Abstract
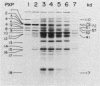
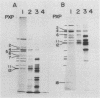
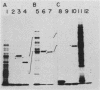
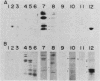
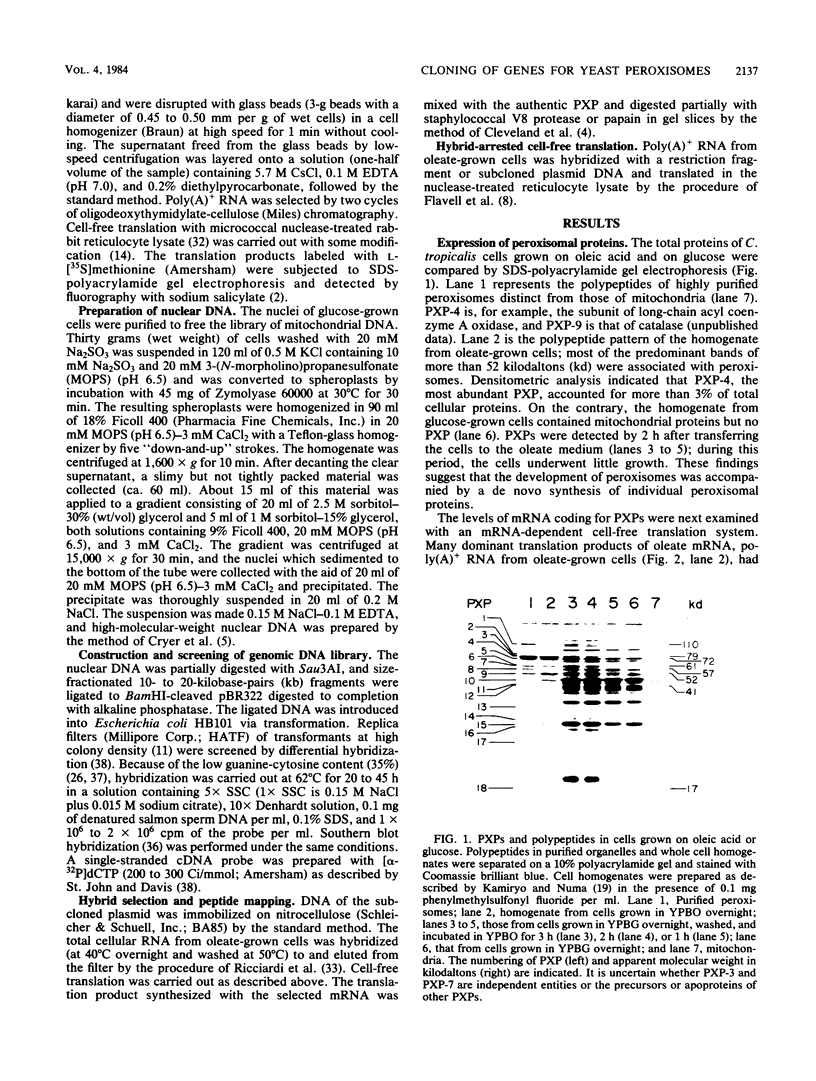
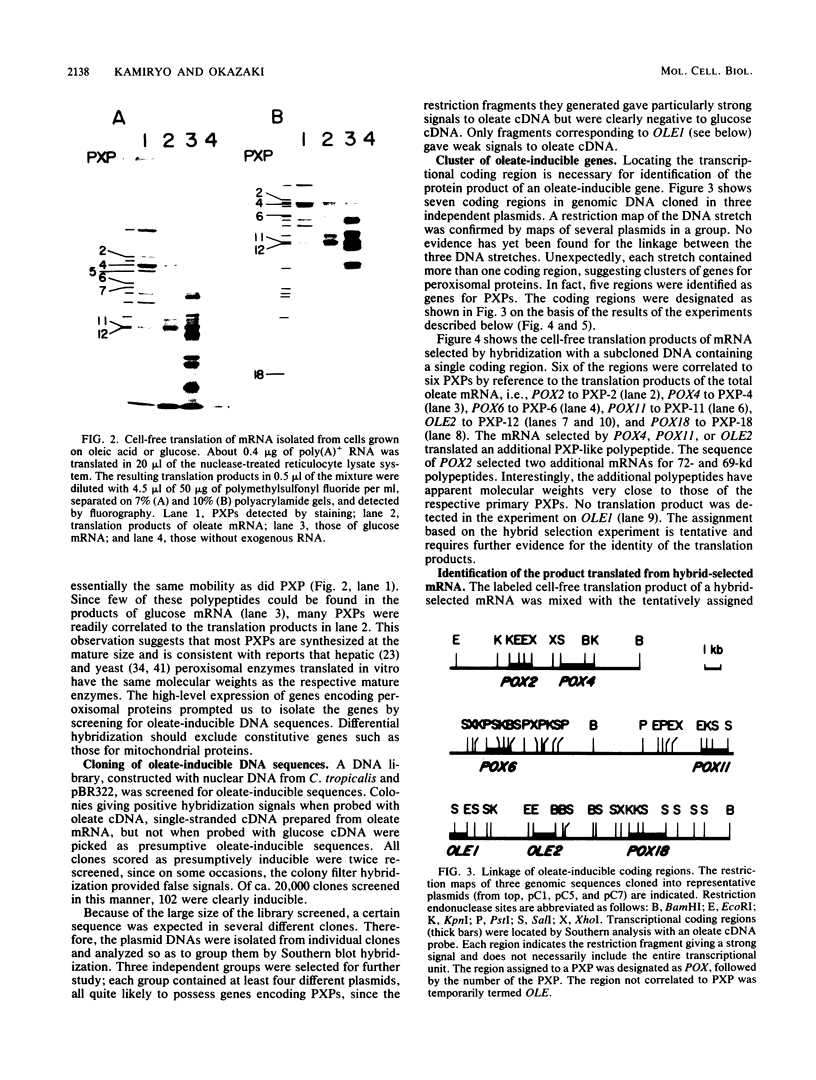
The development of peroxisomes in the cells of Candida tropicalis grown on oleic acid was accompanied by a markedly high expression of peroxisomal proteins. On the basis of this finding, the nuclear DNA library of this yeast was screened by differential hybridization, and 102 clones of oleic acid-inducible sequences were isolated. Seven coding regions were found to form clusters in three stretches of the genomic DNA. Five of the regions were identified as genes for peroxisomal polypeptides (PXPs). The coding sequence for PXP-2 hybrid selected an additional mRNA for PXP-4, the subunit of long-chain acyl coenzyme A oxidase, which was the most abundant PXP. PXP-2 and PXP-4 were close in apparent molecular weight and generated similar peptides when digested with a protease. The gene for PXP-4 was adjacent to that for PXP-2 on the genome and also hybridized to the mRNA coding for PXP-5. These and other similar results suggest that the genes for the peroxisomal proteins of this organism arose by duplication of a few ancestral genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cryer D. R., Eccleshall R., Marmur J. Isolation of yeast DNA. Methods Cell Biol. 1975;12:39–44. doi: 10.1016/s0091-679x(08)60950-4. [DOI] [PubMed] [Google Scholar]
- Dommes P., Dommes V., Kunau W. H. beta-Oxidation in Candida tropicalis. Partial purification and biological function of an inducible 2,4-dienoyl coenzyme A reductase. J Biol Chem. 1983 Sep 25;258(18):10846–10852. [PubMed] [Google Scholar]
- Donahue T. F., Daves R. S., Lucchini G., Fink G. R. A short nucleotide sequence required for regulation of HIS4 by the general control system of yeast. Cell. 1983 Jan;32(1):89–98. doi: 10.1016/0092-8674(83)90499-3. [DOI] [PubMed] [Google Scholar]
- Flavell A. J., Ruby S. W., Toole J. J., Roberts B. E., Rubin G. M. Translation and developmental regulation of RNA encoded by the eukaryotic transposable element copia. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7107–7111. doi: 10.1073/pnas.77.12.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Molecular cloning and characterization of the human beta-like globin gene cluster. Cell. 1980 Apr;19(4):959–972. doi: 10.1016/0092-8674(80)90087-2. [DOI] [PubMed] [Google Scholar]
- Fukui S., Kawamoto S., Yasuhara S., Tanaka A., Osumi M. Microbody of methanol-grown yeasts. Localization of catalase and flavin-dependent alcohol oxidase in the isolated microbody. Eur J Biochem. 1975 Nov 15;59(2):561–566. doi: 10.1111/j.1432-1033.1975.tb02482.x. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hashimoto T. Individual peroxisomal beta-oxidation enzymes. Ann N Y Acad Sci. 1982;386:5–12. doi: 10.1111/j.1749-6632.1982.tb21403.x. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G., Fink G. R. Positive regulation in the general amino acid control of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5374–5378. doi: 10.1073/pnas.80.17.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa S., Kamiryo T., Nakanishi S., Numa S. Cell-free translation and regulation of Candida lipolytica acetyl-coenzyme-A carboxylase messenger RNA. Eur J Biochem. 1980 Feb;104(1):191–198. doi: 10.1111/j.1432-1033.1980.tb04415.x. [DOI] [PubMed] [Google Scholar]
- Kamiryo T., Abe M., Okazaki K., Kato S., Shimamoto N. Absence of DNA in peroxisomes of Candida tropicalis. J Bacteriol. 1982 Oct;152(1):269–274. doi: 10.1128/jb.152.1.269-274.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiryo T. Control of triglyceride synthesis by the intracellular level of long-chain acyl coenzyme A for lipid synthesis. J Bacteriol. 1983 Oct;156(1):447–449. doi: 10.1128/jb.156.1.447-449.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiryo T., Mishina M., Tashiro S. I., Numa S. Candida lipolytica mutants defective in an acyl-coenzyme A synthetase: isolation and fatty acid metabolism. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4947–4950. doi: 10.1073/pnas.74.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiryo T., Nishikawa Y., Mishina M., Terao M., Numa S. Involvement of long-chain acyl coenzyme A for lipid synthesis in repression of acetyl-coenzyme A carboxylase in Candida lipolytica. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4390–4394. doi: 10.1073/pnas.76.9.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiryo T., Numa S. Reduction of the acetyl coenzyme A carboxylase content of Saccharomyces cerevisiae by exogenous fatty acids. FEBS Lett. 1973 Dec 15;38(1):29–32. doi: 10.1016/0014-5793(73)80505-8. [DOI] [PubMed] [Google Scholar]
- Kawamoto S., Nozaki C., Tanaka A., Fukui S. Fatty acid beta-oxidation system in microbodies of n-alkane-grown Candida tropicalis. Eur J Biochem. 1978 Feb;83(2):609–613. doi: 10.1111/j.1432-1033.1978.tb12130.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., Robbi M., Fujiki Y., Wong L. Biogenesis of peroxisomal proteins in vivo and in vitro. Ann N Y Acad Sci. 1982;386:285–300. doi: 10.1111/j.1749-6632.1982.tb21423.x. [DOI] [PubMed] [Google Scholar]
- Meyhack B., Bajwa W., Rudolph H., Hinnen A. Two yeast acid phosphatase structural genes are the result of a tandem duplication and show different degrees of homology in their promoter and coding sequences. EMBO J. 1982;1(6):675–680. doi: 10.1002/j.1460-2075.1982.tb01229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Kamiryo T., Tashiro S., Hagihara T., Tanaka A., Fukui S., Osumi M., Numa S. Subcellular localization of two long-chain acyl-coenzyme-A synthetases in Candida lipolytica. Eur J Biochem. 1978 Sep 1;89(2):321–328. doi: 10.1111/j.1432-1033.1978.tb12533.x. [DOI] [PubMed] [Google Scholar]
- Osumi M., Kazama H. Microbody-associated DNA in Candida tropicalis pK 233 cells. FEBS Lett. 1978 Jun 15;90(2):309–312. doi: 10.1016/0014-5793(78)80393-7. [DOI] [PubMed] [Google Scholar]
- Osumi M. Possible existence of DNA in yeast microbody. J Electron Microsc (Tokyo) 1976;25(1):43–47. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa M., Blobel G. Biosynthesis of peroxisomal enzymes in the methylotrophic yeast Hansenula polymorpha. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6872–6876. doi: 10.1073/pnas.80.22.6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D. T., Lemire J. M., Bostian K. A. Acid phosphatase polypeptides in Saccharomyces cerevisiae are encoded by a differentially regulated multigene family. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2157–2161. doi: 10.1073/pnas.79.7.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- St John T. P., Davis R. W. Isolation of galactose-inducible DNA sequences from Saccharomyces cerevisiae by differential plaque filter hybridization. Cell. 1979 Feb;16(2):443–452. doi: 10.1016/0092-8674(79)90020-5. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Osumi M., Fukui S. Peroxisomes of alkane-grown yeast: fundamental and practical aspects. Ann N Y Acad Sci. 1982;386:183–199. doi: 10.1111/j.1749-6632.1982.tb21416.x. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., van Dijken J. P., Pilon S. A., Harder W. Development of crystalline peroxisomes in methanol-grown cells of the yeast Hansenula polymorpha and its relation to environmental conditions. Arch Microbiol. 1978 May 30;117(2):153–163. doi: 10.1007/BF00402303. [DOI] [PubMed] [Google Scholar]
- Yamada T., Tanaka A., Horikawa S., Numa S., Fukui S. Cell-free translation and regulation of Candida tropicalis catalase messenger RNA. Eur J Biochem. 1982 Dec 15;129(2):251–255. doi: 10.1111/j.1432-1033.1982.tb07046.x. [DOI] [PubMed] [Google Scholar]