Abstract
Objective
Acute kidney injury (AKI) frequently complicates septic shock and independently predicts mortality in this population. Clinical factors alone do not entirely account for differences in risk of AKI between patients. Genetic variants likely explain this differential susceptibility. To identify genetic variants linked to AKI susceptibility, we conducted a high-density genotyping association study in a large population of patients with septic shock.
Design
Retrospective study.
Setting
Tertiary academic medical center.
Patients
1,264 patients with septic shock were analyzed to elucidate clinical risk factors associated with the development of AKI. Among them, 887 Caucasian patients were randomly split into discovery and validation cohorts and genotyped using the Illumina Human CVD BeadChip.
Interventions
None.
Measurements and Main Results
627 of the 1,264 patients with septic shock and 441 of the 887 patients with genotyping data developed AKI within the first 72 hours of ICU admission. Five single nucleotide polymorphisms (SNPs) were associated with AKI in both the discovery and validation cohorts. Two of these were in the BCL2 gene and both were associated with a decreased risk of AKI (rs8094315: OR 0.61, P=0.0002; rs12457893: OR 0.67, P=0.0002, both for combined data). Bcl-2 is involved in the apoptosis pathway, which has previously been implicated in AKI. Another SNP was in the SERPINA4 gene, whose protein product, kallistatin, has been linked to apoptosis in the kidney.
Conclusions
Large-scale genotyping reveals two SNPs in the BCL2 gene and a SNP in the SERPINA4 gene associated with a decreased risk of developing AKI, supporting the putative role of apoptosis in the pathogenesis of AKI.
Keywords: acute kidney injury, apoptosis, BCL2, SERPINA4, genetic susceptibility, sepsis
Introduction
Acute kidney injury (AKI) occurs in over 35% of critically ill patients (1,2). The development of AKI independently predicts mortality in this population (1-6). Sepsis and septic shock are the leading causes of AKI in the intensive care unit (ICU) (6-8) and septic AKI carries a higher mortality than non-septic AKI (8-10) and sepsis or septic shock without AKI (9,11,12). Given the rising incidence of both AKI (6) and sepsis syndromes (13,14), it is becoming increasingly important to identify those septic patients at highest risk of developing AKI.
Certain clinical factors, such as age and severity of illness, have been shown to be associated with AKI in sepsis (11,12,15). However, these do not entirely explain the differential susceptibility to AKI, and genetic differences likely partially explain this variation. There are few studies on genetic polymorphisms in AKI, none of which have used large-scale genotyping. Most of these studies used a broad and varied population, such as ICU admissions with expected stay over forty-eight hours (16), hospitalized patients for whom nephrology consultation was obtained (17,18), or patients with AKI requiring initiation of hemodialysis (19). All of these studies used a candidate gene approach.
Because the pathophysiology of AKI differs based on the etiology (20), the current study focused on a cohort of patients with a common risk factor for AKI. In this cohort of patients with septic shock, we aimed to elucidate which clinical factors were associated with the development of AKI using the Acute Kidney Injury Network (AKIN) criteria (21). We then utilized large-scale genotyping to identify the genetic polymorphisms associated with AKI in this population. We hypothesized that, by using a gene-centric array like the Human-CVD BeadChip that contains dense coverage of genes in various pathways such as inflammation and metabolism, we would find a genetic predisposition to AKI. Some of the results of this study have been previously published in the form of an abstract (22).
Materials and Methods
Study Population
This is a case-cohort study. Subjects were enrolled from an ongoing molecular epidemiology study of patients with acute respiratory distress syndrome (ARDS). Details of this study are described elsewhere (23). Briefly, all admissions to the ICUs at Massachusetts General Hospital (from September 1999 through March 2009) and Beth Israel Deaconess Medical Center (from January 2007 through March 2009) were screened daily for risk factors for development of ARDS, including septic shock. Septic shock was defined according to the ACCP/SCCM Consensus Conference criteria (24). Patients with end-stage renal disease (ESRD) on chronic dialysis were excluded. Additional exclusion criteria included age less than 18 years, diffuse alveolar hemorrhage, chronic lung diseases other than COPD or asthma, directive to withhold intubation, immunosuppression not secondary to corticosteroid use, and treatment with granulocyte colony-stimulating factor. All enrolled subjects with septic shock comprised the cohort for the current study. The Human Subjects Committees approved the study and informed written consent was obtained from subjects or their surrogates.
Baseline characteristics and variables necessary to calculate the Acute Physiology and Chronic Health Evaluation (APACHE) III scores were recorded for all subjects within 24 hours of ICU admission. Daily laboratory values, including serum creatinine, were recorded either until ICU discharge, death, or day 28, whichever came first. Development of AKI was defined based on the AKIN criteria (21). Patients were categorized as having AKI if their serum creatinine increased by more than or equal to 0.3 mg/dl or by a percentage increase of more than or equal to 50% within the first 72 hours of ICU admission. Urine output data was not available.
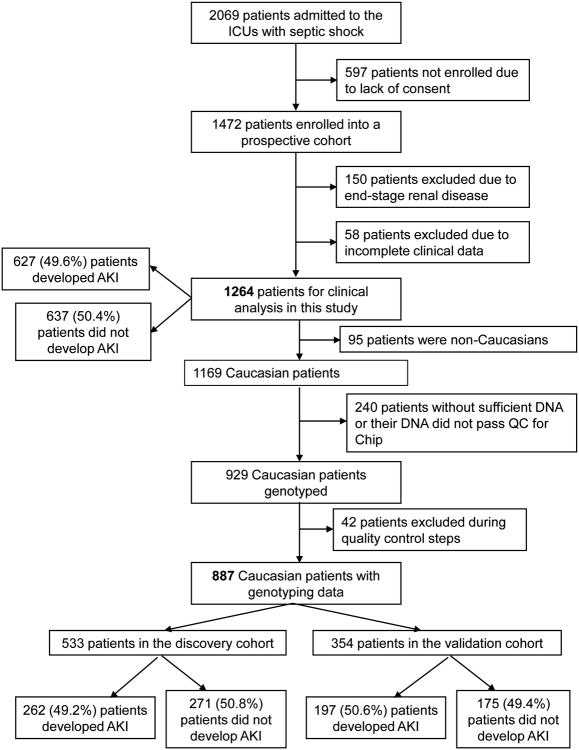
For the genetic analysis, to deal with the issue of multiple testing in a large-scale genotyping study, we randomly split our cohort into discovery (60%) and validation (40%) sets. Although this caused a loss of power, it was done in order to attempt to replicate our results. We then evaluated the relationship of the SNPs and AKI in the cohort as a whole, in order to maximize power. A schematic of the study design is shown in Figure 1.
Figure 1.
Schematic of study design. AKI: acute kidney injury.
Genotyping and Quality Control
All patients had their blood drawn for DNA extraction within 48 hours of ICU admission. Genomic DNA was extracted from whole blood using the Autopure LS robotic workstation (Gentra Systems, Minneapolis, MN) DNA Purification Reagent Kits (Qiagen, Valencia, CA). Five hundred nanograms of DNA per sample were diluted to 50-100 ng/μL for genotyping at the Center for Applied Genomics, Children's Hospital of Philadelphia (Philadelphia, PA), using the Illumina HumanCVD BeadChip (Illumina, San Diego, CA). The Human-CVD BeadChip is a multi-sample genotyping panel including 48,742 markers across approximately 2100 genes associated with cardiovascular, metabolic, and inflammatory syndromes (25). Genotyping personnel were blinded to AKI status. The samples were processed according to Illumina's Infinium II Assay protocols. Briefly, after amplification, fragmentation, precipitation and resuspension, DNA samples were applied to BeadChips for hybridization, followed by enzymatic base extension and fluorescent staining. The intensities of fluorescence were detected by Illumina BeadArray Reader (Illumina, San Diego, CA), and were in turn analyzed using Illumina's BeadStudio Genotyping Module v3.2 for automated genotype calling.
Quality control measures were conducted using the software package PLINK version 1.06 (http://pngu.mgh.harvard.edu/∼purcell/plink/). For SNP quality control, we removed 1,121 non-autosomal single nucleotide polymorphisms (SNPs), 598 SNPs with call rate ≤ 95%, 17,210 SNPs with minor allele frequency < 0.05, and 2,403 SNPs with Hardy-Weinberg test P ≤ 0.0001 in controls. For sample quality control, we removed 42 samples with genotyping call rate ≤ 95%. These steps resulted in a dataset with 27,410 SNPs and 887 samples for final analyses.
Statistical Analysis
Univariate analysis of clinical characteristics was performed by using the chi-squared test for categorical variables and the Wilcoxon test for continuous variables. Characteristics from the univariate analyses with P<0.2 were entered into a multivariate logistic regression model, using a backward selection algorithm with criteria of P>0.05 for elimination. Laboratory values such as serum glucose and bilirubin were not individually included in the multivariate model in order to avoid collinearity, because these values are included in the APACHE III score. Analyses were performed using the SAS statistical software package (version 9.2, SAS Institute Inc., Cary NC).
SNP and haplotype association tests were analyzed using PLINK (26). An additive genetic model was used. In the discovery set, the P-value for significance was arbitrarily set at 0.005. SNPs that were significant in the discovery set were considered to be replicated in the validation set if their P-value was less than 0.05 and if their associated odds ratio was in the same direction as their odds ratio in the discovery set. SNP associations were then adjusted for clinical characteristics that were significant in the multivariate logistic regression model. In the whole cohort used for the genetic analysis, we set the P-value for significance at 5E-4. Because the array has nearly 50,000 SNPs, a standard Bonferroni correction would yield a P-value for significance of 1E-6. However, we feel that correction is too conservative; given that this is a dense gene-centric array, the prior probability that a SNP would be associated with the disease in question is higher than that for a whole-genome array. Similar studies have used less stringent P-values for this array, due to both the over-conservative nature of the Bonferroni correction for a dense gene-centric array, and to sample size limitations (27,28).
Power calculation was performed using QUANTO V1.2.4 (29). Based on the sample size of the discovery set (262 cases), a type I error rate of 0.005, and a MAF of 0.2, there is 88% power to identify a SNP with a OR of 2.0 or 0.5, and a power of 49% to identify a SNP with a RR of 1.5 or 0.67. If the MAF is 0.4, the power for OR =2.0 or 0.5 is 99%, and the power for OR =1.5 or 0.67 is 67%.
Results
Study Patients
During the study period from September 1999 through March 2009, 1,472 patients with septic shock were enrolled in the study. 150 patients were excluded because they were on chronic dialysis and 58 patients were excluded because they did not have serial creatinine data, leaving 1264 patients for the clinical analysis. Cases were 627 (49.6%) patients who developed AKI and controls were 637 (50.4%) patients who did not develop AKI (Figure 1). Baseline characteristics of these patients are shown in Table 1. For patients without body mass index (BMI) data available (n=124), the median was imputed.
Table 1.
Characteristics of the study population.
| Characteristics | AKI (n = 627) | No AKI (n = 637) | P-Value |
|---|---|---|---|
| Age | 64 (52-75) | 65 (53-76) | 0.45 |
| Male | 386 (61.6%) | 368 (57.8%) | 0.17 |
| Caucasian | 585 (93.3%) | 584 (91.7%) | 0.27 |
| APACHE III score | 81 (65-98) | 66 (54-78) | <0.0001 |
| Body mass index (kg/m2) | 0.08 | ||
| Underweight (<18.6) | 27 (4.3%) | 36 (5.7%) | |
| Normal weight (18.6-25) | 182 (29.0%) | 220 (34.5%) | |
| Overweight (25.1-30) | 241 (38.4%) | 228 (35.8%) | |
| Obese (>30) | 177 (28.2%) | 153 (24.0%) | |
| ICUs | <0.0001 | ||
| Medical ICUs | 445 (71.0%) | 366 (57.5%) | |
| Surgical ICUs | 160 (25.5%) | 233 (36.6%) | |
| Others | 22 (3.5%) | 38 (5.9%) | |
| Diabetes | 167 (26.6%) | 173 (27.2%) | 0.83 |
| Liver cirrhosis | 49 (7.8%) | 22 (3.5%) | 0.0008 |
| Pre-ICU hospital stay > 48 hours | 235 (37.5%) | 272 (42.7%) | 0.06 |
| Bacteremia | 171 (27.3%) | 123 (19.3%) | 0.0008 |
| Source of infection | 0.01 | ||
| Lung | 268 (42.8%) | 303 (47.6%) | |
| Abdomen | 92 (14.7%) | 84 (13.2%) | |
| Urinary tract | 41 (6.6%) | 26 (4.1%) | |
| Skin/soft tissue | 44 (7.0%) | 39 (6.1%) | |
| Others | 34 (5.4%) | 56 (8.8%) | |
| Unknown | 36 (5.8%) | 19 (3.0%) | |
| Multiple sources | 111 (17.7%) | 109 (17.1%) | |
| Laboratory values on ICU admission | |||
| White blood cell (103/mm3) | 17.8 (12.1-25.6) | 16.7 (12.2-23.2) | 0.04 |
| Hematocrit (%) | 29.0 (26.1-32.6) | 29.4 (26.7-33.0) | 0.11 |
| Platelet (103/mm3) | 180 (104-261) | 207 (141-304) | <0.0001 |
| Serum glucose (mg/dL) | 186 (148-254) | 171 (139-229) | <0.0001 |
| Serum bilirubin (mg/dL) | 0.9 (0.5-2.1) | 0.7 (0.4-1.4) | <0.0001 |
| Serum creatinine (mg/dL) | 1.8 (1.3-2.9) | 1.0 (0.8-1.4) | <0.0001 |
| Serum albumin (g/dL) | 2.3 (1.8-2.8) | 2.3 (1.9-2.8) | 0.93 |
| Receiving mechanical ventilation within 48 hours of ICU admission | 486 (77.5%) | 484 (76.0%) | 0.52 |
| Presence of ARDS at ICU admission | 91 (14.5%) | 102 (16.0%) | 0.46 |
| Presence of ARDS within 72 hours of ICU admission | 180 (28.7%) | 162 (25.4%) | 0.19 |
| PaO2:FiO2 on day zero of shock | 136 (84-225) | 164 (103-241) | 0.001 |
| Receiving vasopressors for > 48 hours | 393 (62.7%) | 316 (49.6%) | <0.0001 |
| Treatment with activated protein C | 21 (3.4%) | 15 (2.4%) | 0.29 |
Definition of abbreviations: AKI=acute kidney injury; APACHE=Acute Physiology and Chronic Health Evaluation; ICU=intensive care unit; ARDS=acute respiratory distress syndrome.
Data are presented as medians (25th-75th percentiles) or n (%).
Clinical Predictors of AKI
On multivariate analysis, summarized in Table 2, the predictors associated with increased risk of developing AKI included increasing APACHE III score (Odds ratio [OR] 1.03 per one-point increase, P<0.0001), need for vasopressors for longer than 48 hours (OR 1.30, P=0.04), and source of infection (OR for abdominal source 1.53 and OR for unknown source 2.34, both P<0.05; pulmonary source was the reference). BMI either in the overweight (OR 1.35, P=0.047) or obese (OR 1.68, P=0.002) categories was associated with increased risk of AKI. Location in a surgical ICU (OR 0.59, P=0.0004), as compared to a medical ICU, was associated with a decreased risk of AKI. Increasing platelet count (OR 0.998, P<0.0001), and being in the hospital for more than 48 hours before ICU admission (OR 0.74, P=0.021) were associated with a decreased risk of developing AKI. Cirrhosis, although it was significantly different on univariate analysis, was not a significant predictor of AKI in the multivariate analysis.
Table 2.
Multivariate analysis for the development of acute kidney injury.
| OR (95% CI) | P-Value | |
|---|---|---|
| APACHE III score | 1.03 (1.03-1.04) | <0.0001 |
| Body mass index (kg/m2) | ||
| Underweight | 0.85 (0.48-1.53) | 0.596 |
| Normal weight (reference) | 1.0 | |
| Overweight | 1.35 (1.00-1.81) | 0.047 |
| Obese | 1.68 (1.21-2.33) | 0.002 |
| ICUs | ||
| Medical ICUs (reference) | 1.00 | |
| Surgical ICUs | 0.59 (0.44-0.79) | 0.0004 |
| Others | 0.63 (0.35-1.15) | 0.133 |
| Source of infection | ||
| Lung (reference) | 1.00 | |
| Abdomen | 1.53 (1.03-2.27) | 0.036 |
| Urinary tract | 1.73 (0.98-3.06) | 0.060 |
| Skin/soft tissue | 1.66 (0.99-2.80) | 0.056 |
| Others | 0.82 (0.49-1.36) | 0.444 |
| Unknown | 2.34 (1.25-4.39) | 0.008 |
| Multiple sources | 1.02 (0.72-1.45) | 0.905 |
| Platelet (103/mm3) | 0.998 (0.997-0.999) | <0.0001 |
| Pre-ICU hospital stay >48 hours | 0.74 (0.57-0.96) | 0.021 |
| Receiving vasopressors for >48 hours | 1.30 (1.01-1.68) | 0.041 |
Definition of abbreviations: OR=odds ratio; CI=confidence interval; APACHE=Acute Physiology and Chronic Health Evaluation; ICU=intensive care unit.
Genetic Predictors of AKI
For the genetic analysis, there were 887 Caucasian patients with genotyping data from this cohort of septic shock patients, randomly split into discovery (60%) and validation (40%) sets using R software ([version 2.12] from R project [http://www.r-project.org/]) with a random seed of the date of the analysis (refer to Figure 1 for details). There were no significant differences in the incidence of AKI between those with genotyping data and those without (P=0.55). Those with genotyping data were less likely to have a pre-ICU hospital stay longer than 48 hours than those without (38.6% vs. 47.9%, P=0.009) and had a higher glucose within the first 24 hours of ICU admission (181 mg/dL vs. 167 mg/dL, P=0.0003). The remainder of the baseline characteristics did not differ between those with genotyping data and those without. There were also no significant differences in baseline characteristics between the discovery and validation sets (Table E1 in the Supplemental Digital Content).
SNP association analysis in the discovery set, adjusting for age, gender, and APACHE III score, revealed 142 SNPs associated with the development of AKI (Table E2 in the Supplemental Digital Content). These top 142 SNPs were then verified in the validation set.
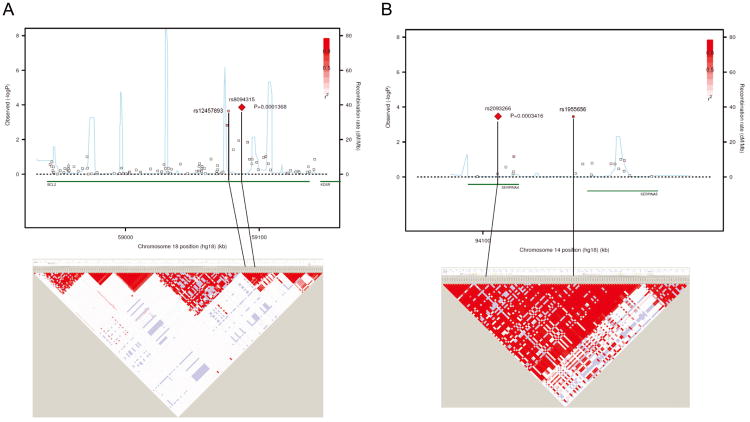
After eliminating SNPs that were not validated or that had odds ratios in opposite directions between the discovery and validation sets, we identified five SNPs associated with AKI (Table 3). Two of these SNPs are in the B-cell CLL/lymphoma 2 (BCL2) gene: rs8094315 (OR 0.62 per additional copy of the minor G allele and P=0.0032 in the discovery set; OR 0.61 and P=0.016 in the validation set) and rs12457893 (OR 0.68 per additional copy of the minor C allele and P=0.0034 in the discovery set; OR 0.71 and P=0.026 in validation set). rs625145 is in the salt-inducible kinase 3(SIK3) gene and is associated with an increased risk of AKI (OR 1.64 per additional copy of the minor T allele and P=0.0028 in the discovery set; OR 1.52 and P=0.029 in the validation set); rs2093266 is in the serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 4 (SERPINA4) gene and is associated with a decreased risk of AKI (OR 0.53 per additional copy of the minor A allele and P=0.0042 in the discovery set; OR 0.55 and P=0.031 in the validation set). The final significant SNP rs1955656 is in the 5′ untranslated region (UTR) of serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 5 (SERPINA5) gene but is in complete linkage disequilibrium with rs2093266. The odds ratios, 95% confidence intervals, and P-values for the combined data are shown in Table 4. These SNPs remained statistically significant after adjusting for APACHE III score, BMI, location, source of infection, platelet count, need for vasopressors for greater than 48 hours, and nosocomial source of infection. Association results and linkage disequilibrium (LD) plots for the BCL2 and SERPINA4-SERPINA5 regions are shown in Figure 2. Haplotype analysis of rs8094315 and rs12457893 on BCL2 revealed that patients carrying the minor alleles of both SNPs (haplotype GC) had a decreased risk of developing AKI (OR 0.61, P=0.000137), compared to other haplotypes. There was no relationship between any of the significant SNPs and 28- or 60-day mortality.
Table 3.
Single nucleotide polymorphisms associated with acute kidney injury.a
| Discovery Set a | Validation Set a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Chr | Position | Gene | Alleleb | Location | MAFc | ORd (95% CI) | P-value | ORd (95% CI) | P-value |
| rs625145 | 11 | 116233146 | SIK3 | A/T | Intron | 0.26/0.19 | 1.64 (1.19-2.28) | 0.0028 | 1.52 (1.04-2.22) | 0.029 |
| rs2093266 | 14 | 94102540 | SERPINA4 | G/A | Intron | 0.07/0.13 | 0.53 (0.34-0.82) | 0.0042 | 0.55 (0.32-0.95) | 0.031 |
| rs1955656 | 14 | 94115128 | SERPINA5 | G/A | 5′ UTR | 0.07/0.13 | 0.52 (0.33-0.81) | 0.0037 | 0.57 (0.33-0.97) | 0.037 |
| rs8094315 | 18 | 59087027 | BCL2 | A/G | Intron | 0.17/0.25 | 0.62 (0.45-0.85) | 0.0032 | 0.61 (0.41-0.91) | 0.016 |
| rs12457893 | 18 | 59077141 | BCL2 | A/C | Intron | 0.42/0.51 | 0.68 (0.52-0.88) | 0.0034 | 0.71 (0.53-0.96) | 0.026 |
Definition of abbreviations: SNP=single nucleotide polymorphism; Chr=chromosome; MAF=minor allele frequency; OR=odds ratio; CI=confidence interval; UTR=untranslated region; BCL2=B-cell CLL/lymphoma 2 gene; SIK3=salt-inducible kinase family 3 gene; SERPINA4= serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 4 gene; SERPINA5= serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 5 gene; APACHE=Acute Physiology and Chronic Health Evaluation.
Adjusted for age, gender, and APACHE III score.
Major allele / minor allele.
Cases/controls.
Odds ratios are for each additional copy of the minor allele.
Table 4.
Adjusted odds ratios and 95% confidence intervals for single nucleotide polymorphisms associated with acute kidney injury.a
| SNP | Gene | OR (Model 1) b | P-value (Model 1) b | OR (Model 2) c | P-value (Model 2) c |
|---|---|---|---|---|---|
| rs625145 | SIK3 | 1.59 (1.24-2.03) | 0.0002 | 1.56 (1.21-2.02) | 0.0006 |
| rs2093266 | SERPINA4 | 0.54 (0.38-0.76) | 0.0003 | 0.54 (0.38-0.76) | 0.0005 |
| rs1955656 | SERPINA5 | 0.54 (0.38-0.76) | 0.0004 | 0.54 (0.38-0.76) | 0.0005 |
| rs8094315 | BCL2 | 0.61 (0.48-0.79) | 0.0001 | 0.61 (0.47-0.79) | 0.0002 |
| rs12457893 | BCL2 | 0.69 (0.57-0.84) | 0.0002 | 0.67 (0.55-0.83) | 0.0002 |
Definition of abbreviations: SNP=single nucleotide polymorphism; OR=odds ratio; CI=confidence interval; BCL2=B-cell CLL/lymphoma 2 gene; SIK3=salt-inducible kinase family 3 gene; SERPINA4= serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 4 gene; SERPINA5= serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 5 gene; APACHE=Acute Physiology and Chronic Health Evaluation.
Odds ratios are based on the entire cohort as a whole and represent the risk per each additional copy of the minor allele.
Model 1 adjusts for age, gender, and APACHE III score.
Model 2 adjusts for APACHE III score, platelet count, body mass index, location, source of infection, nosocomial source of infection, and need for vasopressors for greater than 48 hours.
Figure 2.
Association results and linkage disequilibrium (LD) plots of BCL2 (A) and SERPINA4-SERPINA5 regions (B). The upper panels show P-values for association testing (combined data) by logistic regression analysis, adjusted for age, gender, and Acute Physiology and Chronic Health Evaluation (APACHE) III score. The lower panels present LD plot (using D′) based on single nucleotide polymorphisms with minor allele frequency > 0.05 using HapMap phase 2 individuals of European background. The regional association plots were generated using the web-based tool SNAP version 2.2; the LD plots were generated by Haploview 4.2 software. BCL2: B-cell CLL/lymphoma 2. SERPINA4: serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 4. SERPINA5: serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), membre 5.
Discussion
In this study, we performed large-scale gene-centric genotyping to identify genetic factors associated with the development of AKI in septic shock. By dividing our large cohort into discovery and replication sets, we were able to identify and verify five SNPs associated with AKI development. Three of these five SNPs are in the apoptosis pathway genes: BCL2 and SERPINA4, and a fourth SNP is in complete linkage disequilibrium with the SNP in SERPINA4. These findings remained significant after adjusting for all of the clinical factors associated with AKI identified in the entire cohort.
Bcl-2 plays an integral role in the apoptosis pathway as an anti-apoptosis protein; both apoptosis and Bcl-2 have been shown to be important in AKI, with in vivo and in vitro studies lending strong biological plausibility to our findings (30). Renal biopsies of patients who died from septic shock with AKI demonstrate evidence of apoptosis more frequently than in biopsies of controls (31). In a randomized controlled trial of sixteen patients with gram-negative sepsis, subjects received either extracorporeal therapy with polymyxin-B (PMX-B, meant to reduce plasma levels of lipopolysaccharide [LPS] and thereby decrease activation of proapoptotic pathways) or standard care (32). Subjects who received PMX-B had improved renal function and less need for renal replacement therapy than the control group. When plasma from the septic patients was incubated with cultured human proximal tubular epithelial cells, it caused significant apoptosis. Using plasma obtained 72 hours after enrollment, plasma from PMX-B treated patients caused significantly less apoptosis than plasma from the control group. This series of experiments supports the role of apoptosis in human septic AKI.
In an animal model of LPS-induced acute renal failure, mice who received a caspase inhibitor (meant to downregulate apoptosis) not only had less apoptosis but also were prevented from developing AKI and had less evidence of inflammation (33). Similarly, mice that were given guanosine to prevent apoptosis in a model of ischemic renal failure had less apoptosis and less renal injury (34). In a rat model of ischemic acute renal failure, BCL2 gene expression is increased in the distal tubule of the kidney 24 hours after the injury (35). In rat proximal tubular cells subjected to injury, the mitochondrial fragmentation shown to be an important part of apoptosis is suppressed in BCL2-transfected cells (36).
Our other genetic findings have not been reported previously in AKI, but one of our other significant SNPs is in the SERPINA4 gene, which encodes kallistatin. Kallistatin has anti-apoptotic, vasodilatory, anti-inflammatory, and antioxidant properties (37, 38). In a mouse model of LPS-induced shock, transgenic mice overexpressing kallistatin had a significantly higher survival (39). Kallistatin protects against cardiomyocyte apoptosis in a rat model of myocardial ischemia-reperfusion injury (40), and it suppresses tumor necrosis factor (TNF)-α induced apoptosis in cultured endothelial cells (41). It also decreases apoptotic cells in a rat model of osteoarthritis (42). In the kidney, kallistatin treatment improved renal function in Dahlsalt sensitive rats (43). In humans, kallistatin levels in diabetics correlate with renal dysfunction (44), which is likely a compensatory mechanism.
Another significant SNP, rs1955656, is located in the 5′UTR of SERPINA5, which encodes protein C inhibitor. Protein C inhibitor is expressed in renal tubular cells (45) and has been linked to regulation of renal cell carcinoma metastasis (46), but has not been studied in acute kidney injury. Because this SNP was in complete LD with rs2093266, it is very likely that its association with AKI was driven by the SERPINA4 SNP rs2093266.
Our final significant SNP, rs625145 in the SIK3 gene, has been linked to outcomes in pancreatic cancer (47) but has not been studied in renal disease.
The results of our study reveal several important clinical determinants of AKI. Patients who developed AKI were more likely to have a higher BMI, be admitted to a medical ICU, and have a lower platelet count. The relationship between BMI and development of AKI has been demonstrated in prior large cohort studies (11, 48, 49, 50) although only one of these studies evaluated septic patients with AKI (11). We also found that patients with an abdominal source of infection were more likely to develop AKI than patients with a pulmonary source, which confirms prior data (11, 51).
A possible limitation to our study is the definition of AKI. Because there is no gold standard definition of AKI, we chose to use the AKIN definition, which was developed by an expert panel in 2007 (21). The advantages of the AKIN definition are that it is highly sensitive (including changes in serum creatinine of as little as 0.3 mg/dl, which have been shown to correlate with increased odds of mortality [52]), and that it reduces the need for a baseline serum creatinine by using an acute change within 48 hours. This was particularly useful for our study because we did not have data on baseline renal function in this cohort. The AKIN definition differs from the previously recommended RIFLE definition (53) by not using change in glomerular filtration rate (GFR) and by not requiring a baseline creatinine. However, as the authors of the RIFLE definition note in their paper, “the degree to which serum creatinine changes from baseline will reflect the change in GFR” (53). Despite the differences between the RIFLE and AKIN definitions, both have been shown to be comparably predictive of mortality (54, 55, 56). Unfortunately, we did not have urine output data available for this cohort, so neither the urine output portion of the AKIN definition nor creatinine clearance could be utilized. Similarly, we did not have sufficient serial plasma samples on all 1264 patients in the cohort in order to test other promising biomarkers of AKI, such as cystatin C or neutrophil gelatinase-associated lipocalin (NGAL).
We acknowledge several other potential limitations to our study. First, all large-scale genotyping studies may have false positive findings, and although we validated our results in our larger own cohort and adjusted for many potential confounders, these findings ideally should be replicated in an external cohort. However, our method of replicating by splitting our cohort has been shown to be valid (57, 58). Second, we do not have data on the functional significance of our top SNPs. Intermediate phenotypes, such as mRNA expression or plasma levels of Bcl-2 and kallistatin, were not measured and are beyond the scope of the current study. Third, we did not collect data on incidence of chronic kidney disease in this population (beyond excluding those on chronic dialysis) and therefore could not adjust for this in our model. Fourth, there is a significant relationship between AKI and other acute organ failures in the ICU. While we did not take these individually into account in the multivariable model, markers of other organ failures are part of the APACHE III score and therefore were ultimately adjusted for in the analysis. Unfortunately, therapies for septic shock, such as early goal-directed therapy and timely antibiotic administration, could not be adjusted for as this information was not available in our database. Fifth, we did not take into account interactions between genotypes, although these interactions surely exist. However, this would have added a significant number of tests to our analysis, and we would have been further limited by sample size and power issues. Finally, because genotyping was limited to the Caucasian population to reduce confounding by ethnicity, the genetic findings may not be applicable to other ethnicities.
Conclusions
We performed the first study using large-scale genotyping to evaluate septic shock and AKI. We demonstrated that two SNPs in the BCL2 gene and a SNP in the SERPINA4 gene are associated with a decreased risk of developing AKI. This suggests a significant role of the apoptosis pathway in septic shock complicated by AKI. Additional studies are needed to validate the associations of BCL2 and SERPINA4 polymorphisms with AKI.
Supplementary Material
Table E1. Comparison of baseline characteristics between discovery and validation sets.
Table E2. Top 142 single nucleotide polymorphisms (SNPs) in the discovery set (Model 1).
Acknowledgments
The authors would like to thank Weiling Zhang, Kelly McCoy, Thomas McCabe, Julia Shin, Hanae Fujii-Rios, Ian Taggert, Kezia Ellison, and Yael Tarshish for patient recruitment; Andrea Shafer and Starr Sumpter for research support; Janna Frelich and Julie Delprato for data management; and the patients and staff of the ICUs at Massachusetts General Hospital and Beth Israel Deaconess Medical Center.
Supported by NIH grants R01HL60710, ES00002, and T32HL07874.
Footnotes
The authors have not disclosed any potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ostermann M, Chang RWS. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35:1837–1843. doi: 10.1097/01.CCM.0000277041.13090.0A. [DOI] [PubMed] [Google Scholar]
- 2.Bagshaw SM, George C, Dinu I, et al. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1203–1210. doi: 10.1093/ndt/gfm744. [DOI] [PubMed] [Google Scholar]
- 3.Hoste EAJ, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care. 2006;10:R73–R82. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrantes F, Tian J, Vazquez R, et al. Acute kidney injury criteria predict outcomes of critically ill patients. Crit Care Med. 2008;36:1397–1403. doi: 10.1097/CCM.0b013e318168fbe0. [DOI] [PubMed] [Google Scholar]
- 5.Thakar CV, Christianson A, Freyberg R, et al. Incidence and outcomes of acute kidney injury in intensive care units: A Veterans Administration study. Crit Care Med. 2009;37:2552–2558. doi: 10.1097/CCM.0b013e3181a5906f. [DOI] [PubMed] [Google Scholar]
- 6.Bagshaw SM, George C, Bellomo R, et al. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care. 2007;11:R68–76. doi: 10.1186/cc5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 8.Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2:431–439. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- 9.Bagshaw SM, George C, Bellomo R, et al. Early acute kidney injury and sepsis: A multicentre evaluation. Crit Care. 2008;12:R47–R55. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neveu H, Kleinknecht D, Brivet F, et al. Prognostic factors in acute renal failure due to sepsis: Results of a prospective multicentre study. Nephrol Dial Transplant. 1996;11:293–299. doi: 10.1093/oxfordjournals.ndt.a027256. [DOI] [PubMed] [Google Scholar]
- 11.Bagshaw SM, Lapinsky S, Dial S, et al. Acute kidney injury in septic shock: Clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009;35:871–881. doi: 10.1007/s00134-008-1367-2. [DOI] [PubMed] [Google Scholar]
- 12.Oppert M, Engel C, Brunkhorst FM, et al. Acute renal failure in patients with severe sepsis and septic shock – a significant independent risk factor for mortality: Results from the German Prevalence Study. Nephrol Dial Transplant. 2008;23:904–909. doi: 10.1093/ndt/gfm610. [DOI] [PubMed] [Google Scholar]
- 13.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 14.Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 15.Yegenaga I, Hoste E, Van Biesen W, et al. Clinical characteristics of patients developing ARF due to sepsis/systemic inflammatory response syndrome: Results of a prospective study. Am J Kidney Dis. 2004;43:817–824. doi: 10.1053/j.ajkd.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 16.duCheyron D, Fradin S, Ramakers M, et al. Angiotensin converting enzyme insertion/deletion genetic polymorphism: Its impact on renal function in critically ill patients. Crit Care Med. 2008;36:3178–3183. doi: 10.1097/CCM.0b013e318186a299. [DOI] [PubMed] [Google Scholar]
- 17.Kolyada AY, Tighiouart H, Perianayagam MC, et al. A genetic variant of hypoxia-inducible factor-1α is associated with adverse outcomes in acute kidney injury. Kidney International. 2009;75:1322–1329. doi: 10.1038/ki.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perianayagam MC, Liangos O, Kolyada AY, et al. NADPH oxidase p22phox and catalase gene variants are associated with biomarkers of oxidative stress and adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18:255–263. doi: 10.1681/ASN.2006070806. [DOI] [PubMed] [Google Scholar]
- 19.Jaber BL, Rao M, Guo D, et al. Cytokine gene promoter polymorphisms and mortality in acute renal failure. Cytokine. 2004;25:212–219. doi: 10.1016/j.cyto.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Besschetnova TY, Brooks CR, et al. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31–R38. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank AJ, Sheu CC, Chen F, et al. Clinical and genetic determinants of acute kidney injury in patients with septic shock [abstract] Am J Respir Crit Care Med. 2010;181:A6818. [Google Scholar]
- 23.Gong MN, Thompson BT, Williams P, et al. Clinical predictors of and mortality in acute respiratory distress syndrome: Potential role of red cell transfusion. Crit Care Med. 2005;33:1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 24.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 25.Keating BJ, Tischfield S, Murray SS, et al. Concept, design, and implementation of a cardiovascular gene-centric 50k SNP array for large-scale genomic association studies. PLoS One. 2008;3:e3583. doi: 10.1371/journal.pone.0003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purcell S, Neale B, Todd-Brown K, et al. PLINK: A toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talmud PJ, Drenos F, Shah S, et al. Gene-centric association signals for lipids and apolipoproteins identified via the HumanCVD BeadChip. Am J Hum Genet. 2009;85:628–642. doi: 10.1016/j.ajhg.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zúniga J, Buendía I, Zhao Y, et al. Genetic variants associated with severe pneumonia in A/H1N1 influenza infection. Eur Respir J. 2011 doi: 10.1183/09031936.00020611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gauderman WJ, Morrison JM. QUANTO 1.1: A computer program for power and sample size calculations for genetic-epidemiology studies. 2006 http://hydra.usc.edu/gxe.
- 30.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011 May 11; doi: 10.1038/ki.2011.120. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lerolle N, Nochy D, Guérot E, et al. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic inflammation. Intensive Care Med. 2010;36:471–478. doi: 10.1007/s00134-009-1723-x. [DOI] [PubMed] [Google Scholar]
- 32.Cantaluppi V, Assenzio B, Pasero D, et al. Polymyxin-B hemoperfusion inactivates circulating proapoptotic factors. Intensive Care Med. 2008;34:1638–1645. doi: 10.1007/s00134-008-1124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo R, Wang Y, Minto A, et al. Acute renal failure in endotoxemia is dependent on caspase activation. J Am Soc Nephrol. 2004;15:3093–3102. doi: 10.1097/01.ASN.0000145530.73247.F5. [DOI] [PubMed] [Google Scholar]
- 34.Kelly KJ, Plotkin Z, Dagher PC. Guanosine supplementation reduces apoptosis and protects renal function in the setting of ischemic injury. J Clin Invest. 2001;108:1291–1298. doi: 10.1172/JCI13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gobé G, Zhang XJ, Willgoss DA, et al. Relationship between expression of Bcl-2 genes and growth factors in ischemic acute renal failure in the rat. J Am Soc Nephrol. 2000;11:454–467. doi: 10.1681/ASN.V113454. [DOI] [PubMed] [Google Scholar]
- 36.Brooks C, Wei Q, Cho SG, et al. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chao J, Miao RQ, Chen V, et al. Novel roles of kallistatin, a specific tissue kallikrein inhibitor, in vascular remodeling. Biol Chem. 2001;382:15–21. doi: 10.1515/BC.2001.003. [DOI] [PubMed] [Google Scholar]
- 38.Yin H, Gao L, Shen B, et al. Kallistatin inhibits vascular inflammation by antagonizing tumor necrosis factor-α-induced nuclear factor κB activation. Hypertension. 2010;56:260–267. doi: 10.1161/HYPERTENSIONAHA.110.152330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen LM, Chao L, Chao J. Beneficial effects of kallikrein-binding protein in transgenic mice during endotoxic shock. Life Sci. 1997;60:1431–1435. doi: 10.1016/s0024-3205(97)00094-5. [DOI] [PubMed] [Google Scholar]
- 40.Chao J, Yin H, Yao YY, et al. Novel role of kallistatin in protection against myocardial ischemia-reperfusion injury by preventing apoptosis and inflammation. Hum Gene Ther. 2006;17:1201–1213. doi: 10.1089/hum.2006.17.1201. [DOI] [PubMed] [Google Scholar]
- 41.Shen B, Gao L, Hsu YT, et al. Kallistatin attenuates endothelial apoptosis through inhibition of oxidative stress and activation of Akt-eNOS signaling. Am J Physiol Heart Circ Physiol. 2010;299:H1419–H1427. doi: 10.1152/ajpheart.00591.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsieh JL, Shen PC, Shiau AL, et al. Adenovirus-mediated kallistatin gene transfer ameliorates disease progression in a rat model of osteoarthritis induced by anterior cruciate ligament transection. Hum Gene Ther. 2009;20:147–158. doi: 10.1089/hum.2008.096. [DOI] [PubMed] [Google Scholar]
- 43.Shen B, Hagiwara M, Yao YY, et al. Salutory effect of kallistatin in salt-induced renal injury, inflammation, and fibrosis via antioxidative stress. Hypertension. 2008;51:1358–1365. doi: 10.1161/HYPERTENSIONAHA.107.108514. [DOI] [PubMed] [Google Scholar]
- 44.Jenkins AJ, McBride JD, Januszewski AS, et al. Increased serum kallistatin levels in type 1 diabetes patients with vascular complications. J Angiogenes Res. 2010;2:19–26. doi: 10.1186/2040-2384-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radtke KP, Fernández JA, Greengard JS, et al. Protein C inhibitor is expressed in tubular cells of human kidney. J Clin Invest. 1994;94:2117–2124. doi: 10.1172/JCI117566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wakita T, Hayashi T, Nishioka J, et al. Regulation of carcinoma cell invasion by protein C inhibitor whose expression is decreased in renal cell carcinoma. Int J Cancer. 2004;108:516–523. doi: 10.1002/ijc.11594. [DOI] [PubMed] [Google Scholar]
- 47.Couch FJ, Wang X, Bamlet WR, et al. Association of mitotic regulation pathway polymorphisms with pancreatic cancer risk and outcome. Cancer Epidemiol Biomarkers Prev. 2010;19:251–257. doi: 10.1158/1055-9965.EPI-09-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Druml W, Metnitz B, Schaden E, et al. Impact of body mass on incidence and prognosis of acute kidney injury requiring renal replacement therapy. Intensive Care Med. 2010;36:1221–1228. doi: 10.1007/s00134-010-1844-2. [DOI] [PubMed] [Google Scholar]
- 49.Paolini JBM, Mancini J, Genestal M, et al. Predictive value of abdominal obesity vs. body mass index for determining risk of intensive care unit mortality. Crit Care Med. 2010;38:1308–1314. doi: 10.1097/CCM.0b013e3181d8cd8b. [DOI] [PubMed] [Google Scholar]
- 50.Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology. 2007;107:892–902. doi: 10.1097/01.anes.0000290588.29668.38. [DOI] [PubMed] [Google Scholar]
- 51.Volakli E, Spies C, Michalopoulos A, et al. Infections of respiratory or abdominal origin in ICU patients: what are the differences? Crit Care. 2010;14:R32–R41. doi: 10.1186/cc8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 53.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure – definition, outcome measures, animal models, fluid therapy, and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joannidis M, Metnitz B, Bauer P, et al. Acute kidney injury in critically ill patients classified by AKIN vs RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35:1692–1702. doi: 10.1007/s00134-009-1530-4. [DOI] [PubMed] [Google Scholar]
- 55.Lopes JA, Fernandes P, Jorge S, et al. Acute kidney injury in intensive care unit patients: a comparison between the RIFLE and the Acute Kidney Injury Network classifications. Crit Care. 2008;12:R110–R117. doi: 10.1186/cc6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bagshaw SM, George C, Bellomo R, et al. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1569–1574. doi: 10.1093/ndt/gfn009. [DOI] [PubMed] [Google Scholar]
- 57.Satagopan JM, Elston RC. Optimal two-stage genotyping in population-based association studies. Genet Epidemiol. 2003;25:149–157. doi: 10.1002/gepi.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H, Thomas DC, Pe'er I, et al. Optimal two-stage genotyping designs for genome-wide association scans. Genet Epidemiol. 2006;30:356–368. doi: 10.1002/gepi.20150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table E1. Comparison of baseline characteristics between discovery and validation sets.
Table E2. Top 142 single nucleotide polymorphisms (SNPs) in the discovery set (Model 1).