Abstract
The growing number of cancer survivors worldwide has led to of the emergence of diverse survivorship movements in the United States and Europe. Understanding the evolution of cancer survivorship within the context of different political and healthcare systems is important for identifying the future steps that need to be taken and collaborations needed to promote research among and enhance the care of those living after cancer. We first review the history of survivorship internationally and important related events in both the US and Europe. We then discuss lessons learned from survivorship research broadly, followed by examination of the infrastructure needed to sustain and advance this work, including: platforms for research, assessment tools, and vehicles for the dissemination of findings. We end with future perspectives, identifying the collaborative opportunities for investigators in Europe and the United States to accelerate the pace of survivorship science going forward.
Keywords: cancer, survivorship, research, Europe, United States
Introduction
The dawning of the new millennium ushered in a new era for cancer control globally; one heralded by the rise of interest in the health, functioning and psychosocial well-being of those living through and beyond a cancer diagnosis. The ‘cancer survivorship’ movement started in the United States and is increasingly being championed in diverse countries across Europe. However, to date, survivorship research has occurred in a fragmented fashion with the need for international ventures only now being recognized. In this paper we will (1) review and compare the evolution of the field of cancer survivorship research in the U.S. and in Europe, with illustrative examples, (2) discuss the knowledge generated from this work and the new directions this science is taking and (3) identify resources needed to advance this science. We will also highlight areas where future international collaborations will serve to accelerate the pace of translation from research findings to improvements in care of the growing population of cancer survivors.
Evolution of a Field
Forty years ago, the survival rates for all cancers combined were low.1, 2 Relatively few effective treatment options were available; of those that were, many had serious side effects. Due to advances in recent decades in early detection, effective therapies and supportive care, 5-year survival rates have increased to 50% or more in adults with a history of cancer in the U.S. and in many European countries3 and there are growing numbers of people living with and beyond a diagnosis of cancer.4, 5 In the U.S. and Europe, the greatest improvements in survival were seen for childhood cancers and malignancies of young adults (e.g. Hodgkin’s lymphoma, testicular cancer).6
These advances have led us to begin to ask important questions: What are the persistent problems and late effects in individuals who have survived their cancer? Which survivors are at particular risk for developing late effects? What is the impact of a history of cancer on individuals’ careers, families and wider society? How can late and long-term effects be cost-effectively prevented, detected, and managed?
Evolution of Survivorship Science in the United States
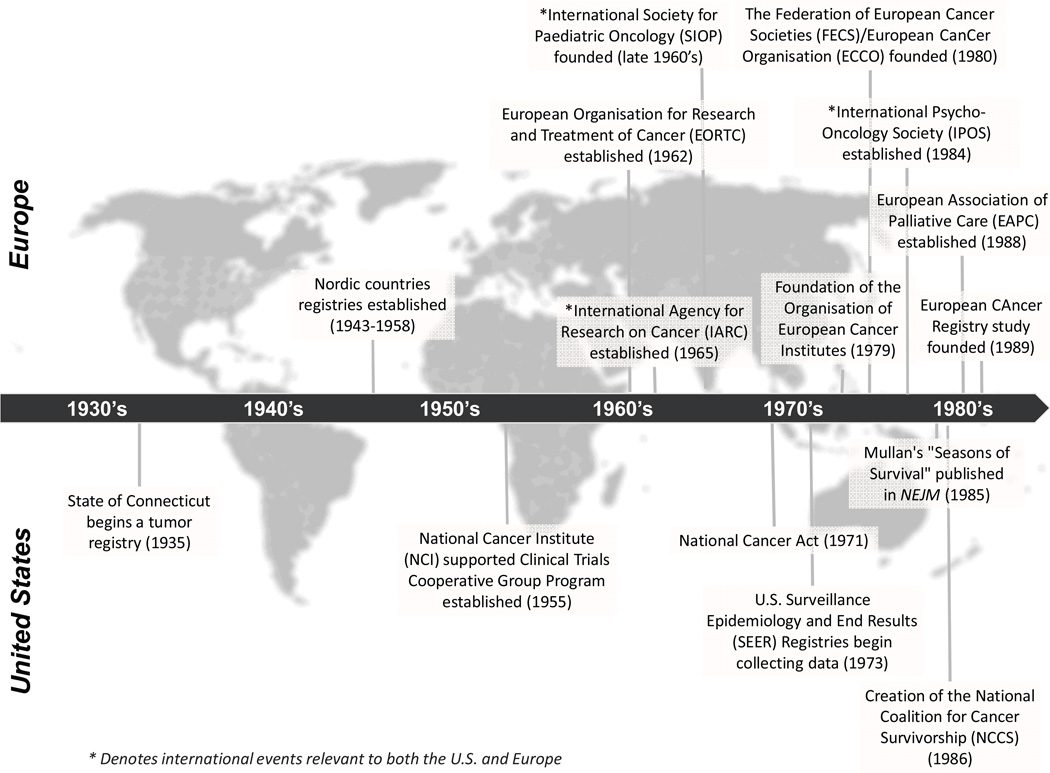
Progress made in cancer prevention and control in the U.S. is often dated from the signing of the National Cancer Act on December 23, 1971 (Figure 1). However, the launch of the survivorship movement in the U.S. is generally linked to two events: a 1985 publication in the New England Journal of Medicine by a young physician, Dr. Fitzhugh Mullan, describing his journey with cancer, which he labeled as the ‘Seasons of Survival,7 and the creation a year later of the National Coalition for Cancer Survivorship (NCCS). At the first meeting of the Coalition in October 1986 in Albuquerque, New Mexico, Mullan and the two dozen founding members proposed a new definition for ‘cancer survivor.’ Up to that time, the term ‘cancer survivor’ was deemed by the medical community to refer to someone who had remained disease-free for a minimum of 5 years. Coalition members reasoned that cancer patients could not wait five years to make decisions about outcomes that would be affected by specific treatment choices (e.g., fertility preservation, receiving a drug that could alter lung capacity or risk for peripheral neuropathy). They proposed that a person should be considered a survivor from the time of diagnosis onward. The revised definition was designed to provide hope and, importantly, to change the medical dialogue such that cancer treatment decisions would be made predicated upon a patient’s preferences and desires regarding life after cancer. While many treated for cancer do not refer to or think of themselves as survivors,8 this language has taken hold broadly in the U.S. It also launched a cascade of activities promoting attention to the unique needs of cancer survivors.
Figure 1. Timeline of important events in the evolution of the field of cancer survivorship.
* Denotes international events relevant to both the U.S. and Europe
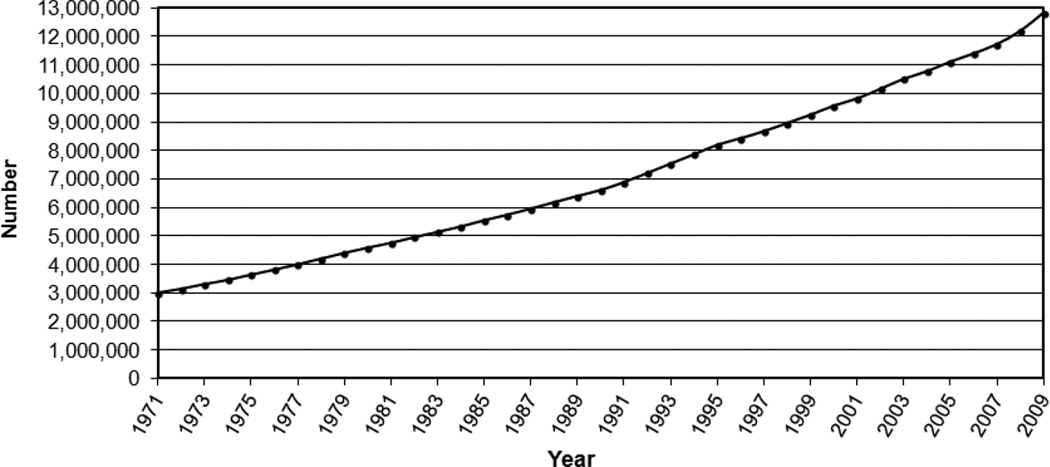
One of the most compelling rationales for cancer survivorship research, namely, the sheer growth in numbers of those living through and beyond cancer in the U.S.,4 has been documented by the Surveillance Epidemiology and End Results (SEER) cancer registries (Figure 2). The SEER registries, which were established by the National Cancer Act and currently cover approximately 28% of the U.S. population (http://seer.cancer.gov/about/factsheets/SEER_brochure.pdf), provide a unique resource for quantifying the growing prevalent population of cancer survivors because they track survival through the balance of life for all cases reported. As of 2012, there were an estimated 13.7 million cancer survivors in the U.S. alone,9 representing approximately 4% of the population.10
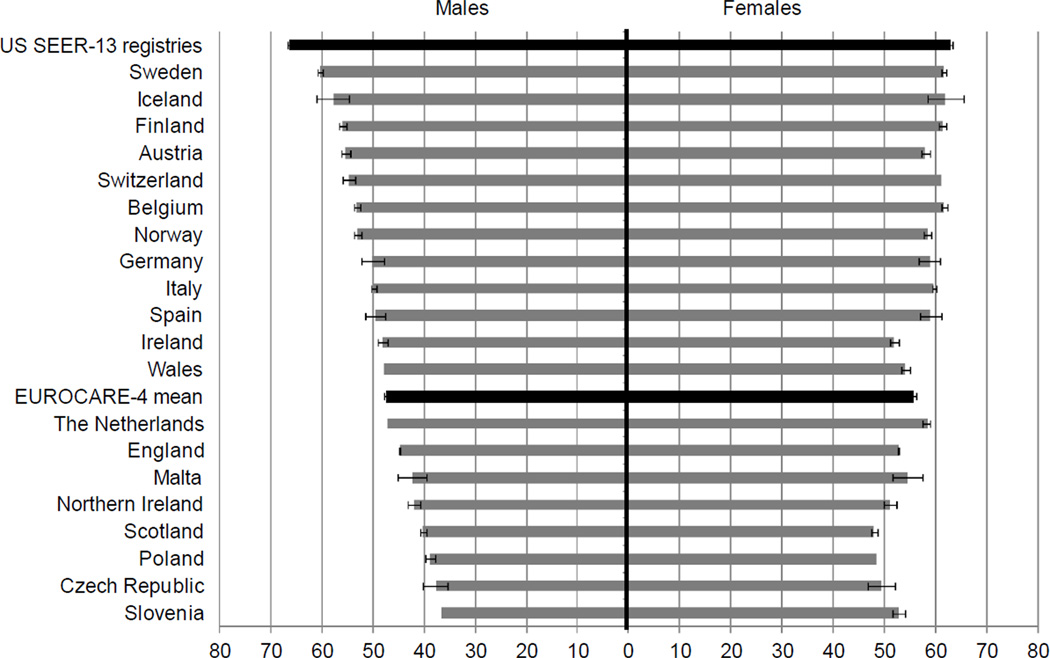
Figure 2. 5-year relative survival of all malignancies diagnosed 2000–2002, stratified by sex.
Data source: Verdecchia A, Francisci S, Brenner H, et al. Recent cancer survival in Europe: a 2000–02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8: 784–796.
SEER= Surveillance, Epidemiology and End Results
Relative survival was calculated as the ratio of absolute survival of patients with cancer to the expected survival of a group of people of the corresponding sex and age in the general population. Registry quality and coverage varied by country; see Verdecchia et al. (2007) for data quality metrics.
Several other key achievements in policy and research support have contributed to the growth of the field of cancer survivorship in the U.S. The Office of Cancer Survivorship (OCS) at the National Cancer Institute (NCI) was established in 1996 to champion and direct research to identify and address the challenges faced by those living long-term after cancer. The American Cancer Society (ACS), established in 1913, funds research in cancer survivorship, and made a major commitment in 2000 to support survivorship science with the initiation of the Study of Cancer Survivors, a large population-based longitudinal study of quality of life.11 Part of the mission statement of the ACS is to diminish suffering from cancer.
In addition, the President’s Cancer Panel (PCP), also established by the National Cancer Act, was tasked with monitoring the progress of the National Cancer Plan. The steady increase in the number of survivors and the lack of information about their health status and needs, became the topic of the annual report of the PCP in 2003–2004.12 This report, and four additional national reports on the challenges to understanding and addressing the care of pediatric13 and adult cancer survivors14–16 brought national visibility to cancer survivorship research. These reports state that cancer survivorship needs to be addressed as a unique place on the cancer control continuum.
Attention to cancer survivors’ health and needs in the U.S. has further benefited from a rich history of patient advocacy and public visibility around cancer. The informed consent movement in the late 1960s promoted attention to the rights of patients regarding information about the nature of their illness, and also their role in treatment decision-making. To be truly ‘informed,’ a patient needs to understand the consequences of choices in care. Since the late 1970s, a number of high profile figures have acknowledged their status as cancer survivors (e.g., Betty Ford, wife of President Ford in 1976, and Lance Armstrong, whose visibility and foundation have had worldwide impact). These disclosures, along with a growing advocacy movement, helped lower cancer-related stigma in the U.S. and prompted a level of public dialogue about this disease. One measure of the impact these conversations have had is that when someone dies of cancer in the U.S. today, obituaries in major city newspapers now cite the cause of death as such, often indicating the specific type of cancer, instead of using the euphemism, ‘died of a lingering illness.’ In the absence of these types of public disclosures and dialogues, cancer still continues to be stigmatized in other countries around the world.
The growing visibility of cancer survivors in the U.S. also led to the creation of a number of organizations championing the research and care of specific populations of cancer survivors, breast cancer advocates leading the way in the early 1990s, but quickly followed by organizations for diversity of cancer sites such as leukemia, colorectal cancer, prostate cancer, bladder cancer, pancreatic cancer, etc. Consumer advocacy was a driver behind the creation of the Office of Cancer Survivorship at the NCI; it has also, at least in the past, functioned to increase federal spending on cancer.17
There is wide variability in healthcare receipt and coverage by region, state and health insurance type in the U.S. Across the U.S., cancer treatment and post-treatment follow-up care are poorly coordinated across multiple providers, settings, and payers. In particular, post-treatment cancer care lacks clear delineation of responsibility among providers, guidance for appropriate tests and treatments, and adequate reimbursement for all aspects of comprehensive care. This is true even for older adult survivors (aged 65 and older) who are eligible for federally run Medicare health care coverage and programs. As a result, there was an initial dearth of attention paid to the needs of post-treatment cancer survivors.15 The well-documented limitations of the U.S. healthcare system present challenges moving forward not only for understanding the multi-level problems experienced by those surviving cancer, but also for systematic implementation of clinical practice changes based on emerging research findings.
Another challenge to understanding and advancing health after cancer in the U.S. relates to limitations around cancer control plans. Individual state cancer plans, supported by the Centers for Disease Control and Prevention (CDC), have been in place since 1998.18 However, the inclusion of goals addressing cancer survivorship issues only occurred in the past decade and only about a third of state plans in 2009 included survivorship sections or chapters (personal communication, Irene Prabhu Das, NCI). Further, recommendations in cancer control plans related to survivorship are often unfunded or underfunded mandates addressed only to the extent annual state level budgets permit. Thus, while the U.S. has in the past decade seen a rapid increase in the attention to cancer survivors and survivorship research and practice, the ability to act on this knowledge is at times stymied by the lack of a uniform delivery system within which to test and implement changes designed to enhance the quality of life and length of survival of all of those diagnosed with cancer.
Evolution of Survivorship Science in Europe
Europe is a complex grouping of 50 countries (including Kazakhstan, in addition to the 49 listed here: http://europa.eu/about-eu/countries/index_en.htm) with >700 million inhabitants, marked cultural, economic and societal variations, and significant variation in the models and levels of health and social welfare provision. Not surprisingly, the field of cancer survivorship research has followed a somewhat different trajectory in Europe. In contrast to the U.S., in Europe the term “cancer survivor” is used less often by individuals with a cancer diagnosis.19, 20 In the European medical literature, this term is typically applied to cancer patients surviving tumor-free at least five years after their diagnosis, as described in the President’s Cancer Panel Report Living Beyond Cancer: a European Dialogue.19 This distinction is reflected in the focus on late and long-term effects in European survivorship studies.
National cancer registries have existed in the Nordic countries for 60–70 years (Denmark: since 1943; Norway: since 1951; Finland: since 1952; Iceland: since 1955; and Sweden: since 1958) and the Netherlands for over 20 years (Figure 1). In other countries (for example, Germany and the United Kingdom) regional cancer registries provide epidemiological data on cancer incidence and mortality. The establishment of both the International Agency for Research on Cancer (IARC) in 1965 and of the EUROpean CAncer REgistry (EUROCARE) in 1989 represented two important steps to generate pan-European data on cancer incidence, mortality and 5-year prevalence (i.e., those surviving at least 5 years after cancer diagnosis). However, the level of national coverage by such regional registries varies widely.21 In 2002, the prevalence of cancer survivors was estimated by a statistical model to be 2% of the total population in Europe22 which represents an increase from 1% in the figures published for 1990 by IARC.23
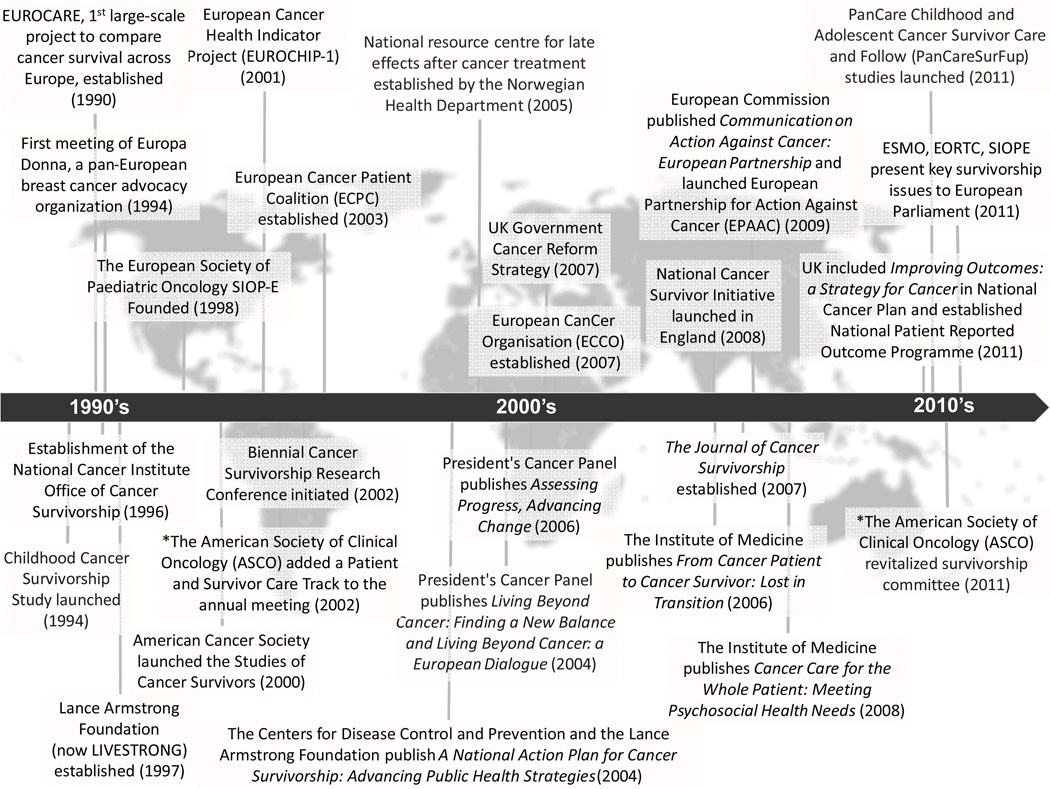
Although Europe lacks organizations specifically devoted to cancer survivorship comparable to those which have evolved in the U.S., certain pan-European organizations representing different segments of cancer care have promoted the field of survivorship science; of note, many of these were established during the last decade (Figure 1). Among these are the European Cancer Patient Coalition (ECPC) which represents the interests of all cancer patient groups, and the European Cancer League, an umbrella organization representing the majority of the national cancer organizations in Europe. A major policy achievement in Europe was the publication of “Communication on Action Against Cancer: European Partnership” in 2009 which highlighted several areas for improvement of cancer care in Europe including a need for stronger collaboration within the EU in cancer survivorship.24 Specifically, the report emphasized the need for identification and dissemination of evidence based practices to reduce the inequalities across the continent. Provision of comparable data on incidence, mortality and prevalence was mentioned explicitly. The European Commission also launched The European Partnership for Action Against Cancer (EPAAC,2009) with the aim, under a common platform, to unify cancer burden indicators (incidence, mortality, survival and prevalence) provided by existing European data collection activities. The Commission also urged all Member states to publish a cancer care plan by the end of 2013. National cancer plans have subsequently been published by 24 out of 27 EU-member states at the time of this publication (www.epaac.eu/national-cancer-plans). Most of these care plans deal with prevention, diagnosis and treatment of cancer. The topic of cancer survivorship appears in approximately half of these plans, under sections referring to survivorship, rehabilitation, supportive and palliative care (beyond end-of-life care), and aftercare.
In most European countries, treatment for cancer is free of charge for the individual patient, but the availability of novel drugs and application of new technologic advances differs. There are also considerable inter-country variations regarding the structure of follow-up care for cancer patients after they have discontinued their cancer treatment. Follow-up care generally falls under the responsibility of medical specialists or family physicians, who often have limited knowledge of long-term follow-up and late complications, which renders systematic medical surveillance of long-term effects difficult.
Looked upon broadly, the concept of cancer survivorship does not seem to have had either a broad or uniform impact on the philosophy or aims of various stakeholders in European contemporary oncology and policy. The one exception to this has been in England which formally launched a National Cancer Survivorship Initiative in September 2008. This latter is currently poised not only to transform medical care for those post-treatment for cancer, but also to test models for the most effective and cost-efficient way to provide this care.25 Across other parts of Europe, some relevant efforts for survivorship research and care, such as providing reliable prevalence data or providing information of after-care such as rehabilitation, are noticeable nevertheless. During the last five years, both ESMO (European Society for Medical Oncology) and ESTRO (European Society for Therapeutic Radiology and Oncology) have included within their annual conferences organized sessions devoted to cancer survivorship. During the ECCO (European CanCer Organization)-ESMO conference in September 2011, medical specialists and representatives from European cancer advocacies, outlined cancer survivors’ needs, including the need for attention to their continued participation in the work force. To the best of our knowledge, the first European conference solely addressing cancer survivorship (European Symposium on Late Complications after Childhood Cancer - ESLCCC) was held in 2007 and now occurs in alternate years. These efforts notwithstanding, the large and increasing number of European cancer survivors and their expected national health burden in the years to come are not sufficiently reflected in the present aims of European efforts to improve cancer care.
Cancer survivorship research in Europe has so far largely been restricted to specified malignancies (childhood cancer, breast cancer, testicular cancer, Hodgkin’s lymphoma) and conducted by a subset of medical specialists (mostly oncologists and pediatricians) and epidemiologists using existing databases and surveys.26, 27 Most of these efforts have depended on time-limited grants. With a few exceptions, research and activities within the field of cancer survivorship have been hampered by the limited involvement of politicians and health care administrators on the national and the European levels. In the last two years, ESMO, the EORTC (European Organization for Research and Treatment of Cancer) and SIOPE (European Society of Pediatric Oncology) presented key issues in cancer survivorship to the European Parliament with the aim to attract European politicians’ attention. So far, it seems that research and care in cancer survivorship has not attracted the attention of European health care researchers and decision makers. A principal challenge to survivorship research in Europe is the limited access to funding, both in terms of financial support and time restrictions. Some improvement has been observed during recent years in some countries, including the establishment of academic positions within the fields of cancer survivorship (e.g., Denmark, Norway, the Netherlands and the United Kingdom) and government financial support of voluntary organizations’ survivorship projects. A five year EU-grant, funded by the 7th Framework Program of the European Commission and awarded in 2010 to the PanCare Childhood and Adolescent Cancer Survivor Care and Follow-up Studies project, indicates an awakening understanding of the importance of cancer survivorship research. A consortium of 16 institutions, PanCareSurFup will carry out research studies into the late effect of treatment for cancer, identify a virtual cohort of childhood cancer survivors for future studies, establish guidelines for follow-up, disseminate the results, and provide training and workshops for stakeholders. The overall goal of this project is to provide health care providers with the information they need to improve the long-term health of every European childhood cancer survivor.
In an effort to illustrate differences within Europe that affect cancer survivorship interest, we have summarized the nature of the cancer registry, care delivery, and in-country governmental activities for three countries familiar to the authors: Norway, the United Kingdom and Italy (Online Appendix A). While all three of these nations have strong registry systems and national health programs, there is considerable variability in national attention to cancer survivorship. Whereas Norway has for a number of years drawn attention to the need for long-term follow up for some cancer survivors (the current National guidelines for breast, prostate, testicular cancer and Hodgkin`s lymphoma contain recommendations for long-term follow-up), England’s National Cancer Survivor Initiative is a relatively new but unique and comprehensive effort to advance survivorship research and care nation-wide. Both Norway (National Resource Center for Studies after Treatment of Cancer Center, established in 2005) and Italy (National Multisite Research Program on Cancer Survivors, launched in 2008)28 invested in research infrastructures to study cancer survivors.
Comparison of the evolution of survivorship research in the U.S. and Europe
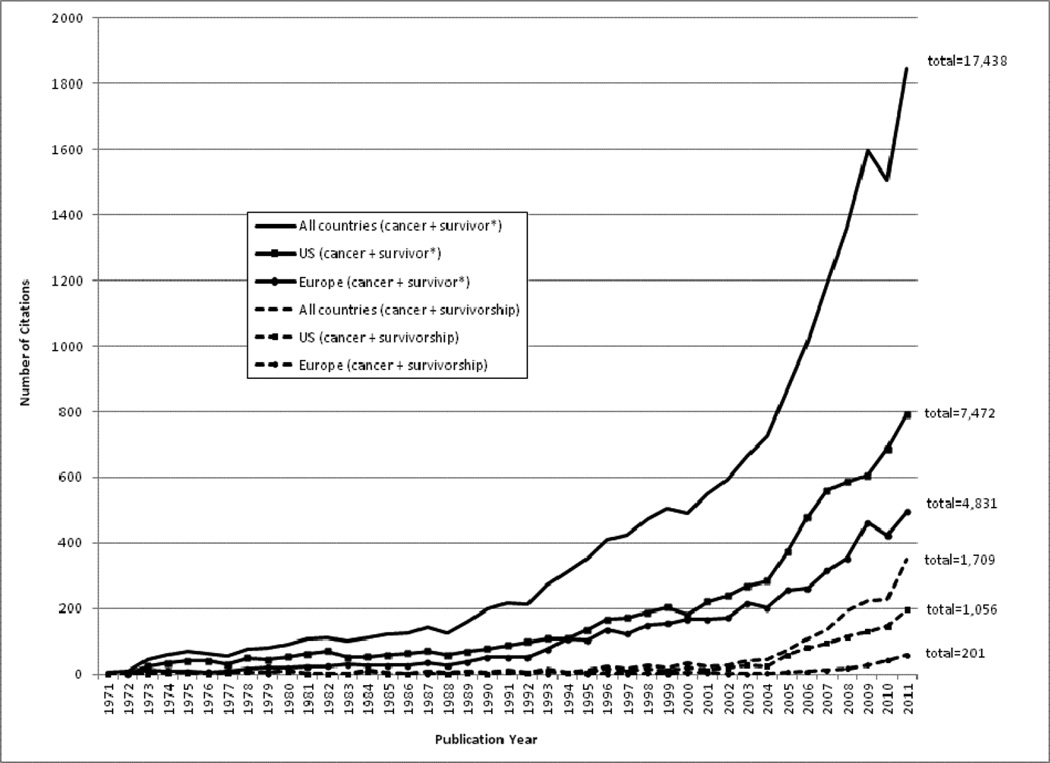
The number of publications dealing with cancer survivorship research has grown dramatically in both the U.S. and Europe (see Figure 3: Publication History). The emerging interest in long-term cancer survivors has paralleled their growing numbers on both sides of the Atlantic.1, 29 Survivorship science has become more sophisticated over time. Studies conducted in the 1970’s and early 1980’s focused on trends in overall survival and development of second malignancies. Subsequently, research into the broader aspects of cancer survivorship was greatly stimulated in both the U.S. and Europe by the dramatic progress in treating pediatric cancers and resulting concerns about the long-term consequences of cancer treatments (for example, Rowland et al.30). Later studies in the 2000’s focused on the incidence and prevalence of persistent and late onset adverse effects, including psychosocial problems,31, 32 and interventions to treat these.33 More recent studies published since 2010 examine the markers and mechanisms of risk for poor outcomes and the cost-effectiveness of current health care provision for reducing preventable morbidity and mortality among long-term survivors.34–36 Challenges encountered on both sides of the Atlantic in providing quality healthcare to a growing population of cancer survivors in the context of shrinking resources are a driving force behind current and emerging research. Further, the lack of attention to and funding for recommendations related to survivorship in cancer control plans in both the U.S. and Europe needs to be addressed. Failure to attend to the major recommendations made by entities in the U.S. and Europe will result in an inability to appropriately support and care for the growing population of survivors globally.
Figure 3. Citations related to cancer survivorship science.
Based on search in SciVerse Scopus database (http://www.scopus.com/home.url), the largest abstract and citation database which covers 17,500 peer-reviewed journals (http://www.info.sciverse.com/scopus). Citations include articles, review articles, conference papers, letters, notes, editorials, and short surveys from 1971 through 2011. The search for cancer + survivor* includes all citations with cancer and surivor, survivors, survivor’s, survivors’, or survivorship in the title or abstract, while the search for cancer + survivorship includes only citations that specifically use the word ‘survivorship.’ Europe was defined by the 27 countries in the European Union (E
Whereas the volume and pace of cancer survivorship research has accelerated rapidly in the past several years, this effect has been more pronounced in the U.S. than in Europe (Figure 3). Three key reasons may account for this difference. First, the 5-year survival rates for several European nations are still <50% (Figure 4).3 In these countries, focusing research on enhancing survival rather than on survivorship outcomes is a reasonable priority; recognizing, nevertheless, that quantity of life and quality of life are both valued survivorship outcomes. Although the overall 5-year relative cancer survival is higher in the U.S. than in several European countries, the U.S. demonstrates poorer overall health than most European nations according to most World Health Organization (WHO) indicators.37 Survivorship researchers and clinicians in Europe and the U.S. are keenly aware that increasing length of survival must be weighed thoughtfully against the human cost of such efforts.38, 39 Second, historically, most European nations have not provided sufficient funding resources for long-term survivorship research.40 In contrast, the U.S. has benefited from strong congressional support for government-led investment in cancer research, including survivorship science. The recent high profile of cancer survivorship in English national health policy and charity activities, with significant service improvement initiatives being centrally and locally commissioned, has not been mirrored by an equal investment in cancer survivorship research despite identification of the need for a systematic comprehensive research program.41 Finally, due to the greater stigma of cancer in some European countries relative to the U.S., there has been less public discourse around and hence more limited political attention paid to cancer survivors’ issues in some European nations.19
Figure 4.
Lessons from Survivorship Research
A number of key lessons have been learned with considerable consistency on both sides of the Atlantic. First, most cancer survivors do well after treatment; they manifest remarkable resilience.42 However, it is also clear that there are few cancer therapies without any adverse effects. A second important finding is that cancer has the potential to affect every aspect of an individual’s life: physical, psychological, social, economic, and existential or spiritual.43 Third, as survivors are followed for longer periods, the emergence of late effects (e.g., second cancers, cardiac failure) sometimes years after discontinuation of cancer treatment is often unexpected and has major impact on survivors’ lives.44 Fourth, cancer survivors need risk-adapted follow-up care that reflects individual challenges, related to the type and treatment of their cancer and their specific other medical and psychosocial needs.45, 46
Taken as a whole, the research conducted in Europe and the U.S. highlights a number of gap areas in our knowledge base. It is unclear who may be at risk for what types of chronic or late occurring effects of cancer and its treatment. While some survivors experience few problems, others with similar disease and treatment may have many. More basic research is required to understand the mechanisms behind and the etiology of the observed long-term effects. Further, limited interventions exist to address many of these (e.g., chronic fatigue, sexual dysfunction, memory problems). Teasing apart what health problems may be secondary to cancer, exacerbated by the diagnosis and treatment, the result of underlying genetic predisposition, a function of environment of lifestyle, and/or simply an effect of aging remains a challenge. As most survivorship research has included tumor-free and /or still young individuals, future studies have to deal with the problems of those living with some form of chronic treatment (e.g., hormonal treatment in breast or prostate cancer) and elderly long-term cancer survivors. Finally, greater appreciation is also needed regarding what medical care should be delivered, by whom, when and to which survivors. Future research should provide the evidence base for models of care for treating the growing population of cancer survivors given a shrinking oncology workforce,47 and including evidence for risk categorization. Further, specific guidance regarding surveillance for late and long-term effects and interventions to address future health status once cancer therapy ends, is needed.
Infrastructure for Survivorship Research
Platforms for Research
A vital barrier to studying survivors is access to this population as a whole and, importantly, detailed information on the treatments they may have received as part of their care. Some research documenting the long-term and late effects of cancer among survivors in both the U.S. and Europe is drawn from data from registries versus patient-contact studies, however, an increasing diversity of platforms (e.g., surveys, epidemiological cohorts, and data linkages) is rapidly emerging within which to conduct survivorship research. Cancer registries are an important primary source for research on survival and persistent and long-term effects after cancer and were the basis for the earliest studies on second malignancies (http://www.epaac.eu/cancer-data-and-information). However, these registries historically do not contain reliable data on follow-up experiences. In particular, patient-reported outcomes (PROs), detailed treatment history (e.g., specific chemotherapeutic agents and doses received) which can be important predictors of late effects, and comorbidities are not systematically collected in these registries. Registries can also be used as a sampling frame for recruitment to studies intended to contact survivors for further assessment, but registry-based recruitment presents challenges in terms of the delays for populating the registry with cancer cases, incomplete or inaccurate contact information for survivors, and non-response to recruitment and survey efforts.48 Despite this, progress is being made and some registries have shown that PROs can be successfully linked to population-based registries (www.profilesregistry.nl).49 Moreover, while many registries have the capacity to capture second malignancies,50 few are capable of tracking recurrent or progressive disease. In certain countries, such as in Nordic countries, some of these short-comings are overcome by linkages to other population-based registries, such as national birth registries or registries on education, income, sick-leave, disability pensions, hospitalizations and use of medications. The Nordic countries and Great Britain, with national health care systems and registries which serve almost 100% of cancer patients, have a unique advantage in conducting population-based survivorship studies as the health and resource utilization of their populations can be tracked. The use of a unique identification number for every citizen in Nordic countries enables researchers to approach cancer survivors even decades after a diagnosis to assess self-reported persisting or late-occurring effects of cancer and its treatment. Surveys among these individuals, especially when coupled with the collection of biological material and physical examination of survivors, can provide the opportunity to examine etiological mechanisms underlying the incidence of late effects among well documented groups of survivors.27, 34 There are two systems in the U.K. and the Netherlands in which patient-reported outcomes are integrated on a routine basis with cancer registry data: the ePOCS system51 and the PROFILES registry.52 The latter also disseminates cancer survivorship data free of charge for academic use (www.profilesregistry.nl). Across all registries, researchers must be aware of the variable quality of the data ascertained.
Access to these types of platforms is more limited in the U.S. where there are multiple healthcare delivery and payer systems and limited communication among these. The one exception is for survivors over the age of 65, the age at which U.S. citizens can enter the government Medicare system. In recent years, linkages between the Medicare and the SEER cancer registry systems make it possible to examine healthcare utilization of the large population of older cancer survivors53. However, complete records of cancer treatments are not available from SEER, and as noted earlier, SEER covers only 28% of the U.S. population. This is a significant limitation for investigators who wish to identify treatment exposures that may be associated with specific types and severity of cancer-related symptoms or conditions. In addition, because there is usually limited information on the health status and behaviors of survivors prior to diagnosis, ascertaining what may be cancer-related effects versus problems or conditions with another etiology is difficult to assess. In an effort to address this challenge and to better understand the relationship between patterns of care and survivorship outcomes, the NCI created the Health Maintenance Organization Cancer Research Network (CRN) (http://crn.cancer.gov/about/CRN_fact_sheet.pdf). A consortium of 14 healthcare delivery systems, covering almost 11 million U.S. individuals, the CRN has the potential to examine such questions as what the impact of different types of service use may have on survivors’ health outcomes, how cancer in one member may affect healthcare status and utilization by other family members, whether patient navigator programs can reduce illness-associated morbidity, questions that some of their European counterparts are already able to answer for their own populations.
Other complementary platforms for survivorship research used by U.S. investigators include national health surveys54 and data from large, prospective epidemiologic cohorts. Examples of these include the National Health Interview Survey (NHIS) an annual in-person, population-based survey of non-institutionalized household members,55 and the Nurses’ Health Study, a large, longitudinal cohort study of the health and well-being of these professionals over their life course.56, 57 While often lacking detailed cancer treatment information, these databases permit comparison of the health and functioning of survivors with that of their peers not affected by cancer.
An additional source of survivor populations used in both the U.S. and Europe include samples drawn from those entered on cancer clinical trials. In many cases, clinical trial cohorts have the unique advantage of permitting access to detailed treatment information, and data on therapies delivered under carefully controlled conditions. However, participation rates in clinical trials among adults in Europe (approximately 5% of the adult cancer population; Personal Communication, Jon Bean, EORTC) and the U.S. (approximately 2 – 3% of the adult cancer population,58 although these numbers are much higher for pediatric cancer patients, most of whom are entered on one or more clinical trials) are low. It is important to note that for many of those diagnosed with cancer, there may be no available trial or they may be ineligible for study entry. The fact that the denominator commonly used to estimate trial participation includes all diagnosed individuals may account for the disturbingly low figures. Furthermore, due to stringent exclusion criteria, only the healthiest patients are entered on these studies, a practice that severely limits generalizability of findings to survivors more broadly. Importantly, co-morbid health conditions, more common among older survivors, often preclude trial inclusion, thus eliminating opportunity to characterize those who may be most vulnerable to experiencing adverse survivorship outcomes. Finally, in addition to these challenges, one study details the barriers to recruiting cancer survivors retrospectively from clinical trials and reported a final participation rate of only 29%, due to difficulty locating patients, lack of institutional commitment, and lack of patient interest.59 Maintaining low drop-out or lost to follow-up rates is critical in efforts to reliably identify those at risk for adverse effects. A number of retention strategies may be needed to ensure long-term participation.
The development and support of cancer survivor specific cohorts for the purpose of advancing survivorship studies remain limited. Despite this, a number of these have been enormously generative including, but not limited to, the longitudinal follow-up of the childhood cancer cohort in the U.S. (Childhood Cancer Survivor Study, CCSS),60 the British Childhood Cancer Survivor Study (BCCSS),26 the American Cancer Society’s Studies of Cancer Survivors cohort study,11 the Health, Eating, Activity and Lifestyle (HEAL) study of breast cancer survivors,61 the repeated examination of breast cancer survivors as done by the Early Breast Cancer Clinical Trial Group organized from Oxford (U.K.) and the European-America studies on long-term effects after testicular cancer.27, 62
Assessment tools
A number of broadly used tools exist in Europe (e.g. EORTC-QLQ-C30) and the U.S. (e.g., FACT system) to evaluate the health-related quality of life of cancer survivors, in particular during active treatment.63 Fewer measures, however, are designed to capture survivors’ outcomes post-treatment, with exceptions such as the Impact of Cancer (IOC) scale.64 Two U.S. efforts over the past several years show promise of helping to fill this gap, and potentially prove useful for international collaboration: the Patient Reported Outcomes Measurement Information System (PROMIS)65 and the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE).66 Both of these NCI-sponsored data collection systems provide a data collection platform for measuring PROs with the purpose of investigating health outcomes. While PRO-CTCAE is currently being tested in the context of clinical trials, the measures are intended to be used for long-term follow-up to identify late-effects of therapy. The modular approach followed by both the EORTC Quality of Life group and the Functional Assessment of Cancer Therapy (FACT) system, in which patients complete a core health-related quality of life assessment tool in combination with disease-specific supplementary tools, may provide a useful basis for the development of survivorship-specific tools.67 Studies suggest that survivors report poorer physical and mental health than individuals without a history of cancer.68, 69 A key lesson learned, as this science has evolved, is that a single summary score of quality of life may fail to reflect the diversity of chronic and late effects experienced by subsets of this population.70 The capacity to describe and compare across diverse countries and cultures these different illness-related outcomes will be important to advancing our knowledge about and ability to effectively care for cancer survivors globally.
Dissemination Vehicles
Fortunately, as the field has grown, so too have outlets for dissemination of the findings of the emerging body of survivorship science. As noted earlier, a number of international groups now host survivorship content at their annual meetings. In 2002, the American Society of Clinical Oncology (ASCO) added a “Patient and Survivor” track to its annual proceedings. This track received increased visibility in 2005 under then President, Dr. David Johnson, a cancer survivor himself. In collaboration with the American Cancer Society, and subsequently, LIVESTRONG and the CDC, the NCI’s Office of Cancer Survivorship has hosted 6 Biennial Cancer Survivorship Research conferences.42 In 2007 the Journal of Cancer Survivorship was launched.71 A number of professional journals have issued special issues focused on cancer survivorship (e.g., Journal of Clinical Oncology, Journal of Pediatric Psychology, The Cancer Journal) or contain separate sections on cancer survivorship in each volume (e.g., Pediatric Blood and Cancer; Cancer Epidemiology, Biomarkers, and Prevention). Two textbooks addressing cancer survivorship have also appeared.72, 73 Supporting the continued presentation and application of pertinent findings resulting from survivorship studies remains a pressing need. History has taught us that knowing about the problems survivors face is insufficient; finding and disseminating evidence-based ways to address these must be an integral part of the science being conducted.14
Future Perspectives
Research for cancer survivors, while no longer in its infancy, is being challenged to keep pace with global changes in demography, the economy, and patterns of cancer- and non-cancer-related morbidity and mortality. The evolution of novel cancer therapies and the means to deliver these are also putting pressure on the scientific community to understand the acute and long-term effects of these innovations on survivors’ health and function. It is clear that to meet these demands for knowledge in a timely fashion, efforts to identify the unique strengths of specific countries to answer given questions, and to foster cross-continental collaboration whenever advantageous, will be at a premium. For example, international collaborative efforts would facilitate increased power to study less common cancers or cancer-related events, answering similar research questions in multiple populations (e.g., by cancer site, healthcare systems, etc.), greater generalizability of research findings, and more efficient use of otherwise disjointed research dollars allotted to similar causes.74 The European Collaborative Group on Cancer Survivorship (ECGCS), (http://www.ecgcs.eu/), founded in April 2012 in Bari, Italy, hopes to do just this by bringing together European survivorship researchers and international advisors from the USA, Canada and Australia in order to share knowledge more efficiently, reduce research fragmentation and overlap and take advantage of larger, multinational cohorts.
Moving forward, models for research will benefit from using experiences in other related areas of healthcare. While cancer may be episodic or cured for some, cancer has become or will for many be a chronic disease, making experiences from other fields within the health care system that deal with chronic disease increasingly relevant. In particular, the premium placed by these models on support of patient self-management of symptoms, on good patient-doctor communication and long-term involvement in medical surveillance may be particularly helpful in structuring long-term survivorship care. Further, systematic use of disability assessment may also be appropriate. For example, the International Classification of Functioning, Disability and Health (ICF) is WHO's framework for measuring health and disability at both individual and population levels.75 The ICF is officially endorsed by all 191 WHO Member States.
A number of collaborative opportunities exist to move this science forward in an efficient and effective manner. Specific areas for future development include:
-
Research
Promotion of international and collaborative research that examines mechanisms underlying development of late effects and their inter-individual variability. This research should include genomic studies, which require large samples and warrant establishment of research collaboration, and should inform the development of targeted preventive and treatment programs.
Performance of continuous surveillance to better understand the prevalence and trajectory of long-term and late effects, as well as yet to be discovered late-effects.
Development of evidence-based models for risk-adapted long-term follow-up for different risk groups of survivors that consider survivor outcomes as well as cost-effectiveness and healthcare systems factors.
Determining which countries have the best resources to answer specific research questions: For example: studies examining different models of care and associated outcomes/costs may be easier to conduct in Europe than the U.S. given varied healthcare systems across nations.
-
Infrastructure Development/Enhancement
Establishment or expansion of national cancer registries with valid exposure data (disease variables and cancer treatment). This must include finding solutions to the challenges associated with harmonizing data across countries/registries due to differences in care delivery, differences in populations covered by health care systems, and different structures of the registries.
Routine linkage and inclusion of patient-reported outcomes data into regional and national cancer registries49, 52
Development of brief, standardized cancer -specific measures to assess patient-reported outcomes of health-related quality of life dimensions, symptoms, health behaviors and co-morbid conditions in cancer survivors.
Coordination of efforts to stimulate the use of common data elements in clinical trials so that findings can be compared or combined.
Establishment of international cohorts which can be followed and assessed at regular intervals during the patient’s life-time with the aim to examine the interaction between cancer survivorship, co-morbidity and aging.
Application of new technologies to make convening key international players and development of new international collaborations more feasible.
-
Policy
Fostering creation of unique international collaborations to share best practices in relation to policy development.
Identification of effective communication strategies to make politicians and stake-holders aware of this rapidly growing area within health care, especially in Europe.
Leveraging the voice of survivors/advocates to advance attention to and funding for research among and care of cancer survivors.
Conclusions
In this paper, we review both the accomplishments and the lingering challenges in survivorship in the context of the growing number of cancer survivors worldwide. By providing details on the state of survivorship in both the United States and Europe, we highlight the need for and emergence of collaborative opportunities across borders. We further hope that this paper will galvanize future research efforts, particularly in the realm of implementing interventions to improve the health and well-being of cancer survivors moving forward. Finally, we were tasked for this paper with describing U.S./European activities around cancer survivorship research. A similar comparative exercise across additional regions, like Asia, Australia, Africa, Central and South America may identify best practices and models to reduce cancer survivors’ morbidity and mortality globally.
Supplementary Material
Footnotes
Disclaimers: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institutes of Health or the Patient-Centered Outcomes Research Institute.
References
- 1.Engholm G, Ferlay J, Christensen N, et al. NORDCAN - a Nordic tool for cancer information, planning, quality control and research. Acta Oncologica. 2010;49:725–736. doi: 10.3109/02841861003782017. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2012. Ca-a Cancer. Journal for Clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Verdecchia A, Francisci S, Brenner H, et al. Recent cancer survival in Europe: a 2000–02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8:784–796. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- 4.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiology Biomarkers Prevention. 2011;20:1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 6.Ries L, Smith MA, Gurney JG, et al. Bethesda, MD: National Cancer Institute, SEER Program; 1999. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. NIH Pub. No. 99-4649. [Google Scholar]
- 7.Mullan F. Seasons of survival- Reflections of a physician with cancer. New England Journal of Medicine. 1985;313:270–273. doi: 10.1056/NEJM198507253130421. [DOI] [PubMed] [Google Scholar]
- 8.Deimling GT, Bowman KF, Wagner LJ. Cancer survivorship and identity among long-term survivors. Cancer Investigation. 2007;25:758–765. doi: 10.1080/07357900600896323. [DOI] [PubMed] [Google Scholar]
- 9.American Cancer Society. Cancer Treatment and Survivorship Facts & Figures 2012–2013. Atlanta, Georgia: American Cancer Society; 2012. [Google Scholar]
- 10.Rowland JH, Mariotto AB, Alfano CM, Pollack LA, Weir HK, White A. Cancer Survivors — United States, 2007. Morbidity and Mortality Weekly Report. 2007;60:269–272. [PubMed] [Google Scholar]
- 11.Smith T, Stein KD, Mehta CC, et al. The rationale, design, and implementation of the American Cancer Society's studies of cancer survivors. Cancer. 2007;109:1–12. doi: 10.1002/cncr.22387. [DOI] [PubMed] [Google Scholar]
- 12.Hoey LM, Ieropoli SC, White VM, Jefford M. Systematic review of peer-support programs for people with cancer. Patient Education and Counseling. 2008;70:315–337. doi: 10.1016/j.pec.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 13.National Research Council. Childhood Cancer Survivorship: Improving Care and Quality of Life. The National Academies Press; 2003. [PubMed] [Google Scholar]
- 14.Adler N, Page A. Cancer Care for the Whole Patient: Meeting Psychosocial Healthcare Needs. Washington DC: Institute of Medicine (IOM); 2008. [Google Scholar]
- 15.Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington DC: National Academies Press; 2006. [Google Scholar]
- 16.Center for Disease Control adn Prevention (CDC), the Lance Armstrong Foundation. A National Action Plan for Cancer Survivorship: Advancing Public Health Strategies. 2004 [Google Scholar]
- 17.Rich IM, Andejeski Y, Alciati MH, et al. Perspective from the Department of Defense Breast Cancer Research Program. Breast Dis. 1998;10:33–45. doi: 10.3233/bd-1998-105-606. [DOI] [PubMed] [Google Scholar]
- 18.Fairley TL, Pollack LA, Moore AR, Smith JL. Addressing Cancer Survivorship Through Public Health: An Update from the Centers for Disease Control and Prevention. Journal of Womens Health. 2009;18:1525–1531. doi: 10.1089/jwh.2009.1666. [DOI] [PubMed] [Google Scholar]
- 19.National Insittutes of Health, National Cancer Institue; 2004. President's Cancer Panel, 2003–2004 Annual Report: Living beyond cancer: A European dialogue. NIH publication no. P996. [Google Scholar]
- 20.Khan NF, Harrison S, Rose PW, Ward A, Evans J. Interpretation and acceptance of the term cancer survivor': a United Kingdom-based qualitative study. European Journal of Cancer Care. 2012;21:177–186. doi: 10.1111/j.1365-2354.2011.01277.x. [DOI] [PubMed] [Google Scholar]
- 21.De Angelis R, Francisci S, Baili P, et al. The EUROCARE-4 database on cancer survival in Europe: data standardisation, quality control and methods of statistical analysis. Eur J Cancer. 2009;45:909–930. doi: 10.1016/j.ejca.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Capocaccia R, Colonna M, Corazziari I, et al. Measuring cancer prevalence in europe: the EUROPREVAL project. Annals of Oncology. 2002;13:831–839. doi: 10.1093/annonc/mdf152. [DOI] [PubMed] [Google Scholar]
- 23.Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. International Journal of Cancer. 2002;97:72–81. doi: 10.1002/ijc.1571. [DOI] [PubMed] [Google Scholar]
- 24.Commission of the European Communities. Communication from the Commission to the European Parliament, the Council, and the European Economic and Social Committee adn the Committee of the Regions on Action Against Cancer: European Partnership. Brussels, Belgium: 2009. [Google Scholar]
- 25.Jefford M, Rowland J, Grunfeld E, Richards M, Maher J, Glaser A. Implementing improved post-treatment care for cancer survivors in England, with reflections from Australia, Canada and the USA. British Journal of Cancer. 2012 doi: 10.1038/bjc.2012.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawkins MM, Lancashire ER, Winter DL, et al. The British Childhood Cancer Survivor Study: Objectives, methods, population structure, response rates and initial descriptive information. Pediatr Blood Cancer. 2008;50:1018–1025. doi: 10.1002/pbc.21335. [DOI] [PubMed] [Google Scholar]
- 27.Sprauten M, Darrah TH, Peterson DR, et al. Impact of long-term serum platinum concentrations on neuro- and ototoxicity in Cisplatin-treated survivors of testicular cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:300–307. doi: 10.1200/JCO.2011.37.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattioli V, Montanaro R, Romito F. The Italian response to cancer survivorship research and practice: developing an evidence base for reform. J Cancer Surviv. 2010;4:284–289. doi: 10.1007/s11764-010-0143-9. [DOI] [PubMed] [Google Scholar]
- 29.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiology, Biomarkers and Prevention. 2011;20:1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowland JH, Glidewell OJ, Sibley RF, et al. Effects of different forms of central nervous system prophylaxis on neuropsychologic function in childhood leukemia. Journal of Clinical Oncology. 1984;12 doi: 10.1200/JCO.1984.2.12.1327. [DOI] [PubMed] [Google Scholar]
- 31.Dahl AA, Haaland CF, Mykletun A, et al. Study of anxiety disorder and depression in long-term survivors of testicular cancer. Journal of Clinical Oncology. 2005;23:2389–2395. doi: 10.1200/JCO.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 32.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. New England Journal of Medicine. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 33.Pinto AC, de Azambuja E. Improving quality of life after breast cancer: dealing with symptoms. Maturitas. 2011;70:343–348. doi: 10.1016/j.maturitas.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Fossa SD, Cvancarova M, Chen L, et al. Adverse Prognostic Factors for Testicular Cancer-Specific Survival: A Population-Based Study of 27,948 Patients. Journal of Clinical Oncology. 2011;29:963–970. doi: 10.1200/JCO.2010.32.3204. [DOI] [PubMed] [Google Scholar]
- 35.Bhatia S. Role of genetic susceptibility in development of treatment-related adverse outcomes in cancer survivors. Cancer Epidemiol Biomarkers Prev. 2011;20:2048–2067. doi: 10.1158/1055-9965.EPI-11-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grunfeld E, Julian JA, Pond G, et al. Evaluating Survivorship Care Plans: Results of a Randomized, Clinical Trial of Patients With Breast Cancer. Journal of Clinical Oncology. 2011;29:4755–4762. doi: 10.1200/JCO.2011.36.8373. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization (WHO) World Health Statistics. 2011
- 38.Niraula S, Seruga B, Ocana A, et al. The price we pay for progress: a meta-analysis of harms of newly approved anticancer drugs. J Clin Oncol. 2012;30:3012–3019. doi: 10.1200/JCO.2011.40.3824. [DOI] [PubMed] [Google Scholar]
- 39.D'Angio GJ. Cure is not enough. J Pediatr Hematol Oncol. 2005;27:508–509. doi: 10.1097/01.mph.0000184573.93093.28. [DOI] [PubMed] [Google Scholar]
- 40.Eckhouse S, Castanas E, Chieco-Bianchi L, et al. European Cancer Research Funding Survey. London, United Kingdom: European Cancer Research Managers Forum; 2005. [Google Scholar]
- 41.National Cancer Survivorship Initiative Research Workstream. Priorities for research on cancer survivorship: MacMillan Cancer Support. 2010:43. [Google Scholar]
- 42.Rowland JH, Baker F. Introduction: resilience of cancer survivors across the lifespan. Cancer. 2005;104:2543–2548. doi: 10.1002/cncr.21487. [DOI] [PubMed] [Google Scholar]
- 43.Alfano CM, Rowland JH. Recovery issues in cancer survivorship: a new challenge for supportive care. Cancer J. 2006;12:432–443. doi: 10.1097/00130404-200609000-00012. [DOI] [PubMed] [Google Scholar]
- 44.van der Pal HJ, van Dalen EC, van Delden E, et al. High Risk of Symptomatic Cardiac Events in Childhood Cancer Survivors. J Clin Oncol. 2012 doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- 45.Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24:5117–5124. doi: 10.1200/JCO.2006.07.0474. [DOI] [PubMed] [Google Scholar]
- 46.Magee CE, Hillan JA, Badger SA, Kennedy RJ, Kirk SJ. Risk stratification as a means of reducing the burden of follow-up after completion of initial treatment for breast cancer. Surgeon. 2011;9:61–64. doi: 10.1016/j.surge.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists : challenges to assuring access to oncology services. J Oncol Pract. 2007;3:79–86. doi: 10.1200/JOP.0723601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pakilit AT, Kahn BA, Petersen L, Abraham LS, Greendale GA, Ganz PA. Making effective use of tumor registries for cancer survivorship research. Cancer. 2001;92:1305–1314. doi: 10.1002/1097-0142(20010901)92:5<1305::aid-cncr1452>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 49.Den Oudsten BL, Traa MJ, Thong MS, et al. Higher prevalence of sexual dysfunction in colon and rectal cancer survivors compared with the normative population: A population-based study. Eur J Cancer. 2012 doi: 10.1016/j.ejca.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Mariotto AB, Rowland JH, Ries LAG, Scoppa S, Feuer EJ. Multiple cancer prevalence: A growing challenge in long-term survivorship. Cancer Epidemiology Biomarkers & Prevention. 2007;16:566–571. doi: 10.1158/1055-9965.EPI-06-0782. [DOI] [PubMed] [Google Scholar]
- 51.Ashley L, Jones H, Thomas J, et al. Integrating cancer survivors' experiences into UK cancer registries: design and development of the ePOCS system (electronic Patient-reported Outcomes from Cancer Survivors) Br J Cancer. 2011;105(Suppl 1):S74–S81. doi: 10.1038/bjc.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van de Poll-Franse LV, Horevoorts N, van Eenbergen M, et al. The Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer. 2011;47:2188–2194. doi: 10.1016/j.ejca.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 53.Potosky AL, Warren JL, Riedel ER, Klabunde CN, Earle CC, Begg CB. Measuring complications of cancer treatment using the SEER-Medicare data. Medical Care. 2002;40:IV-62–IV-68. doi: 10.1097/00005650-200208001-00009. [DOI] [PubMed] [Google Scholar]
- 54.Lerro CC, Stein KD, Smith T, Virgo KS. A systematic review of large-scale surveys of cancer survivors conducted in North America, 2000–2011. J Cancer Surviv. 2012;6:115–145. doi: 10.1007/s11764-012-0214-1. [DOI] [PubMed] [Google Scholar]
- 55.Hewitt M, Breen N, Devesa S. Cancer prevalence and survivorship issues: Analyses of the 1992 National Health Interview Survey. Journal of the National Cancer Institute. 1999;91:1480–1486. doi: 10.1093/jnci/91.17.1480. [DOI] [PubMed] [Google Scholar]
- 56.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 57.Buettner C, Kroenke CH, Phillips RS, Davis RB, Eisenberg DM, Holmes MD. Correlates of use of different types of complementary and alternative medicine by breast cancer survivors in the nurses' health study. Breast Cancer Research and Treatment. 2006;100:219–227. doi: 10.1007/s10549-006-9239-3. [DOI] [PubMed] [Google Scholar]
- 58.Institute of Medicine (IOM) A national cancer clinical trials system for the 21st century: Reinvigorating the NCI Cooperative Group Program. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 59.Ganz PA, Land SR, Antonio C, et al. Cancer survivorship research: the challenge of recruiting adult long term cancer survivors from a cooperative clinical trials group. J Cancer Surviv. 2009;3:137–147. doi: 10.1007/s11764-009-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. Journal of Clinical Oncology. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Irwin ML, Crumley D, McTiernan A, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97:1746–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fossa SD, Gilbert E, Dores GM, et al. Noncancer causes of death in survivors of testicular cancer. J Natl Cancer Inst. 2007;99:533–544. doi: 10.1093/jnci/djk111. [DOI] [PubMed] [Google Scholar]
- 63.Luckett T, King MT, Butow PN, et al. Choosing between the EORTC QLQ-C30 and FACT-G for measuring health-related quality of life in cancer clinical research: issues, evidence and recommendations. Ann Oncol. 2011;22:2179–2190. doi: 10.1093/annonc/mdq721. [DOI] [PubMed] [Google Scholar]
- 64.Zebrack BJ, Ganz PA, Bernaards CA, Petersen L, Abraham L. Assessing the impact of cancer: development of a new instrument for long-term survivors. Psychooncology. 2006;15:407–421. doi: 10.1002/pon.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khanna D, Krishnan E, Dewitt EM, Khanna PP, Spiegel B, Hays RD. Patient-Reported Outcomes Measurement Information System (PROMIS(R)) -- The future of measuring patient reported outcomes in rheumatology. Arthritis Care Res (Hoboken) 2011;63:S486–S490. doi: 10.1002/acr.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Badalucco S, Reed KK. Supporting quality and patient safety in cancer clinical trials. Clinical Journal of Oncology Nursing. 2011;15:263–265. doi: 10.1188/11.CJON.263-265. [DOI] [PubMed] [Google Scholar]
- 67.Arora NK, Hamilton AS, Potosky AL, et al. Population-based survivorship research using cancer registries: a study of non-Hodgkin's lymphoma survivors. J. Cancer Surviv. 2007;1:49–63. doi: 10.1007/s11764-007-0004-3. [DOI] [PubMed] [Google Scholar]
- 68.Weaver KE, Forsythe LP, Reeve BB, et al. Mental and Physical Health-Related Quality of Life among U.S. Cancer Survivors: Population Estimates from the 2010 National Health Interview Survey. Cancer Epidemiology, Biomarkers and Prevention. 2012 doi: 10.1158/1055-9965.EPI-12-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith AW, Reeve BB, Bellizzi KM, et al. Cancer, comorbidities, and health-related quality of life of older adults. Health Care Financing Review. 2008;29:41–56. [PMC free article] [PubMed] [Google Scholar]
- 70.Reeve BB, Potosky AL, Smith AW, et al. Impact of cancer on health-related quality of life of older Americans. J Natl Cancer Inst. 2009;101:860–868. doi: 10.1093/jnci/djp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feuerstein M. Innovations in health care for cancer survivors: let's learn from the past. J Cancer Surviv. 2007;1:177–178. doi: 10.1007/s11764-007-0028-8. [DOI] [PubMed] [Google Scholar]
- 72.Ganz PA. Cancer Survivorship: Today and Tomorrow. New York: Springer; 2007. [Google Scholar]
- 73.Feuerstein M. Handbook of Cancer Survivorship. New York: Springer; 2007. [Google Scholar]
- 74.Elena JW, Travis LB, Simonds NI, et al. Leveraging Epidemiology and Clinical Studies of Cancer Outcomes: Recommendations and Opportunities for Translational Research. JNCI. doi: 10.1093/jnci/djs473. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.World Health Organization (WHO) Geneva, Switzerland: 2001. World Health Organization: International Classification of Functioning, Disability and Health (ICF) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.