Abstract
Transplacental immune regulation refers to the concept that during pregnancy, significant cross-talk occurs between the maternal and fetal immune system with potential long-term effects for both the mother and child. In this study, we made the surprising observation that there is a strong correlation of peripheral blood regulatory T (Treg) cells between the mother and the fetus. In contrast, there is no significant Treg cell correlation between paternal fetal dyads (pairs), suggesting that the specific context of pregnancy, rather than the genetic parental similarity to the fetus, is responsible for this correlation. Gene microarray analysis of Treg cells identified a typical IL-10–dependent signature in maternal and fetal Treg cells. In addition, a direct correlation of serum IL-10 protein levels between maternal fetal dyads was observed. Furthermore, we show that maternal serum IL-10 levels correlate with serum estradiol and estriol, implicating hormonal involvement in this alignment. Interestingly, we show that Treg cells possess higher expression of IL-10 receptor α and that Treg cell IL-10 receptor α expression directly correlates with their Bcl-2 expression. Indeed, in vitro data in both humans and mice demonstrate that IL-10 upregulates Bcl-2 specifically in Treg cells but not non-Treg cells. Our results provide evidence for transplacental regulation of cellular immunity and suggest that IL-10 may influence Treg cell homeostasis through its effect on Treg cell Bcl-2 expression. These novel findings have important implications on immune tolerance in pregnancy and beyond in areas of autoimmunity, allergy, and transplantation.
Introduction
The mother and the fetus are highly interdependent entities that share a close physical and physiological relationship in which the fetus is thought to be subject to significant maternal influences. In contrast, they are separated by placental and fetal membranes, which are unique in humans among other mammals in their developmental timing, anatomy, and function (1).
Immunologically, it is well known that maternal IgG Abs selectively cross the fetal–maternal barrier from early gestation, conveying temporary passive immunity (2). In contrast, cellular components are generally separated by the placenta, with some leakage in both directions without preference toward a specific cell type (3). Nevertheless, maternal regulatory T (Treg) cells have been shown to populate the fetal lymph nodes and are thought to induce fetal immune tolerance toward maternal alloantigens (4). Several other lines of evidence support the notion of transplacental immune regulation during pregnancy. In humans, cord blood cytokine levels have been linked to subsequent development of atopy (5). Maternal exposure to farm environment during pregnancy also reduces atopic sensitization of the offspring (6); this appears to be in part mediated through an increase of fetal Treg cells (7).
In the murine model, maternal Th1-type immunity during pregnancy was shown to decrease the risk of experimental allergic airway disease in the offspring (8). Transplacental passage of allergen specific IgG also protected against asthma in the offspring in an IFN-γ–dependent manner (9). Furthermore, microbial exposure of mice during pregnancy also confers protection against the development of asthma in the offspring (10). Collectively, these studies provide evidence that the prenatal environment in utero has an important role in shaping the fetal immune system. In particular, it would seem that the maternal immune system biases the fetal immune system toward the same polarity. However, exactly which part of the immune system is involved and how this occurs during pregnancy remains largely unresolved.
Foxp3+ Treg cells are a distinct population of Th cells, which play pivotal roles in immune tolerance. Disturbance of the Treg cell population has been linked to multiple immunopathologies, including allergy (11), autoimmunity (12), and cancer (13). Several studies have shown that there is a systemic increase in Foxp3+ Treg cells on the maternal side (14); however, others have shown decreased percentages of CD4+CD25hiFoxp3+ cells (15, 16) These differences are likely due to the different marker combinations used to describe Treg cells. Regardless, the factors leading to this change in Treg cell population during pregnancy are largely unknown, although there is some suggestion of hormonal influence in humans (15) and in mice (17, 18). Whether these influences also affect the fetal side is clearly of great importance in the context of transplacental immune regulation.
On the fetal side, a recent study has shown that fetal T cells may be derived from a hematopoietic stem cell population distinct from adult hematopoietic stem cells and are primed to develop into Treg cells, leading to an increased proportion of Treg cells in the fetus in mid gestation (19). The development of these Treg cells occurs in the thymus, and these Treg cells in turn migrate and become activated in the periphery (20). However, whether maternal factors influence the generation of fetal Treg cells or, indeed, whether fetal influences regulate the maternal Treg cell homeostasis is unknown.
In this study, we present evidence for transplacental regulation of the Treg cell compartment and demonstrate that IL-10, elevated during pregnancy, is involved in this process. We describe in this paper the novel finding that Treg cells are characterized by increased expression of IL-10 receptor α (IL-10RA), hence making them more sensitive to the effects of IL-10. Furthermore, in vitro and ex vivo data suggest that IL-10 regulates Bcl-2 expression in Treg cells, which could contribute to Treg cell survival. These findings provide strong evidence for transplacental regulation of cellular immunity and implicate the important role of IL-10 in Treg cell physiology.
Materials and Methods
Human subjects
Peripheral blood samples were obtained from healthy pregnant patients (n = 98), their partners (n = 17), and nonpregnant volunteers (n = 25). Samples from pregnant women were taken 2–5 h before delivery, and cord blood was obtained at delivery. Mononuclear cells were isolated by Ficoll–Hypaque (Amersham Pharmacia, Piscataway, NJ) gradient centrifugation. For in vitro stimulation assays, the cells were processed fresh; for flow cytometric analysis, the cells were stored at −196°C until use. The Ethics Committee of the Sydney West Area Health Service approved this project according to the Declaration of Helsinki. The human subjects for the serum IL-10 and estradiol (E2) and estriol (E3) correlation were pregnant women (n = 44) independently recruited with blood sampling throughout pregnancy. They all had normal pregnancy outcomes. The South Sydney West Area Health Services Ethics Committee approved this part of the study.
Animals
BALB/c and CBA/J mice were purchased from Harlan Winkelmann (Borchen, Germany). BALB/c-DEREG mice were provided by T. Sparwasser (Institute of Infection Immunology, TWINCORE, Centre for Experimental and Clinical Infection Research, Hannover, Germany) and bred in the animal facilitiy of the Institute for Medical Microbiology, Immunology, and Hygiene (Munich, Germany). All animals were bred and maintained under specific pathogen-free housing conditions. All experiments were performed in accordance with German and international guidelines and were approved by local authorities. For analysis of pregnant mice, CBA/J × Balb/c mating combination was used.
Flow cytometry
Surface Ab and intracellular staining for Foxp3 and cytokines were performed as described previously (9). Data collection was performed on a LSRII (BD Biosciences), FACSCalibur (BD Biosciences), or FACSVerse (BD Biosciences), and data files were analyzed using FlowJo software (Tree Star, San Carlos, CA).
mAbs and reagents
For human cells, the following mAbs and reagents were used: FITC-PE- and eFluor450-anti-CD127, PE- and allophycocyanin-anti-CD25, biotin- and BD V500-anti-CD4, FITC- and allophycocyanin-H7-anti-CD3, BD V500 anti-CD8, and PE-anti–IL-10RA. Intracellular markers AF488- AF647- and PE-anti-Foxp3, BD V450 anti–Bcl-2, PE- and eFluor450-anti–IFN-γ, PerCPeFluor710-anti–IL-22, PE-anti–IL-10, AF647-anti–IL-17, allophycocyanin-anti–IL-4, and biotin-conjugated Abs were developed with streptavidin-PerCP. For in vitro–cultured human cells, dead cells were excluded from the analysis using the Live/Dead Fixable Dead Cell Stain Kit (Invitrogen, Carlsbad, CA). For mouse cells, allophycocyanin anti-mouse CD4, and for intracellular markers, PE anti-mouse Bcl-2 and FITC anti-mouse/rat Foxp3 were used. Ethidium monoazide bromide was used for dead cell exclusion in fixed mouse samples.
FACS
For flow cytometric sorting of human PBMCs, cells were stained using a combination of FITC-anti-CD127, BD V500- or biotin-SA-PerCP-anti-CD4, and PE- or allophycocyanin-anti-CD25. Cells were sorted on a FACSAria cell sorter using our previously published gating strategy (9). For flow cytometric sorting from splenocytes of DEREG BALB/c mice, CD4+Foxp3GFP+ and CD4+Foxp3GFP- cells were sorted on a FACSAria cell sorter. Purity of the sorted populations was ≥95%.
In vitro T cell stimulation assays
A total of 2.5 × 104 flow cytometric sorted Treg cells (CD4+CD127loCD25+) from healthy adult donors were cocultured with 7.5 × 104 non-Treg cells (CD4+CD127hiCD25−) for 3 d and stimulated in the presence or absence of IL-10 (BD Biosciences) (200 U/ml), added on day 0. CFSE (Invitrogen) was used to label Treg or non-Treg cells to facilitate separate analysis of these two subsets. In some experiments, Treg cells were labeled with CFSE, and non-Treg cells were left unlabeled. Percentage of change in Bcl-2 expression was calculated. To determine the percentage of change in Bcl-2 expression and Bcl-2 mean fluorescence intensity (MFI) in the mouse system, 2.5 × 104 sorted Treg cells (CD4+Foxp3GFP+) from BALB/c–DEREG mice were cocultured with 7.5 × 104 non-Treg cells (CD4+Foxp3GFP−). IL-10 (BD Biosciences) (400 U/ml) was added to the cultures on days 0 and 3. On day 6, cells were harvested and analyzed for Bcl-2 expression. For stimulation of both human and murine cells, MicroBead-coated biotinylated anti-CD3 and anti-CD28 (Miltenyi Biotec, Bergisch Gladbach, Germany) was added using a cell to bead ratio of 2:1. For absolute cell numbers of Treg cells in the mouse system, cells were cultured with or without IL-10 (BD Biosciences) (400 U/ml) in the absence of CD3/CD28 stimulation. On day 2, viable cell counts were determined using trypan blue, and cells were analyzed for Bcl-2 expression and Bcl-2 MFI. The absolute number of cell populations of interest was calculated according the percentages determined by flow cytometry.
In vitro IL-10 blocking assay
Splenocytes from female CBA/J mice were cultured with irradiated (30 Gy) male BALB/c splenocytes. Anti-mouse-IL-10 (10 μg/ml) was added on day 0. On day 2, cells were harvested for analysis of Bcl-2 expression.
Cytokine and hormone measurements
Human serum samples were obtained after centrifugation at 1000 × g for 10 min, aliquoted and stored at -80°C. Levels of soluble IL-10, IL-6 and TGF-β in serum were measured by standard ELISA Kits (R&D Systems, Minneapolis). Unconjugated E2 and E3 was measured by enzyme labeled chemiluminescent competitive immunoassay using the IMMULITE 2000 system (Siemens Healthcare Diagnostic Products).
Mouse serum IL-10 level were measured using the in vivo IL-10 capture assay (BD Biosciences). In brief, ELISA was performed from serum from pregnant CBA/J mice, according the manufacturer’s recommendations, 4 h after i.p. injection of 10 μg NA/LE biotin-conjugated anti-mouse IL-10 Ab. IL-10 in supernatant from splenocytes from female CBA/J mice was measured by standard ELISA kit (eBioscience) 2 d after stimulation with irradiated (30 Gy) male lymphocytes from CBA/J (syngeneic) or BALB/c mice (allogeneic).
Preparation of RNA for microarray
Sorted cells were washed and resuspended in cells-to-signal lysis buffer (Ambion, Applied Biosystems, Foster City, CA) and stored at −80°C until further processing. Total RNA was extracted from sorted Treg cells and non-Treg cells using the RNeasy Mini RNA Extraction Kit (Qiagen, Germany). Total RNA quality was assessed using the Agilent RNA 6000 series II Nano Kit (Agilent Technologies, CA). Total RNA (180 ng) from each sample was biotinylated and amplified using the Illumina TotalPrep RNA Amplification Kit (Ambion, TX). Total RNA was reverse transcribed to synthesize the first-strand cDNA, followed by a second-strand synthesis. Double-stranded cDNA was then transcribed and amplified in vitro to synthesize biotin-labeled complementary mRNA (cRNA). cRNA samples (700 ng) were hybridized onto human HT-12_V3 expression beadchips (Illumina, CA) profiling 48,804 transcripts per sample. Bead chips were hybridized at 58°C for 18 h, washed, stained with streptavidin, and scanned using an Illumina-BeadArray-Reader.
Microarray data analysis
Raw data were processed using Beadstudio version 3 (Illumina). Data were exported into BRB-ArrayTools version 3.8, and quantile normalization was applied. Paired t tests were carried out to generate differentially expressed gene lists for further downstream analyses. A p value cutoff of 0.001 was used. Gene lists were exported into GeneGo Metacore (St. Joseph, MI) where tests were carried out for overrepresentation of the gene lists in curated biological pathways. A cutoff of 5% false discovery rate was used to determine significance of overrepresented pathways. Principal component analysis (PCA) was performed on all samples using 101 probes found to be Treg cell and pregnancy specific. The metric used for PCA was centered correlation. Oligonucleotide microarray data have been deposited in National Center for Biotechnology Information’s Gene Expression Omnibus and are accessible through Gene Expression Omnibus Series accession number GSE31976 (http://www.ncbi.nlm.nih.gov/geo/).
Statistical analysis
Statistical analysis was performed using Prism 4.0 software (GraphPad, San Diego, CA). To test for associations, Pearson’s correlation coefficients were calculated. A paired sample t test was performed to compare maternal and fetal serum cytokine levels as well as comparing Treg versus non-Treg cells in human and murine experiments. An unpaired t test was used to perform analysis between pregnant versus nonpregnant samples. For all tests, p < 0.05 was considered significant. Statistical treatments of microarray data are described above.
Results
Alignment of maternal and fetal Treg cells
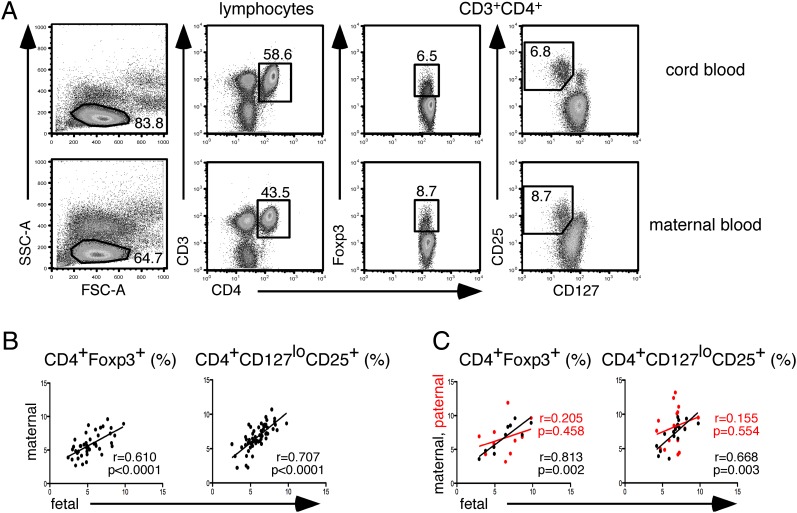
To determine whether there is a relationship in the balance of Treg cells and non-Treg cells in maternal fetal dyads (pairs), we correlated the percentage of peripheral blood Treg cells at term. We found a highly specific and significant correlation in the Treg cell subset, defined as CD4+Foxp3+ (r = 0.61, p < 0.0001) as well as CD4+CD127loCD25+ (r = 0.71, p < 0.0001) (Fig. 1A, 1B), but not with CD4+CD25+Foxp3− activated T cells (r = 0.287, p = 0.165), between the mother and the fetus. In contrast, there was no significant Treg cell correlation between paternal fetal dyads (Fig. 1C), suggesting that the specific context of pregnancy, rather than the genetic parental similarity to the fetus, is responsible for this correlation.
FIGURE 1.
Correlation of peripheral blood maternal, paternal, and fetal Treg cell frequencies. (A) Gating strategy for defining Foxp3+ and CD127lloCD25+ Treg cells within CD4+ T cells. (B) Scatter plot of Treg cell frequencies in term maternal fetal dyads, n = 44 for CD4+ Foxp3+, n = 59 for CD4+CD127loCD25+. (C) Scatterplot comparing paternal (red dots) and maternal (black dots) fetal dyads, n = 11 for CD4+Foxp3+, n = 17 for CD4+CD127loCD25+. In all cases, correlation was calculated using Pearson's correlation coefficient, r, and p values as indicated.
As maternal microchimerism occurs in the fetus, we first decided to determine whether the alignment of Treg cells can be explained by migration of maternal Treg cells into the fetal Treg cell compartment. Using fluorescent in situ hybridization analysis, sorted Treg cells from the cord blood of male fetuses were found to be negative for XX chromosome–bearing cells, which indicates absence of maternal Treg cells in the cord blood. This was confirmed by HLA typing of the fetal Treg cell population from a separate maternal fetal dyad. Given these findings, we hypothesized that in pregnancy the presence of a soluble factor, cytokine or hormone, maintains this specific alignment of maternal and fetal Treg cells.
Gene microarray analysis implicates the role of IL-10 in Treg cell regulation
To investigate the mechanism of Treg cell alignment in the most unbiased fashion, we performed gene expression analysis by microarray on sorted CD4+CD127loCD25+ Treg cells and CD4+CD127hiCD25− non-Treg cells in four healthy term maternal–fetal dyads and four healthy nonpregnant women. To identify genes, which were Treg cell specific and pregnancy specific, we first analyzed differentially expressed genes between Treg and non-Treg cells independently from both nonpregnant and pregnant women. The resulting genes from pregnant and nonpregnant women were then compared. This yielded 93 genes (Fig. 2A; complete list of the 93 genes is listed in Supplemental Table I), which were uniquely expressed in Treg cells in pregnancy.
FIGURE 2.
Gene signatures of maternal Treg cells during pregnancy indicate the involvement of IL-10 signaling pathway. (A) Representative density plots of sorting purities of Treg and non-Treg cells in nonpregnant and pregnant samples. Scatter plots of global gene expression comparing Treg and non-Treg cells in both study groups, followed by a Venn diagram showing the number of differentially expressed genes between Treg and non-Treg cells in nonpregnant (red circle) and pregnant (blue circle) women. A distinct subset of 93 genes is both Treg cell and pregnancy specific. These 93 genes were subjected to pathway analysis, where threshold of determining significant gene sets is <0.005. The IL-10 signaling pathway was the most significant immune response pathway. (B) PCA was performed on the 93 genes. This showed separations between nonpregnant and pregnant groups (principal component 1 [PC1]). Separation was also observed between non-Treg and Treg cells (principal component 2 [PC2]).
Pathway analysis of these 93 genes revealed the IL-10 pathway as the most significant signature among immune regulation pathways. Within the IL-10 signaling pathway, Bcl-2, suppressor of cytokine signaling 2, and suppressor of cytokine signaling 3 were upregulated. On the basis of these 93 genes, we then performed PCA to compare Treg and non-Treg cells derived from maternal and fetal blood and from the blood of nonpregnant individuals. This showed clustering of maternal and fetal Treg cells, with principal component 1 being pregnancy and principal component 2 being cell type (Fig. 2B), consistent with maternal–fetal Treg cell alignment.
Specific alignment of IL-10 at a protein level in maternal–fetal dyads
The results from gene microarray analysis prompted us to investigate IL-10 at the protein level. Interestingly, we found a significant correlation of serum IL-10 levels between the mother and the fetus. No significant correlation was found with other cytokines relevant to Treg cell induction, including TGF-β and IL-6 (Fig. 3A). Furthermore, comparison of maternal and fetal cytokine levels revealed significantly higher levels of IL-10 and TGF-β in cord blood, whereas IL-6 levels were comparable (Fig. 3B). Because IL-2 is important for Treg cell homeostasis (21), we measured serum IL-2 levels and found that in both maternal and cord blood samples, IL-2 levels were below the threshold level of 7 pg/ml.
FIGURE 3.
Correlation of IL-10 at a protein level in maternal-fetal dyads. (A) Scatter plots of serum levels of IL-10 (pg/ml) (n = 37), IL-6 (pg/ml) (n = 33), and TGF-β (ng/ml) (n = 38). (B) Comparison of serum IL-10, IL-6, and TGF-β levels in maternal blood (▪) and cord blood (□). Paired t test was used, p values as indicated. (C) Correlation between E2 and E3 and IL-10 in maternal serum during pregnancy. (D) Scatter plots of percentages of various intracellular cytokines expressed in CD3+CD8− T cells in maternal fetal dyads. IL-10, IFN-γ, and IL-17 (n = 30), IL-4 (n = 19), and IL-22 (n = 20). Correlations between maternal fetal dyads were calculated using Pearson’s correlation coefficient, r, and p values as indicated.
To test whether IL-10 production may be regulated by E2 and/or E3, the predominant estrogens in pregnancy, we measured serum IL-10, E2 and E3 levels in maternal blood, and found a modest but significant correlation between IL-10 and both estrogens (Fig. 3C).
We next examined the intracellular cytokine production by peripheral blood CD4+ T cells in maternal–fetal dyads. There was a significant correlation between maternal and fetal IL-10 production by CD4+ T cells. This correlation was restricted to IL-10 production and not found with other cytokines including IL-4, IFN-γ, IL-17, and IL-22 (Fig. 3D, Supplemental Fig. 1A, 1B). These results indicate there is a synchronized production of IL-10 in maternal–fetal dyads; there is no transplacental correlation of Th1, Th2, and Th17 CD4+ T cell subsets.
Higher expression of IL-10RA in Treg cells and its relationship to Bcl-2 expression
Because the IL-10 signaling pathway appears to be important in Treg cells during pregnancy, we asked whether IL-10RA expression differs between Treg and non-Treg cells. We found that Treg cells had significantly higher expression of IL-10RA (Fig. 4A) compared with non-Treg cells. This suggests that Treg cells may be more sensitive to IL-10 effects compared with non-Treg cells.
FIGURE 4.
IL-10RA expression ex vivo. (A) Representative Histograms showing IL-10RA MFI in CD4+Foxp3+ Treg and CD4+Foxp3− non-Treg cells in cord blood (CB), maternal blood (MB), and in nonpregnant (NP) women. A summary of MFI values within non-Treg and Treg cells are shown as individual dots from the three cohorts, CB (n = 20), MB (n = 19), and NP (n = 18), with the bar representing the mean. Paired t test, p values as indicated. (B) Correlation of IL-10RA and Bcl-2 expression in CD4+Foxp3+ Treg cells. Pearson’s correlation coefficient, r, and p values as indicated. (C) Summary plots with mean values, showing Bcl-2 MFI in Foxp3+ Treg cells in NP versus pregnant individuals (n = 20). (D) Correlation of Bcl-2 MFIs in CD4+Foxp3+ Treg cells between CB and corresponding MB (n = 19). Pearson’s correlation coefficient, r, and p values as indicated.
We then asked whether the level of IL-10RA expression might influence the Bcl-2 expression in Treg cells, because IL-10 is known to upregulate Bcl-2 in certain cell types such as CD34+ hematopoietic progenitor cells (22) and B cells (23). We found a specific correlation between Treg cell IL-10RA and Bcl-2 expression in pregnant mothers and neonates but not in nonpregnant women (Fig. 4B). This suggests that specifically, in pregnancy, the higher serum IL-10 level may control Bcl-2 expression in Treg cells.
Increased Bcl-2 expression by Treg cells during human and murine pregnancy
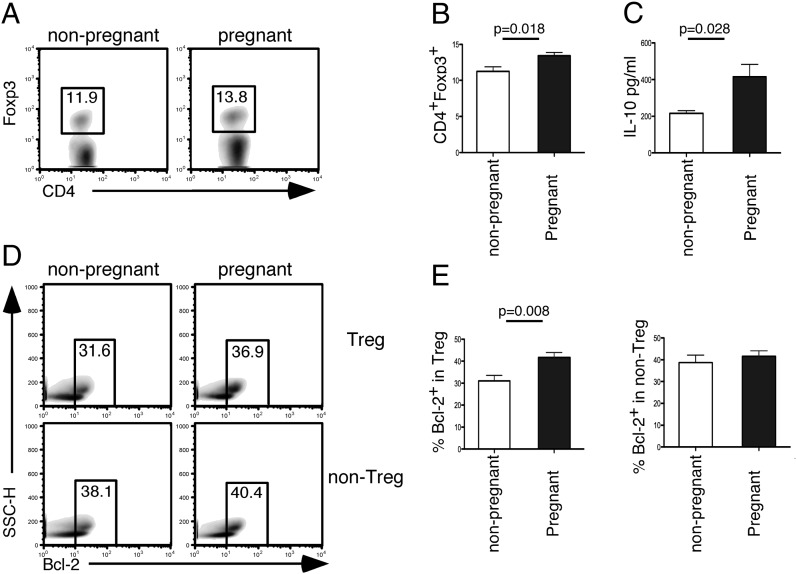
Because the gene microarray analysis showed an upregulation of Bcl-2 mRNA in Treg cells during pregnancy, we investigated whether this was also the case on the protein level. In humans, we found that Bcl-2 expression within Treg cells was significantly increased in pregnancy (Fig. 4C). Furthermore, we found a significant correlation of Bcl-2 expression in Treg cells between the mother and the baby (Fig. 4D). These data demonstrate that Bcl-2 is upregulated in Treg cells in human pregnancy and is tightly regulated between the mother and the baby. In mice, using the allogeneic CBA/J × BALB/c pregnancy model that is known to present normal pregnancies, we also found that pregnant CBA/J mice had significantly higher Treg cell frequency (Fig. 5A, 5B), paralleled by increased serum IL-10 level as measured by in vivo capture assay (Fig. 5C) and more Bcl-2+ cells within the expanding Treg cell population (Fig. 5D, 5E).
FIGURE 5.
Significantly higher percentages of Bcl-2+ cells within the expanding Treg cell population in pregnant CBA/J mice. (A) Representative density plots showing frequencies of CD4+Foxp3+ from splenocytes of nonpregnant versus pregnant CBA/J mice. (B) Summary bar graph (mean ± SEM) showing percentage of CD4+Foxp3+ cells, from nonpregnant (n = 8) or pregnant (n = 7) animals from CBA/J × BALB/c matings on day 8 of pregnancy. (C) Summary bar graph (mean ± SEM) showing IL-10 levels (pg/ml) in serum from nonpregnant (n = 4) or pregnant (n = 4) animals from CBA/J × BALB/c matings on day 8 of pregnancy. (D) Representative density plots of the Bcl-2 gating strategy in nonpregnant versus pregnant Treg and non-Treg cells. The full gating strategy applied is detailed in Supplemental Fig. 2. (E) Summary bar graph (mean ± SEM) of cells in pregnant versus nonpregnant mice. Paired t test, p values as indicated.
IL-10 selectively upregulates Bcl-2 in human and murine Treg cells
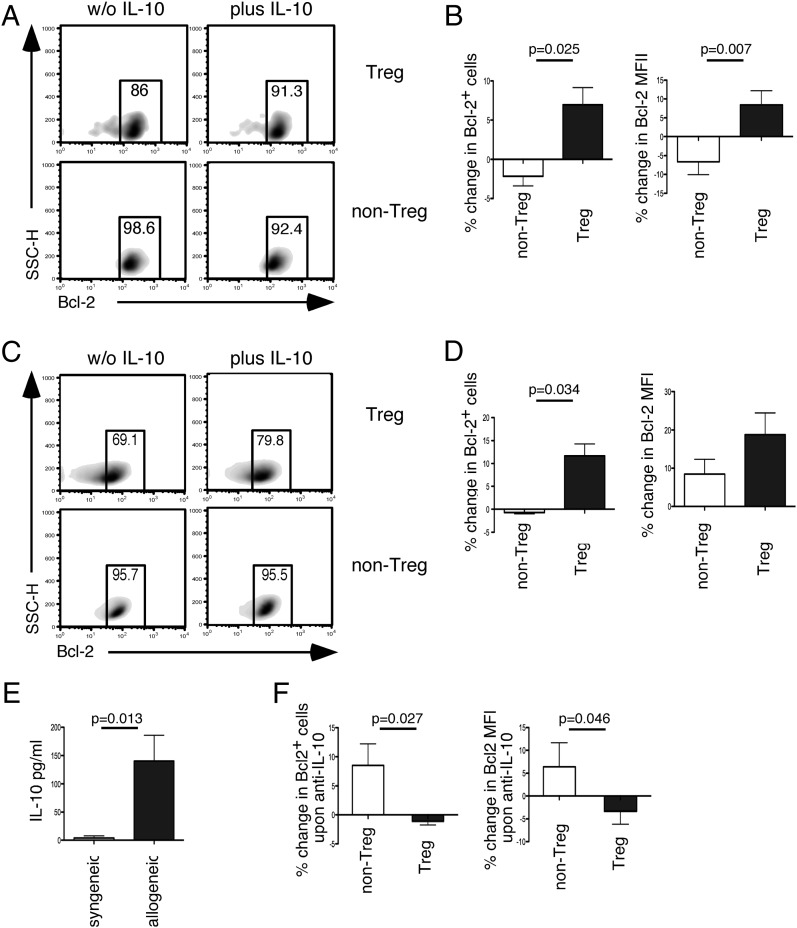
The data presented so far implicate an important role of IL-10 on Bcl-2 expression in Treg cells. We tested this in vitro by coculturing human Treg and non-Treg cells with anti-CD3/CD28 in the presence or absence of IL-10. And indeed, we could observe a significant upregulation of Bcl-2 only in Treg but not non-Treg cells exposed to IL-10 (Fig. 6A, 6B). This result was confirmed using cells isolated from DEREG BALB/c mice where GFP+ Treg cells in coculture with non-Treg cells upregulated their Bcl-2 expression in the presence of IL-10 (Fig. 6C, 6D). In an attempt to isolate the effect of IL-10 on T cells and to avoid excessive cell death because of prolonged culture, cells were cultured in the absence of anti-CD3/CD28 with or without IL-10 for 2 d. In cultures supplemented with IL-10, there was a significant increase in absolute Bcl-2+ Treg cell numbers (means ± SD, 843 ± 203 versus 1876 ± 528; p = 0.027 with paired t test). Furthermore, IL-10 significantly increased Bcl-2 MFI in Treg cells (means ± SD, 14.5 ± 1.5 versus 19.9 ± 1.0; p = 0.015 with paired t test).
FIGURE 6.
Differential effects of IL-10 on Bcl-2 expression in Treg and non-Treg cells in vitro. (A) Representative density plots showing expression of Bcl-2 in the presence or absence of 200 IU/ml IL-10 cocultured human non-CFSE labeled CD4+ CD127loCD25+ Treg and CFSE-labeled CD4+CD127hiCD25− non-Treg cells. Cells were stimulated with CD3/CD28 Abs and analyzed on day 3. (B) Summary bar graph (mean ± SEM) showing percentage of change in Bcl-2+ cells as well as percentage change in Bcl-2 MFI levels within these two subsets. Data are shown from seven independent experiments. (C) Representative flow cytometry staining showing expression of Bcl-2 in cocultured CD4+GFP+ Treg cells and CD4+GFP− non-Treg cells isolated from splenocytes of DEREG BALB/c mice with and without 400 IU/ml IL-10 plus anti-CD3/CD28. (D) Summary bar graphs (mean ± SEM) showing percentage of change in Bcl-2+ cells and percentage of change in Bcl-2 MFI levels. Data are shown from 3 independent experiments. (E) Summary bar graph (mean ± SEM) showing IL-10 level (pg/ml) in supernatant from splenocytes from female CBA/J mice stimulated with irradiated slenocytes from syngeneic CBA/J males or allogeneic BALB/c males. Data show eight and nine animals, respectively, in each group. (F) Summary bar graph (mean ± SEM) showing percentage of change in Bcl-2+ cells upon anti–IL-10 treatment as well as Bcl-2 MFI’s in cells from female CBA/J mice, stimulated with irradiated allogeneic male BALB/c lymphocytes. Data show 11 animals in each group.
To test whether endogenous IL-10 has similar effects on Bcl-2 expression on Treg cells in vitro, we cultured female CBA/J splenocytes with irradiated male BALB/c splenocytes to simulate the allogeneic constellation during normal pregnancies or with irradiated male CBA/J splenocytes as control. IL-10 production was strongly induced in splenocytes from female CBA/J mice when stimulated with allogeneic BALB/c male lymphocytes (Fig. 6E). In these cultures, blocking of endogenous IL-10 production led to a selective decrease in Bcl-2 expression in Treg cells but not in non-Treg cells (Fig. 6F). These results further support the interpretation of our in vivo data that IL-10 contributes to maintenance of Bcl-2 expression in Treg cells.
Discussion
Transplacental immune regulation is a fascinating concept, which has important implications on the development of the immune system; however, the underlying mechanisms of this regulation remain elusive. Our findings summarized in this paper provide evidence of a significant alignment between maternal and fetal cellular immunity, in particular Treg cells, which play an important role for the overall integrity of the immune system. Our findings suggest that this alignment is driven by IL-10, for which serum concentrations correlate between mother and fetus. In vitro and ex vivo human and mouse data implicates a role for IL-10 and its effect on Bcl-2 in the fetal–maternal alignment of Treg cells. These findings highlight an important link between the maternal and the fetal immune system during pregnancy and a previously unrecognized role of IL-10 in Treg cell physiology.
Interestingly, our findings point at a specific role of IL-10 in maintaining the described Treg cell alignment during pregnancy. This is in congruence with the potential immunomodulatory role of pregnancy on the fetal and maternal immune system, because IL-10 is well known to be a pleiotropic cytokine with significant immunomodulatory properties (24). Indeed, there is a significant increase in serum IL-10 levels in normal human pregnancy compared with nonpregnant controls (25). Although IL-10 does not appear to be crucial for the success of pathogen-free pregnancy in the murine model (26), LPS-induced inflammation during pregnancy does lead to fetal demise in IL-10 null mice (27), implicating the important role of IL-10 in normal pregnancy. However, a role of IL-10 in transplacental immune regulation has not been reported so far. Previous studies exploring the effect of maternal influence on development of atopy in offspring have mainly focused on Th1 and Th2 cytokines such as IFN-γ and IL-4 (5, 6, 8, 9). Our finding that serum IL-10 levels were significantly correlated between mother and fetus was surprising, but perhaps related to a previous report showing that maternal IL-10 levels were significantly associated with the offspring’s IL-10 level at 1 y of age (28).
The synchronized nature of IL-10 during pregnancy is further supported by the observation that IL-10 secreting CD4+ T cells are significantly correlated between the mother and the fetus. This is in contrast to IFN-γ–secreting Th1 cells, IL-4 secreting Th2 cells, and IL-17 secreting Th17 cells, which did not correlate. The IL-10 secreting CD4+ Th cells probably do not represent a distinct Th lineage but are likely composed of Foxp3+ Treg cells and IL-10 secreting Tr1 cells. Nevertheless, the fetal maternal alignment of this group of cells suggests a synchronized and regulated production IL-10 during pregnancy. Importantly, it is known that many other cells produce IL-10 during pregnancy, including fetal trophoblasts (29), decidual NK cells, and macrophages (30), all of which could contribute to the elevated IL-10 level in pregnancy.
Although the exact mechanism regulating IL-10 production in pregnancy is unknown, some evidence implicates the influence of pregnancy related hormones, such as human chorionic gonadotropin, E2, and E3, which have been shown to influence IL-10 production (31, 32). However, because E2 and E3 are the predominant estrogens in pregnancy (33), we measured serum IL-10 and E2 and E3 levels in maternal blood and found a modest but significant correlation between serum IL-10 and serum E2 and E3 (Fig. 3D). Indeed, it is possible that maternal and fetal hormonal levels drive the observed IL-10 alignment, because there is significant correlation between maternal and fetal hormonal levels (34, 35). Interestingly, E3, a pregnancy-specific hormone with potent immunosuppressive properties used to treat multiple sclerosis (36), is primarily derived from the fetus (37). This implies that the synchronization of IL-10 production and Treg cell alignment may be in part driven by the fetus. Further studies are required to elucidate the potential role of hormones in the regulation of IL-10 production and the reported fetal–maternal Treg cell alignment.
Another important finding of our study is the role of IL-10 in Treg cell homeostasis. Although much has been noted regarding the role of IL-10 in Treg cell function (38), the influence of IL-10 itself on Treg cells is not well explored. Our results clearly showed that Treg cells have increased expression of the IL-10RA, a finding echoed in a previous study, where CD4+CD25hi cells were noted to have increased expression of IL-10RA (39). This suggests that IL-10 itself maybe important to Treg cell homeostasis and that Treg cells may be more sensitive to IL-10–mediated effects. Indeed, our in vitro data show that in both humans and mice, IL-10 selectively induces Bcl-2 expression in Treg cells when compared with non-Treg cells. Furthermore, blocking endogenous IL-10 production leads to a reduction of Bcl-2–expressing Treg cells. The role of Bcl-2 in Treg cell homeostasis is also supported by previous studies, showing that overexpression of Bcl-2 in CD4+ T cells alters Treg cell homeostasis and leads to an expansion of CD4+CD25+Bcl-2+ Treg cell, resulting in protection against autoimmunity (40); however, the role of IL-10 was not explored in this study. Interestingly, IL-10 was shown to maintain Foxp3 expression in a murine model of inflammatory colitis (41). However, it is difficult to dissect the exact role of IL-10 on Treg cells in vivo, because IL-10 is a pleiotropic cytokine, which affects a broad range of immune and nonimmune cells. In a more recent study using Treg cell–specific IL-10RA knockout mice, Chaudhry et al. (42) showed that IL-10 endowed Treg cells with the ability to suppress Th17 cells but did not affect the stability of Foxp3 expression. Although in this study we have not explored the effect of IL-10 on Foxp3 stability, our results clearly show that IL-10 has control over the expression of the major antiapoptotic molecule, Bcl-2, in human and murine Treg cells.
The fetal–maternal alignment of Treg cells presented in this study is highly relevant as evidence suggests that both the maternal and fetal immune system is significantly affected by pregnancy. It is well known that many autoimmune conditions such as rheumatoid arthritis (43) and multiple sclerosis (44) abate during pregnancy. Although the reason for this is not fully understood, it is tempting to speculate that changes in Treg cell homeostasis during pregnancy may play a role. Another important question that arises from this study is whether the described Treg cell alignment has any role in programming the fetal and/or maternal immune system in the long term. Epidemiological evidence suggests that nulliparous women have an increased risk of developing autoimmune diseases such as systemic lupus erythematosus (45) and multiple sclerosis (46). This suggests that the events occurring during pregnancy, perhaps the described alignment of Treg cells, may modulate the maternal immune system to decrease the risk of future autoimmunity. Interestingly, adverse pregnancy events may predispose the baby to the development of future immunopathology such as autoimmunity and allergy (47). This indicates that normal pregnancy may confer a protective effect for the development of immunopathologies in the offspring. However, whether IL-10 and the Treg cell alignment play a role in such immune programming will require further studies.
In summary, our findings provide new insights into the unique immunological events in human pregnancy. Importantly, we provide evidence for transplacental regulation of cellular immunity and the previously unknown role of IL-10 on Bcl-2 maintenance in Treg cells. These novel findings have important implications on immune tolerance in pregnancy and beyond, in areas of autoimmunity, allergy, and transplantation.
Acknowledgments
We thank the staff of the Centenary Institute Flow Cytometry Facility for excellent assistance with cell sorting. We also thank the midwives at Nepean Hospital and the individuals who participated in this study. We thank Andrew Martin, statistician at the National Health and Medical Research Council Trial Center (University of Sydney), for input in statistical analysis.
This work was supported by the Nepean Medical Research Foundation, University of Sydney and the Australian Women and Children’s Research Foundation, and the Sonderforschungsbereich Transregio 22 of the German Research Council. P.H. was supported by the Royal Australasian College of Physicians Fellows Contributions Scholarship.
The sequences presented in this article have been submitted to the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE31976.
The online version of this article contains supplemental material.
- Bcl-2
- B cell lymphoma 2
- CB
- cord blood
- E2
- estradiol
- E3
- estriol
- IL-10RA
- IL-10 receptor α
- MB
- maternal blood
- MFI
- mean fluorescence intensity
- PCA
- principal component analysis
- Treg
- regulatory T.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Malassiné A., Frendo J. L., Evain-Brion D. 2003. A comparison of placental development and endocrine functions between the human and mouse model. Hum. Reprod. Update 9: 531–539 [DOI] [PubMed] [Google Scholar]
- 2.Firan M., Bawdon R., Radu C., Ober R. J., Eaken D., Antohe F., Ghetie V., Ward E. S. 2001. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of gamma-globulin in humans. Int. Immunol. 13: 993–1002 [DOI] [PubMed] [Google Scholar]
- 3.Loubière L. S., Lambert N. C., Flinn L. J., Erickson T. D., Yan Z., Guthrie K. A., Vickers K. T., Nelson J. L. 2006. Maternal microchimerism in healthy adults in lymphocytes, monocyte/macrophages and NK cells. Lab. Invest. 86: 1185–1192 [DOI] [PubMed] [Google Scholar]
- 4.Mold J. E., Michaëlsson J., Burt T. D., Muench M. O., Beckerman K. P., Busch M. P., Lee T.-H., Nixon D. F., McCune J. M. 2008. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 322: 1562–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macaubas C., de Klerk N. H., Holt B. J., Wee C., Kendall G., Firth M., Sly P. D., Holt P. G. 2003. Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet 362: 1192–1197 [DOI] [PubMed] [Google Scholar]
- 6.Ege M. J., Herzum I., Büchele G., Krauss-Etschmann S., Lauener R. P., Roponen M., Hyvärinen A., Vuitton D. A., Riedler J., Brunekreef B., et al. 2008. Prenatal exposure to a farm environment modifies atopic sensitization at birth. J. Allergy Clin. Immunol. 122: 407–412, e1–e4 [DOI] [PubMed] [Google Scholar]
- 7.Schaub B., Liu J., Hoppler S., Schleich I., Huehn J., Olek S., Wieczorek G., Illi S., von Mutius E. 2009. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J. Allergy Clin. Immunol. 123: 774‑782.e775 [DOI] [PubMed] [Google Scholar]
- 8.Matson A. P., Zhu L., Lingenheld E. G., Schramm C. M., Clark R. B., Selander D. M., Thrall R. S., Breen E., Puddington L. 2007. Maternal transmission of resistance to development of allergic airway disease. J. Immunol. 179: 1282–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polte T., Hennig C., Hansen G. 2008. Allergy prevention starts before conception: maternofetal transfer of tolerance protects against the development of asthma. J. Allergy Clin. Immunol. 122: 1022‑1030.e1025 [DOI] [PubMed] [Google Scholar]
- 10.Conrad M. L., Ferstl R., Teich R., Brand S., Blümer N., Yildirim A. O., Patrascan C. C., Hanuszkiewicz A., Akira S., Wagner H., et al. 2009. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J. Exp. Med. 206: 2869–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palomares O., Yaman G., Azkur A. K., Akkoc T., Akdis M., Akdis C. A. 2010. Role of Treg in immune regulation of allergic diseases. Eur. J. Immunol. 40: 1232–1240 [DOI] [PubMed] [Google Scholar]
- 12.Wing K., Sakaguchi S. 2010. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 11: 7–13 [DOI] [PubMed] [Google Scholar]
- 13.Zou W. 2006. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 6: 295–307 [DOI] [PubMed] [Google Scholar]
- 14.Santner-Nanan B., Peek M. J., Khanam R., Richarts L., Zhu E., Fazekas de St Groth B., Nanan R. 2009. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J. Immunol. 183: 7023–7030 [DOI] [PubMed] [Google Scholar]
- 15.Mjösberg J., Svensson J., Johansson E., Hellström L., Casas R., Jenmalm M. C., Boij R., Matthiesen L., Jönsson J.-I., Berg G., Ernerudh J. 2009. Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17β-estradiol. J. Immunol. 183: 759–769 [DOI] [PubMed] [Google Scholar]
- 16.Tilburgs T., Roelen D. L., van der Mast B. J., de Groot-Swings G. M., Kleijburg C., Scherjon S. A., Claas F. H. 2008. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J. Immunol. 180: 5737–5745 [DOI] [PubMed] [Google Scholar]
- 17.Arruvito L., Sanz M., Banham A. H., Fainboim L. 2007. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J. Immunol. 178: 2572–2578 [DOI] [PubMed] [Google Scholar]
- 18.Schumacher A., Heinze K., Witte J., Poloski E., Linzke N., Woidacki K., Zenclussen A. C. 2013. Human chorionic gonadotropin as a central regulator of pregnancy immune tolerance. J. Immunol. 190: 2650‑2658 [DOI] [PubMed] [Google Scholar]
- 19.Mold J. E., Venkatasubrahmanyam S., Burt T. D., Michaëlsson J., Rivera J. M., Galkina S. A., Weinberg K., Stoddart C. A., McCune J. M. 2010. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. [Published erratum appears in 2011 Science 331: 534] Science 330: 1695–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cupedo T., Nagasawa M., Weijer K., Blom B., Spits H. 2005. Development and activation of regulatory T cells in the human fetus. Eur. J. Immunol. 35: 383–390 [DOI] [PubMed] [Google Scholar]
- 21.Burchill M. A., Yang J., Vang K. B., Farrar M. A. 2007. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol. Lett. 114: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber-Nordt R. M., Henschler R., Schott E., Wehinger J., Behringer D., Mertelsmann R., Finke J. 1996. Interleukin-10 increases Bcl-2 expression and survival in primary human CD34+ hematopoietic progenitor cells. Blood 88: 2549–2558 [PubMed] [Google Scholar]
- 23.Levy Y., Brouet J. C. 1994. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J. Clin. Invest. 93: 424–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosser D. M., Zhang X. 2008. Interleukin-10: new perspectives on an old cytokine. Immunol. Rev. 226: 205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmes V. A., Wallace J. M. W., Gilmore W. S., McFaul P., Alexander H. D. 2003. Plasma levels of the immunomodulatory cytokine interleukin-10 during normal human pregnancy: a longitudinal study. Cytokine 21: 265–269 [DOI] [PubMed] [Google Scholar]
- 26.White C. A., Johansson M., Roberts C. T., Ramsay A. J., Robertson S. A. 2004. Effect of interleukin-10 null mutation on maternal immune response and reproductive outcome in mice. Biol. Reprod. 70: 123–131 [DOI] [PubMed] [Google Scholar]
- 27.Murphy S. P., Fast L. D., Hanna N. N., Sharma S. 2005. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J. Immunol. 175: 4084–4090 [DOI] [PubMed] [Google Scholar]
- 28.Herberth G., Hinz D., Roder S., Schlink U., Sack U., Diez U., Borte M., Lehmann I. 2011. Maternal immune status in pregnancy is related to offspring’s immune responses and atopy risk. Allergy 66: 1065‑1074 . [DOI] [PubMed] [Google Scholar]
- 29.Roth I., Corry D. B., Locksley R. M., Abrams J. S., Litton M. J., Fisher S. J. 1996. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J. Exp. Med. 184: 539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lidström C., Matthiesen L., Berg G., Sharma S., Ernerudh J., Ekerfelt C. 2003. Cytokine secretion patterns of NK cells and macrophages in early human pregnancy decidua and blood: implications for suppressor macrophages in decidua. Am. J. Reprod. Immunol. 50: 444–452 [DOI] [PubMed] [Google Scholar]
- 31.Carbone F., Procaccini C., De Rosa V., Alviggi C., De Placido G., Kramer D., Longobardi S., Matarese G. 2010. Divergent immunomodulatory effects of recombinant and urinary-derived FSH, LH, and hCG on human CD4+ T cells. J. Reprod. Immunol. 85: 172–179 [DOI] [PubMed] [Google Scholar]
- 32.Papenfuss T. L., Powell N. D., McClain M. A., Bedarf A., Singh A., Gienapp I. E., Shawler T., Whitacre C. C. 2011. Estriol generates tolerogenic dendritic cells in vivo that protect against autoimmunity. J. Immunol. 186: 3346–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Touchstone J. C., Murawec T. 1965. Free and conjugated estrogens in blood plasma during human pregnancy. Biochemistry 4: 1612–1614 [DOI] [PubMed] [Google Scholar]
- 34.Farquharson R. G., Klopper A. I. 1984. Unconjugated oestriol in the cord blood of infants delivered by caesarean section at term. Arch. Gynecol. 236: 77–82 [DOI] [PubMed] [Google Scholar]
- 35.Troisi R., Potischman N., Roberts J. M., Harger G., Markovic N., Cole B., Lykins D., Siiteri P., Hoover R. N. 2003. Correlation of serum hormone concentrations in maternal and umbilical cord samples. Cancer Epidemiol. Biomarkers Prev. 12: 452–456 [PubMed] [Google Scholar]
- 36.Sicotte N. L., Liva S. M., Klutch R., Pfeiffer P., Bouvier S., Odesa S., Wu T. C. J., Voskuhl R. R. 2002. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann. Neurol. 52: 421–428 [DOI] [PubMed] [Google Scholar]
- 37.Levitz M., Kadner S., Young B. K. 1984. Intermediary metabolism of estriol in pregnancy. J. Steroid Biochem. 20(4B): 971–974 [DOI] [PubMed] [Google Scholar]
- 38.Rubtsov Y. P., Rasmussen J. P., Chi E. Y., Fontenot J., Castelli L., Ye X., Treuting P., Siewe L., Roers A., Henderson W. R., Jr, et al. 2008. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28: 546–558 [DOI] [PubMed] [Google Scholar]
- 39.Moniuszko M., Bodzenta-Lukaszyk A., Dabrowska M. 2009. Oral glucocorticoid treatment decreases interleukin-10 receptor expression on peripheral blood leucocyte subsets. Clin. Exp. Immunol. 156: 328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.González J., Tamayo E., Santiuste I., Marquina R., Buelta L., González-Gay M. A., Izui S., López-Hoyos M., Merino J., Merino R. 2007. CD4+CD25+ T cell-dependent inhibition of autoimmunity in transgenic mice overexpressing human Bcl-2 in T lymphocytes. J. Immunol. 178: 2778–2786 [DOI] [PubMed] [Google Scholar]
- 41.Murai M., Turovskaya O., Kim G., Madan R., Karp C. L., Cheroutre H., Kronenberg M. 2009. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat. Immunol. 10: 1178–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaudhry A., Samstein R. M., Treuting P., Liang Y., Pils M. C., Heinrich J.-M., Jack R. S., Wunderlich F. T., Brüning J. C., Müller W., Rudensky A. Y. 2011. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34: 566–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barrett J. H., Brennan P., Fiddler M., Silman A. J. 1999. Does rheumatoid arthritis remit during pregnancy and relapse postpartum? Results from a nationwide study in the United Kingdom performed prospectively from late pregnancy. Arthritis Rheum. 42: 1219–1227 [DOI] [PubMed] [Google Scholar]
- 44.Gilmore W., Arias M., Stroud N., Stek A., McCarthy K. A., Correale J. 2004. Preliminary studies of cytokine secretion patterns associated with pregnancy in MS patients. J. Neurol. Sci. 224: 69–76 [DOI] [PubMed] [Google Scholar]
- 45.Ulff-Møller C. J., Jørgensen K. T., Pedersen B. V., Nielsen N. M., Frisch M. 2009. Reproductive factors and risk of systemic lupus erythematosus: nationwide cohort study in Denmark. J. Rheumatol. 36: 1903–1909 [DOI] [PubMed] [Google Scholar]
- 46.Runmarker B., Andersen O. 1995. Pregnancy is associated with a lower risk of onset and a better prognosis in multiple sclerosis. Brain 118: 253–261 [DOI] [PubMed] [Google Scholar]
- 47.Keski-Nisula L., Heinonen S., Remes S., Pekkanen J. 2009. Pre-eclampsia, placental abruption and increased risk of atopic sensitization in male adolescent offspring. Am. J. Reprod. Immunol. 62: 293–300 [DOI] [PubMed] [Google Scholar]