Abstract
Summary
Chronic pain is a state of physical suffering strongly associated with feelings of anxiety, depression and despair. Disease pathophysiology, psychological state, and social milieu can influence chronic pain, but can be difficult to diagnose based solely on clinical presentation. Here, we review brain neuroimaging research that is shaping our understanding of pain mechanisms, and consider how such knowledge might lead to useful diagnostic tools for the management of persistent pain in individual patients.
Keywords: chronic pain; neuroimaging, magnetic resonance imaging, functional
Editor's key points.
Neuroimaging can improve our understanding of pain mechanisms, analgesic action and the placebo effect.
New modelling approaches can explore the dynamic processes influencing pain perception.
Neural mechanisms of the effects of personality and expectancy on pain perception and analgesia have been explored.
Future developments will continue to expand our knowledge of pain mechanisms, allowing translation from laboratory to clinic.
Pain is an unpleasant sensation that is associated with, or described in terms of, a bodily injury.1 Clinicians have long regarded pain as a symptom or warning of disease that should be investigated to expedite treatment of pathology. Unfortunately, medicine does not yet possess every cure, or indeed knowledge of every pathophysiology that can generate pain. Pain can persist despite the best efforts of physicians. Chronic pain is currently defined by the duration of physical symptoms but is, in reality, suffering strongly associated with feelings of anxiety, depression, and despair.2
In the individual, chronic pain is highly influenced by disease pathophysiology, psychological state, and social milieu. The pathogenesis of chronic pain syndromes is often unclear. Research continues to suggest specific patho physiologies that may distinguish between different chronic pain syndromes, for example, fibromyalgia, complex regional pain syndrome. Whether these clinical syndromes can be distinguished as diseases in their own right with specific treatments, or considered as a collection of symptoms that are driven by shared mechanisms, remains unclear. Regardless, psychosocial factors can supervene to influence how pain is perceived or reported by patients, and these factors can operate unconsciously. Their contribution to the chronic pain state further determines appropriate and holistic management of the patient. Hence, there is a desperate need for additional methods that can quantify disease load or psychosocial contributions to the chronic pain state in patients.
In the fifth century BC, Hippocrates declared that pain, like all consciousness, must emerge from brain activity.3 Robust scientific evidence for that philosophical intuition arrived much later (two decades ago) with the demonstration of increased and localized brain activation during pain in humans.4 We now accept that pain may be caused by bodily injury, but as a consciousness, must be generated in the brain.
Functional magnetic resonance imaging (fMRI), positron emission tomography (PET), magnetoencephalography (MEG), and scalp electroencephalography (EEG) are commonly used to study the neural bases of pain. Researchers are also increasingly using other magnetic resonance-based measures (e.g. diffusion tensor imaging, spectroscopy, and volumetric imaging) to assess pain-related changes in the brain's wiring, chemistry, and structure in order to gain further insights into the neurobiology of pain, particularly chronic pain. There are excellent reviews written recently to summarize the findings of neuroimaging studies in healthy individuals and in patients.5,6 Here, we focus on recent neuroimaging studies that continue to shape our understanding of pain in health, disease, and illness. We review the progress in neuroimaging research that is contributing to the development of clinically relevant tools for the management of pain in patients.
The pain ‘neuromatrix’
Neuroimaging studies have now identified several cortical regions in humans that are considered to be important for the perception of pain. The primary and secondary somatosensory cortices (insular and anterior cingulate) and the prefrontal cortices (PFCs) are commonly activated, often bilaterally, and during painful experiences. Furthermore, altered activity in subcortical areas (e.g. brainstem periaqueductal grey (PAG), hypothalamus, amygdala, hippocampus, and cerebellum) is also observed during pain. Thus, activity within several diverse regions of the brain seem to be necessary for the multidimensional experience that is pain.7–9 Some of these regions comprise the so-called ‘pain neuromatrix’,10 a term that is often misused, in which multiple inputs are processed to produce an output (neuro-signature) that is bespoke for an individual depending on context, mood and cognitive state.8 Hence, pain is the product of a widely distributed and variably accessible neural network in the brain, rather than an inevitable consequence of noxious stimulation. Current research suggests that parts of this network can be accessed by non-nociceptive events or inputs to produce ‘pain-like’ experiences (e.g. during empathy for others,11 romantic rejection,12 and social exclusion).13 Hypnotically induced pain in susceptible individuals14 and central post-stroke pain are further examples that illustrate the latent capacity of the brain to elicit pain without concomitant peripheral nociceptive inputs.
Remarkably, none of the brain regions identified above is uniquely associated with pain; they are also involved in many other sensory, motor, cognitive, and emotional functions. Some regions within the so-called pain neuromatrix exhibit significantly correlated activity with sensory or emotional descriptions of the painful experience, suggesting that they have a significant role in pain generation but no brain region has been shown to be exclusively activated during pain experiences.15–18 Disruption of the specific regions within this pain matrix by cortical lesions very rarely remove or ‘numb’ pain completely.19,20 Surgical cingulotomies, performed for intractable pain syndromes, may reduce emotional or motivational aspects of the clinical pain state but leaves intact the capacity for nociceptive pain.21 In fact, prior failure to associate cortical activity or cortical lesions to the experience of pain had encouraged the view that pain had little true cortical representation.22,23 These observations suggest that there is no critical or fixed brain region for pain.
Despite extensive research, an area of the brain that is analogous to the primary visual cortex (i.e. ‘primary pain cortex’) has not been identified. To date, there is no pattern of brain activity indicating pain in an individual with absolute certainty for use by medical, legal, or other regulatory bodies. For example, ‘pain-related’ brain activity from noxious stimulation can occur in patients in minimally conscious states24 but such activations do not necessarily prove pain perception in these individuals. Nonetheless, they suggest that the neuroanatomical substrates for pain are functionally intact and raise the possibility of pain in these uncommunicative subjects with obvious clinical implications.
Imaging nociception
Nociception is defined as the ‘neural process of encoding noxious stimuli’. Although nociception is neither necessary nor sufficient for pain, it is required for protective autonomic or reflexive responses that are essential for our survival. Nociceptors are peripheral sensory neurones which are evolved to respond specifically to high-intensity environmental stimuli that threaten the physical integrity of the organism.25 The neurobiology of these first-order afferents is arguably better understood compared with central neurones onto which they project to transmit information to the brain.26
Investigators have attempted to trace the flow of nociceptive information by examining the temporal sequence of brain activations evoked by noxious stimulation. In humans, the earliest intra-cortical electrical potentials that are evoked by noxious laser stimuli applied to the hand occur simultaneously within the posterior insular region and mid-cingulate cortex.27,28 These regions receive nociceptive input via spino-thalamocortical pathways and are thought to provide a primary interoceptive representation of the physiological condition of the body.29 Activation of the posterior insula during nociception in humans has been shown to be somatopically organized.30 The importance of the posterior insula for nociceptive pain is further suggested by functional imaging studies of pain empathy,11 hypnotically induced pain,14 and recalled pain experiences.31,32 Neural activations during such pains may be similar to that of physically induced pain but there is often attenuation of activation within the posterior insula when pain is reported in the absence of nociceptive stimulation.14 Researchers from independent groups have shown that direct electrical stimulation of the posterior insular region can lead to painful sensations being reported.33,34 The same does not appear to be true of other cortical regions that are also considered part of the pain neuromatrix.23,34 Additionally, the insular cortex has been identified as the most consistently activated region during pain that is induced by nociceptive stimuli.9 These converging data suggest that the posterior insular region might represent a critical neural node through which nociceptive information must be processed. Hence, a ‘nociceptive cortex’ within the operculo-insula region may be postulated, akin to other major senses.
Brain dynamics
As discussed above, the experience of pain requires activity within various regions of the brain. Nevertheless, how pain arises from the flow of information between these different brain regions is largely unknown, as investigating this phenomenon requires a multimodal approach, of which few published studies exist.35 Most studies involve EEG or MEG alone, which record brain activity with high temporal resolution but relatively poor spatial resolution when compared with fMRI. A human scalp EEG study of cortical potentials evoked by highly noxious stimuli has revealed that late, rather than early, laser-evoked responses are associated with conscious detection of noxious stimuli.36 Hence, the consciousness of pain appears to emerge at later stages of such processing when neural information is being integrated across multiple cortical regions.
The processes by which the brain prioritizes nociceptive information over competing sensory stimuli for conscious awareness should also be examined.37 This may explain how the perception of pain arises from nociceptive input. Synchronous neuronal oscillations at gamma-band frequency (>40 Hz) are known to occur when the relevant sensory stimulus is selected for neural representation and perception.38 Using MEG, Gross and colleagues39 demonstrated that painful laser stimuli were associated with stronger gamma-band oscillations (GBO) than unperceived stimuli of equal stimulus intensity, suggesting that the perception of pain emerges when nociceptive input is prioritized in the brain. A more recent EEG study further revealed that, unlike evoked potentials, GBO is not suppressed with repetitive noxious (laser) stimulation.40 In that study, healthy subjects perceived and reported that repeated stimuli were just as painful as the original stimulus. However, the laser-evoked potentials tended to diminish in amplitude with each stimulus repetition. Hence, these potentials appear to signal the novelty, rather than the intensity, of pain experienced.41 Repetition suppression does not occur with GBO; this observation lends further support for the role of GBO in determining the emergence of pain from a noxious stimulus.
Dynamic causal modelling and structural equation modelling are analytical methods that have the potential to further our understanding of pain. These techniques use data from EEG, MEG, fMRI, or PET studies to investigate brain dynamics and cortical coupling,42–44 and are now being applied in the field of pain.45 Such analyses take us beyond a simple spatial representation of pain towards a network-based understanding of this condition that better reflects the dynamic nature of the pain experience.
Prefrontal-limbic control
Numerous imaging studies demonstrate that the PFC can regulate the perception and behavioural expression of pain in humans. The PFC is reciprocally connected to limbic regions and these connections form the neural substrates through which the motivational-emotional aspects of pain can be regulated. The belief that pain from noxious stimulation can be controlled increases tolerance of such painful stimuli; this analgesic effect involves the ventrolateral PFC.46 Individuals can be taught to reinterpret the significance of adverse events so that the negative emotional responses (e.g. fear) can be controlled. During successful reappraisal, the dorsolateral PFC engages the nucleus accumbens and suppresses amygdala activation.47,48 Reducing the fear associated with impending pain via extinction learning requires activation of the ventromedial PFC.49,50 However, anxiety levels can increase if extinction learning fails to lower such fear,51 which can exacerbate a pain experience via para-hippocampal mechanisms.52,53
Frontal-limbic regions are reciprocally connected to the brainstem. This neuroanatomical connection presents another route through which pain can be controlled—via inhibition of neurones within the spinal cord that transmit nociceptive information to the brain. Distraction from pain has been shown to involve activity within the cingulo-frontal cortex, thalamus, and PAG54,55 and, more recently, suppression of spinal cord activity.56 Likewise, placebo analgesia invokes and alters activity in similar regions within the central nervous system.57–59
The brainstem integrator
Neurones in the spinal cord that transmit information regarding noxious events to higher centres within the brain receive descending control from supraspinal regions. This powerful modulatory input, emanating from the frontal-limbic regions, is integrated within the rostral ventromedial medulla (RVM) and PAG; activity within these regions in the brainstem determines whether the nociceptive information is prioritized.60 Depending what the situation demands, these brainstem regions co-ordinate the inhibition or facilitation of nociception. Classical ‘fight or flight’ responses engage immediate inhibitory effects for survival in battlefield or success in competitive sports. After retreat, the same brainstem network can facilitate nociception if physical injuries are sustained. Heightened sensitivity to noxious stimuli (hyperalgesia) is protective during recovery, promoting tissue healing.
Hyperalgesic states
We have demonstrated that, in healthy volunteers, the mescencephalo-pontine reticular formation (MPRF), which projects to the RVM, regulates the expression of hyperalgesia in different central sensitization states. For example, cutaneous capsaicin initiates central sensitization by activation of peripheral nociceptors. The result is hyperalgesia and its maintenance involves MPRF activity.61 Cessation of remifentanil, a potent i.v. opioid can also induce62–64 hyperalgesia.65,66 This opioid-induced phenomenon was investigated in a combined psychophysical-fMRI study.64 MPRF activation was significantly increased only in hyperalgesic subjects and correlated negatively with the degree of hyperalgesia suggesting that brainstem activity regulates the behavioural expression of central sensitization during opioid withdrawal.
Personality and pain
The degree to which limbic and brainstem regions are functionally linked by neural activity can explain the influence of personality on pain thresholds. Using fMRI, we found that insular-brainstem functional connectivity67 was weaker in more anxious individuals, who also had lower pain thresholds when compared with less anxious individuals.68 Hence, the influence of personality traits on pain can be substantiated by mechanisms involving the descending control of pain; therefore, an anxious and pain-vigilant personality might arise from less effective intrinsic regulation of nociception.69
Expectancy effects
Individuals often ‘get what they expect’ from any treatment for pain. Expectancy is often used to explain the placebo effect which is now substantiated by neuroimaging research as a genuine psychobiological event. Mechanisms for placebo analgesia operate within the neural circuit for the descending control of pain which include the ACC, PAG, and the spinal dorsal horn.59 More recently, we investigated whether effects from positive and negative expectancies of opioid analgesia engaged similar brain regions in healthy subjects.70 The experiment involved a fixed and constant infusion of remifentanil. Subjects reported slightly lower pain compared with baseline when the infusion commenced but knowledge of that was hidden. Pain relief became significantly superior when subjects were told that the infusion had commenced. When informed that the infusion had ceased (though in reality the infusion was continued), their ratings of pain returned to baseline. The fMRI data revealed that effects of positive and negative expectancies on opioid analgesia involve distinct neuroanatomical substrates. Expectation of poor analgesic effect was accompanied by activation of the entorhinal cortex, the hippocampal region that mediates the exacerbation of pain through anxiety52 and inhibition (negative activation) of the perigenual ACC (pgACC). Conversely, positive expectation of analgesic effect was associated with increased pgACC activation, suggesting the descending mechanisms of pain inhibition were engaged.
In our study, activations within the primary somatosensory, insular, and mid-anterior cingulate cortices followed subjective reports of pain, with the intensity modulated by expectancy using a fixed remifentanil dose. However, activations in these same regions also correlate with pain modulated by varying doses of remifentanil without psychological manipulations. This raises important questions about whether innate placebo mechanisms in the brain might be altered fundamentally by the presence of the active opioid. Atlas and colleagues71 used a full factorial experimental design in their recent study to investigate potential interaction effects between remifentanil and expectation on pain ratings and also brain activity. They did not find significant interaction effects on pain ratings or brain activity. Pain relief from remifentanil and expectation were indistinguishable based on subjective pain ratings. fMRI did not reveal regions where interaction effects were significant. Instead, expectancy and remifentanil effects were distinguishable because they influenced different brain regions. Additionally, expectancy and remifentanil effects had different onsets. Expectancy effects occurred soon after the subjects became aware the infusion had started, whereas remifentanil effects become prominent only after peak blood concentrations were achieved from the infusion. These studies on drug and expectancy effects suggest that expectancy effects possess distinct functional neuro-anatomies. Further research is needed to explore the possibility of isolating the contribution of placebo or nocebo effects on pain based on neuroimaging data from clinical drug trials.70
The brain and chronic pain
Neuroimaging research in patients with chronic pain have revealed altered structure, function, and neurochemistry in the frontal-limbic-brainstem regions compared with healthy controls underscoring the importance of this descending neuro-circuit for the regulation of pain in humans.
Converging evidence suggests that frontal-limbic dysfunction accompanies chronic pain (Fig. 1). Early fMRI studies have shown altered functional activation of PFC by noxious stimuli in patients with chronic pain. Both hypo-activity72–74 and hyperactivity of the PFC75–77 have been observed. Further evidence of frontal-limbic dysfunction in chronic pain states comes from PET-ligand studies which suggests abnormal opioidergic transmission within frontal-limbic regions in patients with chronic pain (e.g. complex regional pain syndrome,78 central post-stroke pain,79,80 and fibromyalgia).81
Fig 1.
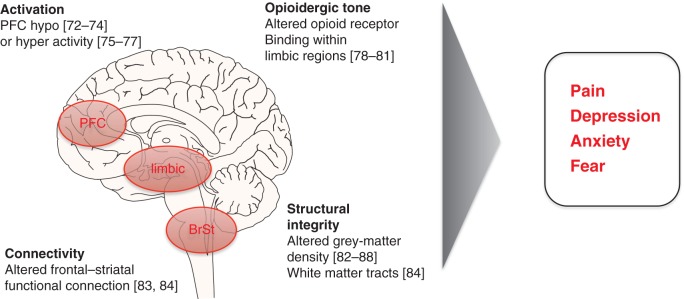
The PFC-limbic-brainstem (BrSt) pathways is involved in the descending modulation of pain. Brain imaging studies (references in brackets) have revealed altered functional activation, structure and neurochemistry of prefrontal and limbic regions in association with symptoms reported by patients with chronic pain.
Numerous investigators have used voxel-based morphometry (VBM) to demonstrate subtle but distinct alterations in grey-matter densities in the brains of patients with chronic pain when compared with healthy controls. Distinct grey-matter changes have been observed in the prefrontal82–84 and limbic regions85–88 in disparate chronic pain syndromes. However, these MR-based changes do not necessarily imply neuronal loss. Furthermore as these MRI data are from cross-sectional studies in patients, they cannot reveal whether the chronic pain state is the cause or consequence of regional grey-matter changes. Nonetheless, in rodent models of neuropathic pain, neuronal remodelling,89 and altered MRI-based measures of grey-matter structures90 occur in the frontal-limbic regions and correlate with ‘anxiety-like’ behaviours after the peripheral nerve lesions. These animal data suggest that the brain can change as a consequence of aberrant input from damaged nociceptive afferents and potentially contribute to the state of chronic pain.
Longitudinal VBM studies in patients with chronic pain now suggest that abnormalities in grey-matter densities within specific brain regions can resolve with improvement in symptoms.91–93 These data have been extended to changes in cortical thickness which is a more quantitative measure that can be compared between studies, unlike grey-matter densities.94 Seminowicz and colleagues demonstrated that the dorsolateral PFC thickened in patients with chronic lower back pain who reported improved symptoms. Additionally, the degree of thickening correlated with the extent of clinical improvement. Interestingly, when compared with controls, patients whose symptoms improved still had thinner cortices in the DLPFC, though the difference did not reach statistical significance. This finding may well reflect incomplete recovery of the pain state in that patient cohort. However, it prompts us to consider whether predisposition to chronic pain can be predicted based on prefrontal structure or function.
The longitudinal fMRI study by Baliki and colleagues83 demonstrated the value of cortical-striatal connectivity in predicting the 1-year outcome of patients with sub-acute back pain. Greater functional connectivity between the PFC and nucleus accumbens predicted pain persistence suggesting that the frontal-striatal connection is involved in the transition from acute to chronic pain. Cortico-striatal connectivity is known to sub-serve a number of neuropsychological functions and is increased during cognitive reappraisal of emotions,47 a skill that may be relevant for coping with chronic pain. Hence, the increased frontal-striatal Fc observed in patients ‘destined’ for chronic pain could represent an adaptive mechanism that was eventually overwhelmed.
Brain dynamics
In recent years, fMRI has revealed that the brain exhibits highly correlated spontaneous and slow (<0.1 Hz) oscillatory activities, even in the absence of any sensory stimulation.95 The biological significance of these ‘resting’ state networks is still unclear.96 It has been suggested that incoming sensory stimulation sculpts the activity and temporal dynamics of these spontaneous networks, reducing the variability of their fluctuations, to allow stable patterns of neural firing within cortical regions that are associated with stimulus perception.97 For example, baseline fluctuations in fMRI activity can influence whether pain arises from noxious stimulation.68,98
Our ability to detect, analyse, and determine changes in the brain's resting state networks (RSNs) has improved considerably in recent years. The default-mode network (DMN) is one such RSN and includes the medial PFC, medial temporal lobes, posterior cingulate cortex, and retrosplenial cortex.99 Studies utilizing fMRI or PET have shown that patients with chronic pain can have abnormal patterns of intrinsic brain activity in the DMN.77,100,101 These studies indicate that resting brain activity within various brain regions is associated in some way with spontaneous pain in this condition.
Activity in the various brain regions that make up the DMN is highly correlated when an individual ‘rests with eyes closed’ and does not perform any explicit task. Hence, the DMN is often associated with ‘mind-wandering’ which philosophical and contemplative traditions believe is associated with unhappiness, in contrast to ‘living in the moment’.102 Meditation is associated with relative deactivation of the main nodes of the DFN (medial prefrontal and posterior cingulate cortices) in experienced meditators.103 Experienced practitioners also report that pain is less unpleasant during meditation. Their subjective reports were associated with decreased activation in PFCs but increased activation in the sensory regions, including the posterior insular.104,105 These observations suggest neural mechanisms that underlie ‘pain modulation’ by meditation comprise increased sensory processing and decreased cognitive control, and contrast with established neural mechanisms for descending control of pain. Such mechanisms may explain the efficacy of psychological approaches that integrate meditative practice and cognition-based therapy for the management of chronic pain.106
The future
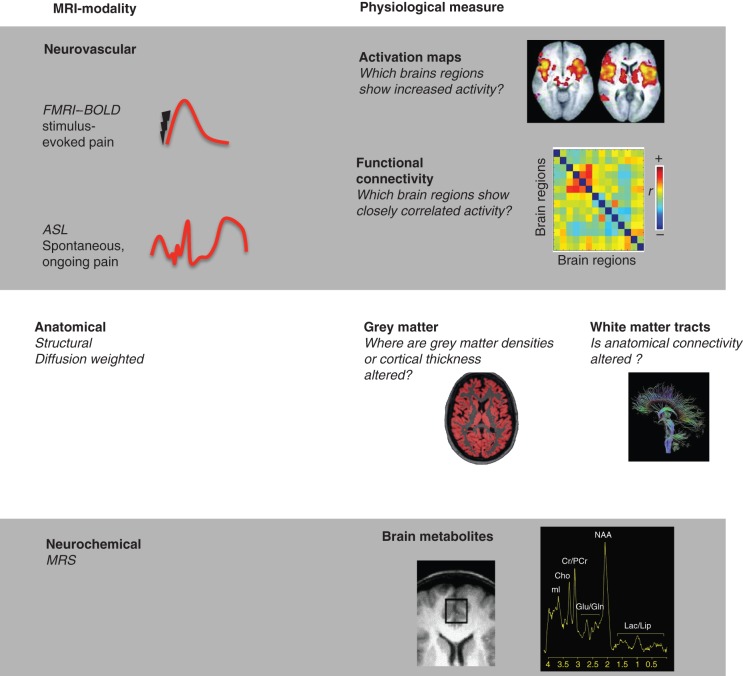
MR-based brain imaging methods are developing rapidly (Fig. 2). Using quantitative arterial spin labelling (ASL), we are now able to measure regional changes in cerebral blood flow that can be associated with tonic, rather than phasic neural events.107–109 This non-invasive technique provides an absolute measure of cerebral perfusion and provides us with the opportunity to identify the neural correlates that underpin the unrelenting, spontaneous and ongoing pain that is the dominant characteristic of chronic pain syndromes. Spinal cord fMRI is now available and further research is being undertaken to enable simultaneous acquisition of neural activities (spinal dorsal horn, brainstem to the cortex) which, in combination with microneurography,102 could allow mapping of the entire ‘pain-pathway’ (nociceptor to brain). Machine-learning algorithms implemented in ‘brain-reading’ studies provide new opportunities for dissecting the neural bases of pain perception.110 Neuroimaging methods are also being developed as clinical tools that reveal the mechanisms that underlie chronic pain in patients where disease pathophysiology and psychosocial influences on pain are unclear based on clinical presentation alone. The challenge now is to improve the sensitivity and specificity of imaging techniques to allow their development as diagnostic tools for the individual patient in our pain clinics.
Fig 2.
MRI-based imaging modalities that measure the brain function, structure, and neurochemistry. Blood oxygenation level dependent (BOLD) fMRI and ASL techniques can map the brain's neurovascular responses that are associated with pain symptoms. These techniques reveal where activation or cerebral perfusion is altered within the brain. More sophisticated connectivity analyses provide information regarding the flow of neural information within the brain. Advanced analyses of anatomical MRI scans can demonstrate subtle changes of grey-matter densities or alterations in the connectivity of white-matter tracts between brain regions. Magnetic resonance spectroscopy quantifies changes in brain metabolites to reveal potential areas of neuroinflammation or neurodegeneration.
Declaration of interest
None declared.
Funding
M.C.L. and I.T. are supported by funding from the National Institute of Health Research Oxford Biomedical Research Centre, Wellcome Trust and Medical Research Council of Great Britain and Northern Ireland. Open Access is funded by the Wellcome Trust.
References
- 1.IASP. Part III: Pain terms: a current list with definitions and notes on usage. In: Merskey M, Bogduk N, editors. Classification of Chronic Pain Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. Seattle: IASP Press; 1994. pp. 209–14. [Google Scholar]
- 2.Morley S. Psychology of pain. Br J Anaesth. 2008;101:25–31. doi: 10.1093/bja/aen123. [DOI] [PubMed] [Google Scholar]
- 3.Adams F. The Law, Oath of Hippocrates, on the Surgery, and on the Sacred Disease. Gloucester, United Kingdom: Dodo Press; 2009. [Google Scholar]
- 4.Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991;251:1355–8. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- 5.Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152:S49–64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tracey I. Can neuroimaging studies identify pain endophenotypes in humans? Nat Rev Neurol. 2011;7:173–81. doi: 10.1038/nrneurol.2011.4. [DOI] [PubMed] [Google Scholar]
- 7.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–91. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Melzack R. Pain and the neuromatrix in the brain. J Dent Educ. 2001;65:1378–82. [PubMed] [Google Scholar]
- 11.Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 12.Kross E, Berman MG, Mischel W, Smith EE, Wager TD. Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci USA. 2011;108:6270–5. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 14.Raij TT, Numminen J, Narvanen S, Hiltunen J, Hari R. Brain correlates of subjective reality of physically and psychologically induced pain. Proc Natl Acad Sci. 2005;102:2147–51. doi: 10.1073/pnas.0409542102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–71. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 16.Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–43. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 17.Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C. Subcortical structures involved in pain processing: evidence from single-trial fMRI. Pain. 2002;99:313–21. doi: 10.1016/s0304-3959(02)00157-4. [DOI] [PubMed] [Google Scholar]
- 18.Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain. 2002;125:1326–36. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- 19.Berthier M, Starkstein S, Leiguarda R. Asymbolia for pain: a sensory-limbic disconnection syndrome. Ann Neurol. 1988;24:41–9. doi: 10.1002/ana.410240109. [DOI] [PubMed] [Google Scholar]
- 20.Ploner M, Freund HJ, Schnitzler A. Pain affect without pain sensation in a patient with a postcentral lesion. Pain. 1999;81:211–4. doi: 10.1016/s0304-3959(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson HA, Davidson KM, Davidson RI. Bilateral anterior cingulotomy for chronic noncancer pain. Neurosurgery. 1999;45:1129–34. doi: 10.1097/00006123-199911000-00023. discussion 34–6. [DOI] [PubMed] [Google Scholar]
- 22.Head H, Holmes G. Sensory disturbances from cerebral lesions. Brain. 1911;34:102–254. [Google Scholar]
- 23.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- 24.Boly M, Faymonville ME, Schnakers C, et al. Perception of pain in the minimally conscious state with PET activation: an observational study. Lancet Neurol. 2008;7:1013–20. doi: 10.1016/S1474-4422(08)70219-9. [DOI] [PubMed] [Google Scholar]
- 25.Woolf CJ, Ma Q. Nociceptors–noxious stimulus detectors. Neuron. 2007;55:353–64. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–36. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frot M, Mauguiere F. Dual representation of pain in the operculo-insular cortex in humans. Brain. 2003;126:438–50. doi: 10.1093/brain/awg032. [DOI] [PubMed] [Google Scholar]
- 28.Frot M, Mauguiere F, Magnin M, Garcia-Larrea L. Parallel processing of nociceptive A-delta inputs in SII and midcingulate cortex in humans. J Neurosci. 2008;28:944–52. doi: 10.1523/JNEUROSCI.2934-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- 30.Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27:201–9. doi: 10.1016/j.neuroimage.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 31.Albanese MC, Duerden EG, Rainville P, Duncan GH. Memory traces of pain in human cortex. J Neurosci. 2007;27:4612–20. doi: 10.1523/JNEUROSCI.0695-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fairhurst M, Fairhurst K, Berna C, Tracey I. An fMRI study exploring the overlap and differences between neural representations of physical and recalled pain. PLoS ONE. 2012;7:e48711. doi: 10.1371/journal.pone.0048711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guenot M, Mauguiere F. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cereb Cortex. 2002;12:376–85. doi: 10.1093/cercor/12.4.376. [DOI] [PubMed] [Google Scholar]
- 34.Mazzola L, Isnard J, Peyron R, Mauguiere F. Stimulation of the human cortex and the experience of pain: Wilder Penfield's observations revisited. Brain. 2012;135:631–40. doi: 10.1093/brain/awr265. [DOI] [PubMed] [Google Scholar]
- 35.Iannetti GD, Niazy RK, Wise RG, et al. Simultaneous recording of laser-evoked brain potentials and continuous, high-field functional magnetic resonance imaging in humans. Neuroimage. 2005;28:708–19. doi: 10.1016/j.neuroimage.2005.06.060. [DOI] [PubMed] [Google Scholar]
- 36.Lee MC, Mouraux A, Iannetti GD. Characterizing the cortical activity through which pain emerges from nociception. J Neurosci. 2009;29:7909–16. doi: 10.1523/JNEUROSCI.0014-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tallon-Baudry C. On the neural mechanisms subserving consciousness and attention. Front Psychol. 2011;2:397. doi: 10.3389/fpsyg.2011.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fell J, Fernandez G, Klaver P, Elger CE, Fries P. Is synchronized neuronal gamma activity relevant for selective attention? Brain Res Brain Res Rev. 2003;42:265–72. doi: 10.1016/s0165-0173(03)00178-4. [DOI] [PubMed] [Google Scholar]
- 39.Gross J, Schnitzler A, Timmermann L, Ploner M. Gamma oscillations in human primary somatosensory cortex reflect pain perception. PLoS Biology. 2007;5:e133. doi: 10.1371/journal.pbio.0050133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang ZG, Hu L, Hung YS, Mouraux A, Iannetti GD. Gamma-band oscillations in the primary somatosensory cortex–a direct and obligatory correlate of subjective pain intensity. J Neurosci. 2012;32:7429–38. doi: 10.1523/JNEUROSCI.5877-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iannetti GD, Hughes NP, Lee MC, Mouraux A. Determinants of laser-evoked EEG responses: pain perception or stimulus saliency? J Neurophysiol. 2008;100:815–28. doi: 10.1152/jn.00097.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fastenrath M, Friston KJ, Kiebel SJ. Dynamical causal modelling for M/EEG: spatial and temporal symmetry constraints. Neuroimage. 2009;44:154–63. doi: 10.1016/j.neuroimage.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 43.Friston K. Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS Biology. 2009;7:e33. doi: 10.1371/journal.pbio.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friston KJ, Dolan RJ. Computational and dynamic models in neuroimaging. Neuroimage. 2010;52:752–65. doi: 10.1016/j.neuroimage.2009.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang M, Mouraux A, Iannetti GD. Parallel processing of nociceptive and non-nociceptive somatosensory information in the human primary and secondary somatosensory cortices: evidence from dynamic causal modeling of functional magnetic resonance imaging data. J Neurosci. 2011;31:8976–85. doi: 10.1523/JNEUROSCI.6207-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiech K, Kalisch R, Weiskopf N, Pleger B, Stephan KE, Dolan RJ. Anterolateral prefrontal cortex mediates the analgesic effect of expected and perceived control over pain. J Neurosci. 2006;26:11501–9. doi: 10.1523/JNEUROSCI.2568-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ochsner KN, Gross JJ. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Curr Dir Psychol Sci. 2008;17:153–8. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 50.Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–43. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: reversal of fear in the human brain. J Neurosci. 2008;28:11517–25. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ploghaus A, Narain C, Beckmann CF, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong J, Gollub RL, Polich G, et al. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. J Neurosci. 2008;28:13354–62. doi: 10.1523/JNEUROSCI.2944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tracey I, Ploghaus A, Gati JS, et al. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22:2748–52. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valet M, Sprenger T, Boecker H, et al. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain—an fMRI analysis. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 56.Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Buchel C. Attention modulates spinal cord responses to pain. Curr Biol. 2012;22:1019–22. doi: 10.1016/j.cub.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 57.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia—imaging a shared neuronal network. Science. 2002;295:1737–40. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 58.Eippert F, Bingel U, Schoell ED, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–43. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 59.Eippert F, Finsterbusch J, Bingel U, Buchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009;326:404. doi: 10.1126/science.1180142. [DOI] [PubMed] [Google Scholar]
- 60.Fields H, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, editors. Textbook of Pain. New York: Churchhill Livingstone; 2006. pp. 125–30. [Google Scholar]
- 61.Lee MC, Zambreanu L, Menon DK, Tracey I. Identifying brain activity specifically related to the maintenance and perceptual consequence of central sensitization in humans. J Neurosci. 2008;28:11642–9. doi: 10.1523/JNEUROSCI.2638-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luginbuhl M, Gerber A, Schnider TW, Petersen-Felix S, Arendt-Nielsen L, Curatolo M. Modulation of remifentanil-induced analgesia, hyperalgesia, and tolerance by small-dose ketamine in humans. Anesth Analg. 2003;96:726–32. doi: 10.1213/01.ANE.0000048086.58161.18. table of contents. [DOI] [PubMed] [Google Scholar]
- 63.Jensen KB, Lonsdorf TB, Schalling M, Kosek E, Ingvar M. Increased sensitivity to thermal pain following a single opiate dose is influenced by the COMT val(158)met polymorphism. PLoS ONE. 2009;4:e6016. doi: 10.1371/journal.pone.0006016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wanigasekera V, Lee MC, Rogers R, Hu P, Tracey I. Neural correlates of an injury-free model of central sensitization induced by opioid withdrawal in humans. J Neurosci. 2011;31:2835–42. doi: 10.1523/JNEUROSCI.5412-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petersen KL, Jones B, Segredo V, Dahl JB, Rowbotham MC. Effect of remifentanil on pain and secondary hyperalgesia associated with the heat–capsaicin sensitization model in healthy volunteers. Anesthesiology. 2001;94:15–20. doi: 10.1097/00000542-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 66.Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49–57. doi: 10.1016/s0304-3959(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 67.Smith SM. The future of FMRI connectivity. Neuroimage. 2012;62:1257–66. doi: 10.1016/j.neuroimage.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 68.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proc Natl Acad Sci USA. 2010;107:355–60. doi: 10.1073/pnas.0906186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fair DA, Dosenbach NUF, Church JA, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA. 2007;104:13507–12. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bingel U, Wanigasekera V, Wiech K, et al. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001244. 70ra14. [DOI] [PubMed] [Google Scholar]
- 71.Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD. Dissociable influences of opiates and expectations on pain. J Neurosci. 2012;32:8053–64. doi: 10.1523/JNEUROSCI.0383-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mayer EA, Berman S, Suyenobu B, et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 73.Jones AK, Derbyshire SW. Reduced cortical responses to noxious heat in patients with rheumatoid arthritis. Ann Rheum Dis. 1997;56:601–7. doi: 10.1136/ard.56.10.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gundel H, Valet M, Sorg C, et al. Altered cerebral response to noxious heat stimulation in patients with somatoform pain disorder. Pain. 2008;137:413–21. doi: 10.1016/j.pain.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 75.Apkarian AV, Thomas PS, Krauss BR, Szeverenyi NM. Prefrontal cortical hyperactivity in patients with sympathetically mediated chronic pain. Neurosci Lett. 2001;311:193–7. doi: 10.1016/s0304-3940(01)02122-x. [DOI] [PubMed] [Google Scholar]
- 76.Gracely RH, Geisser ME, Giesecke T, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–43. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 77.Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165–73. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klega A, Eberle T, Buchholz HG, et al. Central opioidergic neurotransmission in complex regional pain syndrome. Neurology. 2010;75:129–36. doi: 10.1212/WNL.0b013e3181e7ca2e. [DOI] [PubMed] [Google Scholar]
- 79.Willoch F, Schindler F, Wester HJ, et al. Central poststroke pain and reduced opioid receptor binding within pain processing circuitries: a [11C]diprenorphine PET study. Pain. 2004;108:213–20. doi: 10.1016/j.pain.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 80.Maarrawi J, Peyron R, Mertens P, et al. Differential brain opioid receptor availability in central and peripheral neuropathic pain. Pain. 2007;127:183–94. doi: 10.1016/j.pain.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 81.Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27:10000–6. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baliki MN, Petre B, Torbey S, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012 doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–81. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Draganski B, Moser T, Lummel N, et al. Decrease of thalamic gray matter following limb amputation. Neuroimage. 2006;31:951–7. doi: 10.1016/j.neuroimage.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 86.Davis KD, Pope G, Chen J, Kwan CL, Crawley AP, Diamant NE. Cortical thinning in IBS: implications for homeostatic, attention, and pain processing. Neurology. 2008;70:153–4. doi: 10.1212/01.wnl.0000295509.30630.10. [DOI] [PubMed] [Google Scholar]
- 87.Schmidt-Wilcke T, Luerding R, Weigand T, et al. Striatal grey matter increase in patients suffering from fibromyalgia—a voxel-based morphometry study. Pain. 2007;132(Suppl 1):S109–16. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 88.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27:4004–7. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci USA. 2009;106:2423–8. doi: 10.1073/pnas.0809897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seminowicz DA, Laferriere AL, Millecamps M, Yu JS, Coderre TJ, Bushnell MC. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Obermann M, Nebel K, Schumann C, et al. Gray matter changes related to chronic posttraumatic headache. Neurology. 2009;73:978–83. doi: 10.1212/WNL.0b013e3181b8791a. [DOI] [PubMed] [Google Scholar]
- 92.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29:13746–50. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum. 2010;62:2930–40. doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- 94.Seminowicz DA, Wideman TH, Naso L, et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31:7540–50. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 96.Smith K. Neuroscience: idle minds. Nature. 2012;489:356–8. doi: 10.1038/489356a. [DOI] [PubMed] [Google Scholar]
- 97.Schwarzkopf DS, Rees G. Neuroscience. Brain activity to rely on? Science. 2010;327:43–4. doi: 10.1126/science.1184242. [DOI] [PubMed] [Google Scholar]
- 98.Boly M, Balteau E, Schnakers C, et al. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA. 2007;104:12187–92. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–84. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 100.Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. 2012;64:2398–403. doi: 10.1002/art.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–55. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schmelz M, Schmidt R. Microneurographic single-unit recordings to assess receptive properties of afferent human C-fibers. Neurosci Lett. 2010;470:158–61. doi: 10.1016/j.neulet.2009.05.064. [DOI] [PubMed] [Google Scholar]
- 103.Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci USA. 2011;108:20254–9. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gard T, Holzel BK, Sack AT, et al. Pain Attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb Cortex. 2012;22:2692–702. doi: 10.1093/cercor/bhr352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grant JA, Courtemanche J, Rainville P. A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. Pain. 2011;152:150–6. doi: 10.1016/j.pain.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 106.Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152:533–42. doi: 10.1016/j.pain.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 107.Owen DG, Bureau Y, Thomas AW, Prato FS, St Lawrence KS. Quantification of pain-induced changes in cerebral blood flow by perfusion MRI. Pain. 2008;136:85–96. doi: 10.1016/j.pain.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 108.Segerdahl AR, Xie J, Paterson K, Ramirez JD, Tracey I, Bennett DL. Imaging the neural correlates of neuropathic pain and pleasurable relief associated with inherited erythromelalgia in a single subject with quantitative arterial spin labelling. Pain. 2012;153:1122–7. doi: 10.1016/j.pain.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Howard MA, Krause K, Khawaja N, et al. Beyond patient reported pain: perfusion magnetic resonance imaging demonstrates reproducible cerebral representation of ongoing post-surgical pain. PLoS ONE. 2011;6:e17096. doi: 10.1371/journal.pone.0017096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brodersen KH, Wiech K, Lomakina EI, et al. Decoding the perception of pain from fMRI using multivariate pattern analysis. Neuroimage. 2012;63:1162–70. doi: 10.1016/j.neuroimage.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]