Abstract
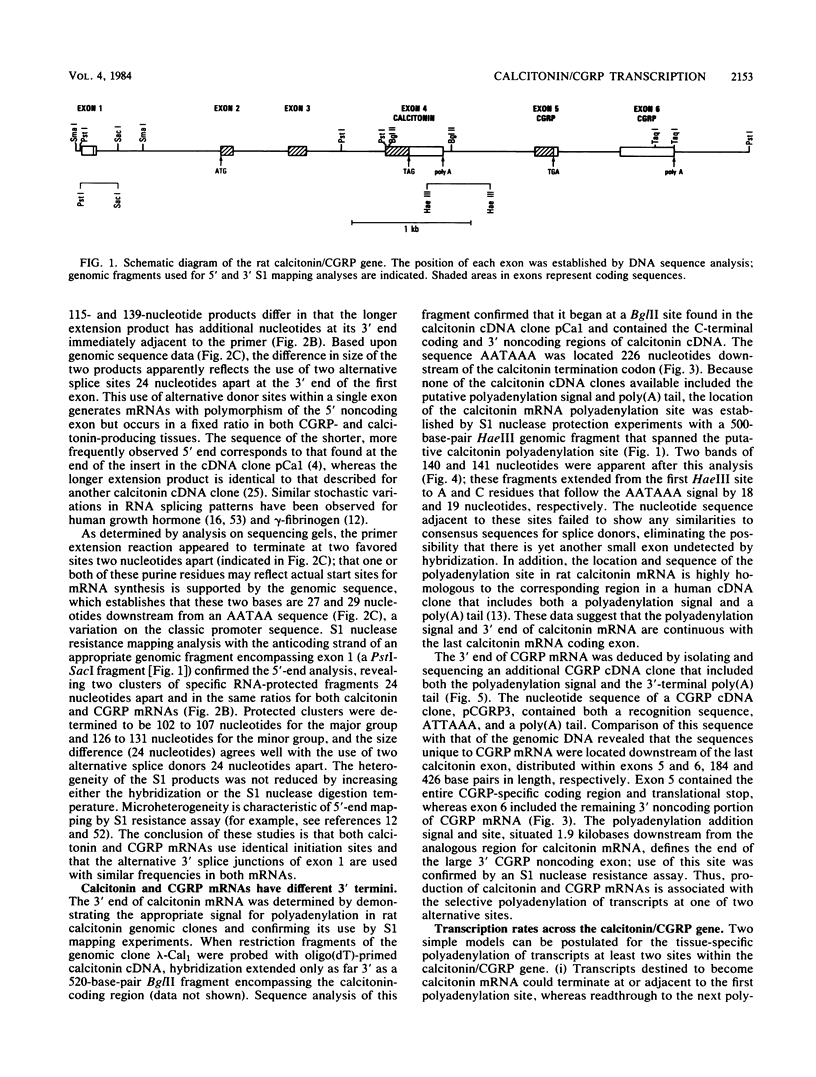
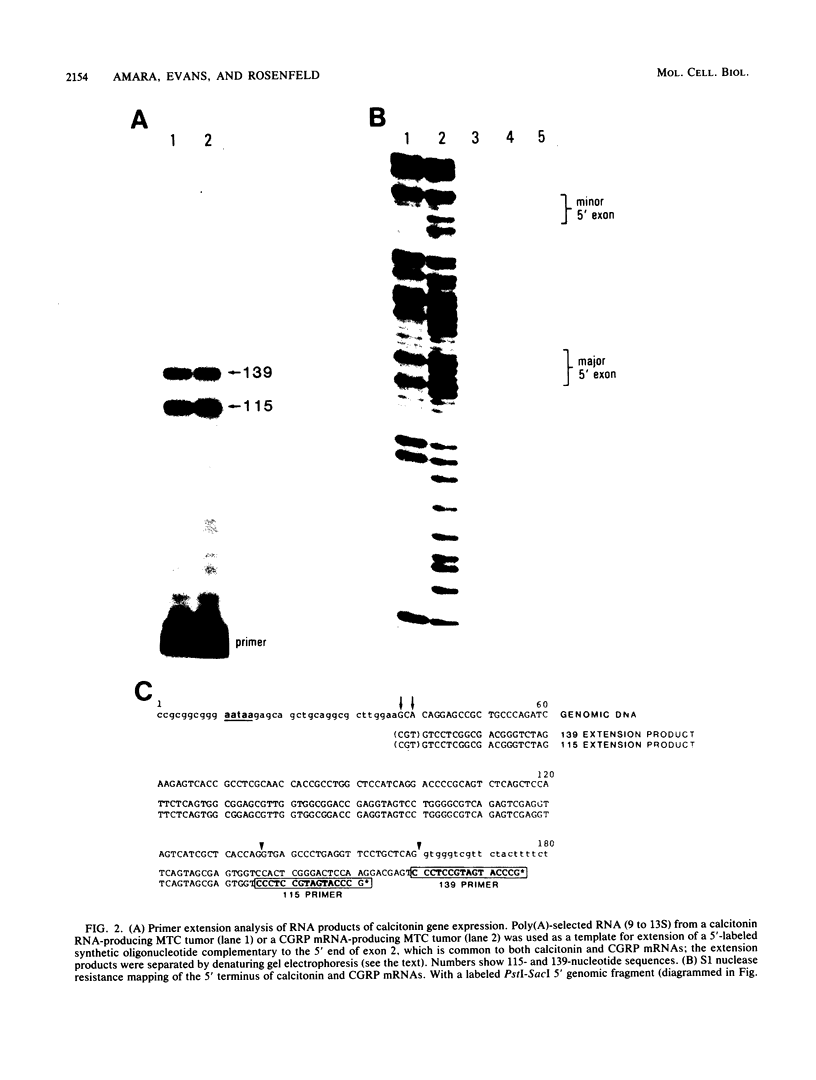
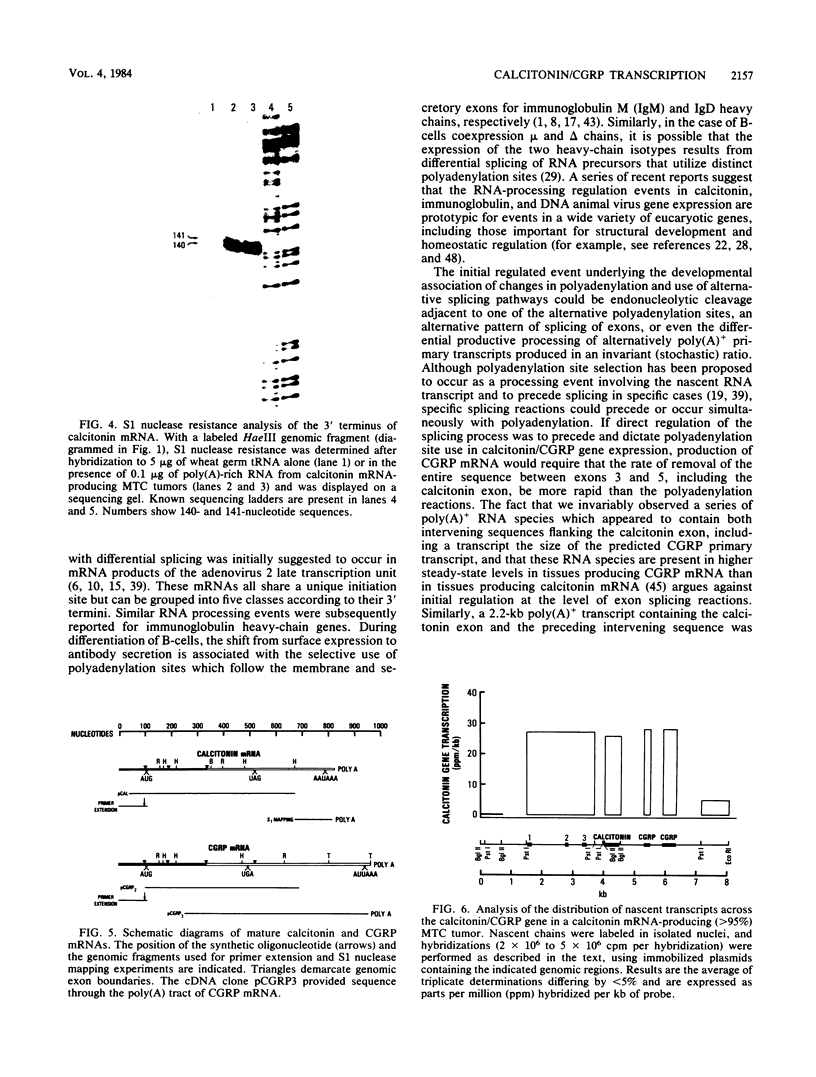
Different 3' coding exons in the rat calcitonin gene are used to generate distinct mRNAs encoding either the hormone calcitonin in thyroidal C-cells or a new neuropeptide referred to as calcitonin gene-related peptide in neuronal tissue, indicating the RNA processing regulation is one strategy used in tissue-specific regulation of gene expression in the brain. Although the two mRNAs use the same transcriptional initiation site and have identical 5' terminal sequences, their 3' termini are distinct. The polyadenylation sites for calcitonin and calcitonin gene-related peptide mRNAs are located at the end of the exons 4 and 6, respectively. Termination of transcription after the calcitonin exon does not dictate the production of calcitonin mRNA, because transcription proceeds through both calcitonin and calcitonin gene-related peptide exons irrespective of which mRNA is ultimately produced. In isolated nuclei, both polyadenylation sites appear to be utilized; however, the proximal (calcitonin) site is preferentially used in nuclei from tissues producing calcitonin mRNA. These data suggest that the mechanism dictating production of each mRNA involves the selective use of alternative polyadenylation sites.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Brunstedt J., Noyes B. E. A general method for detection and characterization of an mRNA using an oligonucleotide probe. J Biol Chem. 1981 Jan 25;256(2):1023–1028. [PubMed] [Google Scholar]
- Alt F. W., Bothwell A. L., Knapp M., Siden E., Mather E., Koshland M., Baltimore D. Synthesis of secreted and membrane-bound immunoglobulin mu heavy chains is directed by mRNAs that differ at their 3' ends. Cell. 1980 Jun;20(2):293–301. doi: 10.1016/0092-8674(80)90615-7. [DOI] [PubMed] [Google Scholar]
- Amara S. G., David D. N., Rosenfeld M. G., Roos B. A., Evans R. M. Characterization of rat calcitonin mRNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4444–4448. doi: 10.1073/pnas.77.8.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara S. G., Jonas V., O'Neil J. A., Vale W., Rivier J., Roos B. A., Evans R. M., Rosenfeld M. G. Calcitonin COOH-terminal cleavage peptide as a model for identification of novel neuropeptides predicted by recombinant DNA analysis. J Biol Chem. 1982 Mar 10;257(5):2129–2132. [PubMed] [Google Scholar]
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Berget S. M., Sharp P. A. Structure of late adenovirus 2 heterogeneous nuclear RNA. J Mol Biol. 1979 Apr 25;129(4):547–565. doi: 10.1016/0022-2836(79)90468-6. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Folk W., Birnstiel M. L. The terminal RNA stem-loop structure and 80 bp of spacer DNA are required for the formation of 3' termini of sea urchin H2A mRNA. Cell. 1983 Dec;35(2 Pt 1):433–440. doi: 10.1016/0092-8674(83)90176-9. [DOI] [PubMed] [Google Scholar]
- Chen-Kiang S., Wolgemuth D. J., Hsu M. T., Darnell J. E., Jr Transcription and accurate polyadenylation in vitro of RNA from the major late adenovirus 2 transcription unit. Cell. 1982 Mar;28(3):575–584. doi: 10.1016/0092-8674(82)90212-4. [DOI] [PubMed] [Google Scholar]
- Cheng H. L., Blattner F. R., Fitzmaurice L., Mushinski J. F., Tucker P. W. Structure of genes for membrane and secreted murine IgD heavy chains. Nature. 1982 Apr 1;296(5856):410–415. doi: 10.1038/296410a0. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R. The spliced structures of adenovirus 2 fiber message and the other late mRNAs. Cell. 1978 Oct;15(2):497–510. doi: 10.1016/0092-8674(78)90019-3. [DOI] [PubMed] [Google Scholar]
- Compere S. J., Palmiter R. D. DNA methylation controls the inducibility of the mouse metallothionein-I gene lymphoid cells. Cell. 1981 Jul;25(1):233–240. doi: 10.1016/0092-8674(81)90248-8. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R., Kant J. A. Organization of the rat gamma-fibrinogen gene: alternative mRNA splice patterns produce the gamma A and gamma B (gamma ') chains of fibrinogen. Cell. 1982 Nov;31(1):159–166. doi: 10.1016/0092-8674(82)90415-9. [DOI] [PubMed] [Google Scholar]
- Craig R. K., Hall L., Edbrooke M. R., Allison J., MacIntyre I. Partial nucleotide sequence of human calcitonin precursor mRNA identifies flanking cryptic peptides. Nature. 1982 Jan 28;295(5847):345–347. doi: 10.1038/295345a0. [DOI] [PubMed] [Google Scholar]
- Crea R., Kraszewski A., Hirose T., Itakura K. Chemical synthesis of genes for human insulin. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5765–5769. doi: 10.1073/pnas.75.12.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- DeNoto F. M., Moore D. D., Goodman H. M. Human growth hormone DNA sequence and mRNA structure: possible alternative splicing. Nucleic Acids Res. 1981 Aug 11;9(15):3719–3730. doi: 10.1093/nar/9.15.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Ford J. P., Hsu M. T. Transcription pattern of in vivo-labeled late simian virus 40 RNA: equimolar transcription beyond the mRNA 3' terminus. J Virol. 1978 Dec;28(3):795–801. doi: 10.1128/jvi.28.3.795-801.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser N. W., Nevins J. R., Ziff E., Darnell J. E., Jr The major late adenovirus type-2 transcription unit: termination is downstream from the last poly(A) site. J Mol Biol. 1979 Apr 25;129(4):643–656. doi: 10.1016/0022-2836(79)90474-1. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Kelly J. D., Cohen E. H. Transcription terminates in yeast distal to a control sequence. Cell. 1983 Jun;33(2):607–614. doi: 10.1016/0092-8674(83)90441-5. [DOI] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Hofer E., Hofer-Warbinek R., Darnell J. E., Jr Globin RNA transcription: a possible termination site and demonstration of transcriptional control correlated with altered chromatin structure. Cell. 1982 Jul;29(3):887–893. doi: 10.1016/0092-8674(82)90450-0. [DOI] [PubMed] [Google Scholar]
- Jacobs J. W., Goodman R. H., Chin W. W., Dee P. C., Habener J. F., Bell N. H., Potts J. T., Jr Calcitonin messenger RNA encodes multiple polypeptides in a single precursor. Science. 1981 Jul 24;213(4506):457–459. doi: 10.1126/science.6264603. [DOI] [PubMed] [Google Scholar]
- Jung A., Sippel A. E., Grez M., Schütz G. Exons encode functional and structural units of chicken lysozyme. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5759–5763. doi: 10.1073/pnas.77.10.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Morahan G., Cowman A. F., Harris A. W. Production of RNA for secreted immunoglobulin mu chains does not require transcriptional termination 5' to the microM exons. Nature. 1983 Jan 6;301(5895):84–86. doi: 10.1038/301084a0. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Takagaki Y., Furuto S., Tanaka T., Nawa H., Nakanishi S. A single gene for bovine high molecular weight and low molecular weight kininogens. Nature. 1983 Oct 6;305(5934):545–549. doi: 10.1038/305545a0. [DOI] [PubMed] [Google Scholar]
- Maki R., Roeder W., Traunecker A., Sidman C., Wabl M., Raschke W., Tonegawa S. The role of DNA rearrangement and alternative RNA processing in the expression of immunoglobulin delta genes. Cell. 1981 May;24(2):353–365. doi: 10.1016/0092-8674(81)90325-1. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Sharp P. A., Gefter M. L. RNA synthesis in isolated nuclei: identification and comparison of adenovirus 2 encoded transcripts synthesized in vitro and vivo. J Mol Biol. 1979 Nov 25;135(1):171–197. doi: 10.1016/0022-2836(79)90346-2. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Sharp P. A., Gefter M. L. Rna synthesis in isolated nuclei processing of adenovirus serotype 2 late messenger rna precursors. J Mol Biol. 1982 Aug 25;159(4):581–599. doi: 10.1016/0022-2836(82)90102-4. [DOI] [PubMed] [Google Scholar]
- Mather E. L., Nelson K. J., Haimovich J., Perry R. P. Mode of regulation of immunoglobulin mu- and delta-chain expression varies during B-lymphocyte maturation. Cell. 1984 Feb;36(2):329–338. doi: 10.1016/0092-8674(84)90226-5. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Inhibition of RNA cleavage but not polyadenylation by a point mutation in mRNA 3' consensus sequence AAUAAA. Nature. 1983 Oct 13;305(5935):600–605. doi: 10.1038/305600a0. [DOI] [PubMed] [Google Scholar]
- Moore C. L., Sharp P. A. Site-specific polyadenylation in a cell-free reaction. Cell. 1984 Mar;36(3):581–591. doi: 10.1016/0092-8674(84)90337-4. [DOI] [PubMed] [Google Scholar]
- Murdoch G. H., Potter E., Nicolaisen A. K., Evans R. M., Rosenfeld M. G. Epidermal growth factor rapidly stimulates prolactin gene transcription. Nature. 1982 Nov 11;300(5888):192–194. doi: 10.1038/300192a0. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Blanchard J. M., Darnell J. E., Jr Transcription units of adenovirus type 2. Termination of transcription beyond the poly(A) addition site in early regions 2 and 4. J Mol Biol. 1980 Dec 15;144(3):377–386. doi: 10.1016/0022-2836(80)90096-0. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E., Jr Steps in the processing of Ad2 mRNA: poly(A)+ nuclear sequences are conserved and poly(A) addition precedes splicing. Cell. 1978 Dec;15(4):1477–1493. doi: 10.1016/0092-8674(78)90071-5. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Wilson M. C. Regulation of adenovirus-2 gene expression at the level of transcriptional termination and RNA processing. Nature. 1981 Mar 12;290(5802):113–118. doi: 10.1038/290113a0. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Roos B. A., Yoon M. J., Frelinger A. L., Pensky A. E., Birnbaum R. S., Lambert P. W. Tumor growth and calcitonin during serial transplantation of rat medullary thyroid carcinoma. Endocrinology. 1979 Jul;105(1):27–32. doi: 10.1210/endo-105-1-27. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Amara S. G., Roos B. A., Ong E. S., Evans R. M. Altered expression of the calcitonin gene associated with RNA polymorphism. Nature. 1981 Mar 5;290(5801):63–65. doi: 10.1038/290063a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Lin C. R., Amara S. G., Stolarsky L., Roos B. A., Ong E. S., Evans R. M. Calcitonin mRNA polymorphism: peptide switching associated with alternative RNA splicing events. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1717–1721. doi: 10.1073/pnas.79.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. G., Mermod J. J., Amara S. G., Swanson L. W., Sawchenko P. E., Rivier J., Vale W. W., Evans R. M. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983 Jul 14;304(5922):129–135. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- Rozek C. E., Davidson N. Drosophila has one myosin heavy-chain gene with three developmentally regulated transcripts. Cell. 1983 Jan;32(1):23–34. doi: 10.1016/0092-8674(83)90493-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Reeder R. H. The nucleotide sequence of the initiation and termination sites for ribosomal RNA transcription in X. laevis. Cell. 1979 Oct;18(2):485–499. doi: 10.1016/0092-8674(79)90066-7. [DOI] [PubMed] [Google Scholar]
- Tosi M., Young R. A., Hagenbüchle O., Schibler U. Multiple polyadenylation sites in a mouse alpha-amylase gene. Nucleic Acids Res. 1981 May 25;9(10):2313–2323. doi: 10.1093/nar/9.10.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeli G., Ohkubo H., Sobel M. E., Yamada Y., Pastan I., de Crombrugghe B. Structure of the promoter for chicken alpha 2 type I collagen gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5334–5338. doi: 10.1073/pnas.78.9.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis M. Growth hormone: deletions in the protein and introns in the gene. Nature. 1980 Apr 10;284(5756):512–512. doi: 10.1038/284512a0. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff E. B. Transcription and RNA processing by the DNA tumour viruses. Nature. 1980 Oct 9;287(5782):491–499. doi: 10.1038/287491a0. [DOI] [PubMed] [Google Scholar]





