Abstract
This study is a retrospective analysis of thalamic neuronal and electromyogram activities between subjects with organic dystonia and a subject with psychogenic dystonia, in whom a thalamotomy was carried out before a diagnosis psychogenic dystonia was made.
The electromyogram signal to noise ratio in the lowest frequency band (<0.76Hz, dystonia frequency – DF) in the electromyogram was not significantly different by diagnosis or muscle (Table 1). The coherence at dystonia frequency for wrist flexors X biceps electromyograms was significantly higher in organic dystonia, while the phase was not apparently different from zero for either diagnosis.
In a thalamic pallidal relay nucleus (ventral oral posterior), neuronal firing rates were not apparently different between psychogenic and organic dystonia. The neuronal signal to noise ratio in ventral oral posterior was significantly higher in organic dystonia than in psychogenic dystonia, while both were greater than in controls with chronic pain. Spike X electromyogram coherence was not apparently different between psychogenic and organic dystonia. The proportion of thalamic cells responding to joint movements was higher in the cerebellar relay nucleus (ventral intermediate) of psychogenic dystonia than organic dystonia.
These results suggest that some features, such as firing rates and thalamic reorganization, are similar in psychogenic and organic dystonia. Other features differ, such as the coherence between the electromyograms from different muscles, and the thalamic neuronal signal to noise ratio, which may reflect pathophysiological factors in organic dystonia.
Keywords: Psychogenic Dystonia, Organic Dystonia, Human thalamus, Neuronal activity, Plasticity, Dystonia related activity
INTRODUCTION
The pathophysiology of psychogenic dystonia (PsyD) is not well understood, and some of the same physiological abnormalities identified in organic dystonia have also been found in PsyD 1. It is possible that some physiological abnormalities do not cause the dystonic movements, but result either from the movements, or from some other common pathophysiological factor. Reorganized forebrain sensory and motor maps have been suggested to result from repetitive movements both in patients with dystonia, and in a monkey model of dystonia 2-4, This concept is consistent with studies demonstrating that repetitive motor activity can lead to reorganization of thalamo-cortical sensory and motor maps in monkeys 5,6. In addition, the activity of thalamic neurons often show significant peaks of activity at the frequency of dystonic movements (dystonia frequency, DF, <0.76 Hz)4.
We have previously reported reorganized thalamic maps and altered dystonia frequency activity in patients undergoing thalamotomy for dystonia 4. Subsequent to surgery, one of these patients was diagnosed as having PsyD. This situation provided a unique opportunity to report descriptively how thalamic neuronal activity in PsyD differs from that recorded in patients with organic dystonia and in ‘controls’ operated for treatment of chronic pain.
METHODS
Results of surgery in the patient with PsyD were included in a report of nine patients with organic dystonia 4. At that time, our patient with PsyD was diagnosed with organic dystonia. Thalamic activity in the psychogenic patient could be analyzed only for the first operation because of the poor quality of recordings in the previously operated thalamic nuclei 4. The electromyogram (EMG) was sampled from myohyoid, deltoid, biceps and wrist flexors in the psychogenic patient, versus flexors and extensors of the elbow and wrist in the other nine patients. Therefore, the thalamic spike X EMG cross-correlation was not previously compared with the other patients 4. These results were also compared with those in three patients with chronic pain secondary to thoracic spinal cord injury. None of these patients had pain or altered motor function in the upper extremity; clinical details are reported in a previous publication7.
All the methods used in this study have been previously described in detail 8-10. Deep sensory cells responded reliably to rapid joint movements and/or squeezing of muscles or tendons, but did not respond to stimulation of the skin deformed by these stimuli. Dystonic movements prevented the sub-classification of deep sensory cells and the identification of cells responding during active movements 4. Therefore, cells responding to cutaneous stimuli provided our most reliable physiological landmark; putative nuclear location was estimated by moving the atlas maps along the anterior commissure-posterior commissure (AC-PC) line to align the anterior border of Vc with the most anterior cell in the region where the majority of cells respond to cutaneous stimulation 4.
Postoperative Analysis
The action potentials of single neurons were discriminated and digitized at 10 kHz by a standard shape-fitting package (Explorer, Brainwave, Thornton, Colorado). The spike train was converted into an equivalent analog signal by using the French-Holden algorithm, a standard technique which preserves the time resolution of the action potentials in the spike train 10-12. The EMG signals were high pass filtered (6 dB) at 20 Hz to eliminate low frequency movement artifact while preserving the raw EMG signal. We had data epochs which were long enough to be analyzed by a standard spectral analysis technique, rather than by a multi-tapper technique which would have been required for shorter epochs 13. Standard techniques were used to take, process and interpret the spectral analysis of the neuron and EMG signals4,10,12.
Patient with Psychogenic Dystonia
The patient was a 33 year old woman with a two year history of dystonia at the time of the first surgery. The dystonia involved the left oro-mandibular structures and the left upper and lower extremity. She had failed treatment with trihexyphenidyl (40 mg per day) and with levodopa/carbidopa. Marked post-operative improvement lasted only 2 months. Following a second, right-sided thalamotomy 18 months after the first, dystonia progressed to involve the other side of the body. Six months later she was seen by a movement disorders neurologist. The diagnosis of PsyD was subsequently made based upon: (1) inconsistencies on examination such as severe tongue involvement only when speech was formally assessed, extreme slowness in performing finger-to-nose testing, but normal speed of similar voluntary movements outside of the context of the examination, and (2) incongruities with organic dystonia including marked acute exaggeration of generalized dystonic postures, such as severe axial extension triggered when she was asked to look upward, and the rapid progression to generalized involvement in an adult without other neurological abnormalities. At that time, she had severe generalized dystonia involving her face, all limbs and axial muscles, and required assistance to walk.
The patient was informed that she might improve considerably with admission to hospital, physiotherapy and withdrawal of the anti-cholinergic medication. Within days of hospitalization, the dystonia had almost completely resolved. At the time of discharge, she had only minimal posturing in the left hand and that subsequently resolved. In view of the rapid and sustained remission, the diagnosis of PsyD was clear. She remained stable on further follow-up and so psychiatric assessment and treatment was not actively pursued.
RESULTS
Analysis of EMG was carried out and demonstrated no apparent difference in signal to noise ratio (SNR) between diagnoses of dystonia (Table 1). The EMG X EMG cross-correlation function could only be compared for wrist flexors × biceps (see Methods). In patients with organic versus psychogenic dystonia, more synchronous EMG activity was observed between these muscles (Table 1).
Table 1.
Summary of results.
| Group | Psychogenic | Organic | Pain |
|---|---|---|---|
| Segments with EMG peak < 0.76Hz among segments per epoch. | 85%, ND Mann Whitney. | 81% | --NA |
| Wrist Flexor x Biceps EMG coherence | 0.23, P<0.01 Mann Whitney. | 0.39 | -- |
| EMG phase | 5.3, ND, Mann Whitney. | -7.9 | -- |
| Vop neuron firing rate | 14.2 Hz, ND, Mann Whitney. | 15.1 Hz | 18.3 Hz, ND to both groups |
| Vop neurons SNR | 2.1, P <0.01, Mann Whitney. | 3.8 | 1.2, P<0.001 for both groups |
| Proportion of Vop neurons with high spike × EMG coherence | 59% for WF. 32% for biceps ND for both muscles, Chi square. |
25% for WF 58% for biceps |
-- |
| Proportion of Vop neurons with phase lead | 69% for WF 43% for biceps ND for both muscles, Chi square. |
100% for WF 43% for biceps |
-- |
| Proportion of cells in Vim and Vop responding to joint movement | 26/50 lumped, P<0.00001 23/31 Vim, P<0.00001 3/19 Vop, ND. Chi square. |
74/334 lumped 62/222 Vim 12/112 Vop |
-- |
| Vim and Vop sensory reorganization | 0.7 mm, ND, Mann Whitney. | 1.1 mm | 0.6 mm, P <0.05 Mann Whitney for the Organic Group only |
Grey shading indicates significant results. P values in the Psychogenic column indicate differences to the Organic group. P values in the Pain column indicate differences to one or both of the other groups. ND indicates that the variable is not significantly different by patient group, but does not in any way prove that the two groups are the same. Abbreviations: EMG – electromyogram, SNR - Signal noise ratio, Vim and Vop – thalamic nuclei Ventralis intermedius and oralis posterior. Other conventions as in the text.
Although RF maps were recorded for cells in the ventral intermediate nucleus (Vim), records of activity during involuntary movements were only available for neurons in ventral oral posterior (Vop) in the patient with PsyD. Therefore, analysis of spike trains and spike X EMG functions were limited to Vop, whereas analysis of somatic sensory properties included neurons in both Vim and Vop. The Vop spike SNR at dystonia frequency was significantly higher in organic dystonia than in PsyD and both were greater than in patients with chronic pain (Table 1). Neither firing rates nor ISIs appeared to be different between these diagnostic groups.
The proportion of neurons with spike X EMG coherence > 0.42 and maximum SNR at dystonia frequency did not appear to be different between PsyD versus organic dystonia, for either wrist flexors or biceps. The phase did not appear to differ between dystonia diagnoses.
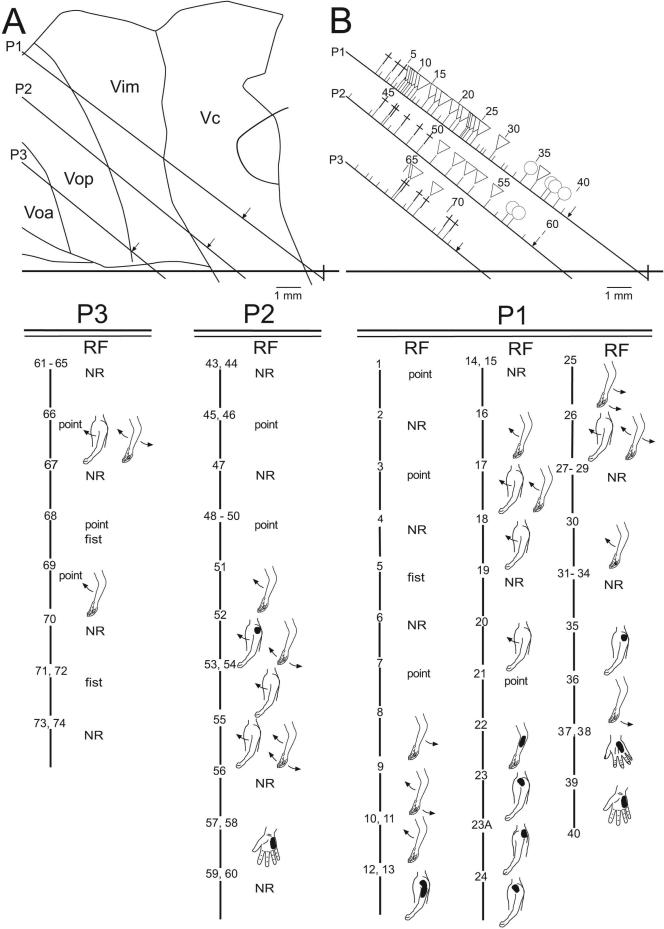
Figure 1, shows the thalamic map of neuronal location and stimulation sites for the patient with PsyD. It is remarkable for the large number of cells with deep receptive fields, which was significantly greater than in patients with chronic pain. The size of the representations of individual joints in Vim (see Figure 1) did not appear to be different between the dystonia diagnoses, although these lengths were less in chronic pain than in organic dystonia.
Figure 1.
Sagittal map of thalamic neuronal location relative to the AC-PC line (horizontal line, PC as indicated) and nuclear boundaries, in the patient with psychogenic dystonia. Electrode trajectories (P3, P2 and P1) are shown by the oblique lines. Locations of recording sites are indicated by ticks to the right of each trajectory. Long tics indicate location of a neuron which responded during sensory stimulation, or active movement, or both; short tics indicate unresponsive neurons. The symbol at a long tic indicates the presence of a neuron responding during active movement (cross), cutaneous stimulation (circle), and deep stimulation (triangle), as described in the methods. Sites are numbered sequentially and numbers are indicated for every fifth tick. Scale as indicated.
Lower panels P1, P2, and P3 show the site number, and receptive field (RF). RF is indicated to the right and below the site number. Note that many cells responded to movement of two joints. For example, one cell responded to extension of both shoulder and elbow. Shading on the figurine indicates the part of the body where mechanical stimulation-evoked cellular activity. All shaded figurines indicate cutaneous stimulation, except for neurons 22 - 24, 35, at which cellular activity was evoked by manipulation of muscle but not by manipulation of the overlying skin. The maximum size of the representation of a part of the body was estimated from the lengths of single electrode trajectories along which all RFs included a single joint, as in a previous study 7. For example, in electrode trajectory P2, passive shoulder movements were represented by neurons 52 to 55, a distance of 2.5 mm.
DISCUSSION
The patient with PsyD has a secure diagnosis since her dystonia remitted with suggestion and physiotherapy, fulfilling Fahn & Williams’ criteria for Documented Psychogenic Dystonia 14. We believe that it is unlikely that she had organic dystonia originally followed by PsyD post-surgery since the appearance of the dystonia was very similar throughout her clinical course, and since the pattern of clinical remission was consistent with PsyD. The results in this manuscript were obtained from this one patient, and the number of cells studied was limited, so the conclusions must be considered tentative until confirmed.
The physiological results suggest that Vop neuronal activity is characterized by higher SNR in organic than psychogenic dystonia, while both are higher than in patients with chronic pain (Table 1). This finding suggests that SNR is an abnormal feature in both types of dystonia, which differs in degree between the two diagnoses. The increased SNR in PsyD may be either a consequence of dystonic movements, or a risk factor which predisposes to the development of dystonia during repetitive movements, or both 2,15. The origin of this change could be related to grouped GPi neuronal discharges in dystonia patients 16,17 which increase with increasing duration and severity of dystonia 16,18. GPi projects to Vop and lesions of either of these structures may interrupt dystonia 4,19. Thalamic neuronal activity was often correlated with EMG activity in both diagnoses, and so may drive movement for both whether it is a consequence of dystonic movements or risk factor for the development of these movements.
The large number of deep sensory cells in PsyD may also be due to a plastic change in the sensory representation resulting from dystonic movements. Specifically, cortical plasticity associated with sensory protocols including associative conditioning lead to greater cortical reorganization than do protocols in which sensory stimuli are not related to conditioning 20,21.
The present study also points to differences between the two types of dystonia. EMG coherence between arm muscles has previously been described in organic dystonia 22,23, excepting focal hand dystonia 24. EMG coherence was lower in PsyD (Table 1). This may be a useful measure to distinguish psychogenic from organic dystonia. Paired associative magnetic stimulation testing of cortical plasticity may also differentiate these conditions 25. Such non-invasive measures may be practical biomarkers for distinguishing psychogenic from organic dystonia, especially given the difficulty differentiating them on clinical grounds alone.
Acknowledgement
This work was supported by the National Institutes of Health – National Institute of Neurological Disorders and Stroke Intramural and Extramural Programs (RO1 NS38493 and ROL NS40059 to FAL). We thank Lance Rowland for excellent technical assistance.
Footnotes
Author Contributions: KK data analysis plus manuscript review and critique; AEL clinical management of the patient with psychogenic dystonia plus manuscript review and critique; MH evaluation of records at the time of surgery plus manuscript review and critique; FAL oversight of data and statistical analysis plus manuscript: writing, review and critique.
References
- 1.Espay AJ, Morgante F, Purzner J, et al. Cortical and spinal abnormalities in psychogenic dystonia. Ann Neurol. 2006;59:825–834. doi: 10.1002/ana.20837. [DOI] [PubMed] [Google Scholar]
- 2.Byl NN, Merzenich MM, Jenkins WM. A primate genesis model of focal dystonia and repetitive strain injury: I. learning-induced dedifferentiation of the representation of the hand in the primary somatosensory cortex in adult monkeys. Neurology. 1996;47:508–520. doi: 10.1212/wnl.47.2.508. [DOI] [PubMed] [Google Scholar]
- 3.Lenz FA, Byl NN. Reorganization in the cutaneous core of the human thalamic principal somatic sensory nucleus (Ventral caudal) in patients with dystonia. J Neurophysiol. 1999;82:3204–3212. doi: 10.1152/jn.1999.82.6.3204. [DOI] [PubMed] [Google Scholar]
- 4.Lenz FA, Jaeger CJ, Seike MS, et al. Thalamic single neuron activity in patients with dystonia: dystonia- related activity and somatic sensory reorganization. J Neurophysiol. 1999;82:2372–2392. doi: 10.1152/jn.1999.82.5.2372. [DOI] [PubMed] [Google Scholar]
- 5.Kaas JH. Plasticity of sensory and motor maps in adult mammals. Annu Rev Neurosci. 1991;14:137–167. doi: 10.1146/annurev.ne.14.030191.001033. [DOI] [PubMed] [Google Scholar]
- 6.Nudo RJ, Milliken GW, Jenkins WM, et al. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenz FA, Kwan HC, Martin R, et al. Characteristics of somatotopic organization and spontaneous neuronal activity in the region of the thalamic principal sensory nucleus in patients with spinal cord transection. J Neurophysiol. 1994;72:1570–1587. doi: 10.1152/jn.1994.72.4.1570. [DOI] [PubMed] [Google Scholar]
- 8.Hua SE, Lenz FA. Posture-related oscillations in human cerebellar thalamus in essential tremor are enabled by voluntary motor circuits. J Neurophysiol. 2005;93:117–127. doi: 10.1152/jn.00527.2004. [DOI] [PubMed] [Google Scholar]
- 9.Lenz FA, Dostrovsky JO, Kwan HC. Techniques for microstimulation and recordings of single units and evoked potentials during stereotactic surgery. J Neurosurg. 1988;68:630–634. doi: 10.3171/jns.1988.68.4.0630. [DOI] [PubMed] [Google Scholar]
- 10.Lenz FA, Tasker RR, Kwan HC, et al. Single unit analysis of the human ventral thalamic nuclear group: correlation of thalamic “tremor cells” with the 3-6 Hz component of parkinsonian tremor. J Neurosci. 1988;8:754–764. doi: 10.1523/JNEUROSCI.08-03-00754.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.French AS, Holden AV. Alias-free sampling of neuronal spike trains. Kybernetic. 1971;8:165–171. doi: 10.1007/BF00291117. [DOI] [PubMed] [Google Scholar]
- 12.Glaser EM, Ruchkin DS. Principles of Neurobiological Signal Analysis. Academic Press; New York: 1976. [Google Scholar]
- 13.Percival DB, Walden AT. Spectral Analysis of Physical Applications. Cambridge University Press; NY,NY: 1993. [Google Scholar]
- 14.Fahn S, Williams DT. Psychogenic dystonia. Adv Neurol. 1988;50:431–455. [PubMed] [Google Scholar]
- 15.Hallett M. The neurophysiology of dystonia. Arch Neurol. 1998;55:601–603. doi: 10.1001/archneur.55.5.601. [DOI] [PubMed] [Google Scholar]
- 16.Lenz FA, Suarez JI, Metman LV, et al. Pallidal activity during dystonia: somatosensory reorganisation and changes with severity. J Neurol Neurosurg Psychiatry. 1998;65:767–770. doi: 10.1136/jnnp.65.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitek JL, Zhang J, Evatt M, et al. GPi pallidotomy for dystonia: clinical outcome and neuronal activity. Adv Neurol. 1998;78:211–219. [PubMed] [Google Scholar]
- 18.Starr PA, Rau GM, Davis V, et al. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson's disease and normal macaque. J Neurophysiol. 2005;93:3165–3176. doi: 10.1152/jn.00971.2004. [DOI] [PubMed] [Google Scholar]
- 19.Ondo WG, Desaloms JM, Jankovic J, et al. Pallidotomy for generalized dystonia [see comments]. Mov Disord. 1998;13:693–698. doi: 10.1002/mds.870130415. [DOI] [PubMed] [Google Scholar]
- 20.Recanzone GH, Merzenich MM, Jenkins WM. Frequency discrimination training engaging a restricted skin surface results in an emergence of a cutaneous response zone in cortical area 3a. J Neurophysiol. 1992;67:1057–1070. doi: 10.1152/jn.1992.67.5.1057. [DOI] [PubMed] [Google Scholar]
- 21.Recanzone GH, Merzenich MM, Jenkins WM, et al. Topographic reorganization of the hand representation in cortical area 3b of owl monkeys trained in a frequency-discrimination task. J Neurophysiol. 1992;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- 22.Tijssen MA, Munchau A, Marsden JF, et al. Descending control of muscles in patients with cervical dystonia. Mov Disord. 2002;17:493–500. doi: 10.1002/mds.10121. [DOI] [PubMed] [Google Scholar]
- 23.Grosse P, Edwards M, Tijssen MA, et al. Patterns of EMG-EMG coherence in limb dystonia. Mov Disord. 2004;19:758–769. doi: 10.1002/mds.20075. [DOI] [PubMed] [Google Scholar]
- 24.Cordivari C, Lees AJ, Misra VP, et al. EMG-EMG coherence in writer's cramp. Mov Disord. 2002;17:1011–1016. doi: 10.1002/mds.10212. [DOI] [PubMed] [Google Scholar]
- 25.Quartarone A, Rizzo V, Terranova C, et al. Abnormal sensorimotor plasticity in organic but not in psychogenic dystonia. Brain. 2009;132:2871–2877. doi: 10.1093/brain/awp213. [DOI] [PMC free article] [PubMed] [Google Scholar]