Abstract
Syntaxin and Munc18 are essential for regulated exocytosis in all eukaryotes. It was shown that Munc18 inhibition of neuronal syntaxin 1 can be overcome by CDK5 phosphorylation, indicating that structural change disrupts the syntaxin-Munc18 interaction. Here we show that this phosphorylation promotes the assembly of Munc18b-Syntaxin 3-SNAP25 tripartite complex and membrane fusion machinery SNARE. Using siRNAs to screen for genes required for regulated epithelial secretion, we identified the requirements of CDK5 and Munc18b in cAMP-dependent gastric acid secretion. Biochemical characterization revealed that Munc18b bears a syntaxin 3-selective binding site located at its most C-terminal 53 amino acids. Significantly, the phosphorylation of Thr572 by CDK5 attenuates Munc18b-Syntaxin 3 interaction and promotes formation of Munc18b-Syntaxin 3-SNAP25 tripartite complex, leading to an assembly of functional Munc18b-Syntaxin 3-SNAP25-VAMP2 membrane fusion machinery. Thus, our studies suggest a novel regulatory mechanism in which phosphorylation of Munc18b operates vesicle docking and fusion in regulated exocytosis.
Keywords: SNARE, syntaxin 3, Munc18b, CDK5, exocytosis
INTRODUCTION
Gastric parietal cells are polarized epithelial cells in which hydrochloric acid secretion is triggered by paracrine, endocrine, and neurocrine pathways [1]. Stimulation of acid secretion typically involves an initial elevation of intracellular calcium and cAMP followed by activation of a cAMP-dependent protein kinase cascade that triggers the translocation and insertion of H, K-ATPase, into apical plasma membranes of parietal cells and results in acid secretion into the glandular lumen [2]. After secretory stimuli, the H,K-ATPase is withdrawn back into the cytoplasm.
Soluble N-ethyl maleimide sensitive factor attachment protein receptors (SNAREs) are crucial for vesicle fusion. In the brain, fusion of synaptic vesicles with the plasma membrane requires three SNARE proteins: syntaxin 1, SNAP25 and VAMP2 [3–5]. The three SNARE proteins form a four-helical bundle, the SNARE complex, which mediates membrane fusion [6]. Our recent studies demonstrate the functional significance of syntaxin 3 (Stx3) [7], VAMP2 [8], and SNAP25 [9] in the parietal cell exocytosis. Despite demonstration of the functional importance of Stx3 in parietal cell secretion [7], it is still unclear how Stx3 is involved in the tubulovesicular membrane dynamics triggered by histamine stimulation.
Several proteins regulate SNARE function in membrane fusion. Of particular importance is the cytosolic protein Munc18 that binds syntaxin. Recent in vivo studies indicate that Munc18 has a pivotal function in vesicle docking and fusion [10,11]. Importantly, depletion of Munc18a in mouse causes a complete loss of neurotransmitter secretion from synaptic vesicles, indicating its essential role in exocytosis [12]. However, it is unclear how Munc18 operates in regulated exocytosis in response to physiological stimulation.
Here we identified the requirements of CDK5, Munc18b and Stx3 for polarized secretion in epithelial cells. Our studies reveal a novel regulatory mechanism by which CDK-mediated phosphorylation of Munc18b operates secretory vesicle docking and fusion in regulated exocytosis.
MATERIALS AND METHODS
Recombinant protein production
Recombinant proteins and their isolation have been described previously [7]. Briefly, Stx, SNAP25 and Munc18b were produced in BL21 Escherichia coli and purified on affinity beads.
Cell Culture and Transfection
Primary cultures of gastric parietal cells from rabbit stomach were produced and maintained as described [13]. Cultures of parietal cells were transfected with a plasmid encoding GFP-tagged Stx3 using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The DNA-lipid mix was added to the plates and incubated for 4 h, followed by a replacement of fresh Opti-MEM. The transfected cells were then maintained in culture at 37° C in the presence of 100 µM cimetidine until use for protein expression assay, immunofluorescence or live cell imaging.
Immunoisolation of SNARE complex from gastric parietal cells
Monoclonal antibody against Munc18b was ordered from BD Biosciences (San Jose, CA, USA), respectively. Antibody was covalently coupled to CNBr Sepharose beads (Sigma) according to the manufacturer's instructions. Triton X-100 extracts of parietal cell plasma membranes were prepared as described by [14] and incubated with antibody-coupled beads. After 2 h of rotations at 4°C, the beads were washed and the bound proteins were fractionated by SDS–PAGE and analyzed by western blotting.
In Vitro Phosphorylation of Munc18b by CDK5
The GST-tagged wild type Munc18b, and non-phosphorylatable (Munc18bT572A) mutant were expressed in E. coli strain BL21 (DE3) and purified by using glutathione beads (Sigma) as previously described [15]. Briefly, 1 liter of LB media was inoculated with bacteria transformed with GST-Munc18b. The expression of protein was induced by addition of 0.5 mM isopropyl-β-D-thiogalactopyranoside at 30°C for 3 hours. After the induction, bacteria were harvested by centrifugation and re-suspended in phosphate-buffered saline (PBS) containing proteinase inhibitors (leupeptin, pepstatin, and chymostatin; 5 µg/ml), and sonicated for four bursts of 10 s each by using a probe-tip sonicator. The lysis solution was clarified by centrifugation for 20 min at 10,000 × g. The soluble fraction was applied to a column packed with glutathione-agarose beads, followed by extensive washes with PBS.
For in vitro phosphorylation assay, aliquots of wild type GST-Munc18b and mutant GST-Munc18bT572A proteins (20 µg each) were incubated with 200 ng of active CDK5 (Upstate Biotechnology, New York) in kinase buffer (25 mM Hepes, pH 7.2, 1 mM DTT, 50 mM NaCl, 2 mM EGTA, 5 mM MgSO4) with 50 µM ATP and 0.5 µCi of [32P]-ATP. The reaction mixtures (50 µl) were incubated at 30°C for 30 min and terminated by adding SDS-PAGE sample buffer. Proteins were then fractionated on SDS-PAGE. The gel was stained with Coomassie Brilliant Blue and quantified by a PhosphoImager (Amersham Biosciences) as previously described [13].
Visualizing GFP-Stx3 dynamics in live parietal cells
Cultured parietal cells expressing GFP-Stx3 were observed in real time in the presence of Dulbecco’s phosphate-buffered saline (Gibco BRL) using a temperature-controlled chamber (Warner Instr. Corp.) at 37°C. Parietal cells were either maintained in a resting state or stimulated, as described above, and images were collected sequentially using a laser-scanning confocal microscope LSM510 Meta (Carl Zeiss, Germany). Digital images were exported into Adobe Photoshop for presentation.
[14C]-Aminopyrine (AP) Uptake Assay
Stimulation of parietal cells was quantified using the AP uptake assay as previously described [8]. Cells were transfected with siRNA for 36 hours before stimulation of histamine and IBMX (100 and 30 µM). AP uptake values were normalized among the various preparations by expressing as a fraction of the stimulated control.
Repression of SNARE proteins with siRNA
The siRNA sequences used for silencing Stx2, Stx3, Munc18b, CDK5 are listed in the supplemental table. As a control, either a duplex targeting cyclophilin or a scrambled sequence was used as described [15]. The 21-mer oligonucleotide RNA duplexes were synthesized by Dharmacon Research, Inc. (Boulder, CO). In trial experiments, different concentrations of siRNA oligonucleotides were used for different treatment times as detailed previously, and transfection efficiency was judged based on the uptake of fluorescein isothiocyanate-conjugated oligonucleotides. The siRNA-mediated protein suppression was judged by Western blot analysis.
Western Blot
Samples were subjected to SDS-PAGE on 6~16% gradient gel and transferred onto nitrocellulose membrane. Proteins were probed by appropriate primary antibodies and detected using ECL (Pierce, IL).
RESULTS AND DISCUSSION
SiRNA screen revealed the importance of CDK5 and Munc18b in parietal cell secretion
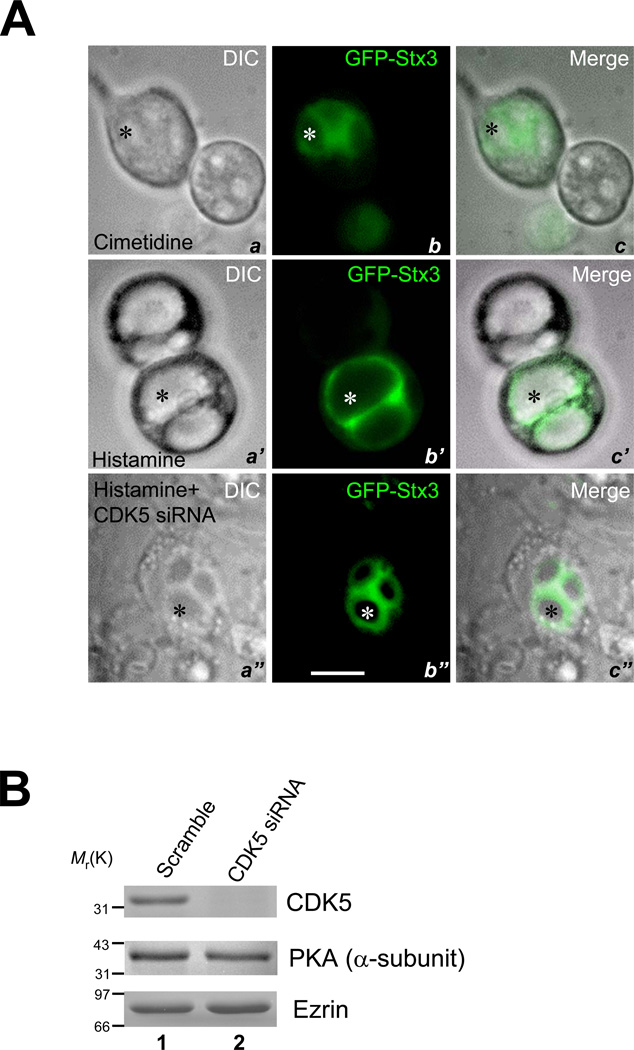
Stx3 is present in the vesicular membrane fraction of gastric parietal cells [14] and is essential for parietal cell activation [7]. To delineate the molecular mechanism underlying the recruitment of H,K-ATPase-containing membrane to the apical membrane during the parietal cell activation, we chose GFP-Stx3 as a reporter. In resting cells, GFP fluorescence is seen throughout the cytoplasm of positively transfected cells and especially in the immediate vicinity of the apical membrane vacuoles (Fig. 1A, a–c; asterisk). The images of maximally stimulated cells (a’–c’) demonstrate that GFP-Stx3 appears on the enlarged apical vacuoles, suggesting that Stx3 is translocated to the apical membrane after stimulation. We used this “cell-based” assay coupled with siRNAs to screen genes essential for parietal cell activation judged by GFP-Stx3 translocation and dilation of the apical vacuoles. The CDK5 protein expression was successfully suppressed by 100 nM siRNA oligonucleotide duplex judged by Western blotting analyses (Fig. 1B). Fig. 1A (a”–c”) shows that suppression of CDK5 prevents histamine-stimulated vacuole swelling due to an inhibition of the partitioning of GFP-Stx3. The GFP fluorescence primarily surrounds the apical membrane vacuoles (b”; asterisk). This becomes clear when the GFP image is merged with DIC optics (c”, asterisk), suggesting that CDK5 may be important for insertion of the proton pump into the apical membrane.
Fig. 1. CDK5 and Munc18b are essential for parietal cell activation.
A. GFP-Stx3 serves as an optical reporter for assessing live gastric parietal cell activation.
Primary cultures of gastric parietal cells were transfected with GFP-Stx3 DNA construct. After 36 h, cells in the presence of 100 µM cimetidine were examined for general morphology and GFP fluorescence. Two adjacent cultured parietal cells are shown with corresponding images of DIC optics (a; DIC), epifluorescence (b; GFP), and their merged image (c). Parietal cells are readily distinguished as large cells with obvious internalized vacuoles of apical membrane (indicated by asterisk). In resting parietal cells (a–c), apical membrane vacuoles are clearly seen in all images surrounded by GFP fluorescence extending into the cytoplasm (b and c).
Another two separate groups were stimulated with 100 µM histamine, either transfected with CDK5 siRNA duplex (100 nM) (a”–c”) or scramble control (a”–c”) 36 hours prior to the stimulation. After a 20 min stimulation, cultured parietal cells were also assessed for general morphology (DIC) and for GFP-Stx3 fluorescence (GFP-syn3). Apical membrane vacuoles are much enlarged in the stimulated state because of the accumulation of HCl and water, whereas the vacuoles are not enlarged when the CDK5 protein is suppressed by siRNA oligonucleotide. There is a high degree of localization of the GFP-Stx3 to the apical membrane vacuoles of secreting cells and a relative diminution of signal in the cytoplasm. Bar: 20 µm.
B. Efficiency of siRNA in suppressing CDK5 protein accumulation.
Cultured parietal cells were transfected with the CDK5 siRNA oligonucleotides for 36 hours and subjected to SDS-PAGE and immunoblotting. Upper panel, immunoblot for CDK5; middle panel, immunoblot for PKA; lower panel, immunoblot for ezrin.
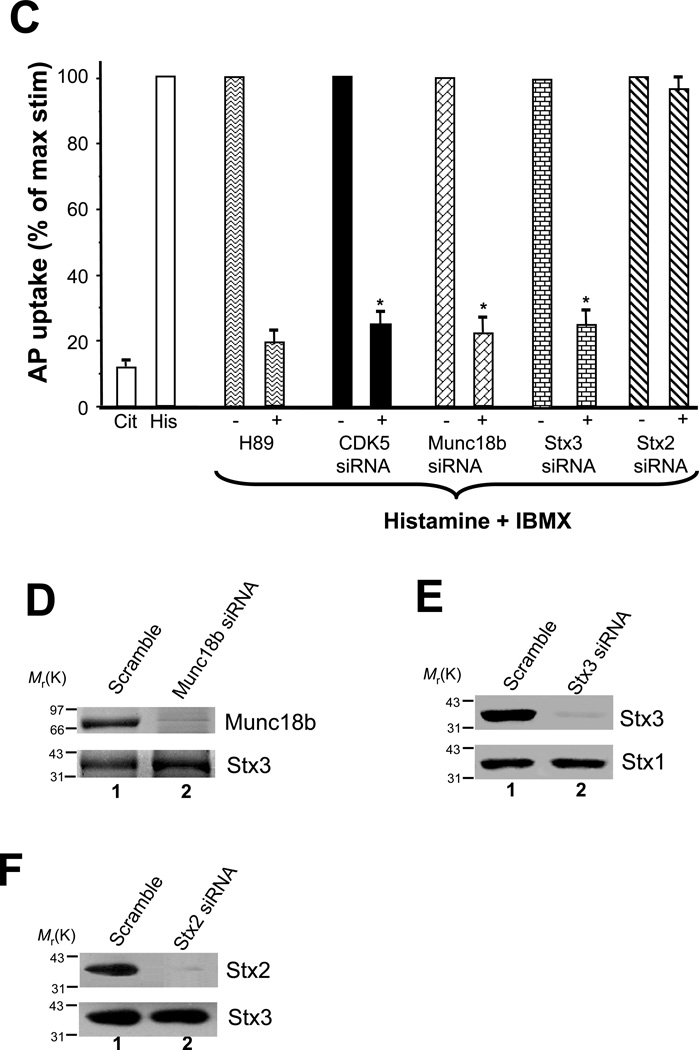
C. Repression of CDK5, Munc18b and Stx3 inhibits acid secretion by cultured parietal cells.
Cultured parietal cells were transfected for 36 h with siRNA duplexes for CDK5, Munc18b, Stx2, Stx3 and scramble oligonucleotides. Additional cultures of non-transfected parietal cells served as untreated control Cells were then incubated for 30 min with cimetidine (Cit) or stimulated in the presence of 100 µM histamine and 30 µM IBMX (His), and the [14C]AP accumulation ratio was measured. The data shown here from four experiments have been normalized by setting the value for the stimulated control to 100% for each experiment. Values are the mean ± S.E. of four independent experiments, each with duplicate determinations. Acid secretion was significantly inhibited in the absence of CDK5, Munc18b and Stx3 (p = 0.003) while depletion of Stx2 did not affect acid secretion. 10 µM H89, a PKA inhibitor, was included as a positive control.
D. Efficiency of Munc18b siRNA.
Cultured parietal cells were transfected with the Munc18b siRNA oligonucleotides for 36 hours and subjected to SDS-PAGE and immunoblotting. Upper panel, immunoblot for Munc18b; lower panel, immunoblot for Stx3.
E. Efficiency of Stx3 siRNA
Cultured parietal cells were transfected with the Stx3 siRNA oligonucleotides for 36 hours and subjected to SDS-PAGE and immunoblotting. Upper panel, immunoblot for Stx3; lower panel, immunoblot for Stx1.
F. Efficiency of Stx2 siRNA.
Cultured parietal cells were transfected with the Stx2 siRNA oligonucleotides for 36 hours and subjected to SDS-PAGE and immunoblotting. Upper panel, immunoblot for Stx2; lower panel, immunoblot for Stx3.
To confirm the role of CDK5 in parietal cell activation, we measured acid secretion using an aminopyrine uptake assay [7]. Parietal cell cultures from each condition were either maintained in a resting state (Cit) or stimulated to maximum secretion with histamine plus IBMX (His). The [14C]-AP uptake was measured as an index of acid secretion (Fig. 1C). To account for variations among four separate preparations, the [14C]-AP uptake ratio was normalized to the control non-infected, stimulated parietal cells that was set at 100% for each experiment. The PKA inhibitor H89 was included as a positive control for confirming the involvement of PKA activation in histamine-stimulated parietal cell secretion. Compared with untransfected controls, [14C]-AP uptakes were not significantly lower in cells transfected with scramble oligonucleotide duplex (95.7 ± 3.7% of stimulated control). However, significant reduction of acid secretion in cells in which CDK5, Munc18b or syntaxin3 was suppressed (Fig. 1C, 1D and 1E). Despite the successful repression of Stx2 protein (Fig. 1F), acid secretion was not greatly impaired (Fig. 1C), suggesting that Stx2 may not participate in parietal cell acid secretion. Thus, we conclude that CDK5, Munc18b and Stx3 are essential for parietal cell acid secretion.
Munc18b contains a Stx3-selective binding activity
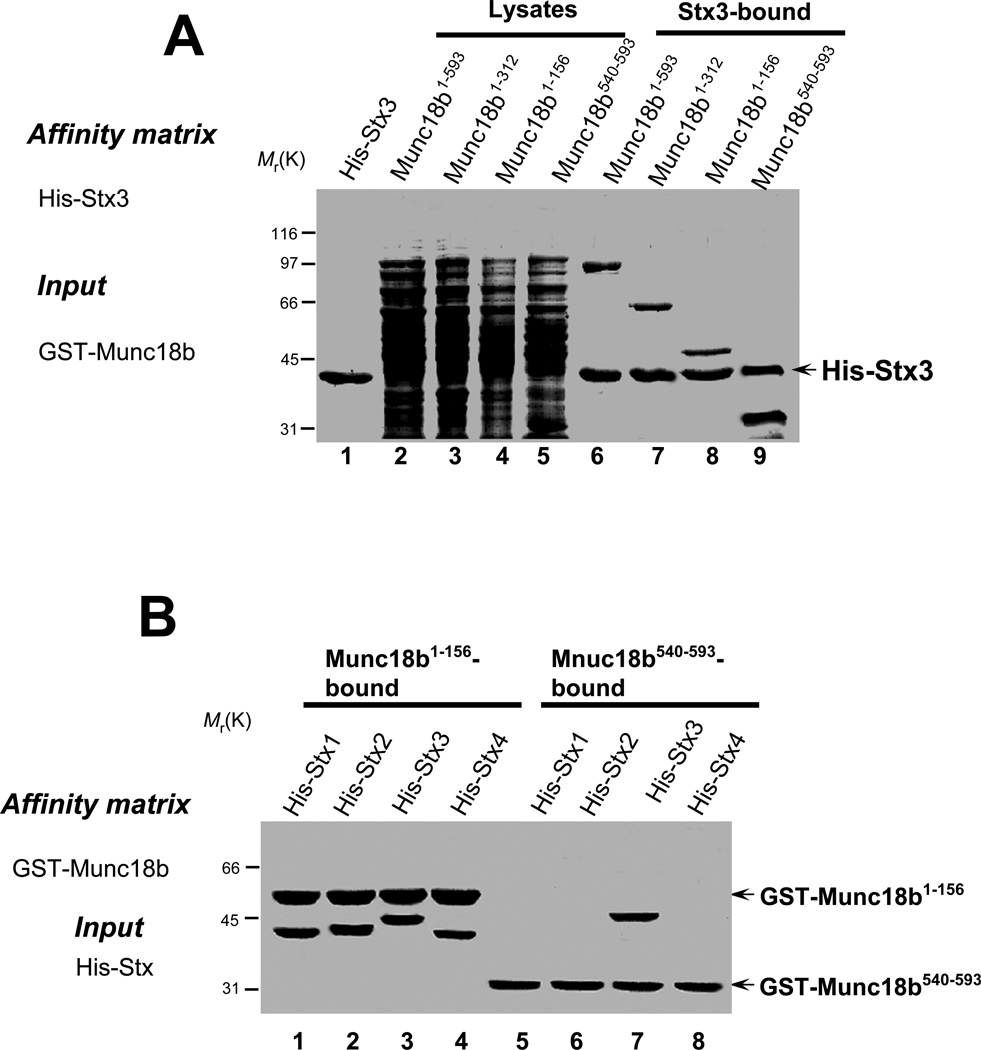
Our previous works demonstrate the functional significance of SNARE proteins in parietal cell secretion [7,9]. To illustrate the molecular function of Munc18b in parietal cell secretion, we carried out biochemical characterization of Munc18b-Stx3 interaction. Using Stx3 as an affinity matrix, our pull-down assay revealed that Munc18b contains two binding sites for Stx3 which are localized to the N-terminal 156 amino acids and the most C-terminal 53 amino acids, respectively (Fig. 2A). Since Munc18b bears a tissue specific expression pattern relative to some Stx isoforms, we asked whether Munc18b contains Stx3-specific binding activity. To this end, the GST-tagged fusion proteins containing N-terminal Munc18b (GST-Munc18b1–156) and C-terminal Munc18b (GST-Munc18b540–593) were used as an affinity matrix to isolate histidine-tagged syntaxin isoforms (Stx1, Stx2, Stx3, and Stx4). As shown in Fig. 2B, the C-terminal Munc18b only absorbs Stx3 while the N-terminal Munc18b binds to all Stx isoforms tested equally. To test whether Munc18b bears selectivity over various Stx isoforms, we conducted a pull-down assay in which purified Stx isoform proteins on Ni agarose beads were incubated with bacterial lysates containing GST-Munc18b. As shown in Fig. 2C, Munc18b was most greatly absorbed by Stx3 followed by Stx2 but poorly by Stx1 protein. Little Munc18b protein was retained on Stx4 beads. These studies indicate that Munc18b-Stx binding activity may be specified by the conformation of Munc18b.
Fig. 2. Munc18b contains a Stx3-selective binding activity regulated by CDK5.
A. Stx3 binds two distinct regions of Munc18b. His-tagged Stx3 on Ni-agarose beads was loaded with bacterial lysates of full-length and various deletion proteins of Munc18b. Bound proteins were fractionated by SDS-PAGE and stained with Coomassie Blue. Note that both Munc18b1–156 and Munc18b540–593 bind Stx3.
B. Munc18b exhibits a Stx3-selective binding activity. GST-tagged Munc18b (both Munc18b1–156 and Munc18b540–593) on glutathione-agarose beads was loaded with various purified His-Stx isoforms. Bound proteins were fractionated by SDS-PAGE and stained with Coomassie Blue. Note that only Stx3 binds to Munc18b540–593 while all isoforms can bind to Munc18b1–156.
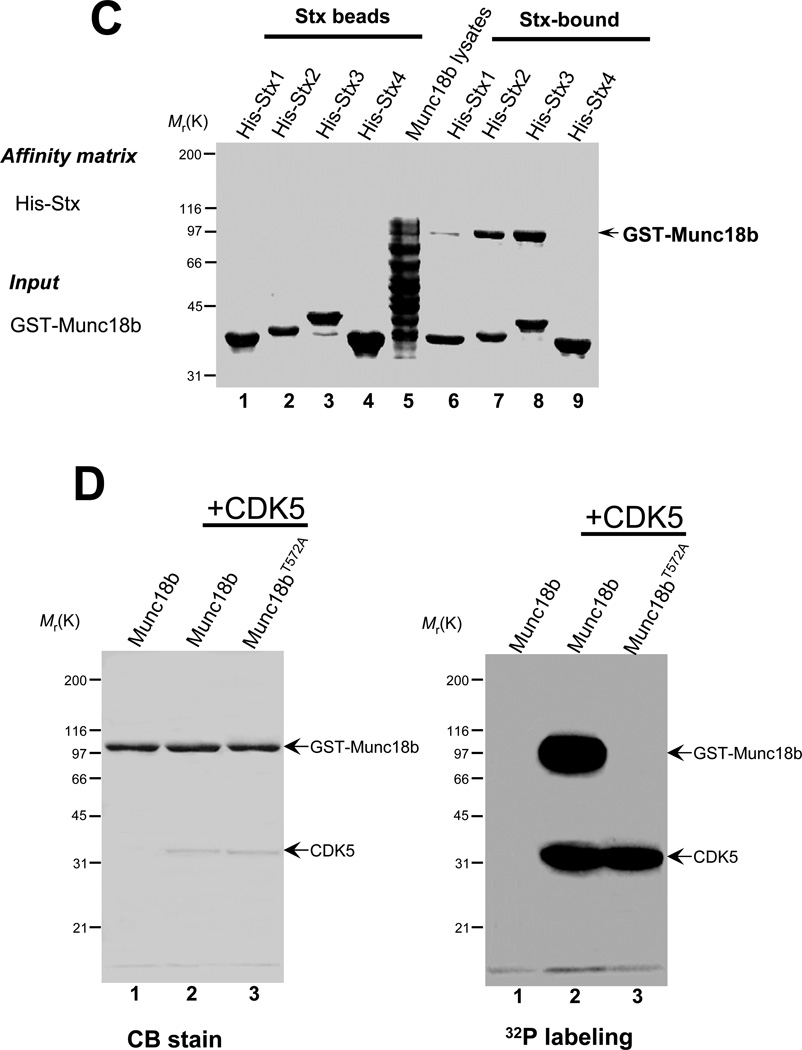
C. Munc18b selectively binds to Stx2 and Stx3 in vitro. His-tagged Stx isoforms on Ni-agarose beads were loaded with bacterial lysates of full-length Munc18b. Bound proteins were fractionated by SDS-PAGE and stained with Coomassie Blue. Note that Munc18b binds poorly on Stx1 and not at all to Stx4.
D. Thr572 of Munc18b is a substrate of CDK5. Bacterially expressed GST-Munc18b fusion proteins, both wild type and mutants (T572A), were purified and phosphorylated in vitro using [32P]-ATP and active CDK5 as described under "Materials and Methods." Samples were separated by SDS-PAGE. Left, Coomassie Brilliant Blue-stained gel of samples of wild type GST-Munc18b alone (lane 1), GST-Munc18b plus CDK5 (lane 2) and GST-Munc18bT572A plus CDK5 (lane 3). Note that roughly equivalent amounts of GST-Munc18b protein were present in the three reactions. right, the same gel was dried and subsequently incubated with x-ray film. Note that there was dramatic incorporation of 32P into wild type but not mutant Munc18b proteins.
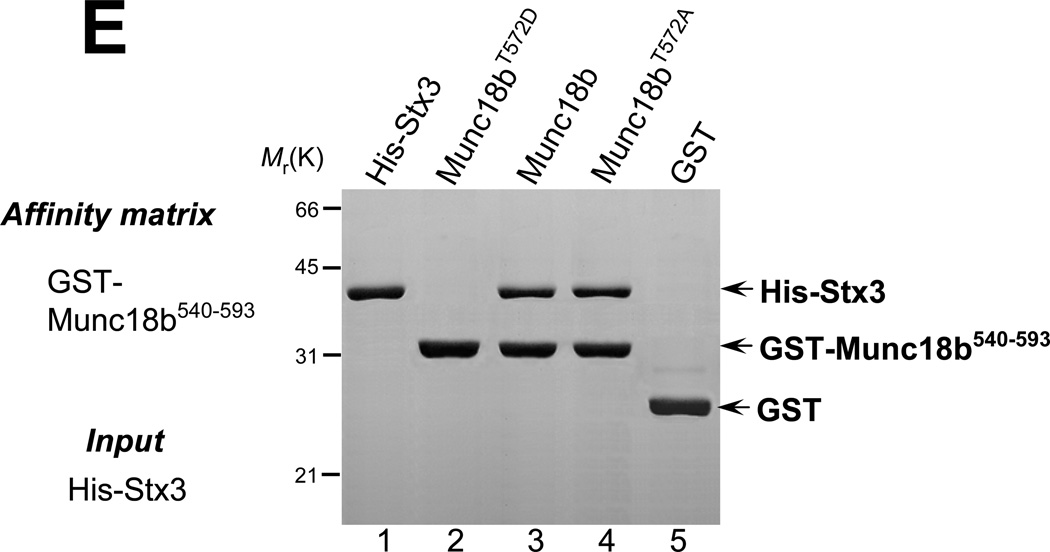
E. CDK5 phosphorylation regulates the C-terminal Munc18b binding to Stx3. GST-tagged Munc18b540–593 proteins (wild type, phospho-mimicking T572D, and nonphosphorylatable T572A) on glutathione-agarose beads was loaded with purified His-Stx3. Bound proteins were fractionated by SDS-PAGE and stained with Coomassie Blue. Note that phospho-mimicking Munc18b540–593 did not bind to Stx3.
CDK5 Phosphorylates Munc18b and Regulates Munc18b-Stx3 interaction
Previous studies show that CDK5 phosphorylates Munc18a and such phosphorylation regulates Munc18a-Stx1 interaction [16]. Since our computational analysis suggests that the CDK5 phosphorylation site is conserved in Munc18b (Thr572), we sought to test if Munc18b is a substrate of CDK5. To this end, we performed an in vitro phosphorylation on recombinant GST-Munc18b fusion proteins, including both wild type protein and non-phosphorylatable mutants in which Thr572 was replaced by alanine (Munc18bT572A). Both GST fusion proteins, wild type andmutant Munc18bT572A, migrate at about the predicted 103 kDa as shown in Fig. 2D (left panel, CB-stain). Incubation of the fusion proteins with [32P]-ATP and CDK5 resulted in the incorporation of 32P into wild type but not Munc18bT572A mutant (Fig. 2D; right panel, 32P-labeling, lanes 2–3). This CDK5-mediated phosphorylation is specific, since incubation of Munc18b with [32P]-ATP in the absence of CDK5 resultedin no detectable incorporation of radioactivity into the wild type protein (Fig. 2D; right panel, lane 1). Thus, we conclude that Thr572 on Munc18b is a substrate of CDK5 in vitro.
To test if CDK5 phosphorylation modulates the Munc18b-Stx3 interaction, we generated phospho-mimicking (T572D) and non-phosphorylatable (T572A) mutants and examined the role of CDK5-mediated Munc18b phosphorylation in Stx3-binding using a pull-down assay. As shown in Fig. 2E, phospho-mimicking Munc18b540–593 failed to absorb histidine-tagged Stx3 (lane 2). Both wild type and non-phosphorylatable Munc18b proteins bind to his-Stx3 protein equally (lanes 3 & 4). No Stx3 protein was found on GST beads. Thus, we conclude that Munc18b-Stx3 interaction is regulated by CDK5 phosphorylation of Thr572.
CDK5 phoshorylation enhances the association of Munc18b with Stx3-SNAP25 complex in vitro
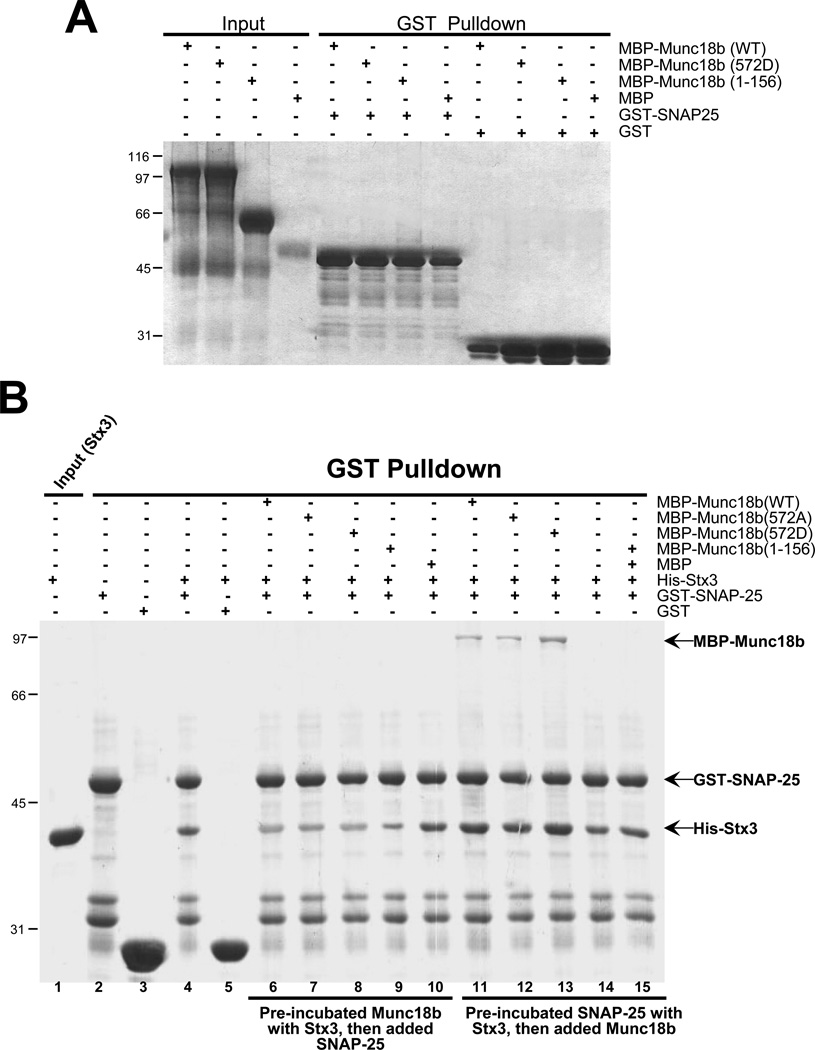
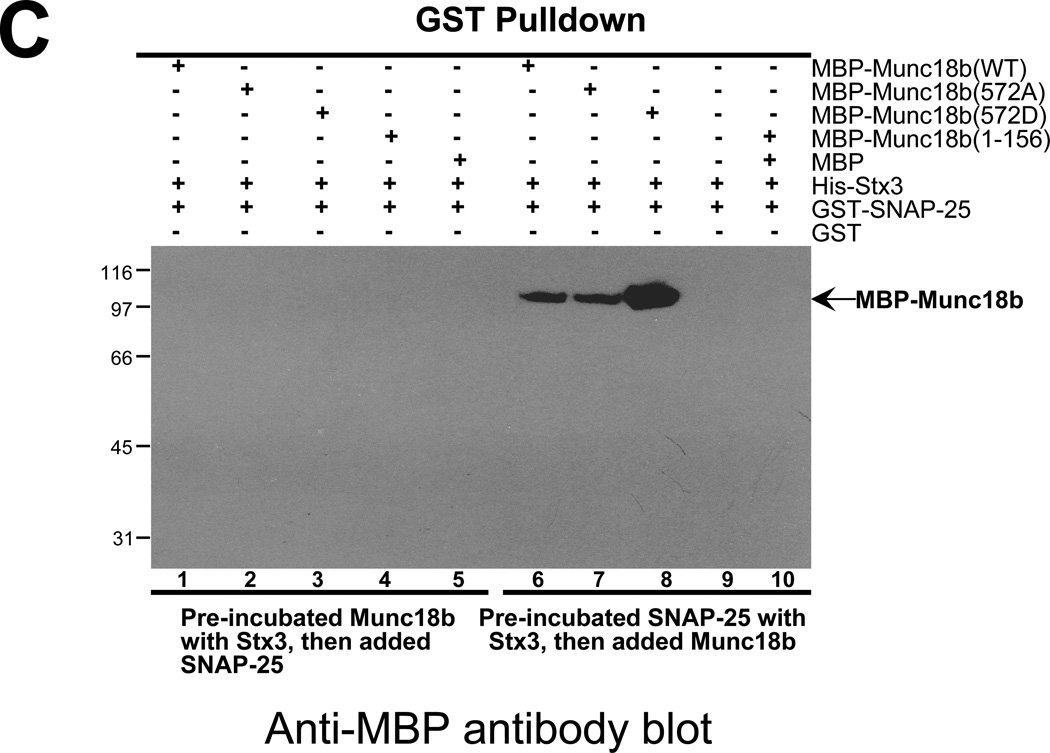
Recent studies demonstrate the importance of Munc18 association with SNARE complex in membrane fusion [17,18]. However, it remains unclear how Munc18-SNARE association is orchestrated and how specific Munc18-SNARE interaction is achieved in regulated exocytosis. To further explore the functional relevance of CDK5-mediated phosphorylation in SNARE complex formation, we asked whether such phosphorylation promotes the association of Munc18 with SNARE complex. While wild type and the phospho-mimicking mutant Munc18b proteins do not bind to SNAP25 directly (Fig. 3A), phospho-mimicking Munc18b protein binds readily to preformed SNAP25-Stx3 complex (Fig. 3B, lane 13). Wild type and non-phosphorylatable Munc18b can also complex with pre-assembled SNAP25-Stx3 complex but less effectively as judged by western blotting analysis of Munc18b protein bound to SNARE (Fig. 3C; lanes 6–7). Thus, we conclude that CDK5-mediated phosphorylation of Thr572 promotes the association of Munc18b with Stx3-SNAP25 complex.
Fig. 3. CDK5 phoshorylation enhances the association of Munc18b with Stx3-SNAP25 complex in vitro.
A. Munc18b bears no SNAP25-binding activity. GST-tagged SNAP25 on glutathione-agarose beads was loaded with purified MBP-tagged full-length and various deletion proteins of Munc18b. Bound proteins were fractionated by SDS-PAGE and stained with Coomassie Blue. Note that neither Munc18b1–156 nor Munc18b540–593 binds SNAP25.
B. CDK5 phosphorylation promotes the binding of Munc18b to the Stx3-SNAP25 complex. GST-tagged SNAP25 on glutathione-agarose beads was either incubated with Stx3 first prior to the loading of purified Munc18b, or incubated with a mixture of Munc18b proteins with Stx3. Bound proteins were fractionated by SDS-PAGE and stained with Coomassie Blue. Note that full-length Munc18b but not Munc18b1–156 binds only to pre-formed Stx3-SNAP25 complex while CDK5-mediated phosphorylation promotes the association of Munc18b to Stx3-SNAP25 complex (lane 13 vs lane 12).
C. Phospho-mimicking Munc18b binds better to the Stx3-SNAP25 complex. Western blotting analysis with MBP antibody demonstrates that CDK5-mediated phosphorylation of T572 promotes the association of full-length Munc18b (lane 8 vs lane 7) but not Munc18b1–156 with performed Stx3-SNAP25 complex in vitro.
CDK5-mediated phosphorylation of Munc18 is essential for SNARE complex formation in vivo
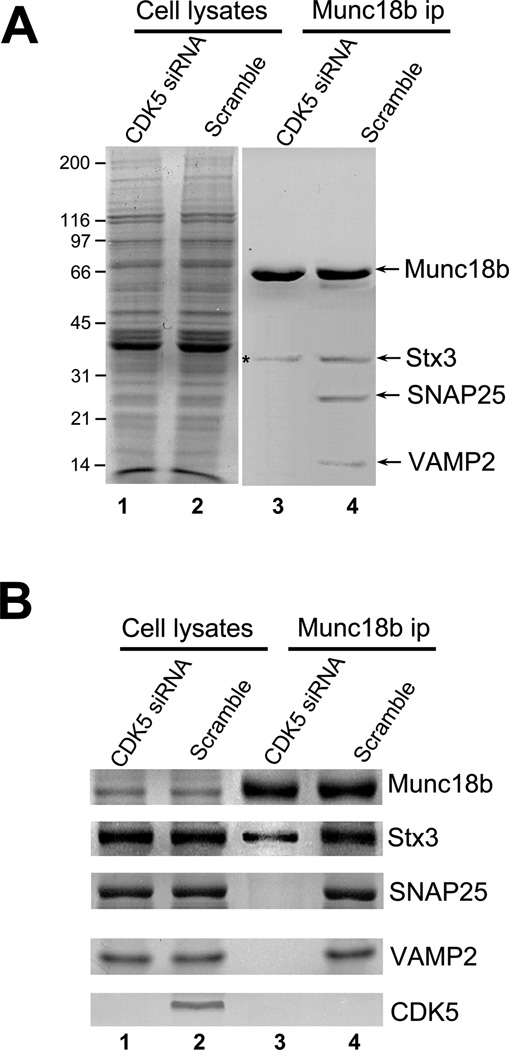
Previous studies show the SNARE complex is essential for parietal cell acid secretion [7, 9]. Since CDK5-mediated phosphorylation facilitates Mucn18b binding to SNAP25-Stx3 complex (Fig. 3C), and CDK5 is required for parietal cell secretion (Fig. 1), we reasoned that suppression of CDK5 would inhibit formation of a stable SNARE complex during parietal cell activation. To this end, we used Munc18b antibody covalently coupled to Sepharose beads to isolate Munc18b and its accessory proteins from parietal cells treated CDK5 siRNA followed by histamine stimulation. Significantly, Munc18b antibody isolated Munc18b complex containing Stx3, SNAP25 and VAMP2 from scramble siRNA transfected-and-histamine-stimulated parietal cells but not CDK5-suppressed-and-histamine-stimulated parietal cells (Fig. 4; lanes 3 and 4). Western blotting analyses confirmed the formation of Munc18-SNARE complex in secreting parietal cells treated with scramble siRNA (Fig. 4B, lane 4) and efficiency in repression of CDK5 (Fig. 4B; lower panel).
Fig. 4. CDK5-mediated phosphorylation of Munc18 is essential for SNARE complex formation in vivo.
A. CDK5 is essential for formation of a stable Munc18b-SNARE complex in secreting parietal cells. Aliquots of cell lysates from histamine-treated cultured parietal cells, pre-transfected with CDK5 siRNA or scramble oligonucleotides, were incubated with CNBr-coupled Munc18b antibody beads. The beads were washed and bound proteins were fractionated by SDS-PAGE and stained with silver. Note that Munc18b antibody beads pull down several polypeptides corresponding to Stx3, SNAP25 and VAMP2 from control siRNA-treated cells.
B. Repression of CDK5 prevents the formation of a stable Munc18b-SNARE complex in parietal cells. Western blotting analyses with various antibodies to SNAREs confirm that CDK5 is essential for the association of Munc18b with performed Stx3-SNAP25-VAMP complex in vivo.
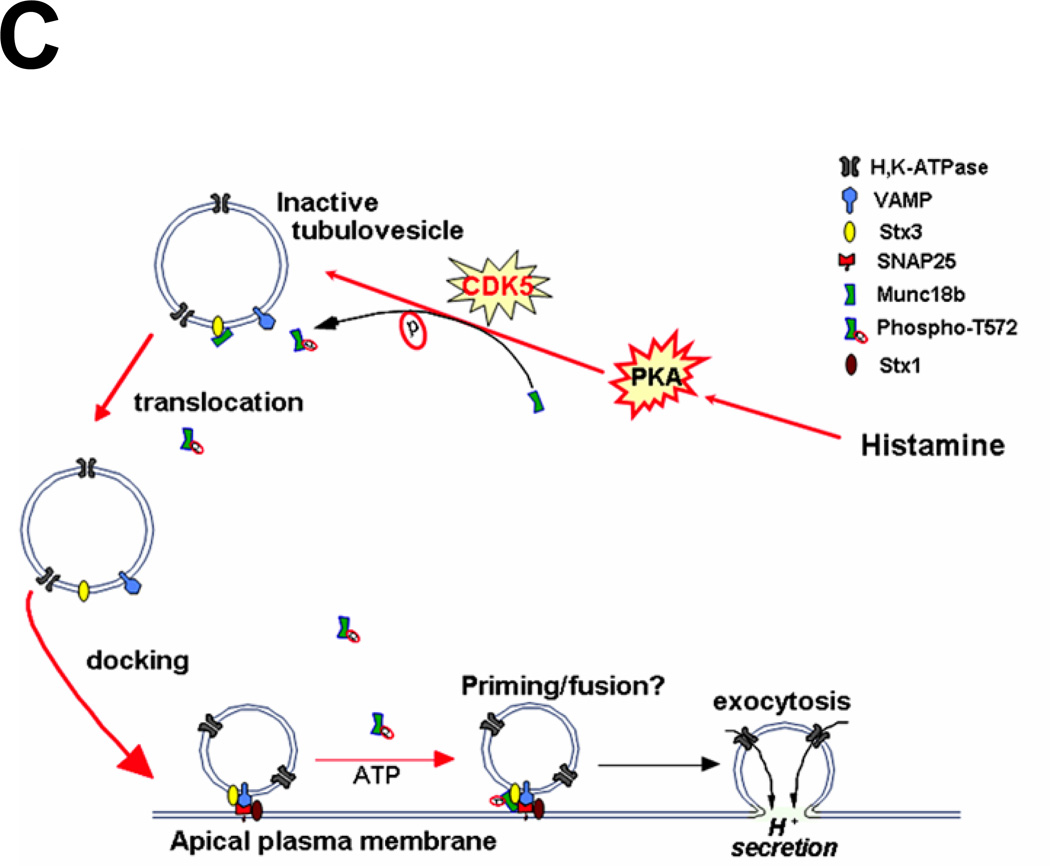
C. Schematic diagram to propose functional role of CDK5-mediated phosphorylation of Munc18b in parietal cell secretion stimulated by histamine.
Our current results provide a coherent picture of the initial steps of SNARE assembly underlying vesicle fusion in hormone-regulated epithelial cell secretion (Fig. 4C). Histamine stimulation initiates a cAMP-dependent signaling cascade and activates CDK5 which phosphorylates Munc18b. This phosphorylation induces a conformational change of Munc18b to modulate its association with Stx3 on the tubulovesicles. This facilitates the vesicle transport and docking at the apical membrane. Phosphorylated Munc18b then promotes or stabilizes the Stx3-SNAP25 complex formation to allow the assembly of functional SNARE fusion machinery for regulated exocytosis. Future studies will directly evaluate the role of Munc18b phosphorylation in gastric acid secretion in real-time.
It was recently found that addition of recombinant Munc18 to partly assembled SNAREs promotes liposomal fusion [17]. In addition, recent studies indicate that a dynamic transition can be made from 'closed' Stx1–Munc18 into an active SNAP25-bound configuration in the presence of arachidonic acid, suggesting an alternative means to activate SNARE complex assembly by inducing conformational change of Stx1 [19]. Our finding that CDK5-mediated phosphorylation of Munc18b functions in promoting SNARE complex formation together with its requirement in regulated acid secretion suggest a novel regulatory mechanism in which Munc18b operates vesicle docking and fusion in hormone-stimulated polarized epithelial secretion.
ACKNOWLEDGEMENTS
This work was supported by Chinese 973 Project Grant 2002CB713700; Chinese Academy of Science Grants KSCX1-YW-R65 and KSCX2-YW-H-10; Chinese Natural Science Foundation Grants 39925018, 30270293, 30500183 (XD), and 90508002; Chinese 863 Project Grant 2001AA215331; Chinese Ministry of Education Grants (20020358051 to XY and IRT0413 to XD); a Georgia Cancer Coalition grant and National Institutes of Health Grants DK56292, CA89019, and CA92080 (to XY).
Abbreviations
- SNAREs
soluble N-ethyl maleimide sensitive factor attachment protein receptors
- Stx
syntaxin
- CDK5
cyclin-dependent kinase 5
- cAMP
cyclic adenosine monophosphate
- SNAP25
synaptosome-associated protein of 25kd
- VAMP 2
vesicle-associated membrane protein 2
- GFP
green fluorescent protein
- DIC
differential interference contrast
- AP
aminopyrine
- Cit
cimetidine
- His
histamine
- IBMX
isobutylmethylxanthine
- PKA
protein kinase A
- GST
glutathione-S-transferase
- WB
western blot
- siRNA
small interfering RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Forte JG, Yao X. The membrane-recruitment-and-recycling hypothesis of gastric HCl secretion. Trends Cell Biol. 1996;6:45–48. doi: 10.1016/0962-8924(96)81009-9. [DOI] [PubMed] [Google Scholar]
- 2.Yao X, Forte JG. Cell biology of acid secretion by the parietal cell. Annu. Rev. Physiol. 2003;65:103–131. doi: 10.1146/annurev.physiol.65.072302.114200. [DOI] [PubMed] [Google Scholar]
- 3.Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly–disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 4.Montecucco C, Schiavo G. Mechanism of action of tetanus and botulinum neurotoxins. Mol Microbiol. 1994;13:1–8. doi: 10.1111/j.1365-2958.1994.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 5.Rizo J, Sudhof T. Snares and Munc18 in synaptic vesicle fusion. Nat Rev Neurosci. 2002;3:641–653. doi: 10.1038/nrn898. [DOI] [PubMed] [Google Scholar]
- 6.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 7.Ammar DA, Zhou R, Forte JG, Yao X. Syntaxin 3 is required for cAMP-induced acid secretion: streptolysin O-permeabilized gastric gland model. Am. J. Physiol. 2002;282:G23–G33. doi: 10.1152/ajpgi.00277.2002. [DOI] [PubMed] [Google Scholar]
- 8.Karvar S, Yao X, Crothers JM, Jr, Liu Y, Forte JG. Localization and function of SNAP-25 and VAMP-2 in functioning gastric parietal cells. J. Biol. Chem. 2002a;277:50030–50035. doi: 10.1074/jbc.M207694200. [DOI] [PubMed] [Google Scholar]
- 9.Karvar S, Yao X, Duman JG, Hybiske K, Liu Y, Forte JG. Intracellular distribution and functional importance of vesicle-associated membrane protein 2 in gastric parietal cells. Gastroenterology. 2002b;123:281–290. doi: 10.1053/gast.2002.34217. [DOI] [PubMed] [Google Scholar]
- 10.Fisher RJ, Pevsner J, Burgoyne RD. Control of fusion pore dynamics during exocytosis by Munc18. Science. 2001;291:875–878. doi: 10.1126/science.291.5505.875. [DOI] [PubMed] [Google Scholar]
- 11.Toonen RF, Kochubey O, de Wit H, Gulyas-Kovacs A, Konijnenburg B, Sorensen JB, Klingauf J, Verhage M. Dissecting docking and tethering of secretory vesicles at the target membrane. EMBO J. 2006;25:3725–3737. doi: 10.1038/sj.emboj.7601256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, Geuze HJ, Sudhof TC. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 13.Zhou R, Zhu L, Kodani A, Hauser P, Yao X, Forte JG. Phosphorylation of ezrin on threonine 567 produces a change in secretory phenotype and repolarizes the gastric parietal cell. J Cell Sci. 2005;118:4381–4391. doi: 10.1242/jcs.02559. [DOI] [PubMed] [Google Scholar]
- 14.Peng XR, Yao X, Chow DC, Forte JG, Bennett MK. Association of syntaxin 3 and VAMP2 with H,K-ATPase-containing tubulovesicles in gastric parietal cells. Mol. Biol. Cell. 1997;8:399–407. doi: 10.1091/mbc.8.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao X, Ding X, Guo Z, Zhou R, Wang F, Long F, Wu F, Bi F, Wang Q, Fan D, Yao X. PALS1 specifies the localization of ezrin to the apical membrane of gastric parietal Cells. J. Biol. Chem. 2005;280:13584–13592. doi: 10.1074/jbc.M411941200. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher AI, Shuang R, Giovannucci DR, Zhang L, Bittner MA, Stuenkel EL. Regulation of exocytosis by cyclin-dependent kinase 5 via phosphorylation of Munc18. J Biol Chem. 1999;274:4027–4035. doi: 10.1074/jbc.274.7.4027. [DOI] [PubMed] [Google Scholar]
- 17.Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Dulubova I, Khvotchev M, Liu S, Huryeva I, Sudhof TC, Rizo J. Munc18-1 binds directly to the neuronal SNARE complex. Proc Natl Acad Sci U S A. 2007;104:2697–2702. doi: 10.1073/pnas.0611318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connell E, Darios F, Broersen K, Gatsby N, Peak-Chew SY, Rickman C, Davletov B. Mechanism of arachidonic acid action on syntaxin-Munc18. EMBO Rep. 2007;8:414–419. doi: 10.1038/sj.embor.7400935. [DOI] [PMC free article] [PubMed] [Google Scholar]