2 (ACE2) is a homologous protease of ACE cloned in 2000 that has emerged as a negative regulator of the RAS, opposing ACE action in the heart, lung and kidneys [41]. ACE2 has been localized to podocytes, and in db/db diabetic mice glomerular expression of ACE2 is reduced whereas glomerular ACE expression is increased [42]. The finding that chronic ACE2 inhibition increases urinary albumin excretion suggests its utility as a potential target for therapeutic interventions to reduce proteinuria and the progression of glomerular disease.
Taken together these data indicate that podocytes are a direct target for Ang II and provide a rationale for AII blockade in the treatment of chronic kidney disease. Further studies are needed to explore the possible protective contribution of ACE2 in diabetic kidney disease.
ANGIOTENSIN II PROMOTES OXIDATIVE STRESS IN THE KIDNEY
All cell types produce reactive oxygen species. H2O2 is the most commonly implicated second messenger for multiple enzyme systems activated by Ang II that use NADPH oxidases as substrates for the production of superoxide anion [43, 44]. NADPH oxidase contains a heterodimeric membrane-bound cytochrome b558 complex whose cytosolic subunits translocate to the cytochrome complex upon agonist stimulation leading to an increase in enzymatic activity [45]. The most abundant NADPH oxidase in the kidney is Nox4, and superoxide generation in response to Ang II and the effect on renal function has been extensively studied. Renal infusion of Ang II in rats reduce renal blood flow, glomerular filtration, sodium excretion and NO levels, all of which were blunted by not only by the AT1R antagonist valsartan but also by the superoxide scavenger tempol and the NADPH oxidase inhibitor apocynin [46]. In the Heymann nephritis model of experimental membranous nephropathy, cytochrome b558 is increased in podocytes [47]. In this model it has been suggested that podocytes express and externalize respiratory-burst enzymes that generate ROS in a manner similar to neutrophilic granulocytes, which could then lead to glomerular damage [47]. In STZ-treated mice Nox4 transcription is increased and renal production of ROS enhanced [48] while in STZ-induced diabetic rats knockdown of Nox4 was associated with decreased ROS production and proteinuria [49]. It had previously been demonstrated in db/db and Akita mice that glucose-induced podocyte-derived ROS promoted podocyte apoptosis, detachment and proteinuria [20]. More recently, in the OVE26 model of type I diabetic kidney disease high glucose was shown to induce ROS generation, Nox1 and 4 mRNA expression, podocyte apoptosis and proteinuria in a cytochrome P450 dependent manner [50]. Additionally advanced oxidation protein products, known to accumulate in diabetic kidney disease and metabolic syndromes, promote NADPH oxidase-dependent podocyte depletion by a p53-Bax apoptotic pathway [51].
In contrast to vascular smooth muscle cells, Ang II activation of NADPH oxidase in the kidney in general and podocytes specifically remains poorly understood. A comprehensive examination of Nox4 expression and downstream effects with podocytes is needed [14].
ANGIOTENSIN II BLOCKADE IN DIABETIC KIDNEY DISEASE – IS MORE REALLY BETTER?
The last two decades have witnessed a significant increase in the use of angiotensin converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) due to the widespread view that these agents slow the progression of diabetic and non-diabetic renal disease for reasons beyond blood-pressure lowering effects [52]. Indeed the Collaborative Study Group showed in 1993 that in insulin-dependent diabetics with proteinuria, the ACEI captopril was associated with a 50% reduction in the risk of combined end points of death, dialysis and transplantation compared to placebo that was independent of blood pressure control [53]. Disappointingly, despite more widespread use of ACEIs and ARBs in the aftermath of this publication, the incidence of diabetes-related ESRD has increased out of proportion to the growth in the number of patients with diabetes [54]. The safety and efficacy of Ang II blockade in diabetic and non-diabetic nephropathy have since been called into question [52, 55]. A subgroup analysis of the ALLHAT diabetic population revealed that more patients treated with lisinopril progressed to ESRD (RR 1.74, 95% CI 1.00–3.01) compared to those treated with chlorthalidone [56]. A large meta-analysis of randomized, controlled trials comparing ACEIs or ARBs with other antihypertensive agents demonstrated no benefit in diabetic nephropathy for ACEI or ARB use on the doubling of serum creatinine, GFR or the progression to ESRD [57]. More recently the ONTARGET investigators have shown that in diabetic and non-diabetic patients the combination of ACEI + ARB is associated with greater doubling of serum creatinine, higher incidence of dialysis and increased mortality compared to monotherapy with ACEI or ARB alone. [58]. These findings contradicted those of the COOPERATE trial [59] which has been recently retracted over findings of scientific misconduct by the lead author [60]. Proponents of dual RAS blockade have counseled caution in interpreting ONTARGET since the population was elderly and only 4% had proteinuria [61, 62]. Additionally, the excess adverse outcomes with dual treatment was largely attributable to acute hemodialysis for transient kidney dysfunction and not from progression of renal disease [62]. Renoprotection has been shown to be greater with higher levels of baseline proteinuria with treatment benefit decreasing at lower levels of proteinuria [63, 64]. Further studies will be needed to enable clinicians to target which patients may benefit from maximal RAS blockade as it appears that at the very least more is not always best for all. Dual blockade may be more appropriate for younger patients with diabetic and non-diabetic nephropathy associated with albuminuria.
CONCLUSIONS
In summary, Ang II plays a critical role in podocyte injury and the progression of diabetic kidney disease. The mechanistic rationale for RAS blockade in the treatment of diabetic and non-diabetic kidney diseases particularly those associated with proteinuria is clear. Future therapeutic trials will need to distinguish the subset of patients most likely to benefit from aggressive RAS blockade. The role of local activation of the RAS in podocytes and resulting alterations in the expression and distribution of slit diaphragm components suggest the potential efficacy of podocyte-specific therapy in the treatment of diabetic kidney disease.
References
- 1.Lenz O, Elliot SJ, Stetler-Stevenson WG. Matrix metalloproteinases in renal development and disease. J Am Soc Nephrol. 2000;11(3):574–81. doi: 10.1681/ASN.V113574. [DOI] [PubMed] [Google Scholar]
- 2.Morano S, Cipriani R, Cerrito MG, et al. Angiotensin-converting enzyme inhibition modulates high-glucose-induced extracellular matrix changes in mouse glomerular epithelial cells. Nephron Exp Nephrol. 2003;95(1):e30–5. doi: 10.1159/000073021. [DOI] [PubMed] [Google Scholar]
- 3.Sharma K, Ziyadeh FN, Alzahabi B, et al. Increased renal production of transforming growth factor-beta1 in patients with type II diabetes. Diabetes. 1997;46(5):854–9. doi: 10.2337/diab.46.5.854. [DOI] [PubMed] [Google Scholar]
- 4.Ziyadeh FN. Different roles for TGF-beta and VEGF in the pathogenesis of the cardinal features of diabetic nephropathy. Diabetes Res Clin Pract. 2008;82(Suppl 1):S38–41. doi: 10.1016/j.diabres.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Blanco S, Vaquero M, Gomez-Guerrero C, Lopez D, Egido J, Romero R. Potential role of angiotensin-converting enzyme inhibitors and statins on early podocyte damage in a model of type 2 diabetes mellitus, obesity, and mild hypertension. Am J Hypertens. 2005;18(4 Pt 1):557–65. doi: 10.1016/j.amjhyper.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Ortega M, Ruperez M, Lorenzo O, et al. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl. 2002;82:S12–22. doi: 10.1046/j.1523-1755.62.s82.4.x. [DOI] [PubMed] [Google Scholar]
- 7.Reiser J, Mundel P. Dual effects of RAS blockade on blood pressure and podocyte function. Curr Hypertens Rep. 2007;9(5):403–8. doi: 10.1007/s11906-007-0074-7. [DOI] [PubMed] [Google Scholar]
- 8.Leon CA, Raij L. Interaction of haemodynamic and metabolic pathways in the genesis of diabetic nephropathy. J Hypertens. 2005;23(11):1931–7. doi: 10.1097/01.hjh.0000188415.65040.5d. [DOI] [PubMed] [Google Scholar]
- 9.Jaimes EA, Hua P, Tian RX, Raij L. Human glomerular endothelium: interplay among glucose, free fatty acids, angiotensin II, and oxidative stress. Am J Physiol Renal Physiol. 2010;298(1):F125–32. doi: 10.1152/ajprenal.00248.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mundel P, Reiser J. Proteinuria: an enzymatic disease of the podocyte? Kidney Int. 2010;77(7):571–80. doi: 10.1038/ki.2009.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mundel P, Kriz W. Structure and function of podocytes: an update. Anat Embryol (Berl) 1995;192(5):385–97. doi: 10.1007/BF00240371. [DOI] [PubMed] [Google Scholar]
- 12.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17(9):428–37. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nat Genet. 2000;24(4):333–5. doi: 10.1038/74139. [DOI] [PubMed] [Google Scholar]
- 14.Stitt-Cavanagh E, MacLeod L, Kennedy C. The podocyte in diabetic kidney disease. Scientific World Journal. 2009;9:1127–39. doi: 10.1100/tsw.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54(6):1626–34. doi: 10.2337/diabetes.54.6.1626. [DOI] [PubMed] [Google Scholar]
- 16.Jefferson JA, Shankland SJ, Pichler RH. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int. 2008;74(1):22–36. doi: 10.1038/ki.2008.128. [DOI] [PubMed] [Google Scholar]
- 17.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99(2):342–8. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steffes MW, Schmidt D, McCrery R, Basgen JM. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int. 2001;59(6):2104–13. doi: 10.1046/j.1523-1755.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- 19.White KE, Bilous RW, Marshall SM, et al. Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes. 2002;51(10):3083–9. doi: 10.2337/diabetes.51.10.3083. [DOI] [PubMed] [Google Scholar]
- 20.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225–33. [PubMed] [Google Scholar]
- 21.Chen HC, Chen CA, Guh JY, Chang JM, Shin SJ, Lai YH. Altering expression of alpha3beta1 integrin on podocytes of human and rats with diabetes. Life Sci. 2000;67(19):2345–53. doi: 10.1016/s0024-3205(00)00815-8. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Ushiyama C, Suzuki S, et al. Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant. 2000;15(9):1379–83. doi: 10.1093/ndt/15.9.1379. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura T, Ushiyama C, Shimada N, et al. Effect of the anti-platelet drug dilazep dihydrochloride on urinary podocytes in patients in the early stage of diabetic nephropathy. Diabetes Care. 2000;23(8):1168–71. doi: 10.2337/diacare.23.8.1168. [DOI] [PubMed] [Google Scholar]
- 24.Petermann AT, Pippin J, Krofft R, et al. Viable podocytes detach in experimental diabetic nephropathy: potential mechanism underlying glomerulosclerosis. Nephron Exp Nephrol. 2004;98(4):e114–23. doi: 10.1159/000081555. [DOI] [PubMed] [Google Scholar]
- 25.Wolf G, Schanze A, Stahl RA, Shankland SJ, Amann K. p27(Kip1) Knockout mice are protected from diabetic nephropathy: evidence for p27(Kip1) haplotype insufficiency. Kidney Int. 2005;68(4):1583–9. doi: 10.1111/j.1523-1755.2005.00570.x. [DOI] [PubMed] [Google Scholar]
- 26.Petermann AT, Pippin J, Durvasula R, et al. Mechanical stretch induces podocyte hypertrophy in vitro. Kidney Int. 2005;67(1):157–66. doi: 10.1111/j.1523-1755.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- 27.Endlich N, Kress KR, Reiser J, et al. Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol. 2001;12(3):413–22. doi: 10.1681/ASN.V123413. [DOI] [PubMed] [Google Scholar]
- 28.Durvasula RV, Petermann AT, Hiromura K, et al. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int. 2004;65(1):30–9. doi: 10.1111/j.1523-1755.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- 29.Durvasula RV, Shankland SJ. Activation of a local renin angiotensin system in podocytes by glucose. Am J Physiol Renal Physiol. 2008;294(4):F830–9. doi: 10.1152/ajprenal.00266.2007. [DOI] [PubMed] [Google Scholar]
- 30.Ma L, Fogo AB. Role of angiotensin II in glomerular injury. Semin Nephrol. 2001;21(6):544–53. doi: 10.1053/snep.2001.26793. [DOI] [PubMed] [Google Scholar]
- 31.Fogo AB. Renal fibrosis and the renin-angiotensin system. Adv Nephrol Necker Hosp. 2001;31:69–87. [PubMed] [Google Scholar]
- 32.Bonnet F, Cooper ME, Kawachi H, Allen TJ, Boner G, Cao Z. Irbesartan normalises the deficiency in glomerular nephrin expression in a model of diabetes and hypertension. Diabetologia. 2001;44(7):874–7. doi: 10.1007/s001250100546. [DOI] [PubMed] [Google Scholar]
- 33.Benigni A, Tomasoni S, Gagliardini E, et al. Blocking angiotensin II synthesis/activity preserves glomerular nephrin in rats with severe nephrosis. J Am Soc Nephrol. 2001;12(5):941–8. doi: 10.1681/ASN.V125941. [DOI] [PubMed] [Google Scholar]
- 34.Macconi D, Ghilardi M, Bonassi ME, et al. Effect of angiotensin-converting enzyme inhibition on glomerular basement membrane permeability and distribution of zonula occludens-1 in MWF rats. J Am Soc Nephrol. 2000;11(3):477–89. doi: 10.1681/ASN.V113477. [DOI] [PubMed] [Google Scholar]
- 35.Huber TB, Gloy J, Henger A, et al. Catecholamines modulate podocyte function. J Am Soc Nephrol. 1998;9(3):335–45. doi: 10.1681/ASN.V93335. [DOI] [PubMed] [Google Scholar]
- 36.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83(1):253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 37.Gloy J, Henger A, Fischer KG, et al. Angiotensin II depolarizes podocytes in the intact glomerulus of the Rat. J Clin Invest. 1997;99(11):2772–81. doi: 10.1172/JCI119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winn MP, Conlon PJ, Lynn KL, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308(5729):1801–4. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 39.Reiser J, Polu KR, Moller CC, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37(7):739–44. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffmann S, Podlich D, Hahnel B, Kriz W, Gretz N. Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol. 2004;15(6):1475–87. doi: 10.1097/01.asn.0000127988.42710.a7. [DOI] [PubMed] [Google Scholar]
- 41.Imai Y, Kuba K, Ohto-Nakanishi T, Penninger JM. Angiotensin-Converting Enzyme 2 (ACE2) in Disease Pathogenesis. Circ J. 2010;74(3):405–10. doi: 10.1253/circj.cj-10-0045. [DOI] [PubMed] [Google Scholar]
- 42.Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17(11):3067–75. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- 43.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74(6):1141–8. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 44.Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept. 2000;91(1–3):21–7. doi: 10.1016/s0167-0115(00)00136-1. [DOI] [PubMed] [Google Scholar]
- 45.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009;302(2):148–58. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez B, Salom MG, Arregui B, Valero F, Fenoy FJ. Role of superoxide in modulating the renal effects of angiotensin II. Hypertension. 2003;42(6):1150–6. doi: 10.1161/01.HYP.0000101968.09376.79. [DOI] [PubMed] [Google Scholar]
- 47.Neale TJ, Ullrich R, Ojha P, Poczewski H, Verhoeven AJ, Kerjaschki D. Reactive oxygen species and neutrophil respiratory burst cytochrome b558 are produced by kidney glomerular cells in passive Heymann nephritis. Proc Natl Acad Sci USA. 1993;90(8):3645–9. doi: 10.1073/pnas.90.8.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Etoh T, Inoguchi T, Kakimoto M, et al. Increased expression of NAD(P)H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibity by interventive insulin treatment. Diabetologia. 2003;46(10):1428–37. doi: 10.1007/s00125-003-1205-6. [DOI] [PubMed] [Google Scholar]
- 49.Gorin Y, Block K, Hernandez J, et al. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280(47):39616–26. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 50.Eid AA, Gorin Y, Fagg BM, et al. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes. 2009;58(5):1201–11. doi: 10.2337/db08-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou LL, Hou FF, Wang GB, et al. Accumulation of advanced oxidation protein products induces podocyte apoptosis and deletion through NADPH-dependent mechanisms. Kidney Int. 2009;76(11):1148–60. doi: 10.1038/ki.2009.322. [DOI] [PubMed] [Google Scholar]
- 52.Onuigbo MA. Analytical review of the evidence for renoprotection by renin-angiotensin-aldosterone system blockade in chronic kidney disease - a call for caution. Nephron Clin Pract. 2009;113(2):c63–70. doi: 10.1159/000228536. [DOI] [PubMed] [Google Scholar]
- 53.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 54.Jones CA, Krolewski AS, Rogus J, Xue JL, Collins A, Warram JH. Epidemic of end-stage renal disease in people with diabetes in the United States population: do we know the cause? Kidney Int. 2005;67(5):1684–91. doi: 10.1111/j.1523-1755.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 55.Onuigbo MA. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant. 2009;25:1344–45. doi: 10.1093/ndt/gfp678. [DOI] [PubMed] [Google Scholar]
- 56.Rahman M, Pressel S, Davis BR, et al. Renal outcomes in high-risk hypertensive patients treated with an angiotensin-converting enzyme inhibitor or a calcium channel blocker vs a diuretic: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Arch Intern Med. 2005;165(8):936–46. doi: 10.1001/archinte.165.8.936. [DOI] [PubMed] [Google Scholar]
- 57.Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet. 2005;366(9502):2026–33. doi: 10.1016/S0140-6736(05)67814-2. [DOI] [PubMed] [Google Scholar]
- 58.Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–59. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 59.Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet. 2003;361(9352):117–24. doi: 10.1016/S0140-6736(03)12229-5. [DOI] [PubMed] [Google Scholar]
- 60.Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Retraction--Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet. 2009;374(9697):1226. doi: 10.1016/S0140-6736(09)61768-2. [DOI] [PubMed] [Google Scholar]
- 61.Abutaleb N. ONTARGET should not be over interpreted. Nephrol Dial Transplant. 25(1):44–7. doi: 10.1093/ndt/gfp633. [DOI] [PubMed] [Google Scholar]
- 62.Ruggenenti P, Remuzzi G. Proteinuria: Is the ONTARGET renal substudy actually off target? Nat Rev Nephrol. 2009;5(8):436–7. doi: 10.1038/nrneph.2009.109. [DOI] [PubMed] [Google Scholar]
- 63.Perico N, Benigni A, Remuzzi G. Present and future drug treatments for chronic kidney diseases: evolving targets in renoprotection. Nat Rev Drug Discov. 2008;7(11):936–53. doi: 10.1038/nrd2685. [DOI] [PubMed] [Google Scholar]
- 64.Ruggenenti P, Perna A, Remuzzi G. Retarding progression of chronic renal disease: the neglected issue of residual proteinuria. Kidney Int. 2003;63(6):2254–61. doi: 10.1046/j.1523-1755.2003.00033.x. [DOI] [PubMed] [Google Scholar]