Abstract
Introduction
Heterotopic ossification (HO), or the abnormal formation of bone in soft tissue, occurs in over 60% of major burn injuries and blast traumas. A significant need exists to improve the current diagnostic modalities for HO which are inadequate to diagnose and intervene on HO at early time-points. Raman spectroscopy has been used in previous studies to report on changes in bone composition during bone development but has not yet been applied to burn induced HO. In this study, we validate transcutaneous, in-vivo Raman spectroscopy as a methodology for early diagnosis of HO in mice following a burn injury.
Methods
An Achilles tenotomy model was used to study HO formation. Following tenotomy, mice were divided into burn and sham groups with exposure of 30% surface area on the dorsum to 60° water or 30° water for 18 seconds respectively. In-vivo, transcutaneous Raman spectroscopy was performed at early time points (5 days, 2 and 3 weeks) and a late time point (3 months) on both the tenotomized and non-injured leg. These same samples were then dissected down to the bone and ex-vivo Raman measurements were performed on the excised tissue. Bone formation was verified with Micro CT and histology at corresponding time-points.
Results
Our Raman probe allowed non-invasive, transcutaneous evaluation of heterotopic bone formation. Raman data showed significantly increased bone mineral signaling in the tenotomy compared to control leg at 5 days post injury, with the difference increasing over time whereas Micro CT did not demonstrate heterotopic bone until three weeks. Ex-vivo Raman measurements showed significant differences in the amount of HO in the burn compared to sham groups and also showed differences in the spectra of new, ectopic bone compared to pre-existing cortical bone.
Conclusions
Burn injury increases the likelihood of developing HO when combined with traumatic injury. In our in-vivo mouse model, Raman spectroscopy allowed for detection of HO formation as early as 5 days post injury. Changes in bone mineral and matrix composition of the new bone were also evidenced in the Raman spectra which could facilitate early identification of HO and allow more timely therapy decisions for HO patients.
Keywords: Heterotopic ossification, Bone, Raman spectroscopy, Micro CT, Achilles tenotomy, Burn injury
1) Introduction
-
1.1)
Heterotopic ossification (HO), the abnormal development of bone in soft tissues, is a clinically devastating sequela of trauma, burn, and orthopaedic surgery which lacks a reliable method for early diagnosis. More than 60% of major burn patients, 65% of major combat injury patients, and 10% of patients who have invasive surgery develop HO.1, 2 Without early detection or intervention, progression of HO can lead to severe long-term effects, including restricted joint mobility, severe pain, and nerve entrapment. Current techniques for diagnosis rely on clinical examination and radiographic imaging modalities that have low sensitivity to the incremental progression of mineralization associated with the early stages of HO.3-5 Therefore, an urgent need exists to develop novel screening techniques to visualize and detect the onset and progression of HO with high sensitivity and specificity. The exact mechanism of HO is still largely unknown, though it is thought to require osteogenic precursor cells, an inciting incident, and a permissive niche.2, 6
-
1.2)
The formation of HO begins within days to weeks of the inciting event, however the exact timing of its earliest occurrence is unknown. Clinically, symptoms are non-specific and consist of swelling, edema, and pain. Patients may have an increase in alkaline phosphatase (AP) levels, however AP levels are also elevated in global trauma and liver disease. Currently, if HO is suspected clinically, radiography is obtained (X-ray or CT). Once visible through these radiographic modalities, however, the disease has already spread beyond the point where it can be treated with oral medications such as non-steroidal anti-inflammatory agents or bisphosphonates such as etindronate.7 Presence of HO requires invasive surgical resection which has significant risk and leaves over 75% of patients with functional deficits. 1, 8-10 Thus, patients would greatly benefit if physicians had access to a point of care, non-invasive, sensitive imaging technique that could be done routinely to detect HO.
-
1.3)
The vast number of imaging modalities used to diagnose HO and the lack of consensus guidelines for HO diagnosis is indicative of the current shortcomings of available imaging technologies. Currently three-phase bone scintigraphy is the most sensitive imaging modality of early HO detection.1 Despite its sensitivity to detect HO, this imaging modality has a high level of variability and false positives which are especially prominent after trauma or fractures where there is a significant amount of inflammation or callus at the injury site. 4, 11 Similarly, ultrasound technology has been shown to detect HO sooner than conventional radiography and can be used in a point of care manner. 3, 12 Like 3-phase scintigraphy, ultrasound cannot distinguish new bone formation from a normally healing fracture callus. Radiographic techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) provide highly detailed anatomic representation of late stage HO, however, these modalities cannot detect early stages of the disease process. Thus, despite being helpful for surgical planning of HO extirpation, current radiographic techniques do not allow for early diagnosis of the disease and the potential institution of therapeutic remedies that can impede or even prevent the process from becoming clinically significant. In summary, current imaging modalities, though helpful in late diagnosis, are inadequate to help clinicians detect early HO development or aid in its interventional treatment.
-
1.4)
We hypothesize that Raman Spectroscopy offers a novel diagnostic technology that is non-invasive, informs surgeons about the extent of the disease process for surgical planning, and also provides the opportunity for early diagnosis which is currently unavailable.13, 14 These unique features may enable early clinical intervention and facilitate focused treatment paradigms designed to halt the progression of HO and its associated morbidities. A single report of the application of Raman spectroscopy to characterize HO2 describes mineralized collagen found in debrided combat wound tissue at a time before it was detectable visually or by other standard modalities. However, this imaging was performed on tissue samples following excision rather than in a non-invasive manner.
-
1.5)
Development of non-invasive imaging techniques to detect early signs of HO would allow for improved prophylactic and treatment strategies. The aim of this study was to establish quantifiable metrics of non-invasive, transcutaneous Raman spectroscopy in the early formation and progression of HO in a mouse model of burn induced HO. We hypothesized that Raman would have the ability to distinguish heterotopic bone from native bone, that the detected signal from the heterotopic bone would intensify with maturity over time, and that these metrics could be validated with Micro CT, ex-vivo Raman, and histology.
2) Methods
2.1) Burn Injury and Achilles Tenotomy Models
-
2.1.1)
The procedure was performed according to a previously established method to produce partial-thickness burn injury.15-17 In brief, 9 male, C57BL/6 mice 7-8 weeks old were anesthetized with a 50 mg/kg intraperitoneal (ip) injection of sodium pentobarbital (Nembutal; Abbott Laboratories, North Chicago, IL). Dorsal hair was closely clipped. Each mouse was placed in an insulated, custom-made mold, which exposed the dorsal region over 30% of the total body surface area. Partial-thickness scald burn injury was achieved by placing the exposed skin of the mouse in a 60°C water bath for 18 seconds (n=3). Sham burn animals received the same treatment except they were immersed in room temperature water (30°C, n=6).
-
2.1.2)
The burn wound was scrub debrided with dry sterile gauze and each animal was resuscitated with 1 mL Ringer’s lactate solution IP injection and 0.5mL subcutaneous injection. After drying, an occlusive dressing of sterile Tegaderm HP (3M HealthCare, St Paul, MN) was applied to prevent wound contamination.
-
2.1.3)
All mice received an Achilles tenotomy on the left leg after burn or sham injury. A 1 cm incision was made on the lateral aspect of the Achilles tendon with a surgical knife. Subsequently, the Achilles tendon was exposed from its origin on the distal end of the gastrocnemius to the insertion at the calcaneus. The Achilles tendon was then divided sharply at its midpoint. The incision was then closed with absorbable sutures. Experiments were performed in accordance with National Institute of Health guidelines and prior approval was obtained from the University of Michigan Animal Care and Use Committee (IACUC #0001553).
2.2) Raman Spectroscopy
-
2.2.1)
The fiber optic probe system used for these experiments has been previously described.14 Spectroscopic measurements were completed at four time points (5 days, 2 weeks, 3 weeks, and 3 months) longitudinally (n=3 burn, n=6 sham). Briefly, the anesthetized mouse is placed on the probe with its left hind leg positioned in the probe assembly. The optical fibers are positioned so that they just contact the animal’s skin. Prior to positioning the mouse for Raman measurement, hair was removed from the leg using a depilatory cream (Nair), and glycerol was topically applied as optical clearing agent.18
-
2.2.2)
Raman measurements were made with a portable system (Rxn-1, Kaiser Optical Systems, Inc., Ann Arbor. MI). A calibration tool (HCA, Kaiser Optical Systems) was used daily for white light correction and calibration of detector wavelength axis. The HCA contains a white light source for calibration of the detector wavelength response and a neon discharge lamp for calibration of the spectrograph wavelength scale. A fluorocarbon excitation fiber was used to correct for skin albedo. A fluorinated ethylene propylene (FEP) cap was placed over the excitation fiber to use as a reference material in the normalization of spectra for comparison.
-
2.2.3)
The in-vivo transcutaneous Raman spectra were collected in standard formats. Spectra were pre-processed for removal of cosmic spikes and correction of spectrograph/detector alignment and grating-induced anamorphic magnification (curvature). Spectra were corrected for the fluorescence background by fitting background to a low order polynomial (Polynomial order = 5). Overlapped bands are fitted to mixed Gaussian-Lorentzian functions. Band heights and areas are measured.
-
2.2.4)
After preprocessing, information was extracted about the various components of bone using bands and band intensity ratios listed in Table 1. Commonly, in bone, Raman spectroscopy positions of mineral and matrix bands and ratios of band heights or band areas are reported. 19 The height of the intense PO4-3 υ1 stretch (958cm-1) was used as a measure of mineral content and the width of this band was used as a measure of mineral crystallinity. The height of the amide I band (1660cm-1) or phenylalanine band (1001cm-1) was used as the measure of matrix content. Mineral to matrix ratio (MTMR) is the intensity of the bone mineral band divided by the intensity of a matrix band. Standard principal components analysis based multivariate methods were used to unmix bone and overlying tissue spectra returned by our measurements. It is important to note that the Raman instrumentation employed in these measurements incorporates fiber-optic probes. The fibers are multimode optical fibers (NA~0.2), which depolarizes light and such no polarization effects are observable. Consequently, the Raman bands are not affected by molecular orientations of bone compositions.20
Table 1.
Raman band assignments and positions for bone, from literature sources.
| Band Assignment | Position, cm-1 |
|---|---|
| Phosphate P-O stretch ν1 | 957-962 |
| Mineral crystallinity | 1/FWHM ν1 |
| Carbonate C-O ν1 | 1071 |
| Hydroxyproline ring | 876 |
| Proline ring | 916 |
| Phenylalanine, ring breathing | 1003 |
| Amide III | 1243-1269 |
| CH2 deformation | 1447-1452 |
| Amide I | 1660-1690 |
| Cross-links ratio | 1660/1690 |
2.3) Micro-Computed Tomography
-
2.3.1)
In parallel with Raman imaging, Micro-CT was obtained longitudinally. At each time point (5 days, 2 weeks, 3 months), mice were imaged by Micro-CT (GE eXplore Locus, GE Healthcare) to assess onset and progression of mineralization (n=3 burn, n=6 sham). Using a calibrated imaging protocol, reconstructed volumes were segmented based on Hounsfield units, anatomical landmarks, and observer identification to define the volume of original cortical bone structures. This volume was subtracted from the image and remaining bone mineral in the soft tissues was quantified for bone volume of HO using standard protocols.5, 21-24 A phantom was used at the time of micro CT imaging to allow for standardization.
2.4) Histologic Sample Collection for ex-vivo Raman
-
2.4.1)
A cross section of a mouse leg was made at the distal end of the tibia and fibula without any fixation or embedding. In short, immediately following euthanasia of mice 3 months post tenotomy with known areas and quantity of HO, the leg was implanted in a paraffin block to provide structural support as sections were made through the fresh tissue and bone with a diamond tipped exact ban-saw. The tissue discs were extracted from the paraffin support and spectra were taken on an ex-vivo Raman platform over a 100 micron area selected through microscopy where HO was known to exist from previous Micro CT analysis. These spectra were collected immediately without interference from prior freezing or fixation chemicals and products.
2.5) Histologic Processing for Picrosirus Red and Pentachrome Stain
-
2.5.1)
Whole lower extremities from proximal tibia distally were denuded of skin and fixed overnight in 0.4% paraformaldehyde in PBS at 4°C. Tissue was taken from equal numbers of burn and sham mice (n=3 burn, n=3 sham). Tissues were then decalcified in 19% ethylenediaminetetracetic acid for 21 days at 4°C, dehydrated through graded ethanol, and paraffin embedded. Sagittal sections through the tibia, fibula and foot of 5 micron width were mounted on Superfrost plus slides (Fisher Scientific, Pittsburg, PA), and dried overnight at 37°C. Sections were subsequently stained with picrosirius red and pentachrome for qualitative observation of heterotopic ossification in relation to the Achilles tenotomy site and original cortical bone.
3) Results
3.1) Raman spectroscopy allows for transcutaneous detection of bone tissue
-
3.1.1)
To establish the ability of Raman spectroscopy to identify and distinguish ectopic bone transcutaneously in a live mouse, we first performed Raman imaging on a set of mice that had known location and amount of HO formation as measured by Micro CT scans at 3 months post tenotomy and burn injuries as described above. An array of optical fibers was situated around the distal tibia of the mouse with the excitation fiber nearest to and touching the area where HO had developed (Fig. 1A). Spectral analysis showed peaks in the bone mineral bands at 958cm-1 (PO43-) and 1070cm-1 (CO32-) respectively which are characteristic of the unique apatite mineralization of bone (Fig. 1B). Peaks at 1660cm-1 were also consistent with expected protein bands, including collagen that would be present in bone tissue. The 1450 cm-1 band is a combination of protein and lipids CH2 wagging vibrations.
Figure 1.

Raman spectroscopy detects bone tissue transcutaneously. A. Apparatus for non-invasive Raman measurement on mice. Excitation fiber (red) and multiple collection fibers (green) allow for transcutaneous in-vivo imaging of mouse. B. Representative Raman spectrum of mouse with known development of HO 3 months after tenotomy and burn injury. Red arrows point to peaks of interest in the Spectra: Phosphate and Carbonate bone mineral components at 958cm-1 and 1070cm-1 respectively, matrix lipids (CH2 deformation) at 1450cm-1, and matrix proteins and collagen (Amide I) at 1660 cm-1. The 1450 cm-1 band is a combination of protein and lipids CH2 wagging vibrations.
3.2) Raman spectroscopy identifies ectopic bone development
-
3.2.1)
Raman measurements were carried out on both the injured and non-injured leg of mice with known HO formation as indicated above and the intensity of the bone mineral signal and the ratio of bone mineral to matrix were examined on both injured and non-injured legs. Increased bone mineral signal was present in the injured leg versus the non-injured leg of burn mice with known HO development 3 months after tenotomy (Fig 2A). Furthermore, in-vivo Raman measurements demonstrated significant differences in the mineral to matrix ratio (MTMR) between the non-injured and the injured leg of these mice with known HO development (Fig 2A, p<0.05). Results were verified by Micro CT and histology (Fig. 2B).
Figure 2.
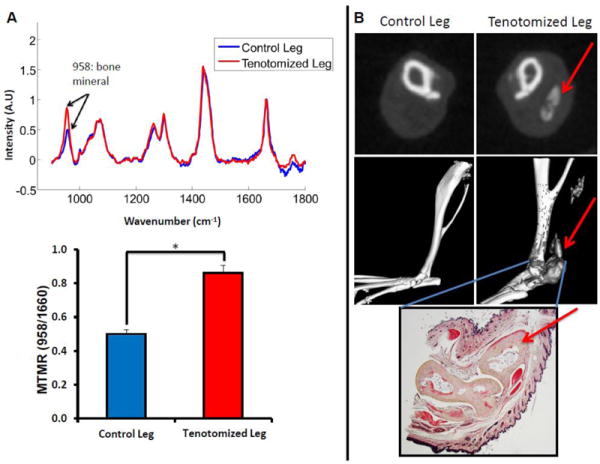
Raman Spectroscopy and cross sectional Micro CT of Achilles tenotomy model on non-injured and on tenotomized leg at 3 months after injury and burn. A. (Top) Raman spectra of tenotomized leg (Red) and non-tentotmized control leg (blue) of a burn mouse that had known HO growth. Spectra are normalized to the protein matrix and collagen band at 1600cm-1 and superimposed to show differences in bone mineral signal at 958cm-1 (Bottom) Mineral to matrix ratio from Raman spectra demonstrates increased mineral content in the tenotomized leg (*, p<0.05) B. Micro CT confirmation of HO growth in the tenotomized leg seen in representative CT slices (Top) and 3D reconstructions (Middle). Red arrows indicate HO formation. Gray areas in the reconstructed image indicate ectopic bone. Histologic verification of ectopic bone growth with pentachrome stain (Bottom) showing bone as pale yellow. Red arrow indicates HO formation.
3.3) At 5 days post-surgery, Raman spectroscopy detects heterotopic bone that is not detected by Micro CT scan
-
3.3.1)
Having established the ability of Raman spectroscopy to identify differences in bone signal and MTMR in mice with known ectopic bone development, the next step was to track the development of HO formation in a proven Achilles tenotomy model to establish the time point at which changes in bone composition are first detectable. In the group of mice followed longitudinally with simultaneous Micro CT and Raman scans, significant differences in MTMR were shown as early as 5 days post tenotomy with concurrent burn injury in animals that would later develop HO that could be confirmed by histology and Micro CT (Fig. 3A, p<0.05). The differences in MMTR also increased at 2 weeks and 3 weeks. Despite our early detection of ectopic bone by Raman, the presence of HO was not detectable by Micro CT at 5 days, but was first detectable at 3 weeks (Fig. 3B). The CT region of HO was subsequently confirmed at this time point by histology (Fig 3B).
Figure 3.

HO formation detected by Raman spectroscopy at 5 days post injury. A. (Left) Raman spectra from a mouse in the Burn group that would develop HO. Spectra are normalized to the FEP material reference band. (Right) Early data from 5 day, 2 week, and 3 week time points show changes via Raman spectroscopy and significant differences in MTMR (*, p<0.05). B. Earliest time point HO detected by CT is 3 weeks. (Left) Micro CT at 5 days and 3 weeks after tenotomy, gray area is newly forming bone with arrow showing nidus of developing HO. (Right) Picrosirius red stain confirming HO at 3 weeks after tenotomy in the same region as seen by Micro CT (arrow).
3.4) Burn injury increases heterotopic bone formation as shown by Raman spectroscopy
-
3.4.1)
To test the ability of transcutaneous Raman spectroscopy to elicit information not only about the presence of HO, but about the quantity of HO development, we compared the spectroscopic measurements to results obtained through Micro CT showing significantly more robust HO development in mice in the burn group than their sham counterparts. Bone signals were detected in both groups at day 5, 2 weeks, and 3 months by Raman spectroscopy (Fig. 4A). Consistent with the location of future HO formation, there was significantly more bone signal and increased MTMR in the burn group than the sham group with differences first appearing at 5 days post injury and increasing over time (Fig. 4B, p<.05). These differences between HO formation in mice who received concurrent burn injury with Achilles tenotomy as detected by Raman spectroscopy were confirmed with Micro CT scans. However, no HO was detected until 3 weeks post-injury via Micro CT. Significant differences between HO development in burn and sham mice were seen at 3 months post-injury via Micro CT (Fig 4C, p<.05). Thus, burn injury increases the detectable HO using our Achilles model and transcutaneous Raman Spectroscopy.
Figure 4.

Raman spectroscopy measurements of bone mineral in Burn and Sham groups at 5 days, 2 weeks, and 3 months. A. Raman spectra around area of tenotomy. Arrows mark bone mineral, lipid, protein, and FEP reference material bands. Spectra are normalized to FEP reference material signal. Blue line demonstrates mice that received achilles tenotomy only. Red line demonstrates mice receiving burn injury plus achilles tenotomy. B. Bone mineral band intensity compared to matrix protein and collagen band intensity (MTMR) shown at 5 days, 2 weeks, and 3 months. Bone mineral ratio normalized to FEP reference band (*, p<.05). C. Micro CT measurements of HO formation in Sham and Burn mice at earliest detectable timepoint (3 weeks) and late time-point (3 months). Red arrow signifies HO growth (gray area, *, p<.05)
3.5) High resolution Raman characterization of heterotopic bone on histologic sections
-
3.5.1)
To better understand the chemical composition of ectopic bone development, carefully cut sections of tenotomy sites with known amounts and location of HO were imaged on a high resolution Raman microscopy platform. In order to yield results representing the overall character of the tissue types, spectra were collected using an imaging mode that took 10 spectra over a 100 micron area (10 frames per image, 10 μm step size). Focusing on a field that could be defined by macroscopically observed bone formation allowed for precise study of the differences between soft tissue, original cortical bone, areas where HO was known to have occurred, and areas where HO could potentially have developed based on Micro CT and macroscopic observation under a dissection microscope. We found that in soft tissue the main band near the 958 cm-1 bone mineral peak is at higher wavenumber and at lower intensity indicating little to no mineral present when normalized to the 1001 cm-1 phenylalanine peak (Fig. 5A). With the formation of HO, the band center shifts toward the value for cortical bone, 958 cm-1. As HO progresses (and with it the amount of HO), regions identified as containing HO demonstrate greater mineralization than those labeled as potential HO in agreement with Micro CT predictions and are distinctive from cortical bone. This is indicated by higher mineral intensity and significant differences in crystallinity (width of bone mineral band) and MTMR (matrix band = phenylalanine) for all three regions (Fig. 5B, p<.05). Thus, we confirmed by traditional histology and by high resolution Raman spectroscopy of histologic sections that we have detected HO transcutaneously. Furthermore, we have shown that heterotopic bone has different mineral crystallinity and MTMR than cortical bone.
Figure 5.

Characterization of HO and cortical bone by high resolution Raman Spectroscopy. A. Mean ex-vivo high resolution Raman spectra of excised microtomed tissue sections from the region near the tenotomy site of a burn mouse, 3 months after injury. Soft tissue (black), Micro CT predicted HO locations (red solid), Micro CT predicted potential HO locations – near the tenotomy site where HO had been seen in other specimens (red dotted), and cortical bone (blue) labeled for both Raman spectra and corresponding circled regions in the optical image (Right). The phosphate (958 cm-1) vibrational region is shown in the left upper inset, dotted line indicating the band center of normal cortical bone. B. Crystallinity and mineral to matrix ratio of microtomed tissue section from burn and tenotomized mouse. Raman spectra are normalized to the 1001 cm-1 phenylalanine peak. (* p<.05)
4) Discussion
-
4.1)
Non-invasive Raman spectroscopy could offer the basic scientist and eventually the clinician a powerful new tool to detect HO and to obtain compositional information about this pathological bone unavailable from established histological and imaging techniques. Though radiologic techniques allow for detection of heterotopic bone at later time-points, they fail to identify the earliest deposits. Furthermore, neither ionizing radiation nor exogenous labels are required for Raman spectroscopy. While histology provides useful information, the acquisition of such specimens requires tissue biopsies which is invasive and impractical clinically. While our current results demonstrate that Raman spectroscopy can be valuable in the study of early stages of HO, it is still too early to define its role in the clinic. Validation of non-invasive Raman spectroscopy for bone in human subjects is underway in our laboratories and we believe this study in mice demonstrates its strengths and potential.
-
4.2)
Certainly, serial CT scans and collection of serial histologic specimens is not viable in a clinical setting. In contrast, Raman spectroscopy provides non-invasive in-vivo analysis of bone composition that can be obtained at multiple time points. Raman spectroscopy might be especially useful for HO and other bone disorders, because it provides information about both bone mineral and matrix composition which in turn are directly related to the health and mechanical competence of the tissue. Furthermore, Raman spectroscopy provides a “fingerprint” that characterizes the bone and might allow clinicians to identify patients that have early HO and are highly likely to develop a more destructive clinical course. 2
-
4.3)
Current early treatment options for HO include anti-inflammatories and bisphosphonates. These agents, despite some successes, have significant side effects and thus are not viable treatments for all patients at risk for HO. 25-30 Furthermore, their efficacy might be limited by the fact that we often diagnose HO beyond the time at which it can be treated. Even more important than the information provided on the pathogenesis of HO is the potential use of Raman spectroscopy to guide treatment timing. If physicians could diagnose HO in its early stages, they might then have the ability to determine whom to target with early prophylaxis and treatment. We propose that some patients or injury patterns are more prone to develop HO than others and that a technology such as Raman spectroscopy that allows early identification of these patients would allow more directed and effective treatments. Studies have not been able to identify a cutoff above of burn severity at which patients become more susceptible to HO formation. Additionally, we do not currently understand what initiates the formation of HO in some patients undergoing elective orthopedic operations and not others who undergo the same operation. Thus, the ability to predict which patients would develop HO by Raman would be a significant clinical breakthrough.
-
4.4)
We demonstrate that transcutaneous Raman spectroscopy allows for the detection of heterotopic bone tissue, shows different signals between ectopic bone and native cortical bone, and detects heterotopic bone as early as five days after injury. This was in comparison to detectable, confirmatory diagnosis with Micro CT imaging, which was first seen at three weeks after formation. We further demonstrate the ability to detect burn-induced augmentations in HO with transcutaneous Raman and verify the progression of HO over time utilizing Micro CT and ex-vivo Raman data. These results expand on previous work done using Raman to characterize HO in excised tissue from human patients developing HO.2 Researchers have demonstrated that injured muscle causes a significant alteration in collagen specific vibrational bands. This change in the collagen “fingerprint” by Raman spectroscopy allowed the identification of “pre-heterotopic ossification.” This analysis, however, required direct analysis of the muscle tissue and was only applied to amputation patients. Greater benefit could be gained from a diagnostic platform that can detect and characterize developing HO transcutaneously, in-vivo in both trauma and burn patients. Future studies will continue to analyze the use of Raman spectroscopy transcutaneously and apply this model to study various treatment options with Raman spectroscopy allowing early analysis and observation of HO formation and the efficacy of mitigation by pharmacologic treatments.
5) Conclusion
-
5.1)
Our findings support the contention that transcutaneous Raman spectroscopy may be utilized as a robust and valuable diagnostic tool to monitor the formation and progression of HO. We provide evidence for the superior detection of early HO when compared to high resolution Micro CT, and validate our results with histology and ex-vivo Raman spectroscopy. Based on its non-invasive nature and potential for early diagnosis, we believe that transcutaneous Raman spectroscopy offers novel and superior advantages when compared to current diagnostic technologies for HO and support continued efforts for potential clinical translation.
-
5.2)
These findings establish a valuable dataset that will allow for future comparisons regarding the utility of various treatment options designed to mitigate the development and progression of HO. Our future aim is to investigate the effects of pharmacologic therapies in the remediation of HO in this burn-induced HO model. By doing so we hope to provide valuable, promising treatment paradigms with potential clinical application to halt the progression of HO and its associated morbidities in affected patients.
Highlights.
Achilles tenotomy with burn injury model to study Heterotopic Ossification in mice
Raman spectroscopy in-vivo and ex-vivo to detect ectopic bone at early time points
Concurrent burn injury increases trauma-induced HO
Raman spectroscopy shows HO as early as 5 days post-injury vs 3 weeks with Micro CT
Presence of HO confirmed with serial Raman spectroscopy, Micro CT, and histology
Acknowledgments
The authors thank Kathy Sweet and Amanda Welton for their technical assistance.
Source of Funding: R01 AR055222,
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med. 2005;37:129–136. doi: 10.1080/16501970510027628. [DOI] [PubMed] [Google Scholar]
- 2.Potter BK, et al. Heterotopic ossification following combat-related trauma. J Bone Joint Surg Am. 2010;92(Suppl 2):74–89. doi: 10.2106/JBJS.J.00776. [DOI] [PubMed] [Google Scholar]
- 3.Cassar-Pullicino VN, et al. Sonographic diagnosis of heterotopic bone formation in spinal injury patients. Paraplegia. 1993;31:40–50. doi: 10.1038/sc.1993.7. [DOI] [PubMed] [Google Scholar]
- 4.Freed JH, Hahn H, Menter R, Dillon T. The use of the three-phase bone scan in the early diagnosis of heterotopic ossification (HO) and in the evaluation of Didronel therapy. Paraplegia. 1982;20:208–216. doi: 10.1038/sc.1982.39. [DOI] [PubMed] [Google Scholar]
- 5.McCreadie BR, Goulet RW, Feldkamp LA, Goldstein SA. Hierarchical structure of bone and micro-computed tomography. Adv Exp Med Biol. 2001;496:67–83. doi: 10.1007/978-1-4615-0651-5_8. [DOI] [PubMed] [Google Scholar]
- 6.Lounev VY, et al. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cullen N, Perera J. Heterotopic ossification: pharmacologic options. J Head Trauma Rehabil. 2009;24:69–71. doi: 10.1097/HTR.0b013e31819a8fcc. [DOI] [PubMed] [Google Scholar]
- 8.Garland DE. Surgical approaches for resection of heterotopic ossification in traumatic brain-injured adults. Clin Orthop Relat Res. 1991:59–70. [PubMed] [Google Scholar]
- 9.Hunt JL, Arnoldo BD, Kowalske K, Helm P, Purdue GF. Heterotopic ossification revisited: a 21-year surgical experience. J Burn Care Res. 2006;27:535–540. doi: 10.1097/01.BCR.0000226023.58438.14. [DOI] [PubMed] [Google Scholar]
- 10.Ring D, Jupiter JB. Operative release of ankylosis of the elbow due to heterotopic ossification. Surgical technique. J Bone Joint Surg Am. 2004;86-A(Suppl 1):2–10. doi: 10.2106/00004623-200403001-00002. [DOI] [PubMed] [Google Scholar]
- 11.Muheim G, Donath A, Rossier AB. Serial scintigrams in the course of ectopic bone formation in paraplegic patients. Am J Roentgenol Radium Ther Nucl Med. 1973;118:865–869. doi: 10.2214/ajr.118.4.865. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka T, Rossier AB, Hussey RW, Ahnberg DS, Treves S. Quantitative assessment of para-osteo-arthropathy and its maturation on serial radionuclide bone images. Radiology. 1977;123:217–221. doi: 10.1148/123.1.217. [DOI] [PubMed] [Google Scholar]
- 13.Okagbare PI, et al. Noninvasive Raman spectroscopy of rat tibiae: approach to in vivo assessment of bone quality. J Biomed Opt. 2012;17:90502–90501. doi: 10.1117/1.JBO.17.9.090502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okagbare PI, Morris MD. Polymer-capped fiber-optic Raman probe for non-invasive Raman spectroscopy. Analyst. 2012;137:77–81. doi: 10.1039/c1an15847c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niederbichler AD, et al. Cardiomyocyte function after burn injury and lipopolysaccharide exposure: single-cell contraction analysis and cytokine secretion profile. Shock. 2006;25:176–183. doi: 10.1097/01.shk.0000192123.91166.e1. [DOI] [PubMed] [Google Scholar]
- 16.Niederbichler AD, et al. Burn-induced heart failure: lipopolysaccharide binding protein improves burn and endotoxin-induced cardiac contractility deficits. J Surg Res. 2011;165:128–135. doi: 10.1016/j.jss.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ipaktchi K, et al. Topical p38 MAPK inhibition reduces bacterial growth in an in vivo burn wound model. Surgery. 2007;142:86–93. doi: 10.1016/j.surg.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulmerich MV, et al. Optical clearing in transcutaneous Raman spectroscopy of murine cortical bone tissue. J Biomed Opt. 2008;13:021108. doi: 10.1117/1.2892687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris MD, Mandair GS. Raman assessment of bone quality. Clin Orthop Relat Res. 2011;469:2160–2169. doi: 10.1007/s11999-010-1692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raghavan M, et al. Quantitative polarized Raman spectroscopy in highly turbid bone tissue. J Biomed Opt. 2010;15:037001. doi: 10.1117/1.3426310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levi B, et al. CD105 protein depletion enhances human adipose-derived stromal cell osteogenesis through reduction of transforming growth factor beta1 (TGF-beta1) signaling. J Biol Chem. 2011;286:39497–39509. doi: 10.1074/jbc.M111.256529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levi B, et al. Dura mater stimulates human adipose-derived stromal cells to undergo bone formation in mouse calvarial defects. Stem Cells. 2011;29:1241–1255. doi: 10.1002/stem.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levi B, et al. Nonintegrating knockdown and customized scaffold design enhances human adipose-derived stem cells in skeletal repair. Stem Cells. 2011;29:2018–2029. doi: 10.1002/stem.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meganck JA, Kozloff KM, Thornton MM, Broski SM, Goldstein SA. Beam hardening artifacts in micro-computed tomography scanning can be reduced by X-ray beam filtration and the resulting images can be used to accurately measure BMD. Bone. 2009;45:1104–1116. doi: 10.1016/j.bone.2009.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002;43:346–353. [PubMed] [Google Scholar]
- 26.van Kuijk AA, Geurts AC, van Kuppevelt HJ. Neurogenic heterotopic ossification in spinal cord injury. Spinal Cord. 2002;40:313–326. doi: 10.1038/sj.sc.3101309. [DOI] [PubMed] [Google Scholar]
- 27.Banovac K. The effect of etidronate on late development of heterotopic ossification after spinal cord injury. J Spinal Cord Med. 2000;23:40–44. doi: 10.1080/10790268.2000.11753507. [DOI] [PubMed] [Google Scholar]
- 28.Romano CL, Duci D, Romano D, Mazza M, Meani E. Celecoxib versus indomethacin in the prevention of heterotopic ossification after total hip arthroplasty. J Arthroplasty. 2004;19:14–18. doi: 10.1016/s0883-5403(03)00279-1. [DOI] [PubMed] [Google Scholar]
- 29.Banovac K, Williams JM, Patrick LD, Haniff YM. Prevention of heterotopic ossification after spinal cord injury with indomethacin. Spinal Cord. 2001;39:370–374. doi: 10.1038/sj.sc.3101166. [DOI] [PubMed] [Google Scholar]
- 30.Thomas BJ, Amstutz HC. Results of the administration of diphosphonate for the prevention of heterotopic ossification after total hip arthroplasty. J Bone Joint Surg Am. 1985;67:400–403. [PubMed] [Google Scholar]


