Abstract
Mechanisms by which mesenchymal-derived tissue lineages participate in amplifying and perpetuating synovial inflammation in arthritis have been relatively underinvestigated and are therefore poorly understood. Elucidating these processes is likely to provide new insights into the pathogenesis of multiple diseases. Leukotriene B4 (LTB4) is a potent proinflammatory lipid mediator that initiates and amplifies synovial inflammation in the K/BxN model of arthritis. We sought to elucidate mechanisms by which mesenchymal-derived fibroblast-like synoviocytes (FLSs) perpetuate synovial inflammation. We focused on the abilities of FLSs to contribute to LTB4 synthesis and to respond to LTB4 within the joint. Using a series of bone marrow chimeras generated from 5-lipoxygenase–/– and leukotriene A4 (LTA4) hydrolase–/– mice, we demonstrate that FLSs generate sufficient levels of LTB4 production through transcellular metabolism in K/BxN serum-induced arthritis to drive inflammatory arthritis. FLSs—which comprise the predominant lineage populating the synovial lining—are competent to metabolize exogenous LTA4 into LTB4 ex vivo. Stimulation of FLSs with TNF increased their capacity to generate LTB4 3-fold without inducing the expression of LTA4 hydrolase protein. Moreover, LTB4 (acting via LTB4 receptor 1) was found to modulate the migratory and invasive activity of FLSs in vitro and also promote joint erosion by pannus tissue in vivo. Our results identify novel roles for FLSs and LTB4 in joints, placing LTB4 regulation of FLS biology at the center of a previously unrecognized amplification loop for synovial inflammation and tissue pathology.
Acute and chronic tissue inflammation employs multiple molecular and cellular pathways to initiate and perpetuate inflammation and injury. Although much information is available on how bone marrow (BM)-derived cells contribute to these processes when recruited to tissues, the role of mesenchymal-derived tissue lineages in generating immune responses and disease pathology remain poorly understood.
The leukotrienes (LTs) are biologically potent metabolites of arachidonic acid (AA) (1, 2). These lipid mediators are known to modulate innate and adaptive immunity by impacting vascular permeability, inducing adhesion molecule expression on vascular endothelium, and activating adhesion molecules (3, 4). Further, LTs increase leukocyte chemoattraction, neutrophil degranulation, smooth muscle contraction, and cytokine secretion (5, 6).
LT synthesis is initiated in leukocytes when AA is released by phospholipases from the cell membranes of activated leukocytes. Only BM-derived myeloid cells, including neutrophils, macrophages, mast cells, and basophils (7–12), possess the enzyme 5-lipoxygenase (5-LO) that sequentially converts AA to 5-hydroperoxyeicosatetraenoic acid and then in a second step to leukotriene A4 (LTA4). LTA4 is metabolized further to either leukotriene B4 (LTB4) or leukotriene C4 (LTC4) by the enzymes LTA4 hydrolase (LTA4H) or LTC4 synthase, respectively. To initiate this process, 5-LO must associate on the nuclear envelope with another myeloid-restricted protein, the 5-lipoxygenase activating protein (11).
Whereas LTA4 synthesis must occur in myeloid cells, its downstream metabolizing enzymes are distributed more widely, with LTA4H expressed in most tissues (10). This distribution of the downstream enzymes affords the opportunity for transcellular biosynthesis (13–17) wherein LTA4 generated by a myeloid cell is transferred intact to a neighboring cell, leading to the formation of either LTB4 (14, 16) or the cysteinyl LTs (13, 17, 18). Through the use of genetically deficient mice and radiation chimeric mice, the contributions of LTB4 or LTC4 generated in a transcellular manner have been shown in dermal inflammation and in the i.p. response to zymosan (19, 20). In each case, transcellular metabolism accounted for 20–25% of either LTB4 or LTC4, respectively, an amount sufficient to sustain the inflammatory response.
Transcellular metabolism of LTA4 to LTB4 has never been shown to contribute to the pathology of any mouse model of human autoimmune disease, in particular autoimmune arthritis. The K/BxN serum-transfer model of joint inflammation is dependent on polymorphonuclear leukocyte (PMN) 5-LO synthetic activity as well as neutrophil recruitment via LTB4. The synovitis in K/BxN arthritis is characterized by pathologic responses in synovial fibroblasts whose aberrant behavior results in synovial lining hyperplasia and erosive pannus formation (21, 22). Although recent studies demonstrate that mesenchymal-derived fibroblast-like synoviocytes (FLSs) actively contribute to the overall level of joint inflammation (21), the mechanisms by which FLSs contribute to the amplification of synovial inflammation, their own recruitment, and the recruitment of PMNs in this model are unknown. In addition, a role for LTB4 in regulating the biology of these cells has not been investigated.
In this article, we define a novel LTB4-mediated amplification loop in which FLS promote significant synovial inflammation in vivo via transcellular synthesis of LTB4. LTB4 stimulates FLS migration and invasion in vitro with comparable efficiency to platelet-derived growth factor (PDGF) and also drives erosive pannus tissue behavior in vivo.
Materials and Methods
Mice
Six- to 10-wk-old mice were used for these studies. Control wild-type (WT) C57BL/6J (B6) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The 5-LO–/– mice and LTA4H–/– mice (kindly provided by Dr. Beverly H. Koller) and LTB4 receptor (BLT)1–/– mice (all N10 back-crosses on the B6 background) (23–25) were maintained as inbred homozygotes. K/BxN mice were maintained as described (26). All of the procedures were approved by the Dana-Farber Cancer Institute Institutional Animal Care and Use Committee.
Generation of BM chimeras
BM transplantation into irradiated recipient mice was performed as described previously (27). Briefly, BM cells were flushed from donor tibia and femur bones with RPMI 1640 (Invitrogen, Grand Island, NY) containing 5% FCS, and marrow plugs were disrupted by trituration to generate a unicellular suspension. The entire BM preparation from one donor was resuspended in 0.5 ml 1× PBS. For transplantation, recipient mice were irradiated with a split dose (500 and 450 cGy) of gamma irradiation, and mice were subsequently supported with oral antibiotic (Baytril; Bayer, Leverkusen, North Rhine-Westphalia, Germany) in drinking water. Each recipient received BM cells harvested from one donor by i.v. tail vein injection. Arthritis experiments were performed after allowing 8 wk for transplant engraftment.
Serum-transfer protocol and arthritis scoring
To induce arthritis, arthritogenic K/BxN serum was transferred to recipient mice as described (26, 28). Briefly, 150 μl serum was administered via an i.p. route on experimental day 0 and day 2. Clinical indices were recorded at 24–48 h intervals. Ankle thickness was measured at the malleolus with the ankle in a fully flexed position using a spring-loaded dial caliper (26). Clinical index was graded as described (29). Briefly, each paw was scored for evidence of inflammation using the scale: 0, no evidence of inflammation; 1, subtle inflammation at one anatomic site (metatarsophalangeal joints, individual phalanx, or localized edema); 2, easily identified swelling involving two anatomic regions but not present diffusely in the paw; 3, swelling present on all of the aspects of the paw. The maximum clinical score is 12.
Measurement of LTB4 levels in inflamed joint tissue
To quantify joint tissue levels of LTB4, we employed a modification of previously reported methods for organic extraction and measurement of LTs in biological specimens (27, 30). Briefly, ankle tissues were harvested, weighed, and mechanically disrupted via homogenization (Brinkmann Instruments, Geneva, Switzerland). Tissue lipids were extracted into buffer containing 50 mM HEPES and 75% methanol. Cellular debris was removed by centrifugation, and lipid-containing clarified supernatants were separated via reversed phase (RP)-HPLC (C18 column; Beckman Coulter, Fullerton, CA) (31). One-minute fractions were collected, lyophilized to remove the organic buffer, resuspended, and analyzed via LTB4 ELISA assay (Cayman Chemical, Ann Arbor, MI) as described (27, 31). Pure LTB4 standards (Cayman Chemical) were used to define LTB4 retention time for RP-HPLC; LTB4 presence was quantified in fractions corresponding to the retention time of the LTB4 standard.
Histological examination
For histomorphometric analysis, ankle tissues were fixed for 24 h in 4% paraformaldehyde in PBS and decalcified with modified Kristensen's solution for 48–72 h (27, 32, 33). Tissues were then dehydrated, embedded in paraffin, sectioned at a thickness of 5 μm, and stained with H&E. Arthritis changes in joint tissues were graded based on a pathological scoring system as described previously (27). To confirm the presence of neutrophils, after Ag retrieval per the manufacturer's protocol, sections were stained with the NIMP R14 Ab (Abcam, Cambridge, MA) or with control rat IgG, followed by detection with a Vectastain ABC HRP kit and diaminobenzidine substrate. NIMP R14 is a neutrophil-specific Ab that recognizes the surface protein Ly6G/Gr-1. Tissue was subsequently counterstained with Gill's II hematoxylin and mounted with Crystal/Mount (Biomeda, Foster City, CA) (33).
Isolation and culture of synovial fibroblasts
Synovial fibroblasts were obtained from donor mouse ankle tissues as described (27). Briefly, after careful removal of skin tissue, the ankle and midfoot was disarticulated distal to the tibia and proximal to the metatarsal by ligament and tendon bisection. Dissected ankle tissues were infiltrated and incubated in type IV collagenase (Worthington Biochemical, Lakewood, NJ) suspended at a concentration of 1 mg/ml in DMEM (Invitrogen) at 37°C for 3 h. Cells in suspension were passed through a sterile 100 μM mesh and washed extensively in media. After overnight culture, nonadherent cells were washed away, and adherent cells were maintained in DMEM supplemented with 10% heat-inactivated FCS (Gemini Bio-Products, Woodland, CA), 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μM 2-ME. After the fourth passage, no CD45+ BM-derived lineage cells were identified by cytofluorometric staining (data not shown), and cells displayed a fibroblast morphology (21).
Ex vivo conversion of LTA4 to LTB4 by FLSs
After the growth medium was removed, FLSs were cultured in HBSS with 1% BSA (Sigma-Aldrich, St. Louis, MO). LTA4 hydrolyzed from LTA4 methyl ester (Cayman Chemical) per the manufacturer's protocol was added to the cell cultures (8 × 104 cells per milliliter) and incubated for 20 min at 37°C in a 5% CO2 incubator (34, 35). The cell supernatants then were collected for detecting the LTB4 production via LTB4 ELISA assay (Cayman Chemical) as described.
Western blotting
FLSs from WT, 5-LO–/–, or LTA4H–/– mice were harvested and lysed in lysis buffer containing 50 mM Tris-HCl (pH 7.2), 275 mM NaCl, 55 mM KCl, 1.0 mM CaCl2, 1% Triton X-100, and 0.5% Nonidet P-40 with protease inhibitor mixture (Sigma-Aldrich), then centrifuged at 12,000 × g for 30 min (36). Samples then were separated on 10% SDS-PAGE and transferred to a polyvinylidene difluoride transfer membrane (PerkinElmer, Boston, MA). These membranes were probed with goat polyclonal LTA4H Ab or rabbit polyclonal heat shock protein 90 Ab (Santa Cruz Biotechnology, Santa Cruz, CA). HRP-conjugated donkey anti-goat IgG or donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) were used as secondary Abs, respectively. The Ab binding was visualized using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer).
Isolation of BM neutrophils
Isolation of mature neutrophils from WT B6, 5-LO–/–, or LTA4H–/– mice BM was performed as described previously (27). Briefly, BM was flushed from donor tibia and femur bones with HBSS (Invitrogen), and marrow plugs were disrupted by trituration to generate a unicellular suspension. The entire BM preparation was resuspended at a concentration of 5 × 107 cells per milliliter in HBSS. Mature neutrophils were separated via centrifugation over a discontinuous Percoll gradient at 500 × g for 30 min at room temperature. The three-step discontinuous Percoll gradient consisted of 55, 65, and 75% (v/v) Percoll (Amersham Biosciences, Piscataway, NJ) in PBS. Mature neutrophils were recovered at the interface of the 65 and 75% fractions. Neutrophil purity (>97%) was determined morphometrically by Diff-Quick staining (IMEB, San Marcos, CA) and by cytofluorometric expression of Gr-1 (27).
Coculture of FLSs and PMNs
After FLSs (8 × 104 cells per milliliter) were cultured for 12 h in DMEM/1% FCS, the media was aspirated and replaced with HBSS with CaCl2 (2 mM) and MgCl2 (0.5 mM). The cells then were stimulated with A23187 (1 μM) either alone or in the presence of neutrophils (8 × 103 cells per milliliter) for 30 min at 37°C, 5% CO2. Cell supernatants were collected. The concentration of LTB4 in the cell supernatants was determined as described above.
Immunofluorescence staining
FLSs were grown on coverslips in DMEM/10% FCS for 24–48 h. After 12 h of incubation in DMEM/1% FCS, cells were stimulated by LTB4 at the indicated concentration at 37°C. PDGF was used as a positive control. Thirty minutes after stimulation, the cells were fixed in 2% paraformaldehyde in PBS for 15 min and permeabilized in 0.5% Triton X-100 (Electron Microscopy Sciences, Hatfield, PA) in PHEM buffer (60 mM PIPES, 25m M HEPES, 10 mM EGTA, and 2 mM MgCl2) for 5 min. Coverslips then were blocked for 1 h in blocking buffer (PHEM buffer supplemented with 5% FBS). After being washed twice with blocking buffer, cells were incubated with Alexa Fluor 555-conjugated anti-cortactin (2 μg/ml in blocking buffer; Millipore, Bedford, MA) for 50 min at room temperature. After being washed twice with blocking buffer, cells then were incubated with Alexa Fluor 488-conjugated phalloidin (1:2000 in blocking buffer; Molecular Probes, Eugene, OR) for 1 h at room temperature. Preparations then were washed three times with blocking buffer, mounted, and analyzed by confocal microscope (ECLIPSE TE2000-U; Nikon, Melville, NY).
Invasion assays
Invasion assays were performed using 12-well transwell filters coated with growth-factor–reduced Matrigel (BD Biosciences, San Jose, CA). WT or BLT1–/– FLSs were plated in the top chamber. Cell invasion was quantified by applying LTB4 or PDGF in the lower chamber and counting the cells on the lower side of the membrane. Invasion values are expressed as the average number of cells per microscopic field. Three microscopic fields per membrane in triplicate experiments were counted. For the pharmacological study, BLT1-specific antagonist CP-105,696 (37) was provided by Pfizer Pharmaceuticals (New York, NY). WT FLSs were pretreated with 5 μM CP-105,696. Thirty minutes later, LTB4-induced invasion assays were performed in the presence of 5 μM CP-105,696 as described above.
Statistical analysis
Results are presented as the mean ± SEM. The statistical significance for comparisons between groups was determined using the Student unpaired two-tailed t test or two-way ANOVA, followed by Bonferroni correction using the Prism software package (version 4.00; GraphPad Software, San Diego, CA). The p values <0.05 were considered significant.
Results
Both transcellular and conventional generation of LTB4 are sufficient to drive K/BxN serum-transfer arthritis
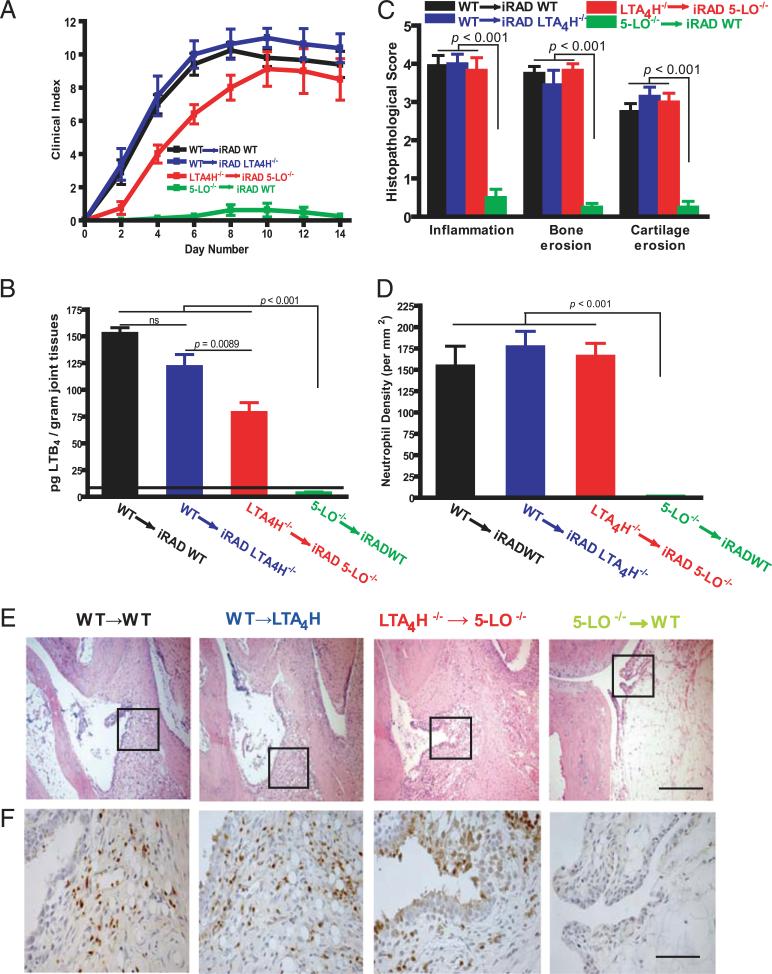
We have shown previously that 5-LO expressed by BM-derived cells controls the synthesis of neutrophil-dependent LTB4 that is central to the pathogenesis of K/BxN serum-transfer arthritis (27). To understand how LTB4 regulates synovial tissue function in this model, we tested the concept that LTB4 generated via transcellular metabolism of neutrophil-derived LTA4 contributes to the pathology of joint inflammation. We initially generated radiation chimeras in which irradiated (iRAD) 5-LO–/– mice were reconstituted with LTA4H–/– BM (LTA4H–/– → iRAD 5-LO–/–). In these mice, transcellular synthesis comprises the only route of LTB4 generation. More specifically, because LTA4 is generated by the enzymatic action of 5-LO on AA in BM-derived cells (Ref. 27 and shown as a control in Fig. 1A, green line), the tissues of the chimeric animals (radioresistant cells) were incapable of generating substrate LTA4 because they lacked 5-LO (Supplemental Fig. 1). However, because they express LTA4H, they retain the capacity to synthesize LTB4 from LTA4 supplied by infiltrating BM-derived neutrophils. Similarly, because they lack LTA4H, BM-derived leukocytes are incapable of generating LTB4. As seen in Fig. 1A, the severity of arthritis observed in the chimeric mice did not differ from that observed in WT controls (red line). We next generated chimeric mice by transplanting irradiated LTA4H–/– mice with WT BM (WT → iRAD LTA4H–/–). Because synovial tissue lacks LTA4H, these mice are incapable of generating LTB4 from transcellular LTA4. These mice demonstrated the same severity of arthritis as the LTA4H–/– → iRAD 5-LO–/–chimeras (Fig. 1A, blue line), indicating that both conventional and transcellular routes of LTB4 generation are sufficient to drive an equivalent degree of clinical and histological synovial inflammation.
FIGURE 1.
Transcellular biosynthesis of LTB4 in K/BxN serum-induced arthritis. A, Clinical index of arthritis severity was monitored daily after arthritogenic K/BxN serum administration to irradiated mice reconstituted with BM from various genotypes. Symbols refer to the donors and to the recipients, respectively (donor genotype → iRAD recipient genotype). Values are the mean ± SEM of data pooled from three independent experiments. (n = 15 mice per group). Differences were significant at p < 0.001 for WT → iRAD WT, WT → iRAD LTA4H–/–, and LTA4H–/– → iRAD 5-LO–/– versus 5-LO–/– → iRAD WT ; p = NS for WT → iRAD WT and WT → iRAD LTA4H–/– versus LTA4H–/– → iRAD 5-LO–/–. B, Quantification of LTB4 production in arthritic joint tissues. Ankle tissues from recipient mice reconstituted with BM from specified genotypes were homogenized, and lipids were extracted at 14 d after K/BxN serum administration. Lipid extracts then were fractionated via RP-HPLC, and fractions corresponding to the retention time for LTB4 were analyzed by ELISA as described in Materials and Methods. Values depicted are picograms per gram of joint tissue (mean ± SEM) of data pooled from three independent experiments. C, Histomorphometric arthritis quantification. Shown are quantitative measurements (mean ± SEM) of inflammation, bone erosion, and cartilage erosion in ankles from each irradiated recipient mice at 14 d after serum administration. Values are the mean ± SEM of data pooled from three independent experiments (n = 15 mice per group). D, Neutrophil enumeration in arthritic synovial tissue. Tissues from experimental mice in Awere stained with anti-Ly6G mAb NIMP-R14, and neutrophil density was quantified histomorphometrically. The data are the mean ± SEM of three independent experiments. The p values in B, C, and D, compared with those detected in 5-LO–/– → iRAD WT, are indicated on the top of each bar. E, Representative histological sections of ankle tissues from mice examined in A. Scale bar, 100 μm. F, Immunohistochemical staining for NIMP R14+ neutrophils quantified in D. Scale bar, 25 μm.
Transcellular LTB4 is a major contributor to synovial pannus-mediated cartilage erosion
To determine whether comparable levels of tissue LTB4 were produced by transcellular metabolism in these BM chimeras, we assayed the concentration of LTB4 in their joint tissues (Fig. 1B). In LTA4H–/– → iRAD 5-LO–/– chimeric mice, where transcellular metabolism comprised the only route of LTB4 generation, we observe 55.3% of WT LTB4 (Fig. 1B, red bar). Histological examination revealed equivalent increases in tissue inflammation, tissue neutrophil infiltration, synovial hyperplasia, and erosion activity in WT → iRAD WT, WT → iRAD LTA4H–/–, and LTA4H–/– → iRAD 5-LO–/– groups (Fig. 1C, 1E). These results indicate that the amounts of synovial LTB4 generated in WT mice are well in excess of that needed to propel the observed clinical and histological damage. Consistent with this observation, the numbers of Gr-1+ neutrophils that infiltrate arthritic joint tissues from LTA4H–/– → iRAD 5-LO–/– mice (Fig. 1D, 1F) were equivalent to WT → iRAD WT mice and to WT → iRAD LTA4H–/– mice, indicating that transcellular LTB4 biosynthesis is sufficient to drive maximal neutrophil recruitment to the inflamed synovium.
FLSs convert exogenous LTA4 to LTB4
Having identified that transcellular biosynthesis of LTB4 by radioresistant cells in joint tissue is sufficient to promote inflammatory arthritis in vivo, because FLSs are the major cell lineage in synovial tissue, we focused on their ability to accomplish transcellular LTB4 biosynthesis. Initially, we confirmed that FLSs express LTA4H but not 5-LO (Fig. 2A, Supplemental Fig. 1). We then showed that FLSs can generate substantial amounts of LTB4 from exogenous substrate LTA4 in a concentration-dependent manner (Fig. 2B). The role of FLS LTA4H was verified by employing FLSs from LTA4H–/– mice that were shown to be incapable of generating LTB4 (Fig. 2C).
FIGURE 2.
Metabolism of exogenous LTA4 in FLSs. A, The expression of LTA4H in cellular lysates from WT, 5-LO–/–, and LTA4H–/– FLSs was analyzed by Western blot. The expression of heat shock protein 90 was used as a control. Results shown are representative of three separate experiments. B, LTB4 formation by FLSs is dependent on the concentration of exogeneous LTA4. WT FLSs (8 × 104 cells per milliliter) were cultured with LTA4 at a concentration of 0.1 or 1 μM for 20 min at 37°C. The formation of LTB4 from exogenous LTA4 was determined by ELISA as described in Materials and Methods. The results represent the mean ± SEM of three experiments. C, FLSs from either WTor LTA4H–/– mice were incubated with 1 μM LTA4 for 20 min at 37°C. LTB4 production in the culture supernatants was measured by ELISA. Results represent the mean ± SEM of three experiments. The p values in B and C are indicated on the top of each bar. The assay limit of detection is denoted by the horizontal dotted line.
FLSs convert neutrophil-derived LTA4 to LTB4
Our previous studies revealed that neutrophils can supply 5-LO activity sufficient to promote arthritis in the K/BxN serum-transfer model (27). Further, neutrophils comprise a major population infiltrating the arthritic joint in both K/BxN arthritis and in human rheumatoid arthritis. In addition, it is well established that neutrophils can generate and release LTA4 in substantial excess to their capacity to form LTB4 (14). Therefore, to gain insight into the physiology of synovial conversion of LTA4 to LTB4, we examined whether interacting neutrophils and FLSs are competent to generate significant amounts of LTB4. As anticipated, mono-cultured neutrophils stimulated with a calcium ionophore generated substantial LTB4, whereas FLSs, which lack 5-LO expression, generate no LTB4 (Fig. 3A). However, in neutrophil/FLS coculture, LTB4 biosynthesis substantially exceeded that observed with neutrophils alone (34 ± 2.6 pg/ml versus 18 ± 2.0 pg/ml, p < 0.05), which is consistent with FLS conversion of neutrophil-derived LTA4 to LTB4. That neutrophils are an obligate source of LTA4 was confirmed in coculture experiments using 5-LO–/– neutrophils where no LTB4 was generated (Fig. 3B). To demonstrate that LTB4 production resulted from transcellular movement of LTA4 from neutrophils to FLSs, we coincubated FLSs with LTA4H–/– neutrophils, which then were stimulated with ionophore. FLSs, which provide the only source of LTA4H in this system, synthesized LTB4 with the same efficiency as WT neutrophils (Fig. 3C).
FIGURE 3.
Transcellular biosynthesis of LTB4 in FLS/neutrophil co-cultures. A, LTB4 formation after stimulation of WT FLSs (8 × 104 cells per milliliter) and WT neutrophils (8 × 103 cells per milliliter) either alone or in coculture after stimulation with 1 μM A23187 for 30 min. B, LTB4 formation after coculture of 5-LO–/– neutrophils and WT FLSs in the presence of 1 μM A23187 for 30 min. C, LTB4 formation after coculture of LTA4H–/– neutrophils and WT FLSs in the presence of 1 μM A23187 for 30 min. LTB4 was analyzed as described in Materials and Methods. Values represent the mean ± SEM of data pooled from three independent experiments. The p values are indicated on the top of the each bar. The assay limit of detection is denoted by the horizontal dotted line.
FLS production of LTB4 is stimulated by TNF
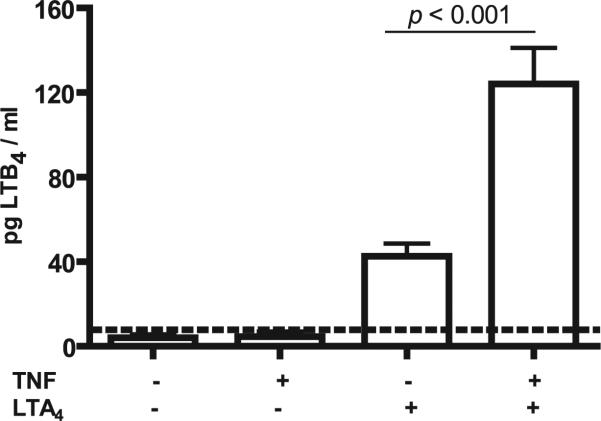
Aside from its self-limiting property via suicide inactivation (15, 38), little is known about the molecular regulation of LTA4H epoxide hydrolase activity. Most current models assume that LTA4H activity is regulated simply by enzyme quantity and the amount of substrate LTA4 available (12, 39), However, most studies of LTB4 production have been performed in myeloid lineage cells. Studies in fibroblasts have used transformed cells and found that increased LTB4 production correlated with elevated LTA4H mRNA and protein levels (40). We investigated whether primary FLS production of LTB4 from exogenous LTA4 could be modulated by inflammatory stimuli. Because TNF plays a significant role in disease pathophysiology in rheumatoid arthritis and in the K/BxN model of arthritis (41, 42), we exposed FLSs to TNF (10 ng/ml) for 2 h prior to supplying exogenous LTA4 and observed a significant (~3-fold) increase in concomitant LTB4 in the supernatant of these stimulated FLSs (Fig. 4).
FIGURE 4.
Metabolism of exogenous LTA4 in FLSs pretreated with TNF. WT FLSs (8 × 104 cells per milliliter) were pretreated with or without TNF (10 ng/ml) for 2 h followed by incubation with exogenous LTA4 (1 μM). LTB4 elaborated into the culture supernatants was quantified by ELISA as described in Materials and Methods. Values are the mean ± SEM of pooled data from three independent experiments. The p values are indicated on the top of each bar. The assay limit of detection is denoted by the horizontal dotted line.
To gain further insight into the molecular basis for the observed increase in LTB4 synthetic capacity in FLSs, we assessed induction of LTA4H protein expression over time in response to TNF stimulation (Supplemental Fig. 2). Interestingly, FLSs treated with TNF for up to 2 h did not show a change in immunoreactive LTA4H protein levels. Because previous studies document that increased mRNA levels correlate with increased LTA4H expression, we assessed FLS LTA4H protein levels in FLSs exposed to transcription inhibitor actinomycin D (5 μg/ml) or RNA polymerase II inhibitor 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (50 μM). As shown in Supplemental Fig. 2, no change in expression of LTA4H was apparent in FLSs whose transcriptional capacity was inhibited, confirming that the stimulation-induced LTB4 synthetic capacity in FLSs proceeds in a manner independent of modulation of LTA4H enzyme levels.
BLT1 expression on radioresistant cells contributes to arthritis severity and pannus tissue invasion into cartilage
Having observed tissue capacity to generate LTB4 in a transcellular fashion, we next investigated a role for LTB4 in modulating tissue behavior in inflammatory arthritis. To establish an impact from LTB4 on tissue behavior in vivo, we transferred WT BM into irradiated mice lacking BLT1 to generate radiation chimeric mice wherein BLT1 remains absent in radioresistant (tissue) cells. In these mice, we find a partial amelioration of clinical signs of arthritis (Fig. 5A). Of particular interest, examination of joint tissues from mice lacking tissue BLT1 expression reveals a striking decrease in synovial pannus erosion into cartilage, whereas other measurements of tissue inflammation and bone erosion were only partially impacted (Fig. 5B). Because pannus erosion into cartilage is thought to be predominantly driven by behavior of FLSs, these in vivo results correlate nicely with our in vitro demonstration that LTB4 directly modifies behavior of this mesenchymal lineage.
FIGURE 5.
BLT1 expression on radioresistant cells contributes to arthritis severity. A, Clinical arthritis severity in radiation chimeric mice: WT → iRAD WT, WT → iRAD BLT1–/–, and BLT1–/– → iRAD BLT1–/–. Shown are the mean ± SEM from one of four independent experiments (n = 5 mice per group per experiment). The p value was determined by two-way ANOVA. B, Histomorphometric quantification of inflammation, bone erosion, or cartilage erosion on day 14 after K/BxN serum transfer in recipient mice. Values are the mean ± SEM of pooled data from three experiments. The p value was determined by the Student unpaired two-tailed t test.
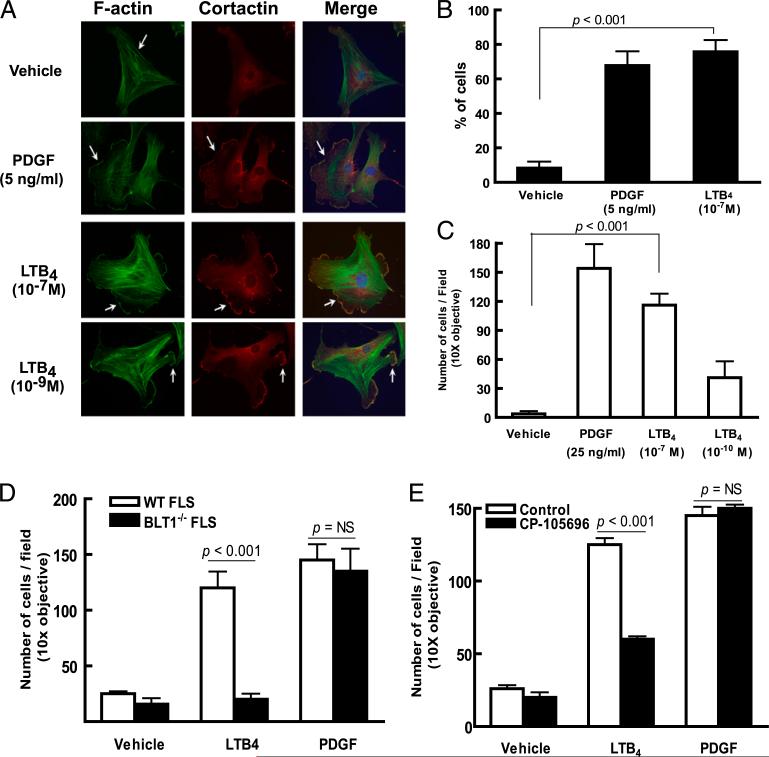
LTB4 promotes synovial fibroblast migratory and invasive behavior
Because the synovial pannus is comprised predominantly of synovial fibroblasts (12, 22) and the tissue extension observed in pannus erosion into articular cartilage is thought to implicitly require the ability of FLSs to migrate and invade through extracellular matrix to accomplish tissue remodeling in synovial pannus, we proceeded with direct examination of the capacity for LTB4 to impact these aspects of synovial fibroblast behavior. After confirming their expression of BLT1 mRNA (Supplemental Fig. 3), we proceeded with functional studies assessing LTB4-stimulated activities in primary FLSs. In contrast to measurements of cytokine (IL-6) production or proliferation, where we observe no impact from administration of LTB4 (Supplemental Figs. 4, 5), we find that LTB4 promotes lamellipodium formation to a degree equivalent to that seen with PDGF, a potent activator of FLSs (21) (Fig. 6A, 6B). We then assessed capacity for LTB4 to promote FLS invasive activity in extracellular matrix-coated transwells and observed a dose-dependent stimulation of FLS invasion again to a degree comparable to that driven by PDGF (Fig. 6C). To confirm the direct activity of LTB4 on primary FLSs, we examined the invasiveness of BLT1–/– and WT FLSs and observe lack of LTB4-driven FLS invasive activity in BLT1–/– FLSs (Fig. 6D). These observations were confirmed via pharmacologic inhibition of BLT1 using the BLT1-specific antagonist CP-105,696 (37) (Fig. 6E).
FIGURE 6.
LTB4 promotes synovial fibroblast migratory and invasive behavior. A, Lamellipodium formation in WT FLSs stimulated by LTB4. WT FLSs were plated on coverslips and stimulated and stained as labeled. Nuclei are visualized with a Hoechst dye (blue). Arrows indicate lamellopodia in FLSs stimulated by LTB4. Data are representative of three independent experiments. B, Percentage of FLSs demonstrating lamellipodium formation. The results represent the mean ± SEM of data pooled from three experiments. The p value was determined by the Student unpaired two-tailed t test. C, LTB4-induced invasion of FLSs. Primary FLSs placed in Matrigel-coated transwell dishes were stimulated as labeled and cells migrating to the lower membrane surface were enumerated. Results shown are the mean ± SEM of data pooled from four independent experiments. D, LTB4-induced invasion in FLSs requires BLT1 expression. WT or BLT1–/– FLSs were placed in Matrigel-coated transwells and stimulated and enumerated as in C. E, BLT1 antagonist CP-105,696 inhibits LTB4-driven FLS invasion. WT FLSs were pretreated with CP-105,696 for 30 min prior to measuring LTB4- or PDGF-driven invasion as in C. Data shown in D and E are the mean ± SEM of data pooled from three experiments. The p value was determined by the Student unpaired two-tailed t test.
Discussion
In contrast to our detailed understanding of proinflammatory leukocyte effector pathways, there remains a relative paucity of mechanistic insight into how tissues participate in the generation of pathologic inflammatory reactions. Most studies of inflammation have characterized secondary mesenchymal responses to paracrine signals emanating from infiltrating immune cells rather than focusing on the role of mesenchymal tissues per se. Examples of these diverse responses include mesenchymal cell proliferation, rearrangement of extracellular matrix (e.g., fibrosis), and vascular hyperplasia. However, there is growing evidence that tissue elements are not simply passive responders in immune or autoimmune reactions. In the context of synovitis, prominent among the growing list of FLS effector pathways elicited in response to immune-generated signals are elaboration of cytokines (e.g., IL-6) (43–45), chemokines (e.g., IL-8 and MCP-1) (36, 46, 47), and eicosanoids (e.g., PGE2 and PGI2) (28, 48, 49). From an amplification standpoint, because their generation can be accomplished via transcellular metabolism of precursor substrates, the eicosanoids provide an ideal context to examine tissue modulation of inflammatory responses. Our observations demonstrate that radioresistant joint tissue and its primary constituent, the mesenchymal synovial fibroblast (FLS), can participate in the pathophysiology of inflammatory arthritis in a primary manner by potently and dynamically metabolizing substrate LTA4 supplied via a transcellular route to generate LTB4. Further, the predominant activity studied for proinflammatory mediators is their impact on BM-derived cells. In this study, we show that the behavior of mesenchymal tissue lineages are impacted directly by LTB4, a mediator traditionally viewed as a leukocyte chemoattractant. LTB4 promoted tissue migration and invasion—activities central to joint destruction in inflammatory arthritis. Thus, in the context of the complex physiology of autoimmune arthritis, these findings provide an example of a primary inflammation-amplifying capacity for tissue via generation of and response to LTB4, a mediator typically ascribed to leukocytes.
The capacity for FLSs and other cells of tissue lineages to interface with leukocytes in a common effector function provides a basis for local fine-tuning of immune responses. For generation of LTB4, there exist several control points for modulating production. Previous studies, confirmed herein, document that LTA4H is rate-limiting in leukocyte LTB4 production (7–10, 12, 35), because excess LTA4 can diffuse from cells for transcellular metabolism. LTA4H undergoes “suicide inactivation” as a consequence of catalyzing the formation of LTB4 (12, 15), further limiting leukocyte capacity for sustained LTB4 generation. Because stimulated neutrophils secrete abundant LTA4 into their local milieu, the capacity for tissue to act as a synthetic reservoir thereby dramatically extends this leukocyte activity—to the point of profound tissue inflammation in the absence of leukocyte LTA4H in our experiments. Interestingly, FLS LTA4H activity can be upregulated by TNF and likely other inflammatory cytokines. This property affords an opportunity for regulated amplification of the highly constrained leukocyte production of LTB4 via dynamic local tissue mechanisms.
The precise mechanism whereby modulation of FLS generation of LTB4 by external stimuli is regulated remains to be elucidated; it is noteworthy that changes in the levels of LTA4H and therefore de novo synthesis of LTA4H are not involved. This contrasts with other mechanisms governing other examples of stimulation-dependent transcellular eicosanoid generation, such as IL-1β-induced overexpression of cyclooxygenase 2 that modulates transcellular biosynthesis of thromboxane A2 between HUVECs and platelets (50). These observations point to as-yet-unappreciated mechanisms regulating LTB4 production in FLSs. Included among the potential mechanisms contributing to this phenotype are regulation of LTA4 substrate accessibility, modulation of LTA4H enzymatic activity, or alteration of LTB4 release from FLSs.
Of equal importance to the observation that radioresistant tissues amplify LTB4 generation is the finding that the behavior of inflamed parenchymal synovial tissue can be modified by LTB4. In the context of inflammatory arthritis, the synovium undergoes profound changes driven by mesenchymal lineage FLSs via mechanisms that remain poorly understood (51–54). The development of synovial pannus, which is primarily responsible for cartilage tissue invasion and which participates in tissue invasion into bone, is a predominant FLS-dependent pathophysiologic event in disease. Our results underscore the regulated nature of the behavior of joint tissues in response to inflammation and provide new molecular insight by identifying LTB4 as a mediator that promotes the behavior of FLSs in these processes. In the interpretation of our results, it is noteworthy that our studies focus on activities elicited by LTB4 stimulation of BLT1. There exists a second known receptor for LTB4 named BLT2. Xu et al. (55) recently reported a predominance of BLT2 over BLT1 mRNA expression in synovium from patients with rheumatoid arthritis. This observation raises the possibility of further LTB4 function elicited in FLSs distinct from the functions that we identify attributable to BLT1 but does not impact on our conclusions. Although further examination of pathways stimulated in FLSs by LTB4 exposure remains warranted, our in vivo data demonstrate that tissue-specific responses to LTB4 influence both the degree of inflammation and the organized pathologic response of the synovial pannus.
Our studies provide a novel approach for understanding how tissues participate locally in autoimmune inflammation pathophysiology via transcellular generation of LTB4. It is distinct from and expands upon the conclusions of previous studies describing the production of eicosanoids by transcellular metabolism in vitro and in vivo. Fitzpatrick et al. (56, 57) reported that LTA4 could be transferred into RBCs and converted to LTB4 by LTA4H present in the cytosol of RBCs. Thereafter, transcellular generation of LTB4 was demonstrated in a number of cell combinations in vitro (13–18, 58–61). In vivo transcellular biosynthesis of LTB4 or LTC4 in response to zymosan injection or to exogenous administration of substrate LTA4 also has been shown (19, 20).
Human inflammatory arthritides, such as rheumatoid arthritis, psoriatic arthritis, gouty arthritis, and others, are characterized by dramatic elevations of neutrophils in synovial fluid and leukocytic tissue infiltration with neutrophil turnover rates estimated at a billion cells per day in a single joint (62–64). Further, LTB4 is elevated markedly in the synovial fluid of patients with rheumatoid arthritis (63). Previous studies by our group have documented a role for neutrophil-derived 5-LO activity as a synthetic source for LTB4 that promotes synovitis in the K/BxN model (27). Our studies now reveal that LTB4 production is substantially more dynamic than previously realized. Consideration of therapeutic inhibition of LTA4H will need to account for activity beyond that present in circulating leukocytes. Our findings also suggest that the therapeutically effective blockade of other mediators, such as TNF, may function in part by impacting joint tissue generation of LTB4.
Supplementary Material
Acknowledgments
We thank Dr. Beverly H. Koller for providing 5-LO–/– and LTA4H–/– mice. We are grateful to Dr. Paul Anderson for insightful discussion. We also acknowledge Teresa Bowman and Nicholas Calderone for expert histotechnical assistance.
This work was supported by grants from the National Arthritis Research Foundation (to M.C.), National Institutes of Health (P01-AI-065858-01 to D.M.L., R01 AI-068871 to R.J.S. and A.M.B., R01 DK74821 to R.J.S., and R01 AI-050892 to A. D.L.), and the Cogan Family Foundation (to D.M.L.).
Abbreviations used in this paper
- AA
arachidonic acid
- B6
C57BL/6J
- BLT
leukotriene B4 receptor
- BM
bone marrow
- FLS
fibroblast-like synoviocyte
- iRAD
irradiated
- 5-LO
5-lipoxygenase
- LT
leukotriene
- LTA4
leukotriene A4
- LTA4H
leukotriene A4 hydrolase
- LTB4
leukotriene B4
- LTC4
leukotriene C4
- PDGF
platelet-derived growth factor
- PMN
polymorphonuclear leukocyte
- RP
reversed phase
- WT
wild-type
Footnotes
Disclosures
M.C. is now employed by Pfizer, Inc. D.M.L. is now employed by Novartis Pharma AG. The other authors have no financial conflicts of interest.
References
- 1.Henderson WR., Jr. The role of leukotrienes in inflammation. Ann. Intern. Med. 1994;121:684–697. doi: 10.7326/0003-4819-121-9-199411010-00010. [DOI] [PubMed] [Google Scholar]
- 2.Murphy RC, Gijón MA. Biosynthesis and metabolism of leukotrienes. Biochem. J. 2007;405:379–395. doi: 10.1042/BJ20070289. [DOI] [PubMed] [Google Scholar]
- 3.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 4.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 5.Werz O. 5-Lipoxygenase: cellular biology and molecular pharmacology. Curr. Drug Targets Inflamm. Allergy. 2002;1:23–44. doi: 10.2174/1568010023344959. [DOI] [PubMed] [Google Scholar]
- 6.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 7.Reid GK, Kargman S, Vickers PJ, Mancini JA, Léveillé C, Ethier D, Miller DK, Gillard JW, Dixon RA, Evans JF. Correlation between expression of 5-lipoxygenase-activating protein, 5-lipoxygenase, and cellular leukotriene synthesis. J. Biol. Chem. 1990;265:19818–19823. [PubMed] [Google Scholar]
- 8.Miller DK, Gillard JW, Vickers PJ, Sadowski S, Léveillé C, Mancini JA, Charleson P, Dixon RA, Ford-Hutchinson AW, Fortin R, et al. Identification and isolation of a membrane protein necessary for leukotriene production. Nature. 1990;343:278–281. doi: 10.1038/343278a0. [DOI] [PubMed] [Google Scholar]
- 9.Dixon RA, Diehl RE, Opas E, Rands E, Vickers PJ, Evans JF, Gillard JW, Miller DK. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990;343:282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- 10.Samuelsson B, Funk CD. Enzymes involved in the biosynthesis of leukotriene B4. J. Biol. Chem. 1989;264:19469–19472. [PubMed] [Google Scholar]
- 11.Mandal AK, Jones PB, Bair AM, Christmas P, Miller D, Yamin TT, Wisniewski D, Menke J, Evans JF, Hyman BT, et al. The nuclear membrane organization of leukotriene synthesis. Proc. Natl. Acad. Sci. USA. 2008;105:20434–20439. doi: 10.1073/pnas.0808211106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folco G, Murphy RC. Eicosanoid transcellular biosynthesis: from cell-cell interactions to in vivo tissue responses. Pharmacol. Rev. 2006;58:375–388. doi: 10.1124/pr.58.3.8. [DOI] [PubMed] [Google Scholar]
- 13.Dahinden CA, Clancy RM, Gross M, Chiller JM, Hugli TE. Leukotriene C4 production by murine mast cells: evidence of a role for extra-cellular leukotriene A4. Proc. Natl. Acad. Sci. USA. 1985;82:6632–6636. doi: 10.1073/pnas.82.19.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimminger F, Sibelius U, Seeger W. Amplification of LTB4 generation in AM-PMN cocultures: transcellular 5-lipoxygenase metabolism. Am. J. Physiol. 1991;261:L195–L203. doi: 10.1152/ajplung.1991.261.2.L195. [DOI] [PubMed] [Google Scholar]
- 15.McGee JE, Fitzpatrick FA. Erythrocyte-neutrophil interactions: formation of leukotriene B4 by transcellular biosynthesis. Proc. Natl. Acad. Sci. USA. 1986;83:1349–1353. doi: 10.1073/pnas.83.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bigby TD, Meslier N. Transcellular lipoxygenase metabolism between monocytes and platelets. J. Immunol. 1989;143:1948–1954. [PubMed] [Google Scholar]
- 17.Edenius C, Heidvall K, Lindgren JA. Novel transcellular interaction: conversion of granulocyte-derived leukotriene A4 to cysteinyl-containing leukotrienes by human platelets. Eur. J. Biochem. 1988;178:81–86. doi: 10.1111/j.1432-1033.1988.tb14431.x. [DOI] [PubMed] [Google Scholar]
- 18.Maclouf J, Murphy RC, Henson PM. Transcellular sulfidopeptide leukotriene biosynthetic capacity of vascular cells. Blood. 1989;74:703–707. [PubMed] [Google Scholar]
- 19.Fabre JE, Goulet JL, Riche E, Nguyen M, Coggins K, Offenbacher S, Koller BH. Transcellular biosynthesis contributes to the production of leukotrienes during inflammatory responses invivo. J. Clin. Invest. 2002;109:1373–1380. doi: 10.1172/JCI14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zarini S, Gijón MA, Ransome AE, Murphy RC, Sala A. Transcellular biosynthesis of cysteinyl leukotrienes in vivo during mouse peritoneal inflammation. Proc. Natl. Acad. Sci. USA. 2009;106:8296–8301. doi: 10.1073/pnas.0903851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DM, Kiener HP, Agarwal SK, Noss EH, Watts GF, Chisaka O, Takeichi M, Brenner MB. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315:1006–1010. doi: 10.1126/science.1137306. [DOI] [PubMed] [Google Scholar]
- 22.Lee DM, Kiener HP, Brenner MB. Synoviocytes. In: Harris ED, Eudd RC, Genovese MC, Firestein GS, Sargent JS, Sludge CB, editors. Kelley's Textbook of Rheumatology. 7th Ed. Elsevier Saunders; Philadelphia: 2005. pp. 175–188. [Google Scholar]
- 23.Goulet JL, Snouwaert JN, Latour AM, Coffman TM, Koller BH. Altered inflammatory responses in leukotriene-deficient mice. Proc. Natl. Acad. Sci. USA. 1994;91:12852–12856. doi: 10.1073/pnas.91.26.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrum RS, Goulet JL, Snouwaert JN, Griffiths RJ, Koller BH. Determination of the contribution of cysteinyl leukotrienes and leukotriene B4 in acute inflammatory responses using 5-lipoxygenase- and leukotriene A4 hydrolase-deficient mice. J. Immunol. 1999;163:6810–6819. [PubMed] [Google Scholar]
- 25.Tager AM, Dufour JH, Goodarzi K, Bercury SD, von Andrian UH, Luster AD. BLTR mediates leukotriene B(4)-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J. Exp. Med. 2000;192:439–446. doi: 10.1084/jem.192.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali JL, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 27.Chen M, Lam BK, Kanaoka Y, Nigrovic PA, Audoly LP, Austen KF, Lee DM. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J. Exp. Med. 2006;203:837–842. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen M, Boilard E, Nigrovic PA, Clark P, Xu D, Fitzgerald GA, Audoly LP, Lee DM. Predominance of cyclooxygenase 1 over cyclooxygenase 2 in the generation of proinflammatory prostaglandins in autoantibody-driven K/BxN serum-transfer arthritis. Arthritis Rheum. 2008;58:1354–1365. doi: 10.1002/art.23453. [DOI] [PubMed] [Google Scholar]
- 29.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 30.Kanaoka Y, Maekawa A, Penrose JF, Austen KF, Lam BK. Attenuated zymosan-induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J. Biol. Chem. 2001;276:22608–22613. doi: 10.1074/jbc.M103562200. [DOI] [PubMed] [Google Scholar]
- 31.Lam BK, Owen WF, Jr., Austen KF, Soberman RJ. The identification of a distinct export step following the biosynthesis of leukotriene C4 by human eosinophils. J. Biol. Chem. 1989;264:12885–12889. [PubMed] [Google Scholar]
- 32.Humason GL. Animal Tissue Techniques. W. H. Freeman; New York: 1967. [Google Scholar]
- 33.Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, Lee DM. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J. Immunol. 2008;180:4885–4891. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gijón MA, Zarini S, Murphy RC. Biosynthesis of eicosanoids and transcellular metabolism of leukotrienes in murine bone marrow cells. J. Lipid Res. 2007;48:716–725. doi: 10.1194/jlr.M600508-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Farias SE, Zarini S, Precht T, Murphy RC, Heidenreich KA. Transcellular biosynthesis of cysteinyl leukotrienes in rat neuronal and glial cells. J. Neurochem. 2007;103:1310–1318. doi: 10.1111/j.1471-4159.2007.04830.x. [DOI] [PubMed] [Google Scholar]
- 36.Nanki T, Nagasaka K, Hayashida K, Saita Y, Miyasaka N. Chemokines regulate IL-6 and IL-8 production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. J. Immunol. 2001;167:5381–5385. doi: 10.4049/jimmunol.167.9.5381. [DOI] [PubMed] [Google Scholar]
- 37.Griffiths RJ, Pettipher ER, Koch K, Farrell CA, Breslow R, Conklyn MJ, Smith MA, Hackman BC, Wimberly DJ, Milici AJ, et al. Leukotriene B4 plays a critical role in the progression of collagen-induced arthritis. Proc. Natl. Acad. Sci. USA. 1995;92:517–521. doi: 10.1073/pnas.92.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGee J, Fitzpatrick F. Enzymatic hydration of leukotriene A4. Purification and characterization of a novel epoxide hydrolase from human erythrocytes. J. Biol. Chem. 1985;260:12832–12837. [PubMed] [Google Scholar]
- 39.Yokomizo T, Uozumi N, Takahashi T, Kume K, Izumi T, Shimizu T. Leukotriene A4 hydrolase and leukotriene B4 metabolism. J. Lipid Mediat. Cell Signal. 1995;12:321–332. doi: 10.1016/0929-7855(95)00041-n. [DOI] [PubMed] [Google Scholar]
- 40.Medina JF, Barrios C, Funk CD, Larsson O, Haeggström J, Rådmark O. Human fibroblasts show expression of the leukotriene-A4-hydrolase gene, which isincreased after simian-virus-40transformation. Eur. J. Biochem. 1990;191:27–31. doi: 10.1111/j.1432-1033.1990.tb19089.x. [DOI] [PubMed] [Google Scholar]
- 41.Aloe L, Probert L, Kollias G, Bracci-Laudiero L, Micera A, Mollinari C, Levi-Montalcini R. Level of nerve growth factor and distribution of mast cells in the synovium of tumour necrosis factor transgenic arthritic mice. Int. J. Tissue React. 1993;15:139–143. [PubMed] [Google Scholar]
- 42.Simmonds RE, Foxwell BM. Signalling, inflammation and arthritis: NF-kappaB and its relevance to arthritis and inflammation. Rheumatology (Oxford) 2008;47:584–590. doi: 10.1093/rheumatology/kem298. [DOI] [PubMed] [Google Scholar]
- 43.Houssiau FA, Devogelaer JP, Van Damme J, de Deuxchaisnes CN, Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988;31:784–788. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- 44.Guerne PA, Zuraw BL, Vaughan JH, Carson DA, Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J. Clin. Invest. 1989;83:585–592. doi: 10.1172/JCI113921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chomarat P, Rissoan MC, Pin JJ, Banchereau J, Miossec P. Contribution of IL-1, CD14, and CD13 in the increased IL-6 production induced by in vitro monocyte-synoviocyte interactions. J. Immunol. 1995;155:3645–3652. [PubMed] [Google Scholar]
- 46.Rathanaswami P, Hachicha M, Wong WL, Schall TJ, McColl SR. Synergistic effect of interleukin-1 beta and tumor necrosis factor alpha on interleukin-8 gene expression in synovial fibroblasts. Evidence that interleukin-8 is the major neutrophil-activating chemokine released in response to monokine activation. Arthritis Rheum. 1993;36:1295–1304. doi: 10.1002/art.1780360914. [DOI] [PubMed] [Google Scholar]
- 47.Koch AE, Kunkel SL, Harlow LA, Johnson B, Evanoff HL, Haines GK, Burdick MD, Pope RM, Strieter RM. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J. Clin. Invest. 1992;90:772–779. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inoue H, Takamori M, Shimoyama Y, Ishibashi H, Yamamoto S, Koshihara Y. Regulation by PGE2 of the production of interleukin-6, macrophage colony stimulating factor, and vascular endothelial growth factor in human synovial fibroblasts. Br. J. Pharmacol. 2002;136:287–295. doi: 10.1038/sj.bjp.0704705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, Pandher K, Lapointe JM, Saha S, Roach ML, et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc. Natl. Acad. Sci. USA. 2003;100:9044–9049. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Camacho M, Vila L. Transcellular formation of thromboxane A(2) in mixed incubations of endothelial cells and aspirin-treated platelets strongly depends on the prostaglandin I-synthase activity. Thromb. Res. 2000;99:155–164. doi: 10.1016/s0049-3848(00)00241-3. [DOI] [PubMed] [Google Scholar]
- 51.Schumacher HR, Kitridou RC. Synovitis of recent onset. A clinicopathologic study during the first month of disease. Arthritis Rheum. 1972;15:465–485. doi: 10.1002/art.1780150502. [DOI] [PubMed] [Google Scholar]
- 52.Kulka JP, Bocking D, Ropes MW, Bauer W. Early joint lesions of rheumatoid arthritis; report of eight cases, with knee biopsies of lesions of less than one year's duration. AMA Arch. Pathol. 1955;59:129–150. [PubMed] [Google Scholar]
- 53.Henderson B, Pettipher ER. The synovial lining cell: biology and pathobiology. Semin. Arthritis Rheum. 1985;15:1–32. doi: 10.1016/0049-0172(85)90007-1. [DOI] [PubMed] [Google Scholar]
- 54.Athanasou NA, Quinn J. Immunocytochemical analysis of human synovial lining cells: phenotypic relation to other marrow derived cells. Ann. Rheum. Dis. 1991;50:311–315. doi: 10.1136/ard.50.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu S, Lu H, Lin J, Chen Z, Jiang D. Regulation of TNFalpha and IL1beta in rheumatoid arthritis synovial fibroblasts by leukotriene B4. Rheumatol. Int. 2010;30:1183–1189. doi: 10.1007/s00296-009-1125-y. [DOI] [PubMed] [Google Scholar]
- 56.Fitzpatrick FA, Morton DR, Wynalda MA. Albumin stabilizes leukotriene A4. J. Biol. Chem. 1982;257:4680–4683. [PubMed] [Google Scholar]
- 57.Fitzpatrick F, Liggett W, McGee J, Bunting S, Morton D, Samuelsson B. Metabolism of leukotriene A4 by human erythrocytes. A novel cellular source of leukotriene B4. J. Biol. Chem. 1984;259:11403–11407. [PubMed] [Google Scholar]
- 58.Claesson HE, Haeggström J. Human endothelial cells stimulate leukotriene synthesis and convert granulocyte released leukotriene A4 into leukotrienes B4, C4, D4 and E4. Eur. J. Biochem. 1988;173:93–100. doi: 10.1111/j.1432-1033.1988.tb13971.x. [DOI] [PubMed] [Google Scholar]
- 59.Feinmark SJ, Cannon PJ. Endothelial cell leukotriene C4 synthesis results from intercellular transfer of leukotriene A4 synthesized by polymorphonuclear leukocytes. J. Biol. Chem. 1986;261:16466–16472. [PubMed] [Google Scholar]
- 60.Ibe BO, Campbell WB. Synthesis and metabolism of leukotrienes by human endothelial cells: influence on prostacyclin release. Biochim. Biophys. Acta. 1988;960:309–321. doi: 10.1016/0005-2760(88)90039-2. [DOI] [PubMed] [Google Scholar]
- 61.Feinmark SJ, Cannon PJ. Vascular smooth muscle cell leukotriene C4 synthesis: requirement for transcellular leukotriene A4 metabolism. Biochim. Biophys. Acta. 1987;922:125–135. doi: 10.1016/0005-2760(87)90146-9. [DOI] [PubMed] [Google Scholar]
- 62.Hollingsworth JW, Siegel ER, Creasey WA. Granulocyte survival in synovial exudate of patients with rheumatoid arthritis and other inflammatory joint diseases. Yale J. Biol. Med. 1967;39:289–296. [PMC free article] [PubMed] [Google Scholar]
- 63.Klickstein LB, Shapleigh C, Goetzl EJ. Lipoxygenation of arachidonic acid as a source of polymorphonuclear leukocyte chemotactic factors in synovial fluid and tissue in rheumatoid arthritis and spondyloarthritis. J. Clin. Invest. 1980;66:1166–1170. doi: 10.1172/JCI109947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edwards SW, Hallett MB. Seeing the wood for the trees: the forgotten role of neutrophils in rheumatoid arthritis. Immunol. Today. 1997;18:320–324. doi: 10.1016/s0167-5699(97)01087-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.