Abstract
Sleep disordered breathing (SDB) is the most common co-morbidity in patients with heart failure (HF) and has a significant impact on quality of life, morbidity, and mortality. A number of therapeutic options have become available in recent years that can improve quality of life and potentially the outcomes of HF patients with SDB. Unfortunately, SDB is not part of the routine evaluation and management of HF, thus it remains untreated in most HF patients. While recognition of the role of SDB in HF is increasing, clinical guidelines for the management of SDB in HF patients continue to be absent. In this article we provide an overview of SDB in HF and propose a clinical care pathway to help clinicians better recognize and treat SDB in their HF patients.
Keywords: heart failure, sleep disordered breathing, clinical care pathway
Introduction
Sleep disordered breathing (SDB) is the most common comorbidity in heart failure (HF), occurring in 50–80% of patients.1–3 SDB accelerates the progression of HF and worsens morbidity and mortality.4,5 Despite its high prevalence and adverse consequences, the diagnosis and treatment of SDB is usually not part of the routine evaluation and management of HF patients. This remains the case despite the presence of effective and accepted therapeutic options to treat SDB in HF that can improve the quality of life of HF patients and potentially impact their outcomes. Clinicians caring for HF patients are not provided with surveillance guidelines for this prevalent comorbidity, which results in a great deal of practice variation. In effect, only a small number of HF patients are ever diagnosed with SDB, and an even smaller number are ever treated.6 In a disease with a high pre-test probability of SDB, it is important that an approach of surveillance rather than screening is adopted in order to provide all likely candidates with access to testing and, potentially, to treatment. In this article, we provide an overview of SDB in HF and present a clinical care pathway to help clinicians begin to better recognize and treat SDB in their HF patients.
What Is Sleep Disordered Breathing?
SDB describes disorders of breathing that occur primarily and often exclusively during sleep. The adverse consequences of SDB often persist throughout the waking hours. SDB is characterized by cycles of significant pauses in breathing with consequent hypoxia and partial neurological arousals that cause disruption to the architecture of sleep. SDB is broadly classified into two types: obstructive sleep apnea (OSA) and central sleep apnea (CSA). OSA is relatively common in both the general and HF populations, whereas CSA is more uniquely associated with HF. However, it is not uncommon to see a mixture of both OSA and CSA in patients with HF. The apnea hypopnea index (AHI), which is defined as the total number of apneas and hypopneas per hour of sleep, is an index of the severity of the SBD (obstructive or central).
Obstructive Sleep Apnea
OSA is found in an estimated 20% of the general population and 35% of patients with HF.3,6 As its name implies, OSA is caused by repeated episodes of partial or complete upper airway obstruction during sleep. Each episode of airway obstruction is associated with decreased or absent air entry into the lungs and subsequent hypoxia despite repetitive, futile respiratory efforts and chest expansion. The airway obstruction is eventually terminated by an arousal from sleep and subsequent recovery of airway patency. These episodes of obstruction, hypoxia, and arousal are a cause of profound intermittent sympathetic activation and pulmonary and systemic vasoconstriction.7,8 Numerous OSA episodes may occur during the course of one night, resulting in curtailment of sleep and deleterious effects on daytime function.
The primary mechanism underlying the development of OSA is pharyngeal collapse due to the loss of pharyngeal dilator muscle and genioglossus tone during sleep.9–11 A tenuous balance between constrictor and dilator forces maintains the patency of the upper airway during normal sleep. OSA occurs when this balance shifts toward the constricting forces. Collapsing factors of the upper airway include pharyngeal edema, cervical congestion, and extra-luminal pressure from the tissue surrounding the airway such as provided by fat deposition in the neck.9,12,13 Aging, male sex, and anatomical variations are also strong contributors to upper airway collapsibility.9,14–16 Dilating forces include primarily the tone of the pharyngeal dilator and genioglossus muscles.9 In normal individuals, the partial withdrawal of dilating muscle tone during sleep is typically insufficient to cause pharyngeal collapse. However, the upper airway of patients with OSA may be anatomically narrowed or dilating muscle tone may and abnormally decreased.9,14 As a result, the normal withdrawal of pharyngeal dilator muscle tone during sleep causes the pharynx to collapse, triggering apnea or hypopnea. The ensuing hypoxia triggers an arousal from sleep.9 Arousals are associated with increased muscle tone and subsequent restoration of upper airway patency.9
OSA is recognized as an important risk factor in the development and progression of a number of cardiovascular disorders, including systemic hypertension, cardiac arrhythmias, myocardial ischemia and infarction, and HF, through its activation of several hemodynamic, neurohumoral, thrombotic, metabolic, and inflammatory mechanisms.17,18 Since OSA-related cardiovascular diseases often have overlapping origins and share many pathophysiologic pathways, isolating the causal role of OSA on each is often difficult. Nonetheless, in patients with HF and OSA, two OSA-related pathophysiologic mechanisms—changes in intrathoracic pressure and increased sympathetic drive—appear to be of particular importance to the progression of HF.
The marked reduction in intrathoracic pressure that results from continued inspiratory efforts against an occluded airway has significant adverse effects on cardiac dynamics and, thus, cardiac output.19 Sudden, large negative swings in intrathoracic pressure increase left ventricular transmural pressure by increasing the difference between intracardiac and extracardiac pressures.20 This increase in transmural pressure results in increased left ventricular afterload.20–22 Negative intrathoracic pressure also enhances venous return to the heart, which increases right ventricular preload. Overfilling of the right ventricle causes the ventricular septum to shift leftward during diastole, which impedes left ventricular filling.23,24 This combination of increased left ventricular afterload and decreased left ventricular preload ultimately leads to a reduction in stroke volume (and thus cardiac output) during each obstructive apneic event.20,25 Since OSA patients can have numerous apneic events each night, cardiac output may be impaired for a substantial portion of the sleep period.
In addition to increasing afterload, increased left ventricular transmural pressure increases wall stress, and therefore myocardial oxygen demand.21 This increased myocardial oxygen demand in conjunction with OSA-related hypoxia may induce myocardial ischemia, impairing myocardial function.20,26 Taken together, these OSA-induced cardiac stresses may contribute over the longer term to ventricular hypertrophy, cardiac remodeling, and failure.19
Obstructive apneas and hypopneas and their associated hypoxia are also potent activators of the sympathetic nervous system (SNS).7,27 The sympathetic activation following each apnea and hypopnea episode results in recurrent vasoconstriction and hypertension during sleep.7 After awakening and cessation of the sleep-related apnea and hypopnea episodes, an increase in baseline sympathetic activity persists for several hours and is associated with a carryover effect of increased in blood pressure into the daytime.7,28
Changes in circulating oxygen levels are first sensed by the peripheral chemoreceptors, especially those in the carotid bodies. Peripheral chemoreceptor stimulation by hypoxia results in both ventilatory and sympathetic activation. This is an innate response in the organism to immediately combat the detrimental effects of hypoxia. The stimulus of the peripheral chemoreceptors by intermittent hypoxia results in prolonged firing and persistence of sympathetic activity.29
The effects of the sympathetic activation response are systemic, but they are particularly important at the level of the kidney. Several studies have found activation of the renal medulla and increased circulating norepinephrine levels in patients with SDB.30–32 Activation of the renin-angiotensin system is critical for the persistence of the blood pressure increase found in SDB patients.33,34 In addition, recent studies suggest that renin-angiotensin activation contributes to further upregulation of the carotid response and augmentation of sympathetic activation to subsequent intermittent hypoxia.35
In sum, the pathologic hemodynamic and vascular effects that result from OSA-induced changes intrathoracic pressure and sympathetic activation place the HF patient with OSA at significantly greater risk for myocardial ischemia, arrhythmias, hypertension, and worsening ventricular dysfunction. Left untreated, these OSA-related effects contribute to the vicious cycle of HF, and ultimately lead to the increased mortality seen in in these patients.17
Central Sleep Apnea
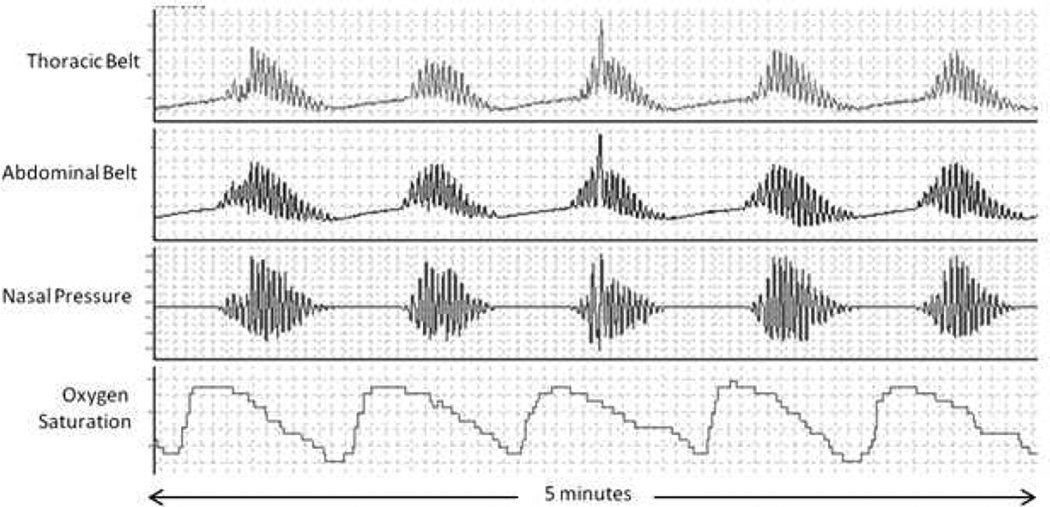
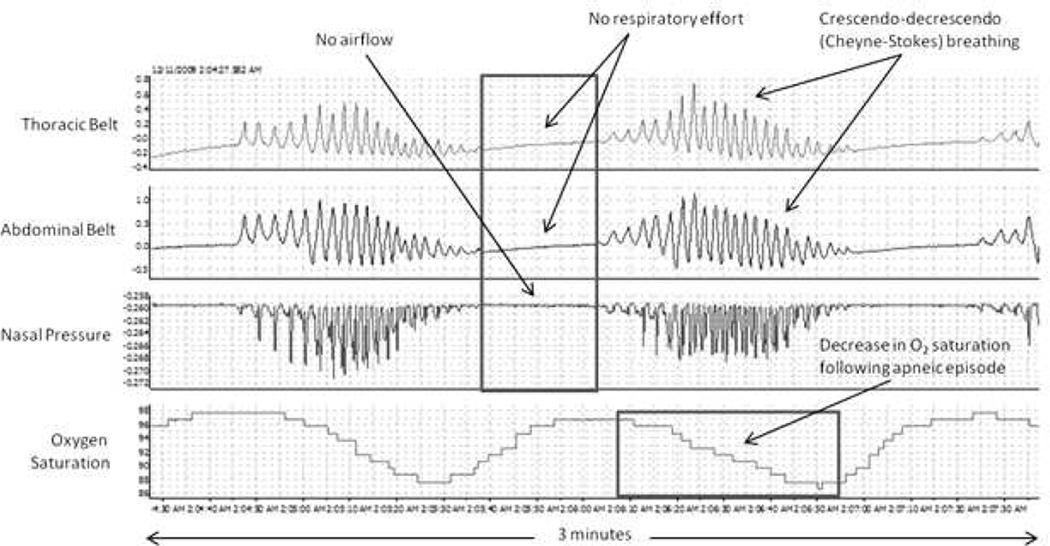
CSA is prevalent in HF, occurring in 30–50% of HF patients with reduced left ventricular ejection fraction (LVEF)3,36–38 and in 18–30% of HF patients with preserved LVEF.39–41 CSA is characterized by the temporary withdrawal of central (brainstem-driven) respiratory drive that results in the cessation of respiratory muscle activity and airflow. CSA in HF patients commonly has a distinctive morphology termed Cheyne-Stokes respiration. CSA with Cheyne-Stokes respiration is recognized by the simultaneous absence of air flow and respiratory effort (central apnea or hypopnea) followed by characteristic hyperventilation in a crescendo-decrescendo pattern (Figure 1).42
Figure 1.
Classic Cheyne-Stokes crescendo-decrescendo respiratory pattern with intervening apneic episodes.
The pathogenesis of CSA in HF is complex and still incompletely understood. However, a substantial body of research suggests that oscillation of the arterial blood carbon dioxide level (PaCO2) above and below the central threshold of ventilation, termed the apneic threshold, plays a key role.43–45 Normally, the PaCO2 is detected by central (brain and brainstem) and peripheral (carotid body) chemoreceptors, such that a rise in the PaCO2 leads to increased respiratory drive. To the contrary, a fall in the PaCO2 leads to reduced respiratory drive. HF patients have increased baseline respiratory drive46 and excessive hyperventilation in response to increased PaCO247 and are commonly hypocapnic; that is, they have a PaCO2 that is chronically low or close to the lower limit of normal.43,48 HF patients with CSA chronically hyperventilate because of both pulmonary congestion, which activates pulmonary irritant receptors that stimulate ventilation, and enhanced central and peripheral chemoreceptor sensitivity.47–51 It is during hyperventilation that the PaCO2 is driven below the apneic threshold, resulting in a central apnea. This apnea persists until the PaCO2 rises significantly above the apneic threshold, stimulating the central and peripheral chemoreceptors and resulting in additional hyperventilation. Hypoxia, which also occurs during the apnea, may arouse the patient from sleep and contribute to this increase in ventilation. With the return of hyperventilation, the cycle begins anew, and the periodic respiratory oscillations (and concomitant PaCO2 oscillations) perpetuate themselves.52,53
Although oscillation of the PaCO2 above and below the apneic threshold appears to be the key mechanism leading to CSA in patients with HF, other factors, namely a diminished cerebrovascular response to changes in CO2 and upper airway instability, may also contribute to respiratory instability and the perpetuation of CSA.54–56 In addition, low cardiac output and prolonged circulation time can contribute to a lag between the central and peripheral chemoreceptor response times and influence the cycle duration of the waxing-waning pattern of periodic breathing seen with CSA.57
Like OSA, the presence of CSA in patients with HF is associated with a set of neurohumoral and hemodynamic responses that are detrimental to the failing heart. Cyclical episodes of apnea, hypoxia, carbon dioxide retention, and arousal provoke periodic elevations in SNS activity that cause tachycardia, peripheral vasoconstriction, sodium retention, and renin-angiotensin system activation.58,59 The consequent increases in myocardial oxygen demand, blood pressure, and blood volume lead to increased myocardial ischemia, preload, and afterload that together further stress the failing heart. Furthermore, increased sympathetic tone may contribute to ventricular irritability and arrhythmias in patients with HF.60 Several studies have found that HF patients with CSA have increased ventricular irritability, which subsequently diminishes with treatment.60,61 In addition, a significant relationship between atrial fibrillation and CSA has also been reported.36
Studies suggest a link between CSA and poor outcome in patients with HF.5,62,63 This appears to be especially true if periodic breathing or Cheyne-Stokes breathing is present during wakefulness or exercise64,65 The presence of CSA has been shown to be an independent risk factor for cardiac hospital readmission, cardiac transplantation, and death in HF patients.5,66,67
Clinical Evaluation of Sleep Disordered Breathing in Heart Failure
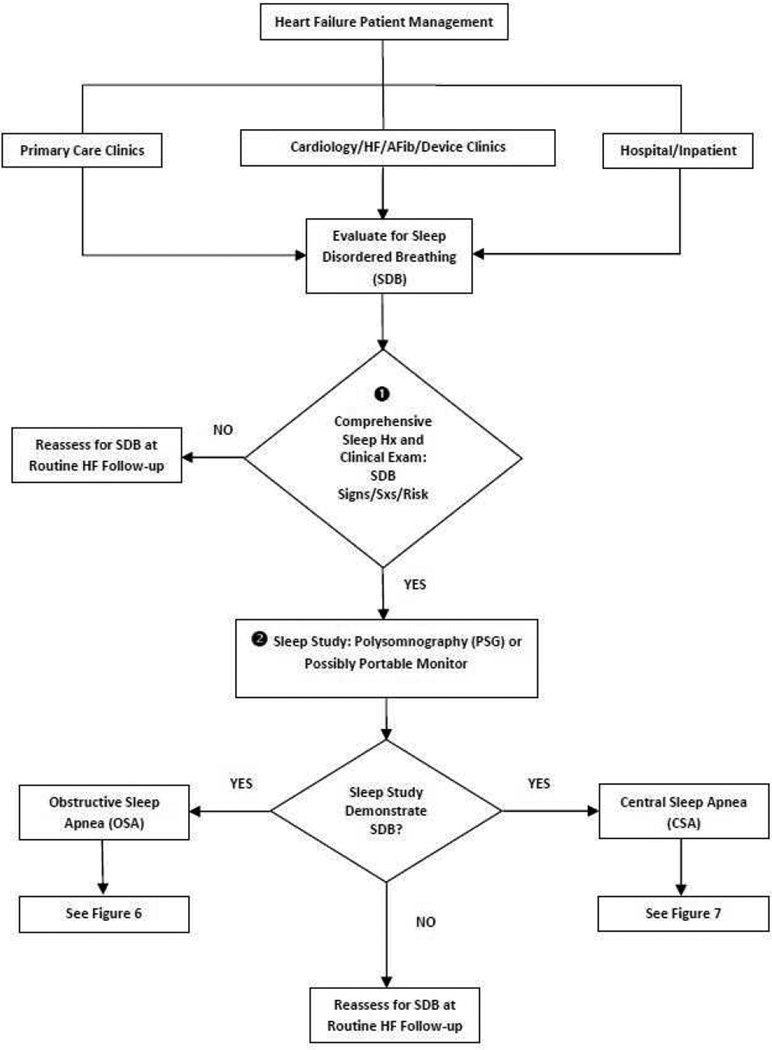
With its high prevalence and association with increased morbidity and mortality in patients with HF, surveillance for SDB should be an important part of routine HF patient care. A clinical care pathway describing the evaluation and diagnosis of SDB in patients with HF is presented in Figure 2 and discussed below.
Figure 2.
Clinical evaluation pathway for sleep disordered breathing in patients with heart failure. Abbreviations: HF, heart failure; AFib, atrial fibrillation; SDB, sleep disordered breathing; PSG, polysomnography; OSA, obstructive sleep apnea; CSA, central sleep apnea.
Comprehensive Sleep History
The clinical care pathway for SDB begins with obtaining a comprehensive sleep history (Figure 2, ➊). Although no patient characteristic or complaint has been found to be predictive of SDB in patients with HF, HF patients with SDB may have one or more symptoms that suggest the presence of SDB, and therefore patients should be carefully questioned about such symptoms. Symptoms related to SDB include habitual snoring, witnessed apneas, unusual daytime or nighttime breathing patterns, gasping/choking episodes, disrupted sleep, daytime sleepiness, paroxysmal nocturnal dyspnea, nocturia, morning headaches, and diminished concentration and memory.17,68 Unfortunately, since many symptoms of SDB are also common to HF, clinicians may mistakenly ascribe SDB symptoms to HF instead. It may be helpful to question the patient’s sleep partner about the patient’s sleep and wake habits, especially regarding episodes of apnea, snoring, frequent arousals, or changes in behavior or mood that can be related to insufficient sleep quality. HF patients admitted to the hospital with acutely decompensated HF should also be carefully assessed for the presence of SDB, since the signs and symptoms of SDB may emerge with worsening HF. Hospitalization provides an ideal opportunity to monitor the patient’s sleep and nocturnal breathing habits for the presence of SDB.69
It is important to note that several short patient questionnaires, such as the Epworth Sleepiness Scale70,71 and the Berlin Questionnaire,72 have been developed to help screen for SDB. While such questionnaires have some utility in screening for SDB in the general population,73,74 they have not been found to be effective in screening for SDB in the HF population.75 They should therefore be used cautiously, if at all, in screening for SDB in HF patients. HF patients should never be excluded from testing for SDB based on a negative questionnaire only.
Clinical Examination
After obtaining a sleep history, a careful physical examination should be performed (Figure 2, ➊). On clinical examination, patients with OSA are often older, male, overweight, and have a history of snoring.1,37 Being significantly overweight is an important risk factor for OSA partly because obese individuals frequently have short, thick necks and peripharyngeal fatty deposits that may contribute to pharyngeal obstruction.76 Obesity, however, appears to be a significantly less important predictor of OSA in individuals older than 60 years. This is due to the superseding effect of aging on decreased airway tone, rendering weight less important.77 Furthermore, obesity has been shown to less important in the presentation of OSA in patients with HF than in those without HF.75 In HF patients with OSA who are not obese, the shift of excess extracellular fluid centrally and rostrally when supine is likely a factor in the pathogenesis of OSA.78,79 HF patients often experience varying degrees of chronic fluid overload. The redistribution of this excess fluid from the legs to the chest and neck when supine during sleep may predispose HF patients with fluid overload to OSA because of the resulting increased peripharygeal edema and cervical congestion that compresses the upper airway and obstructs airflow.78,79
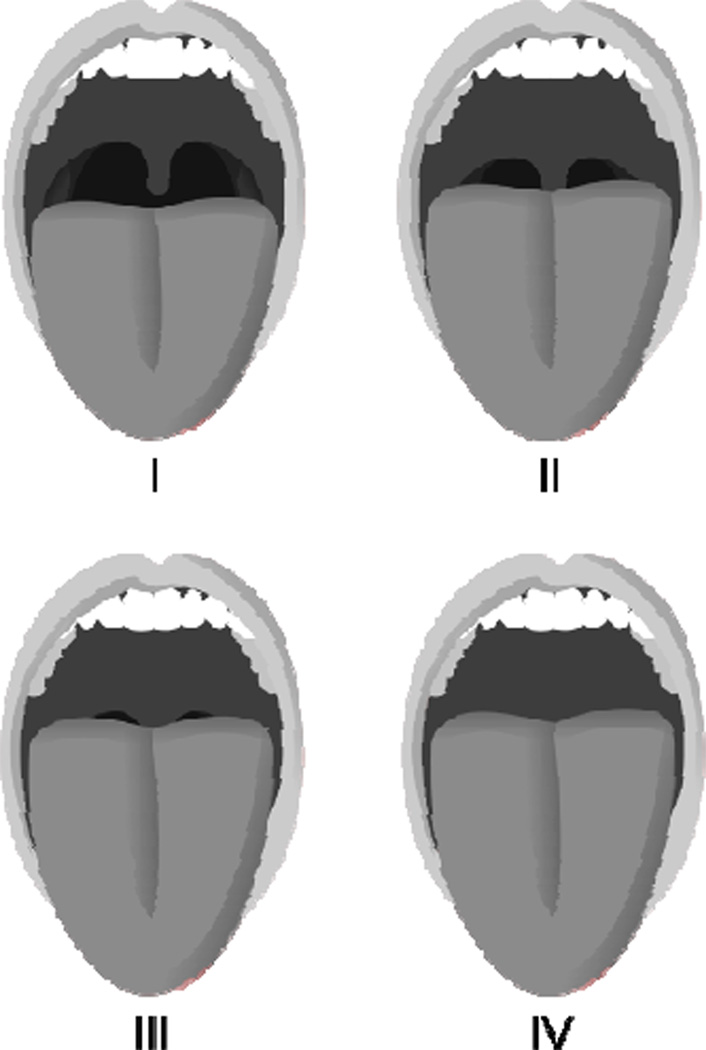
Patients with an anatomically crowded pharynx due to redundant tissue, an elongated soft palate, an enlarged uvula, and/or a small or receding jaw are also at increased risk of OSA as these anomalies reduce the size of the posterior pharyngeal airway.76 The Mallampati airway classification system offers a useful way to assess for anatomical upper airway obstruction and the potential for OSA in the general population, although it has not been studied in HF patients.80 During oropharyngeal assessment, the patient is instructed to open his or her mouth as wide as possible, while protruding the tongue as far as possible. Using the Mallampati airway classification system, the patient’s upper airway is then scored on a scale of I-IV (Figure 3). On average, for every one point increase in the Mallampati score, the odds of having OSA increase more than two-fold.80
Figure 3.
The Mallampati airway classification system (I-IV scale). Class I: soft palate and entire uvula visible; Class II: soft palate and portion of uvula visible; Class III: soft palate visible (may include base of uvula); Class IV: soft palate not visible. (Image courtesy Jmarchn/Wikimedia Commons/Public Domain)
In contrast to HF patients with OSA, HF patients with CSA typically are not overweight and often do not snore.1 Without such overt signs, CSA is much more difficult to identify clinically than OSA. However, certain risk factors have been identified that increase the likelihood of CSA being present in a patient with HF. They include male gender, higher New York Heart Association class, lower LVEF, waking hypocapnia (PaCO2 <38 mmHg), higher prevalence of atrial fibrillation, higher brain natriuretic peptide (BNP) levels, and frequent nocturnal ventricular arrhythmias.1,3,37,81 There should be a high index of suspicion for CSA if any of these findings are present in a patient with HF.
Clinical Testing for Sleep Disordered Breathing
HF patients whose sleep assessment, clinical exam, and medical history suggest the presence of SDB should be referred for further evaluation and testing. One recent study found that only 2% of hospitalized HF patients received screening, and 97% of those tested were diagnosed with SDB.6 The gold standard test for diagnosing SDB is polysomnography (PSG), or the overnight sleep study, which is performed in a sleep laboratory (Figure 2, ➋). During a PSG, multiple physiologic variables are monitored and recorded, including respiratory rate and volume, blood oxygen saturation, airflow through the nose and mouth, heart rate and rhythm, brain electrical activity, eye movement, muscle activity, and limb movement. The PSG is continuously supervised by a trained technician, and analysis requires manual scoring by a sleep specialist.
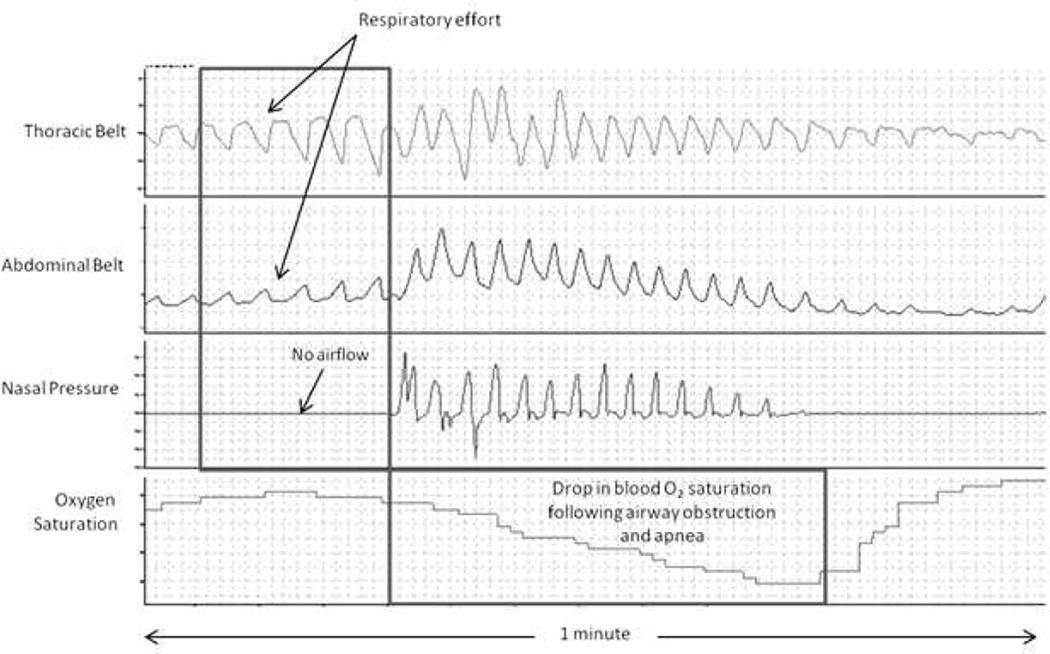
Example PSG recordings of OSA and CSA in patients with HF are shown in Figures 4 and 5. Characteristic findings of OSA on a PSG include increased frequency and severity of obstructive episodes during rapid eye movement (REM) sleep, the absence of airflow through the mouth and nose despite continued respiratory effort-related chest and abdominal motion, and a concomitant drop in the blood oxygen saturation following airway obstruction and apnea. Characteristic findings of CSA on PSG include an onset near the transition into or out of stage one, non-rapid eye movement (NREM) sleep; cycles of deep, rapid, crescendo-decrescendo breathing (i.e., Cheyne-Stokes breathing) followed by periods of hypopnea and/or apnea along with concomitant changes in the blood oxygen saturation; and apneic periods accompanied by the absence of chest or abdominal wall activity.
Figure 4.
Polysomnogram of OSA in a patient with heart failure.
Figure 5.
Polysomnogram of CSA in a patient with heart failure.
Although there is usually a predominance of either obstructive or central apneas in patients with HF, it is possible for both types of apnea to be present in the same individual. In such cases of “mixed” SDB, the PSG recording typically shows a gradual shift from mostly obstructive apneas at the beginning of the night to mostly central apneas towards morning.82,83 Studies of this phenomenon suggest that this shift in apnea type is due to worsening cardiac output as evidenced by increasing time in measured circulatory delay, which correlates with cardiac output.82 The degree to which the initial OSA might contribute to overnight worsening in heart function and the appearance of CSA is unclear and awaits further research.
A clinical PSG report typically includes information about sleep duration; sleep stages; sleep latency (time to sleep onset); sleep efficiency (ratio of time spent asleep (total sleep time) to the amount of time spent in bed); frequency of sudden nocturnal arousals; number of central, obstructive, and mixed apneas/hypopneas; presence of snoring; arrhythmias; leg movements; body position during sleep; and blood oxygen saturation. Another key piece of reported data is the apnea-hypopnea index (AHI) or the respiratory disturbance index (RDI), which describe the severity of SDB. The AHI is defined as the average number of apnea and/or hypopnea episodes that occur during sleep divided by the number of hours of sleep and is expressed in events per hour. The RDI is calculated the same as the AHI; however, the RDI may also include other respiratory disturbances beyond apneas and hypopneas (e.g., snoring arousals, oxygen desaturation events, etc.). Thus, depending upon what respiratory-related events are scored, the RDI may be greater than the AHI. Based on guidelines developed by the American Academy of Sleep Medicine (AASM), the diagnosis of OSA is confirmed if the number of obstructive events (obstructive apneas, hypopneas + respiratory event related arousals) during the sleep study is greater than 15 events/hour, or greater than five events/hour in a patient who reports any of the following: unintentional sleep episodes during wakefulness; daytime sleepiness; non-refreshing sleep; fatigue; insomnia; waking up breath holding, gasping, or choking; or the bed partner describing loud snoring, breathing interruptions, or both during the patient’s sleep.68 OSA severity is defined as mild with an AHI or RDI ≥5 and <15, moderate with an AHI or RDI ≥15 and ≤30, or severe with an AHI or RDI >30.68 For CSA, the PSG is diagnostic if respiratory monitoring demonstrates at least three consecutive cycles of crescendo-decrescendo change in breathing amplitude and one or both of the following: 1) five or more central sleep apneas or hypopneas per sleep hour and/or 2) cyclical crescendo-decrescendo breathing ≥ 10 consecutive minutes.84 Unlike OSA, the AASM has not yet established a severity scale for CSA in HF due to a lack of large scale studies documenting a link between the extent of CSA and morbidity and mortality.84 Clinically, however, the OSA severity scale is often used to describe the severity of CSA as well.3 Thus, CSA severity is typically defined as mild with an AHI or RDI ≥5 and <15, moderate with an AHI or RDI ≥15 and ≤30, or severe with an AHI or RDI >30.
Although the PSG sleep study remains the gold standard for diagnosing SDB, it does have a number of drawbacks, namely that it is relatively expensive (although it is a covered service by Medicare and most private insurance companies when medical need is documented), it requires technical expertise, and it is labor-intensive and time-consuming. Timely access for testing is a problem for many patients as well. Patients may also be unwilling to undergo overnight testing in a monitored sleep laboratory. Therefore, there is an ongoing interest in alternative approaches to PSG, such as portable monitoring, for the diagnostic assessment of patients with suspected SDB.
Portable monitors are designed to be used in an unattended, home-based setting. A number of different types of portable monitors are commercially available, with each device differing in the number and type of sleep-related variables they monitor. Studies of Class III portable monitors (which at a minimum record oxygen, nasal flow, and thoracic and abdominal movement) in both the inpatient and unattended outpatient setting have found them to be accurate when compared to the gold standard conventional PSG for diagnosing SDB associated with HF and for discriminating between CSA and OSA.69,85 However, these devices do not typically measure sleep and may underestimate the severity of the disorder. Portable monitoring thus offers a useful alternative to diagnosing SDB in HF when PSG is unavailable or if the patient is unwilling or unable to undergo testing in a sleep laboratory.
Treating Sleep Disordered Breathing in Heart Failure: What Are the Options?
SDB in patients with HF should be approached as a coexisting chronic disease requiring long-term, multidisciplinary management. The choice of treatment will largely depend on the type and severity of the SDB found on the PSG. Nonetheless, the patient should be encouraged to actively participate in the decision on treatment type and to contribute to the management of his or her own disease. The clinical care pathway describing the treatment of OSA and CSA in patients with HF is presented in Figures 6 and 7 and discussed below.
Figure 6.
Treatment pathway for heart failure patients with obstructive sleep apnea (OSA). Adapted from J Clin Sleep Med. 2009;5:263–276. Abbreviations: ACE, angiotensin converting enzyme; AHI, apnea-hyponea index; CPAP, continuous positive airway pressure; HF, heart failure; OSA, obstructive sleep apnea; SDB, sleep disordered breathing; Tx, treatment
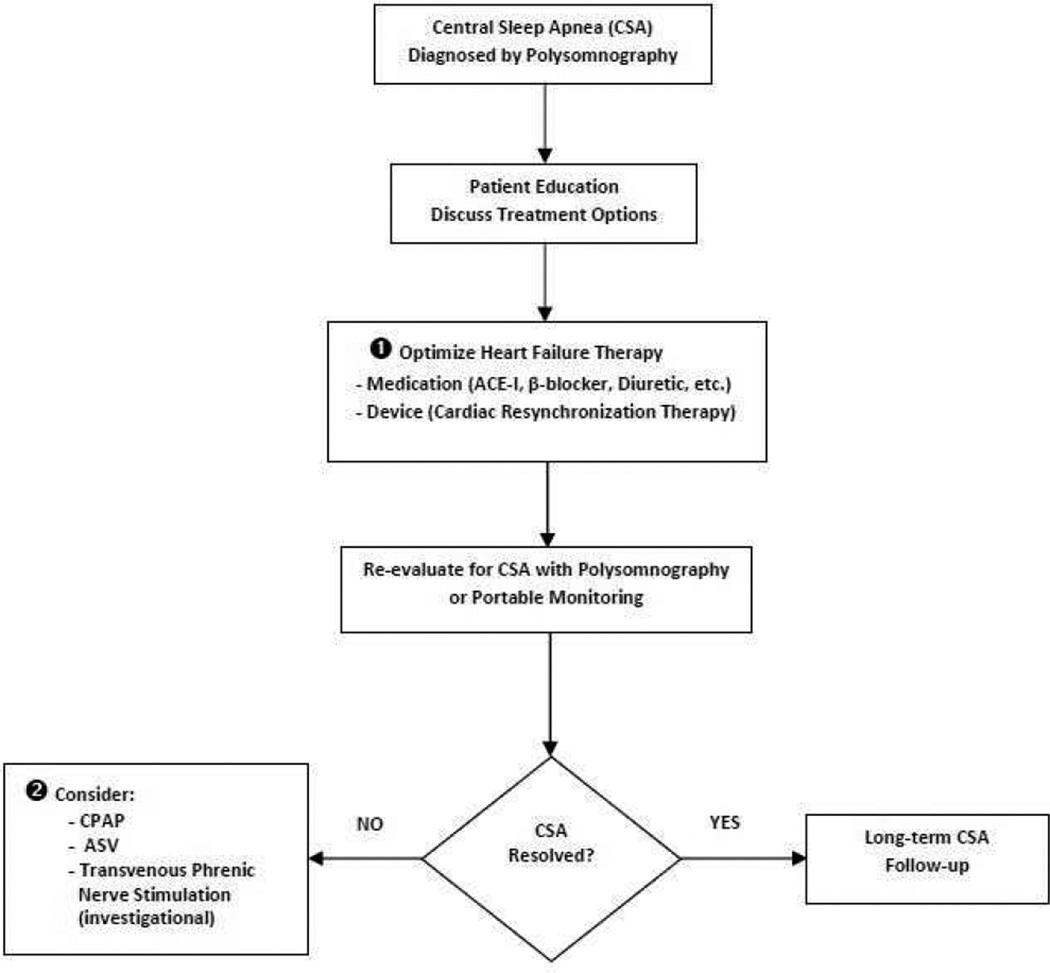
Figure 7.
Treatment pathway for heart failure patients with central sleep apnea (CSA). Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; ASV, adaptive servo-ventilation; CSA, central sleep apnea; CPAP, continuous positive airway pressure
Treatment of Obstructive Sleep Apnea
In HF patients with OSA, treatment should begin with optimizing medical therapy for HF (Figure 6, ➊). Optimal HF therapy includes aggressive diuresis to reduce pulmonary congestion, beta-blockers to blunt the effects of sympathetic overstimulation, and angiotensin-converting enzyme inhibitors to reduce ventricular afterload and improve cardiac output. Achieving fluid homeostasis is key since fluid retention may contribute to peripharygeal edema, cervical congestion, and subsequent airway obstruction in HF patients with OSA when supine.78,79 Recent research has shown that the aldosterone antagonist spironolactone improves postural fluid shifts as well as fluid retention, hypertension, and AHI in patients with OSA86 Thus, in accordance with current HF treatment guidelines, aldosterone antagonists should be part of the comprehensive medical treatment of HF patients with OSA87
For patients whose sleep study shows mild OSA, the addition of simple behavioral or lifestyle modifications may be all that is needed to reduce or abolish the obstructive apneas (Figure 6, ➋). In such cases, weight loss for those who are obese, avoidance of alcohol and sedative medications before bedtime, and simple positional changes, such as avoiding the supine sleeping position, are recommended.68
For patients with moderate to severe OSA, and in cases of mild OSA that are associated with significant daytime sleepiness, continuous positive airway pressure (CPAP) therapy is the primary therapeutic option (Figure 6, ➌).68 CPAP is a type of pneumatic upper airway “splint” that is highly effective in reversing airway obstruction in patients with OSA. With CPAP, the patient is fitted with a tight fitting nasal or facial mask, which is attached to an electric blower that applies a constant positive pressure to the airway to maintain patency. Therapy parameters are established during an overnight CPAP titration sleep study.
In symptomatic individuals, the results of CPAP therapy are often dramatic, with most patients experiencing a rapid return of normal sleep, resolution of their daytime sleepiness, and improvement in their neurocognitive function.88,89 In studies of HF patients with OSA, CPAP therapy has been shown to eliminate recurrent hypoxia, reduce nocturnal blood pressure and heart rate, and improve left ventricular function.90–93 In addition, one small, nonrandomized trial reported that CPAP may even reduce the risk of death and hospitalization among patients with HF and OSA.94 The exact mechanism by which CPAP therapy improves cardiac function in HF patients with OSA is not completely understood, although it may be the result of improved nighttime oxygen saturation, reduced SNS drive, and normalized intrathoracic pressure.90–93
To achieve the therapeutic benefits of CPAP, it must be used consistently and for a prescribed number of hours each night. Unfortunately, patient noncompliance with CPAP therapy is a common problem. Poor compliance (i.e., intermittent use of CPAP) may occur in up to 50% of patients and is often present early in the course of therapy.95 Perceived discomfort, sneezing, nasal discharge or dryness, claustrophobia, and nighttime panic attacks are the most common complaints leading to patient noncompliance. Most of these complaints can be readily addressed through adjustments in mask fit, humidification, and changes in therapy parameters. Careful follow-up for CPAP compliance and problems is therefore required so that an effective therapy routine is established and problems are resolved as needed (Figure 6, ➍).
A number of other therapies have been devised to treat OSA, including custom-made mandibular advancement devices to prevent tongue-related pharyngeal obstruction during sleep, nasal devices worn inside each nostril that create increased expiratory nasal resistance to maintain a patent upper airway, and various surgical procedures designed to enlarge the pharyngeal airway (Figure 6, ➎). However, none of these therapies to date has been found to be as effective as CPAP in reducing or eliminating OSA68 Furthermore, they have not been specifically tested on patients with HF and OSA Thus, these alternative therapies should be reserved for those patients who do not respond to or simply cannot tolerate CPAP therapy.
Treatment of Central Sleep Apnea
At present, there is no consensus regarding the optimal treatment strategy for CSA in patients with HF. A number of different therapies have been proposed, and although several have been shown to be of some benefit in reducing CSA or its symptoms, none have proved curative. Also lacking for most proposed therapies are large scale, prospective, randomized trials establishing their efficacy. At present, therapy focuses on either improving HF or reducing CSA itself.
Since HF is believed to be the fundamental reason for the development of CSA, optimizing treatment of the underlying HF is of foremost importance (Figure 7, ➊). A number of studies have demonstrated that once HF is clinically improved, CSA often improves as well.96–98 Cardiac resynchronization therapy (CRT) should also be considered in indicated patients as several small, nonrandomized studies have shown improvement in CSA with its use.99,100 It is likely that the associated increase in cardiac output is responsible for its effect.
Since CSA persists despite aggressive treatment of HF in most patients, targeted treatment for CSA must be considered (Figure 7, ➋). CPAP has been the most extensively investigated in CSA Early, small, short duration trials of CPAP showed some positive effects on CSA in HF patients, including a reduction in ventricular ectopic beats, a reduction in nocturnal urinary and daytime plasma norepinephrine levels (a marker of SNS activity), improved quality of life, reduced daytime sleepiness, and a trend towards reduced mortality and cardiac transplantation.61,66,101,102 In order to confirm these early findings, the Canadian Positive Airway Pressure Trial for Patients with Congestive Heart Failure and Central Sleep Apnea (CANPAP) was performed.103 CANPAP was a large, prospective, multicenter study that randomized 258 optimally treated HF patients with an LVEF< 40% and CSA with an AHI >15 to receive either CPAP or no CPAP. The primary endpoint of the study was heart transplant-free survival. Secondary endpoints included the effects of CPAP on LVEF, quality of life, exercise tolerance, number of hospitalizations, and levels of plasma norepinephrine and atrial natriuretic peptide.
Results from CANPAP were mixed. On average, the AHI was reduced from 40 to 19 events/hour after 3 months of CPAP therapy, and this reduction was associated with improved nocturnal oxygenation, exercise tolerance, and LVEF, and lower plasma norepinephrine levels. Nonetheless, CPAP did not have any effect on the primary endpoint, transplant-free survival. Interestingly, there was also an early divergence in survival rates that suggested early worse outcomes in the CPAP-treated group. After a mean follow-up of two years, however, the primary outcome was identical in the treated and control groups.
CANPAP, however, suffered from a number of issues that made its results difficult to interpret. Key among them was poor patient compliance with CPAP therapy, which after one year, was being used for only 3.6 hours per night. Poor compliance may have been why the CPAP patients experienced significantly less reduction in their AHI than in earlier CPAP trials. This finding raised the question whether the failure of CPAP to more completely reverse CSA during the trial may have been the reason why the study failed to meet its primary endpoint, transplant-free survival. To evaluate this possibility, a post-hoc analysis of the CANPAP data was performed.104 CANPAP patients were stratified into 3 groups: control subjects; those treated with CPAP with suppression of CSA to an AHI <15; and those treated with CPAP without suppression of CSA (AHI > 15). It was found that in the 57% of CANPAP patients in whom CPAP reduced the AHI <15, transplant-free survival was statistically significantly improved compared with the control group (no CPAP), while among the 43% of CANPAP patients in whom CPAP did not reduce the AHI <15, the transplant-free survival did not improve compared to the control group (no CPAP). These results appeared to confirm the hypothesis that adequate suppression of CSA might lead to improved transplant-free survival.
Overall, CANPAP has been widely viewed as a flawed study, with CPAP therapy being shown to have unpredictable, and in some cases adverse, effects on HF patients with CSA. It has been suggested that because CPAP increases intrathoracic pressure, it may cause adverse effects on both right and left ventricular preload and afterload that ultimately worsen rather than improve cardiac function.105 It has also been proposed that these adverse hemodynamic effects may have contributed to the early mortality in the CANPAP trial.105
Because of poor patient compliance and the potential adverse hemodynamic effects with CPAP therapy, a newer and potentially better tolerated and effective type of noninvasive ventilatory support, called adaptive pressure support servoventilation (ASV) has been developed and is currently undergoing clinical evaluation. ASV was designed to address several aspects of the respiratory disturbance, including ventilatory overshoot and undershoot (Figure 1). ASV, like CPAP, delivers a baseline continuous positive airway pressure; however, ASV devices are also equipped with sensitive sensors that can detect central apneas and deliver several breaths at the tidal volume and respiratory rate previously determined to match the patient’s minute ventilation during stable breathing. The goal of ASV therapy is to prevent the increase in PaCO2 during apnea and the hyperventilation that follows, thereby breaking the periodic breathing cycle.
Research suggests that ASV may be better tolerated than CPAP, which is likely due to its use of ventilation algorithms that provide different levels of pressure support based on the type of sleep disordered breathing detected by the device.106–108 This results in greater regulation of amount of airflow blown into the patient, making it more comfortable. Nonetheless, the patient still must wear a mask, which may be difficult for those who are short of breath at baseline. Small studies of ASV have shown it to be more effective than CPAP in reducing CSA in HF.106–108 Additionally, these small studies demonstrated that ASV improves LVEF and quality of life.106,107 Large, multinational trials are currently underway to see if ASV will also improve morbidity and mortality in HF patients with CSA.
Recently, another new investigational treatment for CSA has been introduced, and it may hold some promise. It involves a totally implantable lead-based system that provides unilateral transvenous stimulation of the phrenic nerve to regulate breathing during a central apneic event. The stimulation system is similar in size and appearance to a standard pacemaker and is likewise implanted in either the right or left pectoral region. The system utilizes two transvenous leads: one for neurostimulation and one for sensing. As a totally implantable, device-based therapy, it may be better tolerated than CPAP or ASV in HF patients and thus improve patient compliance with treatment.109
Early clinical experience with this technology has been encouraging. In one small multicenter pilot study, 16 HF patients (ejection fraction 30±12%) with documented CSA by PSG underwent acute placement of a neurostimulation lead into either the right brachiocephalic vein or the left pericardiophrenic vein. Patients then underwent polysomnography over two nights to compare sleep characteristics during a control night (no phrenic stimulation) to a therapy night (with phrenic stimulation during episodes of CSA). Overall, unilateral transvenous phrenic nerve stimulation resulted in significant improvement in major indices of CSA severity, including the AHI, central apnea index, 4% oxygen desaturation index, and arousal index.110 Active research of the system is currently ongoing to evaluate its longer-term safety and efficacy.
Conclusion
Both OSA and CSA are common in HF. Mounting clinical evidence shows that both conditions accelerate the progression of HF and worsen morbidity and mortality. Unfortunately, OSA and CSA are not widely recognized by clinicians, so they are not routinely considered in the evaluation and treatment of HF. This is especially true for CSA, where its more subtle findings are often lost in the signs and symptoms that typically accompany HF.
The diagnosis and treatment of SDB in HF requires a multidisciplinary approach, as the expertise of primary care, heart failure, and sleep medicine clinicians is needed to care for these challenging patients. A high index of suspicion for SDB should exist when an HF patient presents with the clinical features or risk factors of SDB. In these patients, an overnight sleep study should be performed.
The treatment of OSA in HF with CPAP is well established; however, the optimal treatment of CSA in patients with HF remains unclear. When CSA is found in a patient with HF, optimizing medical therapy, and when appropriate, using device-based therapy (e.g., CRT), are of foremost importance. In cases where CSA persists despite aggressive treatment of HF, other interventions, such as CPAP, should be considered. Additionally, ASV and phrenic nerve stimulation offer promising new ways to treat CSA in HF, but large-scale, long-term, randomized, controlled trials are still needed to further evaluate their potential clinical impact.
ACKNOWLEDGMENTS
The authors wish to thank Janice Hoettels, PA-C, MBA, for her assistance with the preparation of the manuscript.
Funding: Funding was provided by Respicardia, Inc., Minnetonka, MN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Drs. Khayat and Abraham are paid consultants to Respicardia, Inc. Ms.Clark is employed by Respicardia, Inc. Ms. Rathman is a speaker and consultant to Medtronic, Inc., a consultant to St. Jude Medical, and a member of the speakers bureau for Osuka. All other authors have no conflicts of interest to report.
REFERENCES
- 1.Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, Wexler L, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–2159. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 2.Paulino A, Damy T, Margarit L, Stöica M, Deswarte G, Khouri L, et al. Prevalence of sleep-disordered breathing in a 316-patient French cohort of stable congestive heart failure. Arch Cardiovasc Dis. 2009;102:169–175. doi: 10.1016/j.acvd.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Töpfer V. Sleep disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–257. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Parker JD, Newton GE, Floras JS, Mak S, Chiu K-L, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49:1625–1631. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 5.Lanfranchi PA, Braghiroli A, Bosimini E, Mazzuero G, Colombo R, Donner CF, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99:1435–1440. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 6.Javaheri S, Caref EB, Chen E, Tong KB, Abraham WT. Sleep apnea testing and outcomes in a large cohort of Medicare beneficiaries with newly diagnosed heart failure. Am J Respir Crit Care Med. 2011;183:539–546. doi: 10.1164/rccm.201003-0406OC. [DOI] [PubMed] [Google Scholar]
- 7.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonsignore MR, Marrone O, Insalaco G, Bonsignore G. The cardiovascular effects of obstructive sleep apneas: analysis of pathogenic mechanisms. Eur Respir J. 1994;7:786–805. doi: 10.1183/09031936.94.07040786. [DOI] [PubMed] [Google Scholar]
- 9.Fogel RB, Malhotra A, White DP. Sleep. 2. Pathophysiology of obstructive sleep apnea/hypopnea syndrome. Thorax. 2004;59:159–163. doi: 10.1136/thorax.2003.015859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fogel RB, Malhotra A, Pilar G, Edwards JK, Beauregard J, Shea SA, et al. Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Mechanisms of muscle control. Am J Respir Crit Care Med. 2001;164:2025–2030. doi: 10.1164/ajrccm.164.11.2102048. [DOI] [PubMed] [Google Scholar]
- 11.Fogel RB, Trinder J, Malhotra A, Stanchina M, Edwards JK, Schory KE, et al. Within breath control of genioglossal muscle activation in humans: effect of sleep-wake state. J Physiol. 2003;550:899–910. doi: 10.1113/jphysiol.2003.038810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu K-L, Ryan CM, Shiota S, Ruttanaumpawan P, Arzt M, Haight JS, et al. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. Am J Respir Crit Care Med. 2006;174:1378–1383. doi: 10.1164/rccm.200607-927OC. [DOI] [PubMed] [Google Scholar]
- 13.Bucca CA, Brussino L, Battisti A, Mutani R, Rolla G, Mangiardi L, et al. Diuretics in obstructive sleep apnea with diastolic heart failure. Chest. 2007;132:440–446. doi: 10.1378/chest.07-0311. [DOI] [PubMed] [Google Scholar]
- 14.Bradley TD, Brown IG, Grossman RF, Zamel N, Martinez D, Phillipson EA, et al. Pharyngeal size in snorers, non-snorers, and patients with obstructive apnea. N Engl J Med. 1986;315:1327–1331. doi: 10.1056/NEJM198611203152105. [DOI] [PubMed] [Google Scholar]
- 15.Eikermann M, Jordan AS, Chamberlin NL, Gautam S, Wellman A, Lo Y-L, et al. The influence of aging on pharyngeal collapsibility during sleep. Chest. 2007;131:1702–1709. doi: 10.1378/chest.06-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malhotra A, Huang Y, Fogel RB, Pillar G, Edwards JK, Kikinis R, et al. The male predisposition to pharyngeal collapse. Importance of airway length. Am J Respir Crit Care Med. 2002;166:1388–1395. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 17.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. Circulation. 2008;118:1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 18.Bradley TD, Floras JS. Sleep apnea and heart failure: Part I: obstructive sleep apnea. Circulation. 2003;107:1671–1678. doi: 10.1161/01.CIR.0000061757.12581.15. [DOI] [PubMed] [Google Scholar]
- 19.Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol. 2011;57:119–127. doi: 10.1016/j.jacc.2010.08.627. [DOI] [PubMed] [Google Scholar]
- 20.Bradley TD, Hall MJ, Ando S, Floras JS. Hemodynamic effects of simulated obstructive apneas in humans with and without heart failure. Chest. 2001;119:1827–1835. doi: 10.1378/chest.119.6.1827. [DOI] [PubMed] [Google Scholar]
- 21.Parker JD, Brooks D, Kozar LF, Render-Teixeira CL, Homer RL, Bradley TD, et al. Acute and chronic effects of airway obstruction on canine left ventricular performance. Am J Respir Crit Care Med. 1999;160:1888–1896. doi: 10.1164/ajrccm.160.6.9807074. [DOI] [PubMed] [Google Scholar]
- 22.Tolle FA, Judy WV, Yu PL, Markand ON. Reduced stroke volume related to pleural pressure in obstructive sleep apnea. J Appl Physiol. 1983;55:1718–1724. doi: 10.1152/jappl.1983.55.6.1718. [DOI] [PubMed] [Google Scholar]
- 23.Shiomi T, Guilleminault C, Stoohs R, Schnittger I. Leftward shift of the interventricular septum and pulsus paradoxus in obstructive sleep apnea syndrome. Chest. 1991;100:894–902. doi: 10.1378/chest.100.4.894. [DOI] [PubMed] [Google Scholar]
- 24.Brinker JA, Weiss JL, Lappe DL, Rabson JL, Summer WR, Permutt S. Leftward septal displacement during right ventricular loading in man. Circulation. 1980;61:626–633. doi: 10.1161/01.cir.61.3.626. [DOI] [PubMed] [Google Scholar]
- 25.Yumino D, Kasai T, Kimmerly D, Amirthalingam V, Floras JS, Bradley TD. Differing effects of obstructive and central sleep apneas on stroke volume in patients with heart failure. Am J Respir Crit Care Med. 2013;187:433–438. doi: 10.1164/rccm.201205-0894OC. [DOI] [PubMed] [Google Scholar]
- 26.Franklin KA, Nilsson JB, Sahlin C, Naslund U. Sleep apnea and nocturnal angina. Lancet. 1995;345:1085–1087. doi: 10.1016/s0140-6736(95)90820-x. [DOI] [PubMed] [Google Scholar]
- 27.Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003;177:385–390. doi: 10.1046/j.1365-201X.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- 28.Narkiewicz K, Kato M, Phillips BC, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332–2335. doi: 10.1161/01.cir.100.23.2332. [DOI] [PubMed] [Google Scholar]
- 29.Peng Y-J, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prabhakar NR, Kumar GK, Peng Y-J. Sympatho-adrenal activation by chronic intermittent hypoxia. J Appl Physiol. 2012;113:1304–1310. doi: 10.1152/japplphysiol.00444.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mills PJ, Kennedy BP, Loredo JS, Dimsdale JE, Ziegler MG. Effects of continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apnea. J Appl Physiol. 2006;100:343–348. doi: 10.1152/japplphysiol.00494.2005. [DOI] [PubMed] [Google Scholar]
- 32.Heitmann J, Ehlenz K, Penzel T, Becker HF, Grote L, Voigt KH, et al. Sympathetic activity is reduced by nCPAP in hypertensive obstructive sleep apnoea patients. Eur Respir J. 2004;23:255–262. doi: 10.1183/09031936.04.00015604. [DOI] [PubMed] [Google Scholar]
- 33.Witkowski A, Prejbisz A, Florczak E, Kadziela J, Sliwinski P, Bieleri P, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–565. doi: 10.1161/HYPERTENSIONAHA.111.173799. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension. 1999;34:309–314. doi: 10.1161/01.hyp.34.2.309. [DOI] [PubMed] [Google Scholar]
- 35.Peng Y-J, Raghuraman G, Khan SA, Kumar GK, Prabhakar NR. Angiotensin II evokes sensory long-term facilitation of the carotid body via NADPH oxidase. J Appl Physiol. 2011;111:964–970. doi: 10.1152/japplphysiol.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Javaheri S. Sleep disorders in systolic heart failure: a prospective study of 100 male patients. The final report. Int J Cardiol. 2006;106:21–28. doi: 10.1016/j.ijcard.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 37.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 38.MacDonald M, Fang J, Pittman SD, White DP, Malhotra A. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med. 2008;4:38–42. [PMC free article] [PubMed] [Google Scholar]
- 39.Herrscher TE, Akre H, Øverland B, Sandvik L, Westheim AS. High prevalence of sleep apnea in heart failure outpatients: even in patients with preserved systolic function. J Card Fail. 2011;17:420–425. doi: 10.1016/j.cardfail.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11:602–608. doi: 10.1093/eurjhf/hfp057. [DOI] [PubMed] [Google Scholar]
- 41.Chan J, Sanderson J, Chan W, Lai C, Choy D, Ho A, et al. Prevalence of sleep-disordered breathing in diastolic heart failure. Chest. 1997;111:1488–1493. doi: 10.1378/chest.111.6.1488. [DOI] [PubMed] [Google Scholar]
- 42.The International Classification of Sleep Disorders, Revised. 2nd ed. Westchester IL: The American Academy of Sleep Medicine; 2005. [Google Scholar]
- 43.Naughton M, Benard D, Tam A, Rutherford R, Bradley TD. Role of hyperventilation in the pathogenesis of central sleep apneas in patients with congestive heart failure. Am Rev Respir Dis. 1993;148:330–338. doi: 10.1164/ajrccm/148.2.330. [DOI] [PubMed] [Google Scholar]
- 44.Hanly P, Zuberi N, Gray R. Pathogenesis of Cheyne-Stokes respiration in patients with congestive heart failure: relationship to arterial PCO2. Chest. 1993;104:1079–1084. doi: 10.1378/chest.104.4.1079. [DOI] [PubMed] [Google Scholar]
- 45.Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp Physiol. 2005;90:13–24. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- 46.Xie A, Skatrud JB, Puleo DS, Rahko PS, Dempsey JA. Apnea-hypopnea threshold for CO2 in patients with congestive heart failure. Am J Respir Crit Care Med. 2002;165:1245–1250. doi: 10.1164/rccm.200110-022OC. [DOI] [PubMed] [Google Scholar]
- 47.Solin P, Roebuck T, Johns DP, Walters EH, Naughton MT. Peripheral and central ventilatory responses in central sleep apnea with and without congestive heart failure. Am J Respir Crit Care Med. 2000;162:2194–2200. doi: 10.1164/ajrccm.162.6.2002024. [DOI] [PubMed] [Google Scholar]
- 48.Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med. 1999;341:949–954. doi: 10.1056/NEJM199909233411304. [DOI] [PubMed] [Google Scholar]
- 49.Yu J, Zhang F, Fletcher EC. Stimulation of breathing by activation of pulmonary peripheral afferents in rabbits. J Appl Physiol. 1998;85:1485–1492. doi: 10.1152/jappl.1998.85.4.1485. [DOI] [PubMed] [Google Scholar]
- 50.Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999;99:1574–1579. doi: 10.1161/01.cir.99.12.1574. [DOI] [PubMed] [Google Scholar]
- 51.Lorenzi-Filho G, Azevedo ER, Parker JD, Bradley TD. Relationship of carbon dioxide tension in arterial blood to pulmonary wedge pressure in heart failure. Eur Respir J. 2002;19:37–40. doi: 10.1183/09031936.02.00214502. [DOI] [PubMed] [Google Scholar]
- 52.Bradley TD, Floras JS. Sleep apnea and heart failure: Part II: central sleep apnea. Circulation. 2003;107:1822–1826. doi: 10.1161/01.CIR.0000061758.05044.64. [DOI] [PubMed] [Google Scholar]
- 53.Brack T. Cheyne-Stokes respiration in patients with congestive heart failure. Swiss Med Wkly. 2003;133:605–610. doi: 10.4414/smw.2003.10268. [DOI] [PubMed] [Google Scholar]
- 54.Xie A, Skatrud JB, Khayat R, Dempsey JA, Morgan B, Russell D. Cerebrovascualr response to carbon dioxide in patients with congestive heart failure. Am J Respir Crit Care Med. 2005;172:371–378. doi: 10.1164/rccm.200406-807OC. [DOI] [PubMed] [Google Scholar]
- 55.Alex CG, Onal E, Lopata M. Upper airway occlusion during sleep in patients with Cheyne-Stokes respiration. 1986;133:42–45. doi: 10.1164/arrd.1986.133.1.42. [DOI] [PubMed] [Google Scholar]
- 56.Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during sleep apnea. J Appl Physiol. 1995;78:1806–1815. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
- 57.Hall MJ, Xie A, Rutherford R, Ando S, Floras JS, Bradley TD. Cycle length of periodic breathing in patients with and without heart failure. Am J Respir Crit Care Med. 1996;154:376–381. doi: 10.1164/ajrccm.154.2.8756809. [DOI] [PubMed] [Google Scholar]
- 58.Somer VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol. 1989;67:2101–2106. doi: 10.1152/jappl.1989.67.5.2101. [DOI] [PubMed] [Google Scholar]
- 59.Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26:1257–1263. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- 60.Leung RS, Diep TM, Bowman ME, Lorenzi-Filho G, Bradley TD. Provocation of ventricular ectopy by Cheyne-Stokes respiration in patients with heart failure. Sleep. 2004;27:1337–1343. doi: 10.1093/sleep/27.7.1337. [DOI] [PubMed] [Google Scholar]
- 61.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000;101:392–397. doi: 10.1161/01.cir.101.4.392. [DOI] [PubMed] [Google Scholar]
- 62.Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Resp Crit Care Med. 1996;153:272–276. doi: 10.1164/ajrccm.153.1.8542128. [DOI] [PubMed] [Google Scholar]
- 63.Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49:2028–2034. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 64.Andreas S, Hagenah G, Moller C, Werner GS, Kreuzer H. Cheyne-Stokes respiration and prognosis in congestive heart failure. Am J Cardiol. 1996;78:1260–1264. doi: 10.1016/s0002-9149(96)00608-x. [DOI] [PubMed] [Google Scholar]
- 65.Ponikowski P, Anker SD, Chua TP, Francis D, Banasiak W, Poole-Wilson PA, et al. Oscillatory breathing patterns during wakefulness in patients with chronic heart failure: clinical implications and role of augmented peripheral chemosensitivity. Circulation. 1999;100:2418–2424. doi: 10.1161/01.cir.100.24.2418. [DOI] [PubMed] [Google Scholar]
- 66.Sin DD, Logan AG, Fitzgerald FS, Liu PP, Bradley TD. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation. 2000;102:61–66. doi: 10.1161/01.cir.102.1.61. [DOI] [PubMed] [Google Scholar]
- 67.Khayat R, Abraham W, Patt B, Brinkman V, Wannemacher J, Porter K, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Cardiac Fail. 2012;18:534–540. doi: 10.1016/j.cardfail.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Epstein LJ, Kristo D, Strollo PJ, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management, and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 69.Khayat RM, Jarjoura D, Patt B, Yamokoski T, Abraham WT. In-hospital testing for sleep disordered breathing in hospitalized patients with decompensated heart failure-report of prevalence and patient characteristics. J Card Fail. 2009;15:739–746. doi: 10.1016/j.cardfail.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 71.Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994;17:703–710. doi: 10.1093/sleep/17.8.703. [DOI] [PubMed] [Google Scholar]
- 72.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 73.Gottlieb DJ, Whitney CW, Bonekat WH, Iber C, James GD, Lebowitz M, et al. Relation of sleepiness to respiratory disturbance index, the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159:502–507. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- 74.Punjabi NM, Bandeen-Roche K, Young T. Predictors of objective sleep tendency in the general population. Sleep. 2003;26:678–683. doi: 10.1093/sleep/26.6.678. [DOI] [PubMed] [Google Scholar]
- 75.Arzt M, Young T, Finn L, Skatrud JB, Ryan CM, Newton GE, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166:1716–1722. doi: 10.1001/archinte.166.16.1716. [DOI] [PubMed] [Google Scholar]
- 76.Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 77.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003;289:2230–2237. doi: 10.1001/jama.289.17.2230. [DOI] [PubMed] [Google Scholar]
- 78.Friedman O, Bradley TD, Chan CT, Parkes R, Logan AG. Relationship between overnight rostral fluid shift and obstructive sleep apnea in drug-resistant hypertension. Hypertension. 2010;56:1077–1082. doi: 10.1161/HYPERTENSIONAHA.110.154427. [DOI] [PubMed] [Google Scholar]
- 79.Redolfi S, Yumino D, Ruttanaumpawan P, Yau B, Su MC, Lam J, et al. Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. Am J Respir Crit Care Med. 2009;179:241–246. doi: 10.1164/rccm.200807-1076OC. [DOI] [PubMed] [Google Scholar]
- 80.Nuckton TJ, Glidden DV, Browner WS, Claman DM. Physical examination: Mallampati score as an independent predictor of obstructive sleep apnea. Sleep. 2006;29:903–908. doi: 10.1093/sleep/29.7.903. [DOI] [PubMed] [Google Scholar]
- 81.Calvin AD, Somers VK, van der Walt C, Scott CG, Olson LJ. Relation of natriuretic peptide concentrations to central sleep apnea in patients with heart failure. Chest. 2011;140:1517–1523. doi: 10.1378/chest.10-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tkacova R, Niroumand M, Lorenzi-Fihlo G, Bradley TD. Overnight shift from obstructive to central apneas in patients with heart failure: role of PCO2 and circulatory delay. Circulation. 2001;103:238–243. doi: 10.1161/01.cir.103.2.238. [DOI] [PubMed] [Google Scholar]
- 83.Tkacova R, Wang H, Bradley TD. Night-to-night alterations in sleep apnea type in patients with heart failure. J Sleep Res. 2006;15:321–328. doi: 10.1111/j.1365-2869.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 84.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 85.Quintana-Gallego E, Villa-Gill M, Caromona-Bernal C, Botebol-Benhamou G, Martinez-Maritinez A, Sanchez-Armengol A, et al. Home respiratory polygraphy for diagnosis of sleep-disordered breathing in heart failure. Eur Respir J. 2004;24:443–448. doi: 10.1183/09031936.04.00140603. [DOI] [PubMed] [Google Scholar]
- 86.Gaddam K, Pimenta E, Thomas SJ, Cofield SS, Oparil S, Harding SM, et al. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum Hypertens. 2009;24:532–537. doi: 10.1038/jhh.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 88.Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343:572–575. doi: 10.1016/s0140-6736(94)91522-9. [DOI] [PubMed] [Google Scholar]
- 89.Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Drey IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:461–467. doi: 10.1164/ajrccm.159.2.9803121. [DOI] [PubMed] [Google Scholar]
- 90.Tkacova R, Rankin F, Fitzgerald FS, Cloras JS, Bradley TD. Effects of continuous positive airway pressure on obstructive sleep apnea and left ventricular afterload in patients with heart failure. Circulation. 1998;98:2269–2275. doi: 10.1161/01.cir.98.21.2269. [DOI] [PubMed] [Google Scholar]
- 91.Malone S, Liu PP, Holloway R, Rutherford R, Xie A, Bradley TD. Obstructive sleep apnea in patients with dilated cardiomyopathy: effects of continuous positive airway pressure. Lancet. 1991;338:1480–1484. doi: 10.1016/0140-6736(91)92299-h. [DOI] [PubMed] [Google Scholar]
- 92.Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, Kubo T, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 93.Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169:361–366. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 94.Kasai T, Narui K, Dohi T, Yanagisawa N, Ishiwata S, Ohno M, et al. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest. 2008;133:690–696. doi: 10.1378/chest.07-1901. [DOI] [PubMed] [Google Scholar]
- 95.American Thoracic Society. Indications and standards for use of nasal continuous positive airway pressure (CPAP) in sleep apnea syndrome. Am J Respir Crit Care Med. 1994;150:1738–1745. doi: 10.1164/ajrccm.150.6.7952642. [DOI] [PubMed] [Google Scholar]
- 96.Dark DS, Pingleton SK, Kerby GR, Crabb JE, Gollub SB, Glatter TR, et al. Breathing pattern abnormalities and arterial oxygen desaturation during sleep in congestive heart failure syndrome: improvement following medical therapy. Chest. 1987;91:833–836. doi: 10.1378/chest.91.6.833. [DOI] [PubMed] [Google Scholar]
- 97.Walsh JT, Andrews R, Starling R, Cowley AJ, Johnston ID, Kinnear WJ. Effects of captopril and oxygen on sleep apnoea in patients with mild to moderate congestive heart failure. Br Heart J. 1995;73:237–241. doi: 10.1136/hrt.73.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baylor P, Tayloe D, Owen D, Sander C. Cardiac failure presenting as sleep apnea. Elimination of apnea following medical management of cardiac failure. Chest. 1988;94:1298–1299. doi: 10.1378/chest.94.6.1298. [DOI] [PubMed] [Google Scholar]
- 99.Sinha AM, Skobel EC, Breithardt OA, Norra C, Markus KU, Creuer C, et al. Cardiac resynchronization therapy improves central sleep apnea and Cheyne-Stokes respiration in patients with chronic heart failure. J Am Coll Cardiol. 2004;44:68–71. doi: 10.1016/j.jacc.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 100.Kara T, Novak M, Nykodym J, Bybee KA, Meluzin J, Orban M, et al. Effect of cardiac resynchronization therapy on sleep disordered breathing in patients with systolic heart failure. Chest. 2008;134:87–93. doi: 10.1378/chest.07-2832. [DOI] [PubMed] [Google Scholar]
- 101.Naughton MT, Liu PP, Bernard DC, Goldstein RS, Bradley TD. Treatment of congestive heart failure and Cheyne-Stokes respiration during sleep by continuous positive airway pressure. Am J Respir Crit Care Med. 1995;151:92–97. doi: 10.1164/ajrccm.151.1.7812579. [DOI] [PubMed] [Google Scholar]
- 102.Tkacova R, Liu PP, Naughton MT, Bradley TD. Effect of continuous positive airway pressure on mitral regurgitant fraction and atrial natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 1997;30:739–745. doi: 10.1016/s0735-1097(97)00199-x. [DOI] [PubMed] [Google Scholar]
- 103.Bradley TD, Logan AG, Kimoff RJ, Sériès F, Morrison D, Ferguson K, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 104.Arzt M, Floras JS, Logan AG, Kimoff RJ, Sériès F, Morrison D, et al. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP) Circulation. 2007;115:3173–3180. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 105.Javaheri S. CPAP should not be used for central sleep apnea in congestive heart failure. J Clin Sleep Med. 2006;2:399–402. [PubMed] [Google Scholar]
- 106.Philippe C, Stoica-Herman M, Drouot X, Raffestin B, Escourrou P, Hittinger L, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92:337–342. doi: 10.1136/hrt.2005.060038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kasai T, Usui Y, Yoshioka T, Yanagisawa N, Takata Y, Narui K, et al. Effect of flow-triggered adaptive servo-ventilation compared with continuous positive airway pressure in patients with chronic heart failure with coexisting obstructive sleep apnea and Cheyne-Stokes respiration. Circ Heart Fail. 2010;3:140–148. doi: 10.1161/CIRCHEARTFAILURE.109.868786. [DOI] [PubMed] [Google Scholar]
- 108.Teschler H, Dohring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164:614–619. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 109.Augostini R. A novel approach to the treatment of central sleep apnea in patients with heart failure. Herzschrittmacherther Elektrophysiol. 2012;23:9–13. doi: 10.1007/s00399-011-0165-7. [DOI] [PubMed] [Google Scholar]
- 110.Ponikowski P, Javaheri S, Michalkiewicz D, Bart BA, Czarnecka D, Jastrzebski M, et al. Transvenous phrenic nerve stimulation for the treatment of central sleep apnea in heart failure. Eur Heart J. 2012;33:889–894. doi: 10.1093/eurheartj/ehr298. [DOI] [PMC free article] [PubMed] [Google Scholar]