Abstract
Patients with schizophrenia (SP) exhibit deficits in both attentional reorienting and inhibition of return (IOR) during visual tasks. However, it is currently unknown whether these deficits are supramodal in nature and how these deficits relate to other domains of cognitive dysfunction. In addition, the neuronal correlates of this pathological orienting response have not been investigated in either the visual or auditory modality. Therefore, thirty SP and 30 healthy controls (HC) were evaluated with an extensive clinical protocol and functional magnetic resonance imaging (fMRI) during an auditory cuing paradigm. SP exhibited both increased costs and delayed IOR during auditory orienting, suggesting a prolonged interval for attentional disengagement from cued locations. Moreover, a delay in the development of IOR was associated with cognitive deficits on formal neuropsychological testing in the domains of attention/inhibition and working memory. Event-related fMRI showed the characteristic activation of a frontoparietal network (invalid trials > valid trials), but there were no differences in functional activation between patients and HC during either attentional reorienting or IOR. Current results suggest that orienting deficits are supramodal in nature in SP, and are related to higher-order cognitive deficits that directly interfere with day-to-day functioning.
Keywords: schizophrenia, attention, auditory, bottom-up, orienting, fMRI
1. Introduction
Schizophrenia can be conceptualized as a disorder of informational processing (Braff and Light, 2004, Callaway and Naghdi, 1982). Salient stimuli are initially detected in the environment by automatic, involuntary preattentional processes. The preattentional system acts as a filter for detecting novel stimuli while ignoring redundant, repetitive stimuli. Neurophysiological tests such as mismatch negativity, sensory gating (P50), and prepulse inhibition have measured preattentional deficits in discrete neural circuits in patients with schizophrenia (Patterson et al., 2008, Salisbury et al., 2002, Swerdlow et al., 2008). After salience detection, controlled, voluntary attentional processes take over with further decision-making and higher-level cognitive processes such as occur during the auditory oddball discrimination task (P300) and the continuous performance task (Bowen et al., 1994, Jeon and Polich, 2003). Behavioral and neuronal deficits on attention-based tasks have also been documented (Kiehl et al., 2005, Laurens et al., 2005). Importantly, it has been posited that these preattentional and attentional deficits may lead to higher-level cognitive deficits and functional impairment that is characteristic of schizophrenia (Light and Braff, 2005, Wynn et al., 2005, Wynn et al., 2010).
The ability to orient attention to different locations in space spans the continuum of both automatic (e.g., bottom-up) and controlled (e.g., top-down) attentional information processing. During bottom-up orienting, cues predict target location at chance levels (Jonides and Irwin, 1981, Mondor and Breau, 1999, Mondor and Bryden, 1992, Spence and Driver, 1994). When the stimulus onset asynchrony (SOA) between the cue and target is short (< 250 ms), reaction times are faster (i.e., facilitated) for validly (i.e., cue and target occur in same spatial location) compared to invalidly (i.e., cue and target in different spatial locations) cued stimuli as a result of attentional reorienting (Arrington et al., 2000, Corbetta et al., 2000, Mayer et al., 2004a, Mayer et al., 2007). Attentional reorienting following invalid visual and auditory cues has been associated with increased activation in the supplementary motor area, frontal eye fields, and inferior parietal lobes (Arrington, Carr, 2000, Corbetta, Kincade, 2000, Mayer, Harrington, 2007, Mayer et al., 2004b, Thiel et al., 2004). At longer SOAs (> 400 ms), valid cues delay reaction times relative to the invalid cues (Mondor, 1999, Mondor and Breau, 1999, Spence and Driver, 1998, Tassinari et al., 2002). The increased cost associated with valid cues at longer SOAs has been called inhibition of return (IOR), and is thought to be mediated by a distributed network including the bilateral frontal eye fields, parietal areas, temporal-parietal junction, and superior colliculus (Corbetta, Kincade, 2000, Lepsien and Pollmann, 2002, Mayer, Harrington, 2007, Sapir et al., 1999). IOR may serve as a mechanism for promoting processing of novel stimuli in the environment (Posner, 1980, Posner and Cohen, 1984) or may result from the inhibition of saccades (Klein, 2000). Thus, failure of IOR has several real-world implications such as sensory over-stimulation and difficulties sustaining directed attention in patients with schizophrenia (Gouzoulis-Mayfrank et al., 2007).
Previous investigators have compared the bottom-up control of visual-spatial attention in patients with schizophrenia (SP) and healthy control (HC) subjects at different states of illness. At shorter SOAs, patients with schizophrenia exhibited increased costs (longer reaction times) associated with right visual field stimuli (Carter et al., 1992, Maruff et al., 1995a, Posner et al., 1988, Sapir et al., 2001, Wigal et al., 1997), which was interpreted as indicating left hemisphere deficits in SP. However, other studies were unable to replicate this specific left hemisphere pathology (Daban et al., 2004, Gold et al., 1992, Liotti et al., 1993). Instead, the most consistent finding has been diminished (Gouzoulis-Mayfrank, Balke, 2007, Gouzoulis-Mayfrank et al., 2004), delayed (Kebir et al., 2010, Larrison-Faucher et al., 2002), or “profoundly disturbed” (Gouzoulis-Mayfrank et al., 2006a) IOR in SP. For example, unmedicated SP exhibited diminished IOR, and this deficit was not present in healthy relatives or prodromal subjects (Gouzoulis-Mayfrank, Balke, 2007). Patients with schizophrenia experiencing an acute psychotic exacerbation had impaired IOR, which persisted after considerable clinical improvement (Gouzoulis-Mayfrank, Heekeren, 2004). In addition, diminished IOR did not correlate with illness duration, type of medication, or number of psychotic episodes (Gouzoulis-Mayfrank, Arnold, 2006a), suggesting that it may be a state- and medication-independent biomarker of schizophrenia (Gouzoulis-Mayfrank, Balke, 2007). Investigators have also replicated diminished IOR with pharmacological models (glutamate N-methyl-D-aspartic acid antagonists and serotonin 5-HT2A agonists) of attentional deficits of schizophrenia (Daumann et al., 2008, Gouzoulis-Mayfrank et al., 2006b). However, to date no studies have investigated whether these IOR deficits extend to the auditory modality, and whether behavioral disturbances are also associated with functional abnormalities.
Thus, the current study utilized event-related functional magnetic resonance imaging (fMRI) to examine deficits in auditory reorienting and IOR in a large cohort of SP and matched HC. We hypothesized that patient reaction time data would indicate increased costs for attentional reorienting at shorter SOAs, as well as a diminished or a delayed IOR response. We also hypothesized that these behavioral disturbances would manifest as a reduced hemodynamic response within the frontoparietal network, providing evidence of neuronal dysfunction during these two fundamental orienting responses.
2. Methods
2.1. Subjects
Patient recruitment occurred in the larger context of a Center Of Biomedical Research Excellence funded program examining the neural mechanisms of cognitive impairments in schizophrenia. Thirty patients with schizophrenia (SP; 7 females and 23 males; 37.17 +/− 13.15 years old) and 30 gender and age-matched healthy controls (HC; 35.97 +/− 13.21 years old) were recruited from University of New Mexico Mental Health Center inpatient and outpatient psychiatric clinics, the Albuquerque Veterans Administration Medical Center and other local hospitals and clinics. One SP and one HC were outliers (greater than three standard deviations) relative to their cohort on several fMRI motion parameters and were eliminated from subsequent analyses. Additionally, one SP and one HC were removed from subsequent analyses for poor task performance (less than 70% accuracy and mean reaction times greater than three standard deviations), leaving data from 28 SP and 28 HC for final analyses.
Inclusion criteria for patient selection included diagnosis of schizophrenia and chronological age of 18 to 65 years. Each patient completed the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (First et al., 2002b) for diagnostic confirmation and evaluation for co-morbidities. All SP were clinically stable and treated with a variety of antipsychotic medications; therefore, an olanzapine equivalent was calculated to compare across antipsychotic medications (Gardner et al., 2010). SP with a history of neurological disorders including head trauma (loss of consciousness > 5 minutes), mental retardation, current use of mood stabilizers, or history of active substance dependence or abuse (except for nicotine) within the past year were excluded. All SP had a negative toxicology for drugs of abuse at the start of the study. HC were recruited from the same geographic location and completed the SCID – Non-Patient Edition to rule out Axis I conditions (First et al., 2002a).
Additional exclusionary criteria for HC included family history of a psychotic disorder in a first-degree relative, history of more than one lifetime depressive episode, history of depression or antidepressant use within the last six months, or history of lifetime antidepressant use of more than one year. Smoking history was assessed in terms of mean years of smoking, number of cigarettes smoked per day, and time of day of first cigarette (Fagerstrom, 1978). Nicotine modulates attentional orienting and represents a potential confound in this investigation (Thiel and Fink, 2008, Thiel et al., 2005). Therefore, smokers were asked to refrain from smoking for two hours before neuroimaging (confirmed by blowing zero carbon monoxide into a portable monitor) to reduce the peak effects of nicotine (Harris et al., 2004). The University of New Mexico Human Research Review Committee approved this study, and all participants provided written informed consent prior to study enrollment.
2.2 Clinical Assessment
Each subject completed medical history, neurological exam, and pregnancy test (if applicable) during their initial visit. SP completed symptom ratings with the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987), Calgary Depression Scale for Schizophrenia (Addington et al., 1993), and the Clinical Global Impression (Guy, 1976). Neuropsychological testing was grouped by cognitive domain as follows: attention/inhibition [Connors Continuous Performance Test (commissions and hit reaction times) and MATRICS Continuous Performance Test – Identical Pairs (overall T)], learning and memory [Hopkins Verbal Learning Test-Revised short and delayed recall], working memory [MATRICS Wechsler Memory Scale (Letter-Number Span and Spatial Span)], processing speed [MATRICS Brief Assessment of Cognition in Schizophrenia (Symbol Coding)], Wechsler Adult Intelligence Scale - IV (Symbol Search), and the Trail Making Test part A], and executive function [Word Fluency (FAS), MATRICS Neuropsychological Assessment Battery (Mazes), and Category Fluency (Animal Naming)].
2.3. Experimental Design and Task
Subjects practiced the exogenous auditory orienting task (Mayer, Harrington, 2007) prior to performing this task in a 3 Tesla Siemens Trio scanner. Cues presented to the left or right ear (100 ms, 2000 Hz tone pip) correctly predicted the location of the targets (100 ms, 1000 Hz tone pip) in 50% of the trials. Subjects were informed that the cues were not predictive of the target location prior to scanning. The cue and target tones had 10 ms linear onset-offset ramps to minimize clicks. Presentation software (Neurobehavioral Systems) presented the auditory stimuli via Avotec Silent Scan 3100 Series System. The order of valid (cue predicted location of target) and invalid trials varied pseudorandomly. Subjects pressed a button with their right middle finger for tones on the right headphone and a button with their right index finger for tones on their left. The SOA between cue and target was 200, 400, 600 or 800 ms. These SOAs were designed to effectively capture both facilitation (200 ms SOA) and IOR (800 ms), as well as approximate the crossing of the two functions (400 and 600 ms SOA). The inter-trial interval was 4, 6, or 8 seconds to both facilitate sampling of the hemodynamic response (Burock et al., 1998) as well as minimize non-linear additivity across trials (Glover, 1999). Subjects completed a total of 168 trials (42 trials per SOA) across three separate runs.
2.4. Imaging data acquisition
Structural images were collected with a magnetization-prepared 180 degrees radio-frequency pulses and rapid gradient-echo (MP RAGE) sequence [TE’s (echo time) = 1.64, 3.5, 5.36, 7.22, and 9.08 ms, TR (repetition time) = 2.53 s, flip angle = 7°, NEX (number of excitations) = 1, slice thickness = 1 mm, FOV (field of view) = 256 mm, and resolution = 256 × 256]. Functional images were collected with a single-shot, gradient-echo echoplanar pulse sequence [TE = 29 ms, TR = 2000 ms, flip angle = 75°, FOV = 240 mm, voxel size: 3.75 × 3.75 × 4.55 mm] with a total of 162 sequential echo-planar images per run and a total of 3 runs. The first image of each run was eliminated secondary to T1 equilibrium effects leaving a total of 483 images for the final analyses.
2.5. Imaging data analysis
First level and group statistics were carried out using the Analysis of Functional Neuroimages (AFNI) (Cox, 1996). First, time series were spatially registered to the second echoplanar image of the first run in two- and three-dimensional space to reduce the effects of motion, temporally interpolated to correct for slice time acquisition differences and de-spiked. These images were then resliced to 3 mm3, converted to a standard stereotaxic coordinate space (Talairach and Tournoux, 1988), and blurred using a 6 mm Gaussian full-width half-maximum filter. Deconvolution performed on a voxel-wise basis generated one hemodynamic response function (HRF) spanning the first 16 seconds post-stimulus onset for each condition (valid and invalid trials at each SOA of four SOAs). Six rigid-body motion parameters were entered as regressors of no interest to minimize the effect of head motion. The third and the fourth images (4.0 to 8.0 seconds post-stimulus onset, corresponding to the peak of the HRF) were averaged and divided by the baseline to obtain an estimate of percent signal change.
A voxel-wise, 2 × 2 × 4 (Diagnosis × Validity × SOA) linear mixed-effect model was then performed on this spatially normalized percent signal change data. This approach is preferable to standard ANOVA in that it allows one to model heteroscedasticity across groups, which is frequently present in SP versus HC. All voxel-wise analyses were corrected for multiple comparisons based on Gaussian Random Field Theory as implemented in the FSL package (z-threshold of 2.3 and p < 0.05).
3. Results
3.1 Neuropsychological/Clinical Measures
There were no significant (p > 0.10) group differences on age, education attainment, or primary caregiver education attainment (Table 1). There was a significant difference between groups on frequency of smokers (χ2 = 4.462, p = 0.035), with 11 current smokers in the patient group and four current smokers in the control group. Clinical characteristics of the patient group can be found in Table 2, and suggested that the sample had mild to moderate symptomatology (CGI severity = 3.25 +/− 1.01, PANSS positive symptom score = 14.57 +/− 5.61; PANSS negative symptom score = 12.93 +/− 4.34) and affective disturbance (Calgary Depression Scale = 4.14 +/− 4.72). The mean total olanzapine equivalent dosage was 15.82 +/− 14.43 milligrams/day, and the antipsychotics for individual patients are listed in Table 3.
Table 1.
Demographic and cognitive variable for patients with schizophrenia (SP) and healthy controls (HC).
| SP mean (SD) | HC mean (SD) | p value | Cohen’s d | |
|---|---|---|---|---|
|
| ||||
| Demographics | ||||
| Age | 36.75 (13.31) | 35.68 (12.26) | p = 0.755 | 0.09 |
| Education Level* | 4.14 (1.51) | 4.71 (1.46) | p = 0.156 | −0.39 |
| Parental Education | 4.29 (2.24) | 4.68 (1.66) | p = 0.478 | −0.20 |
| Cognition Indices | ||||
| Attention/Inhibition | 44.33 (6.99) | 52.40 (3.70) | p = 0.000 | −1.46 |
| Learning/Memory | 37.04 (8.68) | 44.38 (9.78) | p = 0.006 | −0.81 |
| Working Memory | 41.46 (10.67) | 50.54 (8.38) | p = 0.001 | −0.96 |
| Processing Speed | 39.27 (9.72) | 55.12 (5.52) | p = 0.000 | −2.03 |
| Executive | 41.07 (7.58) | 49.88 (5.01) | p = 0.000 | −1.39 |
| Intelligence | ||||
| VIQ | 99.48 (13.69) | 108.15 (11.40) | p = 0.016 | −0.70 |
| PIQ | 104.93 (18.27) | 116.00 (10.31) | p = 0.009 | −0.76 |
| WTAR | 104.30 (12.99) | 111.58 (12.04) | p = 0.039 | −0.59 |
Abbreviations: VIQ = Verbal Intelligence Quotient; PIQ = Performance Intelligence Quotient; WTAR = Wechsler Test of Adult Reading
Education levels as follows: 1 = 6th grade education, 4 = high school graduate, 6 = college graduate
Table 2.
Patient clinical characteristics
| Patient Clinical Characteristics | Mean (SD) |
|---|---|
|
| |
| Age on onset | 20.04 (8.03) |
| Illness duration | 16.22 (12.91) |
| PANSS positive symptoms | 14.57 (5.61) |
| PANSS negative symptoms | 12.93 (4.34) |
| Calgary Depression | 4.14 (4.72) |
| Clinical Global Impression | 3.25 (1.01) |
| Olanzapine equivalents | 15.82 (14.43) |
Abbreviation: PANSS = Positive and Negative Syndrome Scale
Table 3.
Antipsychotics
| Antipsychotics* | Dosage Range** |
|---|---|
|
| |
| Aripiprazole (n = 3) | 10 – 15 mg |
| Clozapine (n = 4) | 50 – 400 mg |
| Fluphenazine (oral, n = 1) | 10 mg |
| Haloperidol (oral, n = 1) | 5 mg |
| Haloperidol decanoate (n = 2) | 50 mg |
| Olanzapine (n = 1) | 20 mg |
| Perphenazine (n = 1) | 8 mg |
| Quetiapine (n = 1) | 200 – 800 mg |
| Risperidone (oral, n =6) | 1 – 4 mg |
| Risperidone consta (n =6) | 12.5 – 50 mg |
| Thiothixene (n = 1) | 60 mg |
| Ziprasidone (n = 1) | 160 mg |
Eight of the 28 patients were treated with multiple antipsychotics. This table lists either the long acting injection or the antipscyhotic with the higher olanzapine equivalents.
mg/day or dose of long acting injection
The neuropsychological results are presented in Table 1. A MANOVA was performed to examine group differences in the composite indices of attention/inhibition, learning and memory, working memory, processing speed, and executive functioning. The multivariate effect of diagnosis was significant (F5,47 = 12.60, p = 0.000) as were the univariate effects of attention/inhibition (F1,51 = 27.31, p = 0.000), learning and memory (F1,51 = 8.38, p = 0.006), working memory (F1,51 = 11.80, p = 0.001), processing speed (F1,51 = 52.69, p = 0.000), and executive functioning (F1,51 = 24.71, p = 0.000). Examination of the mean showed that SP scored lower than HC on all indices. A second MANOVA indicated that patients had significantly lower intelligence quotients than controls (F2,50 = 4.58, p = 0.015), with significant between-subjects univariate effects for both performance (F1,51 = 7.37, p = .009) and verbal (F1,51 = 6.25, p = 0.016) intelligence. SP had lower estimates of pre-morbid intelligence based on a test of word reading (Wechsler Test of Adult Reading, t1,51 = 2.11, p = 0.039), but SP and HC were matched on parental educational level (p = 0.48), a less biased estimate of premorbid educational attainment (Saykin et al., 1991). Effect sizes across the majority of measures were large (i.e., greater than 0.75), suggesting robust cognitive deficits in the SP group.
3.2. Behavioral performance
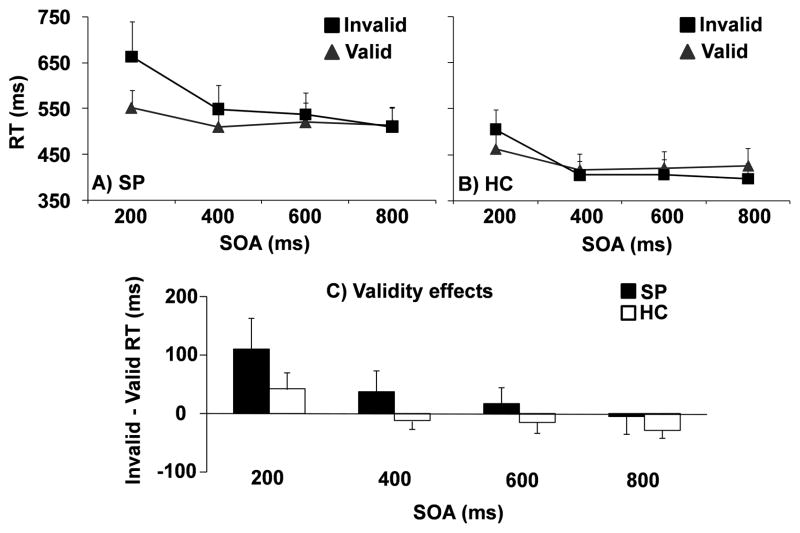
A 2 × 2 × 4 (diagnosis × validity × SOA) linear mixed model analysis was performed on the reaction-time data to evaluate task performance (Figure 1A and 1B). The reaction time (RT) analysis indicated that the three-way interaction was not significant (p > 0.10). However, significant main effect of diagnosis (F1,54 = 19.30, p = 0.000) was observed, with SP evincing significantly slower RT (mean = 544.66 +/− 106.73 ms) than HC (mean = 430.26 +/− 87.15 ms). There was also a significant main effect of validity (F1,54 = 5.77, p = 0.02), with subjects showing significantly slower RT for invalid (mean = 497.00 +/− 128.06 ms) compared to valid (mean = 477.92 +/− 104.27 ms) trials. The main effect of SOA was also significant (F3,54 = 30.65, p = 0.000), with reaction times decreasing as a function of SOA (545.74 ms +/−134.17 at the 200 ms SOA to 462.05 ms +/− 107.17 at the 800 ms SOA).
Figure 1.
Mean reaction time (RT) in milliseconds (ms) at each stimulus onset asynchrony (SOA) for patients (SP; Panel A) and controls (HC; Panel B). Grey triangles were used to represent valid trials and black squares for invalid trials. Panel C exhibits validity effects (invalid RT – valid RT) at each SOA for patients (SP; black) and controls (HC; white). Error bars correspond to two times the standard error of the mean for all data.
In addition, there was a significant validity by diagnosis interaction (F1,54 = 7.42, p = 0.009) and validity by SOA interaction (F3,54 = 9.97, p = 0.000). Simple effects tests suggested that the validity by diagnosis interaction was the result of SP having significantly longer reaction times on invalid compared to valid trials (t1,27 = −2.88, p = .008, Cohen’s d = −0.37) while HC had no significant differences between valid and invalid trial types (p = 0.728). For the validity by SOA interaction, simple effects indicated the presence of facilitation between invalid (mean = 584.28 +/− 179.85 ms) and valid (mean = 507.21 +/− 103.08 ms) trials (t1,55 = − 4.89, p = 0.000, Cohen’s d = −0.53) at the 200 ms SOA, with a trend towards IOR (valid > invalid) at the 800 ms SOA (p = 0.068, Cohen’s d = 0.14). There were no differences between valid and invalid reaction times at the 400 and 600 ms SOAs (p > 0.10).
A 2 × 4 (diagnosis × SOA) linear mixed model analysis was also performed on the validity effects (invalid RT – valid RT; Figure 1C). Examination of validity effects clearly indicated that HC showed the expected pattern of facilitation at the 200 ms SOA, followed by the transition to IOR at the longer SOA periods. In terms of between subjects efffects, a main effect for diagnosis (F1,54 = 7.42, p = 0.009) was observed with SP exhibiting larger validity effects (40.68 +/− 74.69 ms) relative to HC (−2.55 +/− 38.39 ms). The main effect of SOA was also significant (F3,54 = 9.97, p = 0.000), with larger validity effects at shorter (200 ms SOA: 77.07 +/− 118.06 ms) relative to longer (800 ms SOA: −15.78 +/− 63.49 ms) SOAs secondary to IOR. Although the diagnosis by SOA interaction term was not significant (p = 0.497), t-tests were conducted to examine a priori predictions of greater costs for attentional reorienting (larger validity effects) for SP at the 200 ms SOA and a diminished or a delayed IOR. Results indicated that SP had significantly greater costs compared to HC at the 200 ms SOA (t1,54 = −2.25, p = 0.029), and a delay in the development of IOR at the 400 (t1,36.13 = −2.53, p = 0.016) and 600 (t1,44.2 = −1.85, p = 0.071) ms SOAs. There was no difference between groups at the 800 ms SOA (p > 0.10).
To test if the deficits in attentional orienting could explain the impairment in high-level cognitive functions in SP, we computed the correlations between the validity effects and the neuropsychological indices separately for SP and HC. The correlations between validity effects and neuropsychological indices were not significant for HC. In contrast, SP had significant correlations between attention/inhibition and the 400 (r = −0.52, p = 0.006) and 800 (r = −0.38, p = 0.039) ms validity effects, as well as working memory and the 400 (r = − 0.38, p = 0.049) and 800 (r = −0.42, p = 0.029) ms validity effects, suggesting that delayed IOR was associated with poor performance.
Finally, we also assessed whether medication load (olanzapine equivalents) was associated with the orienting deficits observed in SP. Cumulative dose of antipsychotics was not associated with the validity effects at any of the SOAs (p > 0.05).
3.3. Functional imaging results
Two MANOVAs were performed to assess group differences in rotational and translational motion. The multivariate effect of rotational motion was significant (F3,52 = 3.38, p = 0.025), with patients showing significantly greater rotation in pitch (around the y-axis; F1,54 = 8.76, p = 0.005) and a trend for greater rotation in roll (around the x-axis; F1,54 = 3.67, p = 0.061) during univariate tests. The multivariate effect of translational motion was not significant (p > 0.10).
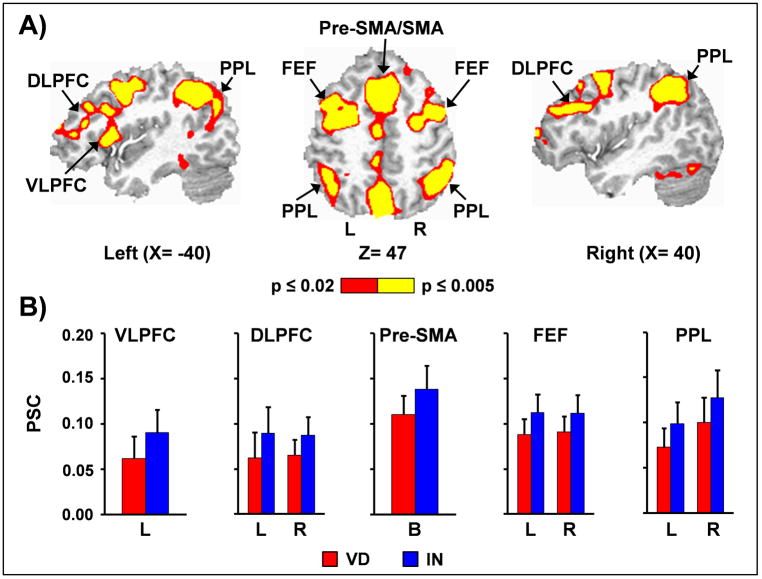
The reorienting of attention (main effect of validity; invalid > valid trials) was associated with widespread bilateral activity in several regions including pre-supplementary/supplementary motor area and anterior cingulate gyrus (BAs 6/8/9/24/32), frontal eye fields (BA 6), dorsolateral prefrontal cortex (BAs 9/10/46), posterior superior temporal gyrus (BAs 13/21/22/42), inferior parietal lobule (BAs 7/39/40), cingulate and posterior cingulate gyrus (BAs 23/24/31), precuneus/cuneus (BAs 7/19), primary and secondary visual cortex (BAs 17/18/19/30/37), subthalamic and thalamic nuclei, striatum and cerebellum (Figure 2). In addition, there was also increased activation within the left ventrolateral prefrontal cortex and insula (BAs 10/13/44/45/46/47) for invalidly cued trials. No regions exhibited greater activation for validly cued trials.
Figure 2.
Invalid trials were associated with widespread bilateral activity in several regions including (Panel A) pre-supplementary/supplementary motor area (Pre-SMA/SMA), frontal eye fields (FEF), dorsolateral prefrontal cortex (DLPFC), and posterior parietal lobe (PPL). The left ventrolateral prefrontal cortex (VLPFC) was also active during invalidly cued trials. Slice locations (X and Z) are presented according to the Talairach atlas, and activations were color-coded (red versus yellow) according to p-value magnitude. Percent signal change (PSC) data is presented in Panel B for valid (VD; red bars) and invalid (IN; blue bars) trials for selected left (L), right (R) and bilateral (B) regions, with error bars equivalent to two times the standard error of the mean.
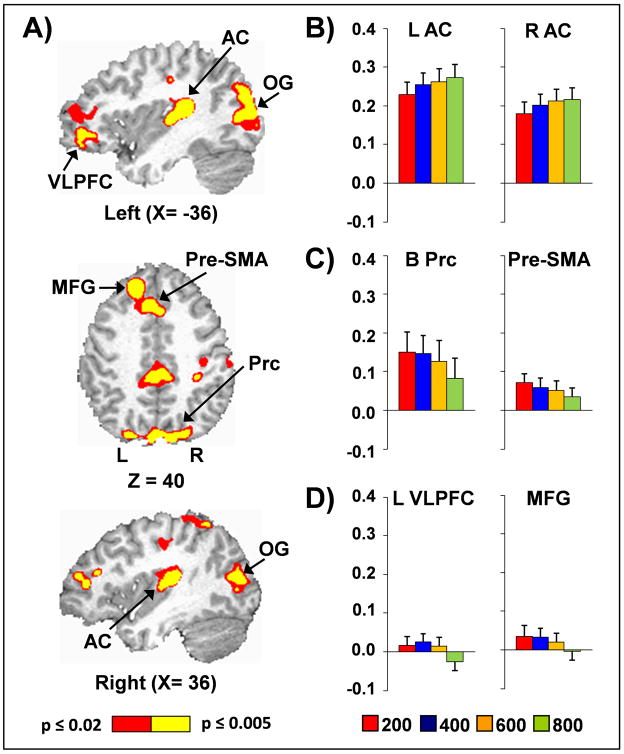
The main effect of SOA was significant in several regions, with three distinct patterns of activation noted (Figure 3). First, within the bilateral primary and secondary auditory cortex (BAs 13/41/42/43/22), activation increased as a function of SOA. In contrast, activation within bilateral pre-supplementary motor area, superior frontal gyrus and anterior cingulate gyrus (BAs 6/8/9/24/32), right inferior parietal lobule (BA 40), and bilateral thalamus and striatum tended to decrease while remaining positive with increasing SOA. Finally, the left middle frontal gyrus (BAs 8/9), left ventrolateral prefrontal cortex (BAs 10/11/46/47), bilateral precuneus extending into cingulate cortex (BAs 5/7/30/31), and bilateral visual cortex/cuneus (BAs 17/18/19) demonstrated decreased activation as a function of SOA, with deactivation also present at longer SOA intervals.
Figure 3.
Panel A presents regions exhibiting a main effect of stimulus onset asynchrony (SOA), with slice locations (X and Z) presented according to the Talairach atlas. Panels B-D present the percent signal change (PSC) data from selected regions at the 200 (red), 400 (blue), 600 (orange) and 800 (green) ms SOA intervals. In Panel B, the main effect of SOA was associated with increasing activation as a function of SOA in the bilateral primary and secondary auditory cortex (AC). Panel C shows how activation within the bilateral precuneus (B Prc) and pre-supplementary motor area (Pre-SMA) decreased as a function of SOA. Finally, activation within the bilateral middle frontal gyrus (MFG), bilateral occipital gyri (OG; percent signal change data not presented) and left ventrolateral prefrontal cortex (L VLPFC) decreased as a function of SOA, with deactivation present at longer SOAs (Panel D).
The SOA by validity interaction was significant in the bilateral cerebellum and medial temporal lobe (hippocampus and parahippocampal gyrus) extending into the temporal-occipital cortex (BAs 19, 20, 21, 35, 36 and 37). Follow-up simple effects tests indicated that activation was greater for valid compared to invalid trials at the 200 ms SOAs, whereas activation was greater for the invalid than valid trials at both the 400 and 800 ms SOAs (all p < 0.01).
The main effect of diagnosis and all interactions involving the diagnosis term were not significant following false positive correction. To test whether this negative finding resulted from larger variability in the functional data in SP relative to HC, we used Levene’s test for equality of variance on eight regions of interest derived from data collapsed across all SOA’s (frontoparietal orienting network derived from invalid versus valid comparisons). None of the regions of interest exhibited significant differences in mean percent signal change or variance between the two groups (p > 0.10).
4. Discussion
In the current study, patients with schizophrenia (SP) exhibited robust cognitive deficits on a wide range of formal cognitive tests including measures of intelligence, attention/inhibition, learning and memory, working memory, processing speed, and executive functioning relative to controls. Multi-dimensional cognitive impairment is a consistent finding in schizophrenia research (Heinrichs and Zakzanis, 1998, Mesholam-Gately et al., 2009, Sponheim et al., 2010), and underscores the important role that cognitive dysfunction plays in determining functionality for patients (Green et al., 2004). In addition, current findings indicate even more basic deficits on a task measuring stimulus-driven (i.e., bottom-up) shifts of auditory attention. Whereas HC exhibited the expected pattern of facilitation followed by IOR on this bottom-up task, SP exhibited increased deficits in disengaging auditory attention (invalid > valid) at short cue-target intervals, as well as a delayed development of IOR (valid > invalid) at longer cue-target intervals.
Previous studies on visual orienting have suggested both increased reorienting effects (Chirio et al., 2010, Maruff et al., 1995b, Posner, Early, 1988, Wigal, Swanson, 1997) as well as delayed IOR (Amado et al., 2009, Gouzoulis-Mayfrank, Arnold, 2006a, Gouzoulis-Mayfrank, Balke, 2007, Gouzoulis-Mayfrank, Heekeren, 2004, Kebir, Ben Azouz, 2010, Larrison-Faucher, Briand, 2002, Nestor et al., 2010, Sapir et al., 2007, Sapir, Henik, 2001, Wigal, Swanson, 1997) in SP. Collectively, current and previous results suggest that these spatial orienting deficits are supramodal in nature, and likely indicative of more basic deficits in attentional processes. However, the exact nature of these orienting deficits remains a topic of debate. As previously discussed, theories of specific left hemisphere deficits in SP have proven challenging to replicate (Daban, Krebs, 2004, Gold, Randolph, 1992, Liotti, Dazzi, 1993), and not all studies report disengagement effects (Sapir, Henik, 2001). A recent study (Kebir, Ben Azouz, 2010) demonstrated that SP eventually exhibit IOR at extended delays (SOA between 700 and 800 ms), suggesting that orienting deficits are more characteristic of a delay in IOR rather than a diminished/absent IOR response. Perhaps the most parsimonious explanation for the majority of findings is that SP require a prolonged interval to disengage their attention from cues, which potentially results in larger reorienting effects at short SOAs as well as a delay in the development of IOR.
The aberrant disengagement of attention in SP likely interferes with controlled, top-down attentional information processing, rendering SP more susceptible for ignoring distracting/redundant stimuli (Gouzoulis-Mayfrank, Balke, 2007). Support for this theory can be seen in the relationship between validity effects (invalid RT – valid RT) on our relatively simple orienting task and higher-order cognitive functions measured through traditional neuropsychological exams. Specifically, patients who exhibited a delay in IOR also performed worse on traditional neuropsychological measures of attention and inhibition relative to the patients who exhibited a more normal pattern of orienting. More importantly, these basic deficits in disengaging attention to simple sensory stimuli also appear to have deleterious consequences for actively manipulating information (working memory), potentially limiting the amount of information that can actively be processed and/or consolidated at any given time.
Functional results indicated that a distributed network of regions in the frontal oculomotor sites, dorsal lateral and ventral lateral prefrontal cortex, the posterior parietal lobe, and the cerebellum were activated during the disengagement of attention across all SOAs (i.e., main effect of validity). Thus, while the spatial topography of activation of the disengagement network is similar to our previous findings, key differences emerged on the direction of findings across the different SOA intervals (i.e. interaction of validity and SOA). Specifically, we have previously reported activation of a similar frontoparietal-cerebellar network during the disengagement of attention (invalid > valid) at short SOAs (Mayer et al., 2009a, Mayer, Harrington, 2007), followed by a reversal of activity (valid > invalid) for several of these structures during auditory IOR (Mayer, Harrington, 2007). It is likely that differences across these studies were partially driven by the inclusion of SP, who exhibited increased reaction times for invalid trials at three of the four SOAs. Thus, the continued presence of behavioral reorienting across the majority of SOA likely influenced the underlying neuronal circuitry, producing a pattern of neuronal activity that we previously observed only under short cue-target durations in only healthy control samples.
In spite of the behavioral differences in attentional reorienting and IOR, we did not observe any functional differences between SP and HC either within the large-scale fronto-parietal network (Arrington, Carr, 2000, Corbetta, Kincade, 2000, Mayer, Harrington, 2007) or within other regions critical to attentional orienting. These null effects did not seem to be the result of increased variability of the hemodynamic response or secondary to medication load in SP. Thus, current results suggest that SP do not invoke activity in additional regions to compensate for their behavioral deficits, which occurs in both normal aging (Cabeza et al., 2002) as well as clinical disorders such as psychotic major depression (Garrett et al., 2011) and mild traumatic brain injury (Mayer et al., 2009b). The lack of compensatory activity in SP may be related to higher-level attentional deficits and/or executive dysfunction. Alternatively, current results may also indicate that differences in neuronal signaling and metabolism, at the spatial scale examined by BOLD fMRI (Attwell et al., 2010, Logothetis, 2008), may not be adequate for capturing underlying orienting deficits. Finally, we note that it is difficult to interpret neuroimaging findings in light of differential behavioral performance on the task across the two groups.
In addition, current quantitative analyses focused on the peak of the hemodynamic response function (HRF), whereas as a qualitative examination suggested several consistent abnormalities that occurred later in the HRF. Specifically, SP hemodynamic response typically required several additional seconds to return to baseline (full-width at half maximum) and did not exhibit the characteristic post-undershoot (Buxton et al., 2004) relative to HC for several of the frontal regions implicated in attentional reorienting. Previous studies have also indicated significant abnormalities in hemodynamic response latencies in SP (~500 ms), reflecting differences in neuronal activity, neurovascular coupling or cerebral vasculature (Amenta et al., 2002, Ford et al., 2005). In addition, HRF abnormalities may also be secondary to medication effects or other clinical co-morbidities. As these results were not predicted, they require replication in an independent sample. However, regardless of etiology current and previous findings suggest caution when using a model that assumes an identical HRF shape across SP and HC groups (i.e., convolving stimulus timecourses with a “canonical” HRF).
6. Conclusion
This study was the first behavioral and functional imaging investigation of auditory orienting in schizophrenia. Our behavioral results were consistent with previous visual orienting paradigms in schizophrenia, suggesting that the orienting deficits in SP are supramodal in nature. In addition, patients who exhibited a delay in the development of IOR also exhibited more deficits on higher-order cognitive tests. In contrast to behavioral findings, our imaging results did not demonstrate any differences in the frontoparietal network in SP relative to HC. Future work on auditory orienting in SP should stratify patients based on the presence/absence of delayed IOR and include direct measures of neuronal activation to better characterize this dysfunction.
Research Highlights.
Patients had delayed inhibition of return (IOR) during auditory orienting
Results suggest prolonged attentional disengagement from cued locations in patients
Delayed IOR was associated with cognitive deficits in neuropsychological testing
fMRI results showed the characteristic activation of a fronto-parietal network
Patients had no significant differences in functional activation during IOR
Abbreviations
- SP
patients with schizophrenia
- HC
healthy controls
- IOR
inhibition of return
- fMRI
functional magnetic resonance imaging
- SOA
stimulus onset asynchrony
- SCID
the Structured Clinical Interview for DSM-IV Axis I Disorders
- PANSS
Positive and Negative Syndrome Scale
- CCPT
Connors Continuous Performance Test
- HRF
hemodynamic response function
- TE
echo time
- TR
repetition time
- FOV
field of view
- ANOVA
analysis of variance
- MANOVA
multivariate analysis of variance
References
- Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. The British journal of psychiatry. 1993:39–44. [PubMed] [Google Scholar]
- Amado I, Bourdel MC, Daban C, Poirier MF, Loo H, Bouhours P, et al. Preattentional processes and disorganization in schizophrenia: Influence of a 6-week risperidone treatment. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33:1107–12. doi: 10.1016/j.pnpbp.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Amenta F, Ricci A, Tayebati SK, Zaccheo D. The peripheral dopaminergic system: morphological analysis, functional and clinical applications. Ital J Anat Embryol. 2002;107:145–67. [PubMed] [Google Scholar]
- Arrington CM, Carr TH, Mayer AR, Rao SM. Neural mechanisms of visual attention: object-based selection of a region in space. Journal of cognitive neuroscience. 2000;12 (Suppl 2):106–17. doi: 10.1162/089892900563975. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–43. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen L, Wallace CJ, Glynn SM, Nuechterlein KH, Lutzker JR, Kuehnel TG. Schizophrenic individuals’ cognitive functioning and performance in interpersonal interactions and skills training procedures. Journal of psychiatric research. 1994;28:289–301. doi: 10.1016/0022-3956(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA. Preattentional and attentional cognitive deficits as targets for treating schizophrenia. Psychopharmacology. 2004;174:75–85. doi: 10.1007/s00213-004-1848-0. [DOI] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport. 1998;9:3735–9. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. NeuroImage. 2004;23 (Suppl 1):S220–33. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Callaway E, Naghdi S. An information processing model for schizophrenia. Archives of general psychiatry. 1982;39:339–47. doi: 10.1001/archpsyc.1982.04290030069012. [DOI] [PubMed] [Google Scholar]
- Carter CS, Robertson LC, Chaderjian MR, Celaya LJ, Nordahl TE. Attentional asymmetry in schizophrenia: controlled and automatic processes. Biological psychiatry. 1992;31:909–18. doi: 10.1016/0006-3223(92)90117-i. [DOI] [PubMed] [Google Scholar]
- Chirio M, Krebs MO, Waismann R, Vanelle JM, Olie JP, Amado I. Attention and visual orienting in siblings, schizophrenic patients, and controls: impairment in attentional disengagement. Journal of clinical and experimental neuropsychology. 2010;32:449–54. doi: 10.1080/13803390903146949. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature neuroscience. 2000;3:292–7. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Daban C, Krebs MO, Bourdel MC, Willard D, Loo H, Olie JP, et al. Effects of atypical neuroleptics on alertness and visual orienting in stabilized schizophrenic patients: a preliminary study. Int J Neuropsychopharmacol. 2004;7:255–63. doi: 10.1017/S1461145704004250. [DOI] [PubMed] [Google Scholar]
- Daumann J, Heekeren K, Neukirch A, Thiel CM, Moller-Hartmann W, Gouzoulis-Mayfrank E. Pharmacological modulation of the neural basis underlying inhibition of return (IOR) in the human 5-HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology. 2008;200:573–83. doi: 10.1007/s00213-008-1237-1. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–41. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. New York: New York State Psychiatric Institute, Biomedical Research; 2002a. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York, New York: New York State Psychiatric Institute, Biomedical Research; 2002b. [Google Scholar]
- Ford JM, Johnson MB, Whitfield SL, Faustman WO, Mathalon DH. Delayed hemodynamic responses in schizophrenia. NeuroImage. 2005;26:922–31. doi: 10.1016/j.neuroimage.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. The American journal of psychiatry. 2010;167:686–93. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- Garrett A, Kelly R, Gomez R, Keller J, Schatzberg AF, Reiss AL. Aberrant brain activation during a working memory task in psychotic major depression. The American journal of psychiatry. 2011;168:173–82. doi: 10.1176/appi.ajp.2010.09121718. [DOI] [PubMed] [Google Scholar]
- Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. NeuroImage. 1999;9:416–29. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- Gold JM, Randolph C, Coppola R, Carpenter CJ, Goldberg TE, Weinberger DR. Visual orienting in schizophrenia. Schizophrenia research. 1992;7:203–9. doi: 10.1016/0920-9964(92)90013-u. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Arnold S, Heekeren K. Deficient inhibition of return in schizophrenia-further evidence from an independent sample. Progress in neuro-psychopharmacology & biological psychiatry. 2006a;30:42–9. doi: 10.1016/j.pnpbp.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Balke M, Hajsamou S, Ruhrmann S, Schultze-Lutter F, Daumann J, et al. Orienting of attention in unmedicated patients with schizophrenia, prodromal subjects and healthy relatives. Schizophrenia research. 2007;97:35–42. doi: 10.1016/j.schres.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, Stoll M, Stock C, Daumann J, et al. Inhibition of return in the human 5HT2A agonist and NMDA antagonist model of psychosis. Neuropsychopharmacology. 2006b;31:431–41. doi: 10.1038/sj.npp.1300882. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Voss T, Moerth D, Thelen B, Meincke U. Blunted inhibition of return in schizophrenia-evidence from a longitudinal study. Progress in neuro-psychopharmacology & biological psychiatry. 2004;28:389–96. doi: 10.1016/j.pnpbp.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophrenia research. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC: US Dept of Health, Education and Welfare; 1976. [Google Scholar]
- Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, et al. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology. 2004;29:1378–85. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–45. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- Jonides J, Irwin DE. Capturing Attention. Cognition. 1981;10:145–50. doi: 10.1016/0010-0277(81)90038-x. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kebir O, Ben Azouz O, Rabah Y, Dellagi L, Johnson I, Amado I, et al. Confirmation for a delayed inhibition of return by systematic sampling in schizophrenia. Psychiatry research. 2010;176:17–21. doi: 10.1016/j.psychres.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Stevens MC, Celone K, Kurtz M, Krystal JH. Abnormal hemodynamics in schizophrenia during an auditory oddball task. Biological psychiatry. 2005;57:1029–40. doi: 10.1016/j.biopsych.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends in cognitive sciences. 2000;4:138–47. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Larrison-Faucher A, Briand KA, Sereno AB. Delayed onset of inhibition of return in schizophrenia. Progress in neuro-psychopharmacology & biological psychiatry. 2002;26:505–12. doi: 10.1016/s0278-5846(01)00298-6. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Kiehl KA, Ngan ET, Liddle PF. Attention orienting dysfunction during salient novel stimulus processing in schizophrenia. Schizophrenia research. 2005;75:159–71. doi: 10.1016/j.schres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Pollmann S. Covert reorienting and inhibition of return: an event-related fMRI study. Journal of cognitive neuroscience. 2002;14:127–44. doi: 10.1162/089892902317236795. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Archives of general psychiatry. 2005;62:127–36. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- Liotti M, Dazzi S, Umilta C. Deficits of the automatic orienting of attention in schizophrenic patients. Journal of psychiatric research. 1993;27:119–30. doi: 10.1016/0022-3956(93)90056-8. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–78. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Maruff P, Hay D, Malone V, Currie J. Asymmetries in the covert orienting of visual spatial attention in schizophrenia. Neuropsychologia. 1995a;33:1205–23. doi: 10.1016/0028-3932(95)00037-4. [DOI] [PubMed] [Google Scholar]
- Maruff P, Malone V, Currie J. Asymmetries in the covert orienting of visual spatial attention to spatial and non-spatial cues in Alzheimer’s disease. Brain. 1995b;118 (Pt 6):1421–35. doi: 10.1093/brain/118.6.1421. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Dorflinger JM, Rao SM, Seidenberg M. Neural networks underlying endogenous and exogenous visual-spatial orienting. NeuroImage. 2004a;23:534–41. doi: 10.1016/j.neuroimage.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Franco AR, Harrington DL. Neuronal modulation of auditory attention by informative and uninformative spatial cues. Human brain mapping. 2009a;30:1652–66. doi: 10.1002/hbm.20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Harrington DL, Stephen J, Adair JC, Lee RR. An event-related fMRI Study of exogenous facilitation and inhibition of return in the auditory modality. Journal of cognitive neuroscience. 2007;19:455–67. doi: 10.1162/jocn.2007.19.3.455. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Elgie R, Gasparovic C, Phillips JP, et al. Auditory orienting and inhibition of return in mild traumatic brain injury: a FMRI study. Human brain mapping. 2009b;30:4152–66. doi: 10.1002/hbm.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Seidenberg M, Dorflinger JM, Rao SM. An event-related fMRI study of exogenous orienting: supporting evidence for the cortical basis of inhibition of return? Journal of cognitive neuroscience. 2004b;16:1262–71. doi: 10.1162/0898929041920531. [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–36. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Mondor TA. Predictability fo the cue-target relation and time-course of auditory inhibition of return. Perception & psychophysics. 1999;61:1501–9. doi: 10.3758/bf03213113. [DOI] [PubMed] [Google Scholar]
- Mondor TA, Breau LM. Facilitative and inhibitory effects of location and frequency cues: evidence of a modulation in perceptual sensitivity. Perception & psychophysics. 1999;61:438–44. doi: 10.3758/bf03211964. [DOI] [PubMed] [Google Scholar]
- Mondor TA, Bryden MP. Orienting of auditory spatial attention: effects of a lateralized tone cue. Neuropsychologia. 1992;30:743–52. doi: 10.1016/0028-3932(92)90043-l. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Klein K, Pomplun M, Niznikiewicz M, McCarley RW. Gaze cueing of attention in schizophrenia: individual differences in neuropsychological functioning and symptoms. J Clin Exp Neuropsychol. 2010;32:281–8. doi: 10.1080/13803390902984472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JV, Hetrick WP, Boutros NN, Jin Y, Sandman C, Stern H, et al. P50 sensory gating ratios in schizophrenics and controls: a review and data analysis. Psychiatry research. 2008;158:226–47. doi: 10.1016/j.psychres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Bouma H, Bouwhuis DG, editors. Attentional and Performance Control of Language Processing. London: Lawrence Erlbaum Associates Publishers; 1984. pp. 531–56. [Google Scholar]
- Posner MI, Early TS, Reiman E, Pardo PJ, Dhawan M. Asymmetries in hemispheric control of attention in schizophrenia. Archives of general psychiatry. 1988;45:814–21. doi: 10.1001/archpsyc.1988.01800330038004. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Archives of general psychiatry. 2002;59:686–94. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- Sapir A, Dobrusin M, Ben-Bashat G, Henik A. Neuroleptics reverse attentional effects in schizophrenia patients. Neuropsychologia. 2007;45:3263–71. doi: 10.1016/j.neuropsychologia.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Sapir A, Henik A, Dobrusin M, Hochman EY. Attentional asymmetry in schizophrenia: disengagement and inhibition of return deficits. Neuropsychology. 2001;15:361–70. doi: 10.1037//0894-4105.15.3.361. [DOI] [PubMed] [Google Scholar]
- Sapir A, Soroker N, Berger A, Henik A. Inhibition of return in spatial attention: direct evidence for collicular gneration. Nature neuroscience. 1999;2:1053–4. doi: 10.1038/15977. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, et al. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Archives of general psychiatry. 1991;48:618–24. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Spence C, Driver J. Covert spatial orienting in audition: Exogenous and endogenous mechanisms. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:555–74. [Google Scholar]
- Spence C, Driver J. Auditory and audiovisual inhibition of return. Perception & psychophysics. 1998;60:125–39. doi: 10.3758/bf03211923. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, Jung RE, Seidman LJ, Mesholam-Gately RI, Manoach DS, O’Leary DS, et al. Cognitive deficits in recent-onset and chronic schizophrenia. Journal of psychiatric research. 2010;44:421–8. doi: 10.1016/j.jpsychires.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199:331–88. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Tassinari G, Campara D, Benedetti C, Berlucchi G. The contribution of general and specific motor inhibitory sets to the so-called auditory inhibition of return. Exp Brain Res. 2002;146:523–30. doi: 10.1007/s00221-002-1192-8. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Fink GR. Effects of the cholinergic agonist nicotine on reorienting of visual spatial attention and top-down attentional control. Neuroscience. 2008;152:381–90. doi: 10.1016/j.neuroscience.2007.10.061. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Cerebral correlates of alerting, orienting and reorienting of visuospatial attention: an event-related fMRI study. NeuroImage. 2004;21:318–28. doi: 10.1016/j.neuroimage.2003.08.044. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Nicotine modulates reorienting of visuospatial attention and neural activity in human parietal cortex. Neuropsychopharmacology. 2005;30:810–20. doi: 10.1038/sj.npp.1300633. [DOI] [PubMed] [Google Scholar]
- Wigal SB, Swanson JM, Potkin SG. Lateralized attentional deficits in drug-free and medicated schizophrenic patients. Neuropsychologia. 1997;35:1519–25. doi: 10.1016/s0028-3932(97)00087-0. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Sergi MJ, Dawson ME, Schell AM, Green MF. Sensorimotor gating, orienting and social perception in schizophrenia. Schizophrenia research. 2005;73:319–25. doi: 10.1016/j.schres.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Sugar C, Horan WP, Kern R, Green MF. Mismatch negativity, social cognition, and functioning in schizophrenia patients. Biological psychiatry. 2010;67:940–7. doi: 10.1016/j.biopsych.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]