Abstract
Grey-matter volumetric and cognitive deficits in young, high-risk relatives of schizophrenia patients may be vulnerability markers of the illness. Although these markers may be correlated, it is unclear if their distributions in relatives overlap. We examined convergence of these markers in 94 young first and second-degree relatives (HR) and 81 healthy controls. Subjects were assessed using WCST, CPT-IP and Benton–Hamscher tests and on grey-matter volumes of brain regions related to language, attention and executive function using FreeSurfer to process T1-MR-images. K-means clustering using cognitive performance scores split relatives into sub-samples with better (HR+C, n=35) and worse (HR−C, n=59) cognition after controlling for age and gender. All regional volumes and language related regional laterality-indices were compared between HR−C, HR+C and control subjects, controlling for age, gender and intra-cranial volume. Volumes of caudate nuclei, thalami, hippocampi, inferior frontal gyri, Heschl’s gyri, superior parietal cortices, supramarginal gyri, right angular gyrus, right middle frontal gyrus and right superior frontal gyrus, leftward laterality of supramarginal and inferior frontal gyri and rightward laterality of the angular gyrus were reduced in HR−C compared to controls. Volumes of Heschl’s gyri, left supramarginal gyrus, inferior frontal gyri, hippocampi and caudate nuclei HR−C were smaller in HR−C compared to HR+C. HR+C showed deficits compared to controls only for the superior parietal and right angular volumes. Premorbid neuroanatomical and laterality alterations in schizophrenia may selectively manifest in cognitively compromised relatives. Overlapping structural and cognitive deficits may define a hyper vulnerable sub-sample among individuals at familial predisposition to schizophrenia.
Introduction
Schizophrenia may involve heritable alterations of peri-adolescent neurodevelopment (DeLisi, 1997). Patients share these alterations with their genetically predisposed relatives, who are high-risk for psychotic disorders (DeLisi, 1997). Relatives may progress to psychotic disorders spanning either phenotypic extreme depending on the degree of familial diathesis. Relatives with highest vulnerability may develop severe, early-onset psychotic disorders (Kumra et al., 1998, 2002, 2001). Eleven to fifteen percent may develop adult-onset schizophrenia, while 40% may transition to less severe schizophrenia spectrum disorders (Erlenmeyer-Kimling et al., 1997, 1995). These variable clinical outcomes of genetically susceptible relatives may reflect the latent genetic heterogeneity of schizophrenia spectrum illnesses (Delisi, 2009; DeLisi and Fleischhaker, 2007; DeLisi et al., 1987; Jablensky and Kalaydjieva, 2003; Kremen et al., 2004). The heterogeneous risk-profile suggests that some “hyper-vulnerable” relatives may be at enhanced risk for future psychotic disorders, compared to relatives in general (Diwadkar et al., 2006; Velthorst et al., 2009). Cognitive and neuroanatomical deficits in relatives may increase their susceptibility for future psychosis and a non-uniform distribution of these premorbid vulnerability markers within relatives may explain the relatives’ uneven risk-profile for future psychotic disorders. Deficits of attention, language and executive-function may predict future psychopathology in relatives (Cornblatt, 2002; Diwadkar et al., 2006; Eack et al., 2008; Johnstone et al., 2005; Kremen et al., 1994; Sarfati and Hardy-Bayle, 2002). Grey-matter volumetric and lateralization abnormalities in relatives correlate with these cognitive markers (Antonova et al., 2005, 2004; Bhojraj et al., 2009; Cooke et al., 2008; Lymer et al., 2006; Premkumar et al., 2008a,b; Toulopoulou et al., 2004). Neuroanatomical deficits may also independently confer risk for psychosis (Fornito et al., 2008; Job et al., 2005, 2002, 2003, 2006; Kumari and Cooke, 2006; Lawrie et al., 2008, 1999; Sun et al., 2009; Wood et al., 2008), and are related to symptoms and electrophysiological alterations of schizophrenia (Kumari et al., 2008a,b). Convergence of neuroanatomical and cognitive markers may confer risk in excess of that entailed by familial diathesis alone, and may identify “hyper-vulnerable” relatives.
Studies in relatives, albeit independently demonstrating cognitive deficits (Keshavan et al., 2004) and hippocampal, thalamic, basal gangliar, cingulate, frontal and temporal cortical deficits (Job et al., 2005, 2003; Keshavan et al., 2002; Lawrie et al., 2008; Wood et al., 2008), have not assessed the overlap of cognitive and structural alterations.
We classified a sample of high-risk relatives into cognitively-worse and cognitively-better sub-samples based on attention, verbal fluency and executive function performance scores. Hippocampal, thalamic, caudate superior-temporal, Heschl’s, supramarginal, angular, inferior frontal, superior frontal and middle frontal and superior parietal cortical grey-matter volumes, and inferior frontal, supramarginal, angular, Heschl’s and superior temporal gyral laterality indices were assessed, given their relation to the assessed cognitions and their implication in schizophrenia (Antonova et al., 2005, 2004; Bhojraj et al., 2009; Crow, 2000; Premkumar et al., 2008a,b; Toulopoulou et al., 2004). We hypothesized the cognitively worse group to show greater neuroanatomical alterations compared to the cognitively better group.
Methods
Participants
The study was conducted at the Western Psychiatric Institute and Clinic, Pittsburgh. Participants were 94 adolescent and young adult relatives of (HR) of schizophrenia probands [77 first-degree relatives (66 offspring and 11 siblings) and 17 second-degree relatives] and 64 healthy controls (HC). Relatives of parents with schizophrenia or schizoaffective disorder were recruited by approaching patients in the clinic and through advertisements. HC were recruited through advertisements in the same community as HR. Clinical assessments of HC and HR and parental diagnoses of schizophrenia or schizoaffective disorder used the structured clinical interviews for DSM-IV diagnoses (SCID) (First et al., 1995) and were confirmed using consensus meetings led by senior diagnosticians (M.K and D.M). None of the HR were diagnosed with psychotic or other psychiatric illnesses and none had received antipsychotic medications. Participants with an IQb80, lifetime evidence of a psychotic disorder, exposure to antipsychotic medications or anti-depressant medications, substance use disorder within the last month, neurological or medical condition were excluded. All participants signed informed consent after the study was fully explained to them. For participants <18 years of age, the consent was provided by the parent or guardian, and the subjects provided informed assent. The study was approved by the University of Pittsburgh Institutional Review Board.
Structural MRI assessments
MRI scans were obtained on subjects using a GE 1.5T whole body scanner (GE Medical Systems, Milwaukee, Wisconsin). The scans were three-dimension spoiled gradient recalled (SPGR), acquired in a steady-state pulse sequence (124 coronal slices, 1.5 mm cortical thickness, TE=5 ms, TR=25 ms, acquisition matrix=256×192, FOV=24 cm, flip angle 40°). The detailed scanning procedure has been described in detail in our previous publication (Gilbert et al., 2001). T1-images were processed using FreeSurfer. Images with significant motion artifacts (significant motion artifacts may be related to excessive head movement of the subject in the scanner. These render the obtained T1 MRI image un-processable by FreeSurfer due to poor image quality) were not included in the study. FreeSurfer (Bhojraj et al., 2009; Goghari et al., 2007) is one of the few (Yates et al., 2006) semi-automated methods used to examine relatives and has established validity with automatic and manual methods (Pengas et al., 2009; Tae et al., 2008). FreeSurfer has three automated stages, each followed by editing by an experienced brain morphometrician (A.F) blind to subject identity, clinical diagnoses and cognitive performance. AF analyzed all images to maintain uniformity. The first stage performs motion correction, using two or more T1 image sequences and skull stripping (Segonne et al., 2004). If inspection reveals the strip to be inaccurate, the images were edited by AF in FreeSurfer or were imported into FSL and the strip was performed using the Brain Extraction Tool (Smith et al., 2004). The stripped images, checked for accuracy, were subjected to grey-white segmentation (Fischl et al., 2002; Han et al., 2002) [second stage of FreeSurfer]. The third stage of FreeSurfer automatically parcellates the a priori regions of interest (hippocampus, superior–temporal, Heschl’s, supramarginal, angular, inferior frontal, superior frontal and middle frontal gyri, superior parietal cortex, thalami and caudate-nucleii in our study) based on gyral anatomical landmarks and performs cortical grey-matter volume measurements (Desikan et al., 2006).
Cognitive assessments
Perseverative errors on the Wisconsin Card Sorting Test [WCST] (Heaton et al., 1993), and the verbal and visual d’ scores on the Continuous Performance test [CPT-IP version] (Cornblatt et al., 1988) were used to assess executive function and attention respectively. Verbal fluency measures were assessed using a letter task (number of words generated in 20 seconds that start on C, F and L alphabets) and a category task (e.g. names of animals, fruits and vegetables) (Benton and Hamscher, 1978). Clinical interviewers were blind to imaging and cognitive data. A.F was blind to clinical and cognitive data. The cognitive test administrators were blind to imaging and clinical data.
Statistical analyses
We used the K-means clustering algorithm to delineate a sub-sample (cluster) within HR showing poor performance on tests of attention, executive functioning and verbal-fluency. The K-means algorithm divides a given sample into an a priori decided number of sub-samples (two in this study) intended to differ on predefined measures (four cognitive performance scores in this study). The K-means method plots all subjects (HR in this study) in n-coordinate space (n=number of predefined measures) based on their scores for the n measures. It successively classifies each subject into distinct clusters so as to minimize the squared Euclidean distances between subjects within the same cluster and to maximize the distance between clusters (Pollard, 1981). The algorithm terminates if the within cluster variance reduction and between cluster variance maximization cannot be further improved. This optimizes the between-cluster difference for the predefined measures. The statistical significance of this classification is assessed by comparing the clusters on the predefined measures using F statistics obtained through ANOVA comparisons. Raw scores for the percentage of perseverative errors on the WCST, visual d’ scores and verbal d’ scores on the CPT-IP and total (letter+category) verbal fluency scores were z-transformed. These transformed scores were specified as the predefined measures to delineate two sub-samples within the HR sample. This allowed us to identify a subgroup within relatives showing convergent deficits of these cognitive tests. F statistics for comparisons of these scores across the formed clusters allowed significance testing of the presence of a distinct cognitively compromised subgroup within the sample of relatives. F statistics of ANCOVA tests were used to control for the confounding effect of age and gender on the clustering.
Grey-matter volumes of a priori hypothesized brain regions were compared between the two clusters and healthy controls using ANCOVA controlling for intra cranial volume (ICV), gender and age.
Results
HC did not differ from HR on age [(Mean±SD in years) controls (16.6±4.5), HR (15.4±3.6), t=1.79, p=0.1], handedness (Chi-square=0.1, p=0.7), race (Chi-square=0.33, p=0.57) and gender (controls: 42% males, HR: 53% males, Chi-square=1.6, p=0.20). Homogeneity of variances (Levene’s test p>0.1) assumptions were met for following ANCOVA tests.
The K-means method split the n=94 HR into subgroups of n=59 with low cognitive performance (designated HR−C) and n=35 performing better on the cognitive tests (designated HR + C) compared to HR−C. HR−C had lower verbal fluency and d’ scores compared to both HC and HR+C. HR+C had verbal fluency and visual d’ scores lower than those in controls and greater than HR−C (see Table 1, Fig. 1). This statistically significant clustering of HR into “cognitively compromised” (HR−C) and ‘cognitively intact’ (HR+C) subgroups was not confounded by age and gender, as HR−C had poorer scores after controlling for these.
Table 1.
Comparisons of cognitive performance across groups.
| Cognitive scores | HR−C Vs HR+C |
HR−C Vs HC |
HR+C Vs HC |
|---|---|---|---|
| F (1,92),p | F(1,137),p | F(1,114), p | |
| Percentage of perseverative errors | p>0.2 | p>0.2 | p>0.2 |
| Verbal d’ scores | 7.04, 0.005 | 9.15, 0.001 | p>0.2 |
| Visual d’ scores | 6.44, 0.012 | 8.23, 0.003 | 4.01, 0.048 |
| Total verbal fluency scores | 3.80, 0.055 | 7.89, 0.003 | 4.77, 0.033 |
F statistics and p values for ANCOVA tests comparing cognitive performance scores controlling for age and gender. Results suggest that the K-means algorithm split the HR sample into two subsets significantly differing on cognitive performance independent of the effect of age and gender (see Fig. 1).
Fig. 1.
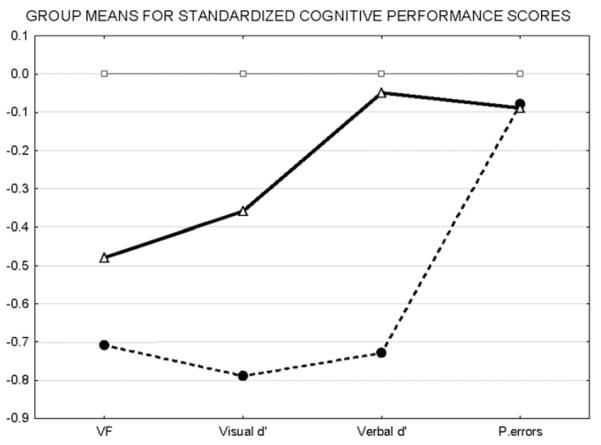
Group means for standardized cognitive performance scores. We identified a sub-sample within relatives with poorer (HR−C) cognition compared to the remaining relatives (HR+C). Raw scores for cognitive performance for each domain were converted to z-scores by standardizing them to the control mean. Mean z-scores for HC-C (dashed line), HC+C (thick line) and controls are plotted on the Y-axis for each domain mentioned on the X-axis. HR−C are seen to show deficits for most performance scores compared to both HC and HR+C. HR+C appear to be intermediate between HC and HR−C on cognitive performance.
The caudate nuclei, thalami, hippocampi, inferior frontal gyri, Heschl’s gyri, superior parietal cortices, supramarginal gyri, right angular gyrus, right middle frontal gyrus and right superior frontal gyrus were reduced in HR−C compared to controls. The Heschl’s gyri, left supramarginal gyrus, inferior frontal gyri, hippocampi and caudate nucleii were reduced in HR−C compared to HR+C. HR+C showed trend-level deficits compared to controls only for the superior parietal cortices and right angular gyrus (see Table 2 and Fig. 2). The superior temporal cortices, left superior, left middle frontal and left angular regions showed no deficits. The leftward lateralization of the supramarginal gyrus and the rightward lateralization of the angular gyrus seen in HC were reduced in HR−C while the leftward lateralization of the inferior frontal gyrus in HC was reversed in HR−C (see Table 3 and Fig. 3). No laterality deficits were noted for HR+C compared to other groups.
Table 2.
Grey-matter volume comparisons.
| Region | HR−C Vs HR+C |
HR−C Vs HC |
HR+C Vs HC |
|---|---|---|---|
| F(1,91 ),p | F( 1,136),p | F(1,113), p | |
| Intra cranial volume | 1.79, 0.195 | 0.9, 0.693 | 1.2, 0.429 |
| Left Heschl’s gyrus | 3.81, 0.053 | 2.99, 0.08 | p>0.2 |
| Right Heschl’s gyrus | 4.92, 0.032 | 11.22, 0.000 | p>0.2 |
| Left supramarginal gyrus | 3.28, 0.071 | 20.06, 0.000 | p>0.2 |
| Right supramarginal gyrus | p>0.2 | 6.05, 0.015 | p>0.2 |
| Right angular gyrus | p>0.2 | 18.1, 0.000 | 3.12, 0.070 |
| Left superior parietal gyrus | p>0.2 | 3.43, 0.065 | 3.62, 0.059 |
| Right superior parietal gyrus | p>0.2 | 8.23, 0.004 | 4.22, 0.039 |
| Right middle frontal gyrus | p>0.2 | 6.78, 0.010 | p>0.2 |
| Right superior frontal gyrus | p>0.2 | 3.96, 0.048 | p>0.2 |
| Left inferior frontal gyrus | 7.32, 0.001 | 12.32, 0.000 | p>0.2 |
| Right inferior frontal gyrus | 4.05, 0.042 | 3.32, 0.071 | p>0.2 |
| Left hippocampus | 4.18, 0.039 | 8.79, 0.000 | p>0.2 |
| Right hippocampus | 4.11, 0.041 | 8.02, 0.000 | p>0.2 |
| Left caudate | 4.71, 0.036 | 9.09, 0.000 | p>0.2 |
| Right caudate | 6.45, 0.011 | 14.21, 0.000 | p>0.2 |
| Left thalamus | p>0.2 | 5.67, 0.018 | p>0.2 |
| Right thalamus | p>0.2 | 6.68, 0.012 | p>0.2 |
F statistics and p values for ANCOVAs controlling for ICV, age and gender comparing brain regional grey matter volumes between groups. HR−C are seen to show volumetric deficits compared to HC and HR+C groups. HR+C showed trend-level deficits compared to HC for right angular and superior parietal cortices. (see Fig. 2).
Fig. 2.
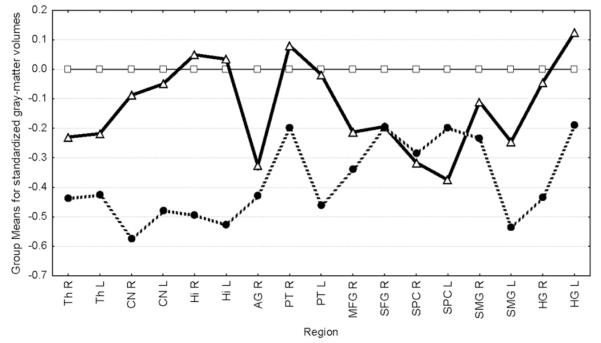
Group means for standardized grey-matter volumes. Grey-matter volumes for each region were standardized to the control mean. Mean standardized scores for HC-C (dashed line), HC + C (thick line) and controls are plotted on the Y-axis for each region mentioned on the X-axis. HR−C are seen to show deficits for most regions compared to both HC and HR+ C. HR+ C appear to be intermediate between HC and HR−C for volumes. Th=Thalamus, CN=caudate nuclei, AG=angular gyrus, SMG=supramarginal gyrus, PT=pars triangularis of the inferior frontal gyrus, HI=hippocampus, MFG=middle frontal gyrus, SFG=superior frontal gyrus, HG =Heschl’s gyrus. All regions were reduced in HR−C compared to controls.
Table 3.
Laterality index comparisons.
| Region | HR−C Vs HR+C |
HR−C Vs HC |
HR+C Vs HC |
|---|---|---|---|
| F (1,92),p | F(1,137),p | F(1,114), p | |
| Heschl’s gyrus | p>0.2 | p>0.2 | p>0.2 |
| Supramarginal gyrus | 4.22, 0.040 | p>0.2 | p>0.2 |
| Angular gyrus | 6.08, 0.014 | p>0.2 | p>0.2 |
| Inferior frontal gyrus | 4.32, 0.037 | p>0.2 | p>0.2 |
F statistics and p values for ANCOVAs controlling for gender and age comparing brain regional laterality indices between groups. HR−C are seen to show laterality deficits compared to HC (see Fig. 3).
Fig. 3.
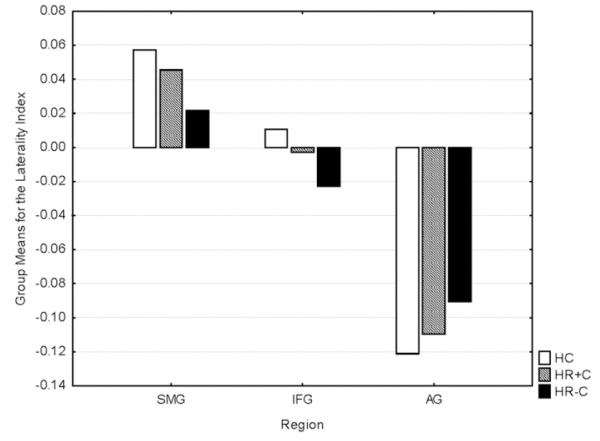
Group means of laterality indices. Mean laterality indices (on Y-axis) for each group for each region are plotted. IFG=pars triangularis of the inferior frontal gyrus, SMG=supramarginal gyrus AG=angular gyrus.
Discussion
We found volumetric and laterality deficits in adolescent and young adult relatives of schizophrenia patients to overlap with cognitive impairment. As predicted, relatives with poorer cognition (HR−C) had volumetric reductions compared to controls for all assessed regions and compared to relatives with better cognition (HR+C) for the majority of assessed regions. HR+C showed only minimal volumetric deficits compared to controls and no deficits compared to HR−C. These results suggest that structural deficits in HR may be inter-related with cognitive deficits and may hence be restricted to those HR with poor cognition. The caudate nuclei, thalami, hippocampi, inferior frontal gyri, Heschl’s gyri, superior parietal cortices, supramarginal gyri, right angular gyrus, right middle frontal gyrus and right superior frontal gyrus volumes were reduced in HR−C compared to controls. The Heschl’s gyri, left supramarginal gyrus, inferior frontal gyri, hippocampi and caudate nucleii were reduced in HR−C relative to HR+C. Alterations of the prefrontal regions, Heschl’s gyrus, thalamus and hippocampus, may convey risk for transition to psychosis in relatives (Job et al., 2005, 2003, 2006; Lawrie et al., 2008; Sun et al., 2009; Takahashi et al., 2009). These regions were altered in HR−C compared to HR+C and HC and were not altered in HR+C compared to either HR−C or HC. Neuroanatomical deficits may contribute to the enhanced risk for future psychosis, shown in relatives manifesting attention, language and executive function deficits (Cornblatt, 2002; Diwadkar et al., 2006; Eack et al., 2008; Johnstone et al., 2005; Kremen et al., 1994; Sarfati and Hardy-Bayle, 2002). To our knowledge, previous studies have not reported deficits of the superior parietal cortex in high-risk relatives. The leftward lateralization of the inferior frontal gyrus in HC was reversed in HR−C while the leftward laterality of the supramarginal gyrus and the rightward laterality of the angular gyrus in HC were reduced in HR−C. Laterality deficits were not seen in HR+C. We have found similar lateralization alterations of the inferior frontal, angular and the supramarginal gyri and a correlation of laterality deficits and verbal fluency deficits in a subset of the currently studied HR (Bhojraj et al., 2009). Laterality deficits may be restricted to HR−C. Laterality deficits in patients may effect a breach of the hemispheric segregation of language processing possibly causing psychotic symptoms including language dysfunction and auditory verbal hallucinations in schizophrenia patients (see (Bhojraj et al., 2009) for a review).
Structural alterations and cognitive deficits may both vary with the degree of genetic-loading for schizophrenia (Hall et al., 2008; McIntosh et al., 2007, 2006). The existence of distinct HR−C and HR+C sub-samples may reflect the latent genetic heterogeneity of schizophrenia spectrum illnesses (Delisi, 2009; DeLisi and Fleischhaker, 2007; DeLisi et al., 1987; Jablensky and Kalaydjieva, 2003; Kremen et al., 2004).
Although our findings suggest overlapping neuroanatomical and cognitive deficits in relatives, lack of prospective data regarding psychotic symptoms precludes their implications for predicting psychosis. Longitudinal studies using a convergent approach, such as the one in our study, during the ‘transition’ of relatives to psychosis may ascertain whether relatives showing coincident neuroanatomical and cognitive deficits have a heightened risk for psychosis compared to relatives, in general. We did not assess possible predictors of schizophrenia like sub-threshold psychotic symptoms, affective symptoms, memory deficits and schizotypal features (Diwadkar et al., 2006; Eack et al., 2008). Including these as clustering variables may have improved the definition of the hyper-vulnerable (HR−C) sub-sample. Subtle group differences, such as those between HR+C and HR−C and between HR+C and HC may have escaped detection possibly due to the low power due to the moderate sample size. The selection of a priori examined regions, based on their relation to the assessed cognitive domains may be somewhat simplistic, as the functional significance of brain regions is currently debatable.
We found overlapping neuroanatomical and neurocognitive deficits within a sub-sample of at-risk relatives. The possible hyper-vulnerability conferred by coincident structural and cognitive deficits on this sub-sample needs to be examined further. Identifying putative hyper-vulnerable relatives may help define targets of preventive strategies for schizophrenia (Johnstone et al., 2002).
Acknowledgments
We thank Konasale M Prasad and Shaun M Eack for their help with various aspects of this study. Funding source: National Institute of Mental Health (MH 64023 and 01180 to MK); National Alliance for Research on Schizophrenia and Depression (Independent Investigator award to MK); National Alliance for Research on Schizophrenia and Depression and General Clinical Research Center (GCRC) (M01 RR00056 to MK).
Footnotes
Disclosure/conflict of interest statement This research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr. Res. 2004;70:117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Antonova E, Kumari V, Morris R, Halari R, Anilkumar A, Mehrotra R, Sharma T. The relationship of structural alterations to cognitive deficits in schizophrenia: a voxel-based morphometry study. Biol. Psychiatry. 2005;58:457–467. doi: 10.1016/j.biopsych.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Benton A, Hamscher K. Multilingual Aphasia Examination Manual (revised) Iowa City. University of Iowa; Iowa: 1978. [Google Scholar]
- Bhojraj T, Francis A, Rajarethinam R, Eack S, Kulkarni S, Prasad K, Montrose D, Dworakowski D, Diwadkar V, Keshavan M. Verbal fluency deficits and altered lateralization of language brain areas in individuals genetically predisposed to schizophrenia. Schizophr. Res. 2009 doi: 10.1016/j.schres.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke MA, Fannon D, Kuipers E, Peters E, Williams SC, Kumari V. Neurological basis of poor insight in psychosis: a voxel-based MRI study. Schizophr. Res. 2008;103:40–51. doi: 10.1016/j.schres.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA. The New York high risk project to the Hillside recognition and prevention (RAP) program. Am. J. Med. Genet. 2002;114:956–966. doi: 10.1002/ajmg.b.10520. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The continuous performance test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Res. 1988;26:223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Invited commentary on: functional anatomy of verbal fluency in people with schizophrenia and those at genetic risk. The genetics of asymmetry and psychosis. Br. J. Psychiatry. 2000;176:61–63. doi: 10.1192/bjp.176.1.61. [DOI] [PubMed] [Google Scholar]
- DeLisi LE. Is schizophrenia a lifetime disorder of brain plasticity, growth and aging? Schizophr. Res. 1997;23:119–129. doi: 10.1016/S0920-9964(96)00079-5. [DOI] [PubMed] [Google Scholar]
- Delisi LE. Searching for the true genetic vulnerability for schizophrenia. Genome Med. 2009;1:14. doi: 10.1186/gm14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE, Fleischhaker W. Schizophrenia research in the era of the genome, 2007. Curr. Opin. Psychiatry. 2007;20:109–110. doi: 10.1097/YCO.0b013e328049558f. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Goldin LR, Gershon ES. Studies of biological factors associated with the inheritance of schizophrenia: a selective review. J. Psychiatr. Res. 1987;21:507–513. doi: 10.1016/0022-3956(87)90099-9. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Montrose DM, Dworakowski D, Sweeney JA, Keshavan MS. Genetically predisposed offspring with schizotypal features: an ultra high-risk group for schizophrenia? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:230–238. doi: 10.1016/j.pnpbp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Eack SM, Prasad KM, Montrose DM, Goradia DD, Dworakowski D, Miewald J, Keshavan MS. An integrated psychobiological predictive model of emergent psychopathology among young relatives at risk for schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:1873–1878. doi: 10.1016/j.pnpbp.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Squires-Wheeler E, Adamo UH, Bassett AS, Cornblatt BA, Kestenbaum CJ, Rock D, Roberts SA, Gottesman II. The New York High-Risk Project. Psychoses and cluster A personality disorders in offspring of schizophrenic parents at 23 years of follow-up. Arch. Gen. Psychiatry. 1995;52:857–865. doi: 10.1001/archpsyc.1995.03950220067013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Adamo UH, Rock D, Roberts SA, Bassett AS, Squires-Wheeler E, Cornblatt BA, Endicott J, Pape S, Gottesman II. The New York High-Risk Project. Prevalence and comorbidity of axis I disorders in offspring of schizophrenic parents at 25-year follow-up. Arch. Gen. Psychiatry. 1997;54:1096–1102. doi: 10.1001/archpsyc.1997.01830240052008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Williams J, Gibbon M. Structured clinical interview for DSM-IV (SCID) American Psychiatric Association; Washington, DC: 1995. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fornito A, Yung AR, Wood SJ, Phillips LJ, Nelson B, Cotton S, Velakoulis D, McGorry PD, Pantelis C, Yucel M. Anatomic abnormalities of the anterior cingulate cortex before psychosis onset: an MRI study of ultra-high-risk individuals. Biol. Psychiatry. 2008;64:758–765. doi: 10.1016/j.biopsych.2008.05.032. [DOI] [PubMed] [Google Scholar]
- Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS. Thalamic volumes in patients with first-episode schizophrenia. Am. J. Psychiatry. 2001;158:618–624. doi: 10.1176/appi.ajp.158.4.618. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Rehm K, Carter CS, MacDonald AW. Sulcal thickness as a vulnerability indicator for schizophrenia. Br. J. Psychiatry. 2007;191:229–233. doi: 10.1192/bjp.bp.106.034595. [DOI] [PubMed] [Google Scholar]
- Hall J, Whalley HC, Moorhead TW, Baig BJ, McIntosh AM, Job DE, Owens DG, Lawrie SM, Johnstone EC. Genetic variation in the DAOA (G72) gene modulates hippocampal function in subjects at high risk of schizophrenia. Biol. Psychiatry. 2008;64:428–433. doi: 10.1016/j.biopsych.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Han X, Xu C, Braga-Neto U, Prince JL. Topology correction in brain cortex segmentation using a multiscale, graph-based algorithm. IEEE Trans. Med. Imaging. 2002;21:109–121. doi: 10.1109/42.993130. [DOI] [PubMed] [Google Scholar]
- Heaton R, Chelune G, Talley J, Kay G, Curtiss G. Wisconsin Card Sorting Test Manual—Revised and Expanded. Psychological Assessment Resources. Inc.(PAR); Odessa, FL: 1993. [Google Scholar]
- Jablensky AV, Kalaydjieva LV. Genetic epidemiology of schizophrenia: phenotypes, risk factors, and reproductive behavior. Am. J. Psychiatry. 2003;160:425–429. doi: 10.1176/appi.ajp.160.3.425. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Structural gray matter differences between first-episode schizophrenics and normal controls using voxel-based morphometry. Neuroimage. 2002;17:880–889. [PubMed] [Google Scholar]
- Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Voxel-based morphometry of grey matter densities in subjects at high risk of schizophrenia. Schizophr. Res. 2003;64:1–13. doi: 10.1016/s0920-9964(03)00158-0. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25:1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, McIntosh AM, Owens DG, Johnstone EC, Lawrie SM. Grey matter changes can improve the prediction of schizophrenia in subjects at high risk. BMC Med. 2006;4:29. doi: 10.1186/1741-7015-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone EC, Lawrie SM, Cosway R. What does the Edinburgh high-risk Study tell us about schizophrenia? Schizophrenia: from prediction to prevention. Am. J. Med. Genet. 2002;114:906–912. doi: 10.1002/ajmg.b.10304. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Ebmeier KP, Miller P, Owens DG, Lawrie SM. Predicting schizophrenia: findings from the Edinburgh High-Risk Study. Br. J. Psychiatry. 2005;186:18–25. doi: 10.1192/bjp.186.1.18. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Dick E, Mankowski I, Harenski K, Montrose DM, Diwadkar V, DeBellis M. Decreased left amygdala and hippocampal volumes in young offspring at risk for schizophrenia. Schizophr. Res. 2002;58:173–183. doi: 10.1016/s0920-9964(01)00404-2. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Montrose DM, Stanley JA, Pettegrew JW. Premorbid characterization in schizophrenia: the Pittsburgh High Risk Study. World Psychiatry. 2004;3:163–168. [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Seidman LJ, Pepple JR, Lyons MJ, Tsuang MT, Faraone SV. Neuropsychological risk indicators for schizophrenia: a review of family studies. Schizophr. Bull. 1994;20:103–119. doi: 10.1093/schbul/20.1.103. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Seidman LJ, Faraone SV, Toomey R, Tsuang MT. Heterogeneity of schizophrenia: a study of individual neuropsychological profiles. Schizophr. Res. 2004;71:307–321. doi: 10.1016/j.schres.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Kumari V, Cooke M. Use of magnetic resonance imaging in tracking the course and treatment of schizophrenia. Expert Rev. Neurother. 2006;6:1005–1016. doi: 10.1586/14737175.6.7.1005. [DOI] [PubMed] [Google Scholar]
- Kumari V, Fannon D, Geyer MA, Premkumar P, Antonova E, Simmons A, Kuipers E. Cortical grey matter volume and sensorimotor gating in schizophrenia. Cortex. 2008a;44(9):1206–1214. doi: 10.1016/j.cortex.2007.11.007. (Oct, Electronic publication 2008 Jan 20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Peters ER, Fannon D, Premkumar P, Aasen I, Cooke MA, Anilkumar AP, Kuipers E. Uncontrollable voices and their relationship to gating deficits in schizophrenia. Schizophr. Res. 2008b;101:185–194. doi: 10.1016/j.schres.2007.12.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumra S, Jacobsen LK, Lenane M, Zahn TP, Wiggs E, Alaghband-Rad J, Castellanos FX, Frazier JA, McKenna K, Gordon CT, Smith A, Hamburger S, Rapoport JL. Multidimensionally impaired disorder”: is it a variant of very early-onset schizophrenia? J. Am. Acad. Child Adolesc. Psychiatry. 1998;37:91–99. doi: 10.1097/00004583-199801000-00022. [DOI] [PubMed] [Google Scholar]
- Kumra S, Shaw M, Merka P, Nakayama E, Augustin R. Childhood-onset schizophrenia: research update. Can. J. Psychiatry. 2001;46:923–930. doi: 10.1177/070674370104601004. [DOI] [PubMed] [Google Scholar]
- Kumra S, Nicolson R, Rapoport J. Childhood-onset schizophrenia. The Early Stages of Schizophrenia. 2002. p. 161.
- Lawrie SM, Whalley H, Kestelman JN, Abukmeil SS, Byrne M, Hodges A, Rimmington JE, Best JJ, Owens DG, Johnstone EC. Magnetic resonance imaging of brain in people at high risk of developing schizophrenia. Lancet. 1999;353:30–33. doi: 10.1016/S0140-6736(98)06244-8. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, McIntosh AM, Hall J, Owens DG, Johnstone EC. Brain structure and function changes during the development of schizophrenia: the evidence from studies of subjects at increased genetic risk. Schizophr. Bull. 2008;34:330–340. doi: 10.1093/schbul/sbm158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymer GK, Job DE, William T, Moorhead J, McIntosh AM, Owens DG, Johnstone EC, Lawrie SM. Brain-behaviour relationships in people at high genetic risk of schizophrenia. Neuroimage. 2006;33:275–285. doi: 10.1016/j.neuroimage.2006.06.031. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead WJ, Harrison LK, Whalley HC, Johnstone EC, Lawrie SM. Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006;141B:76–83. doi: 10.1002/ajmg.b.30254. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Baig BJ, Hall J, Job D, Whalley HC, Lymer GK, Moorhead TW, Owens DG, Miller P, Porteous D, Lawrie SM, Johnstone EC. Relationship of catechol-O-methyltransferase variants to brain structure and function in a population at high risk of psychosis. Biol. Psychiatry. 2007;61:1127–1134. doi: 10.1016/j.biopsych.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Pengas G, Pereira JM, Williams GB, Nestor PJ. Comparative reliability of total intracranial volume estimation methods and the influence of atrophy in a longitudinal semantic dementia cohort. J. Neuroimaging. 2009;19:37–46. doi: 10.1111/j.1552-6569.2008.00246.x. [DOI] [PubMed] [Google Scholar]
- Pollard D. Strong consistency of k-means clustering. Ann. Stat. 1981:135–140. [Google Scholar]
- Premkumar P, Fannon D, Kuipers E, Cooke MA, Simmons A, Kumari V. Association between a longer duration of illness, age and lower frontal lobe grey matter volume in schizophrenia. Behav. Brain Res. 2008a;193:132–139. doi: 10.1016/j.bbr.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Premkumar P, Kumari V, Corr PJ, Fannon D, Sharma T. Neuropsychological function-brain structure relationships and stage of illness: an investigation into chronic and first-episode schizophrenia. Psychiatry Res. 2008b;162:195–204. doi: 10.1016/j.pscychresns.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Sarfati Y, Hardy-Bayle MC. Could cognitive vulnerability identify high-risk subjects for schizophrenia? Am. J. Med. Genet. 2002;114:893–897. doi: 10.1002/ajmg.b.10251. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sun D, Phillips L, Velakoulis D, Yung A, McGorry PD, Wood SJ, van Erp TG, Thompson PM, Toga AW, Cannon TD, Pantelis C. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr. Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tae WS, Kim SS, Lee KU, Nam EC, Kim KW. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology. 2008;50:569–581. doi: 10.1007/s00234-008-0383-9. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Yung AR, Soulsby B, McGorry PD, Suzuki M, Kawasaki Y, Phillips LJ, Velakoulis D, Pantelis C. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch. Gen. Psychiatry. 2009;66:366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- Toulopoulou T, Grech A, Morris RG, Schulze K, McDonald C, Chapple B, Rabe-Hesketh S, Murray RM. The relationship between volumetric brain changes and cognitive function: a family study on schizophrenia. Biol. Psychiatry. 2004;56:447–453. doi: 10.1016/j.biopsych.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Velthorst E, Nieman DH, Becker HE, van de Fliert R, Dingemans PM, Klaassen R, de Haan L, van Amelsvoort T, Linszen DH. Baseline differences in clinical symptomatology between ultra high risk subjects with and without a transition to psychosis. Schizophr. Res. 2009;109:60–65. doi: 10.1016/j.schres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Pantelis C, Velakoulis D, Yucel M, Fornito A, McGorry PD. Progressive changes in the development toward schizophrenia: studies in subjects at increased symptomatic risk. Schizophr. Bull. 2008;34:322–329. doi: 10.1093/schbul/sbm149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates SL, Barach A, Gingell S, Whalley HC, Job D, Johnstone EC, Best JJ, Lawrie SM. Parcellating the temporal lobes from magnetic resonance images using generic software in subjects at high risk of developing schizophrenia. Psychiatry Res. 2006;147:197–212. doi: 10.1016/j.pscychresns.2006.01.012. [DOI] [PubMed] [Google Scholar]


