Abstract
Previous evidence suggests that distinct fronto-parietal regions may be involved in representing action kinematics (means) and action results (outcome) during action observation. However, the evidence is contradictory with respect to the precise regions that are critical for each type of representation. Additionally unknown is the degree to which ability to detect action means and outcome during observation is related to action production performance. We used a behavioral task to evaluate the ability of healthy and left-hemisphere stroke participants to detect differences between pairs of videos that dissociated object-related action means (e.g., wiping with circular or straight movement) and/or outcome (e.g., applying or removing detergent). We expected that deficits in detecting action means would be associated with spatiomotor gesture production deficits, whereas deficits in detecting action outcome would predict impairments in complex naturalistic action. We also hypothesized a posterior to anterior gradient in the regions critical for each type of representation, disproportionately affecting means and outcome encoding, respectively. Results indicated that outcome – but not means – detection predicted naturalistic action performance in stroke participants. Regression and voxel lesion-symptom mapping analyses of lesion data revealed that means – but not outcome – coding relies on the integrity of the left inferior parietal lobe, whereas no selective critical brain region could be identified for outcome detection. Thus, means and outcome representations are dissociable at both the behavioral and neuroanatomical levels. Furthermore, the data are consistent with a degree of parallelism between action perception and production tasks. Finally, they reinforce the evidence for a critical role of the left inferior parietal lobule in the representation of action means, whereas action outcome may rely on a more distributed neural circuit.
Keywords: Action representation, Means, Outcome, Stroke, Object-related action, Voxel-based lesion-symptom mapping
1. Introduction
Understanding how individuals achieve complex, multi-step actions in everyday life settings is one of the main challenges of action and cognition research. Towards this aim, a number of influential models have proposed that complex actions have their basis in a hierarchical goal structure (Bekkering, Wohlschläger, & Gattis, 2000; Bernstein, 1996; Cooper & Shallice, 2000, 2006; Farag et al., 2010; Norman & Shallice, 1986; Schneider & Logan, 2007; Shallice & Burgess, 1996; Stanton, 2006). In such frameworks, planning complex actions requires the maintenance of temporally distant goals in accordance with a desired outcome (e.g. making a cup of coffee) while planning and executing a series of action subgoals (e.g. grasping the cup, pouring coffee, adding cream, etc.). In many such models, planning and control is achieved via actions at distinct levels of representation, including a kinematic level (e.g., closing the fingers in a specified configuration), an object-goal level (e.g., grasp a cup) and an outcome level (e.g., drink the coffee) (Hamilton & Grafton, 2008).
A number of lines of evidence indicate that kinematic representations of action ‘means’ may be behaviorally distinguished from action outcomes. For example, human participants (unlike chimpanzees) are able to efficiently represent both kinematics and outcomes when the two appear to be inconsistent (Kaneko & Tomonaga, 2012). Moreover, motor priming from action observation has been reported as a result of either action means or outcome congruency between observed and executed actions, depending on the characteristics of the task (Ocampo, Painter, & Kritikos, 2012). Execution of the action of drinking from a cup can be facilitated by perceiving an action such as drinking from a cup using a power grip (same outcome) or making a toast with a cup using a precision grip (same means) compared to an action such as making a toast with a cup using a power grip (different means and outcome). In imitation tasks, imitation of movement detail is most accurate in no-goal conditions, and disrupted by the presence of goals (Wild, Poliakoff, Jerrison, & Gowen, 2010). These data are consistent with the separability of means and goal representations in a number of tasks requiring movement execution.
Additional support for the distinction between means and outcome action representations comes from neuroanatomical dissociations during production tasks. Planning action means appears to recruit posterior brain areas, especially in the left inferior parietal lobule (IPL), whereas maintaining intended outcomes relies on the prefrontal cortex (Dehaene & Changeux, 1997; Miller & Cohen, 2001). In the neuropsychological literature, left inferior parietal lesions are associated with ideomotor apraxia, a deficit in representing the spatial and temporal aspects of skilled object manipulation (Buxbaum, 2001; Buxbaum, Kyle, Grossman, & Coslett, 2007; Jax, Buxbaum, & Moll, 2006), whereas prefrontal lesions (of either hemisphere) have been associated with so-called “frontal apraxia” or “action disorganization syndrome”, a deficit in the ability to sequence high-level action goals in temporally extended tasks, including naturalistic action (Cooper, Schwartz, Yule, & Shallice, 2005; Sirigu et al., 1996; Zalla, Plassiart, Pillon, Grafman, & Sirigu, 2001; Zanini, Rumiati, & Shallice, 2002; Zanini, 2008).
Neuroimaging data also indicate that different regions of the fronto-parietal network involved in hand action planning are recruited for planning means-related versus outcome-related aspects of actions (Johnson-Frey, Newman-Norlund, & Grafton, 2005). For example, in an EEG study, Van Schie and Bekkering (2007) asked participants to move a ball by one of two means (e.g. two different grips) to achieve one of two possible outcomes (e.g., posting the ball at two different target locations). Results indicated that the means level of action planning was associated with parietal brain activity whereas the outcome level was associated with left anterior prefrontal activity. Similar conclusions were reached by de Baene, Albers, and Brass (2012) in an fMRI study that dissociated the type of cognitive task participants performed on abstract stimuli and the stimulus-response mapping they used to respond to the task. Taken together, neuropsychological and neuroimaging data collected in production tasks suggest a division of labor within the fronto-parietal network for action planning at different hierarchical levels, with a greater involvement of parietal and frontal regions in means and outcome planning, respectively.
Evidence for similar neuroanatomic dissociations of means and outcome representations in perceptual tasks not requiring motor execution is relatively limited and inconsistent. Performance of frontal patients in sequencing visually-presented actions suggests that these patients are also particularly impaired at representing higher-levels action goals in perceptual tasks (Zalla, Pradat-Diehl, & Sirigu, 2003), although dissociations between action means and outcome perception have not been directly tested in patient studies. At least one functional neuroimaging study indicates that the inferior frontal gyrus (IFG) may be selectively recruited for outcome but not means detection (de Lange, Spronk, Willems, Toni, & Bekkering, 2008), whereas other recent studies have reported that the IFG, along with the IPL are involved in means but not outcome detection (Hesse, Sparing, & Fink, 2009; Ogawa & Inui, 2012).
In an elegant series of fMRI experiments, Hamilton and Grafton (Grafton & Hamilton, 2007; Hamilton & Grafton, 2006, 2008) used a repetition suppression (RS) paradigm to investigate the neural bases of means and outcomes representations during action observation. They found RS effects in different fronto-parietal regions for action means and outcome levels. Hamilton and Grafton (2008) studied RS effects using objects (e.g. box with sliding lid) shown in 4 hand-action videos that differed in means or outcome (e.g. opening or closing the box by pushing the lid with a finger or pulling with both finger and thumb). RS for repeated outcome was observed in the right hemisphere in both the IFG and the IPL (see also Ortigue, Sinigaglia, Rizzolatti, & Grafton, 2010 for similar results in EEG) along with the post-central gyrus extending into the anterior intraparietal sulcus. Conversely, RS for repeated means was reported in left posterior regions including the left middle intraparietal sulcus, left lateral occipital cortex, and left superior temporal sulcus, but the identified voxels did not survive statistical correction for multiple comparisons.
Taken together, the neuropsychological and neuroimaging results are consistent with the conclusion that distinct regions within the fronto-parietal network may be involved in action means and outcome encoding during action observation, but are unclear with respect to the precise regions mediating each level of representation. Moreover, performance in perceptual tasks has not been linked to action production performance. That is, means and outcome representation levels have been assessed in either perceptual tasks (passive viewing or various judgments on action videos) or production tasks, never both. Consistent with the prominent Mirror Neuron System (MNS) hypothesis (Rizzolatti & Craighero, 2004), there is evidence that at least some aspects of action recognition recruit the same neural circuit as action execution (but see Hickok & Hauser, 2010; Kalénine, Buxbaum, & Coslett, 2010; Kilner, 2011; Mahon & Caramazza, 2008). In its strong form, the MNS predicts parallels between means and outcome processing in detection and production tasks, but this prediction has not been directly addressed to date. In addition, dissociations between means and outcome levels of action representations have not been tested in brain-lesioned patients. Hence the cerebral regions that are critical for means and outcome coding (as opposed to merely activated in means and outcome processing tasks) remain largely unknown.
The present study was conducted in left brain-lesioned participants with two main goals. The first was to evaluate the consequence of means and outcome coding impairments during action observation on action production performance. In other words, we aimed at evaluating the relationship between means and outcome detection performance, on the one hand, and the ability to perform isolated gestures (e.g. drinking from cup) and multi-step actions (e.g. making a cup of coffee) on the other. We expected the level of the representational deficit in perception to map onto the level of action execution difficulties. In particular, we hypothesized that deficits in encoding action means during action observation would be associated with spatiomotor gesture production deficits, whereas deficits in action outcome coding would predict impairments in complex naturalistic action. The second goal was to determine the left hemisphere regions that are critical for means and outcome correct detection. Based on the main action production findings from the neuropsychological and neuroimaging literature, we hypothesized a posterior to anterior gradient in action representations. More specifically, we expected deficits at the lower representational level, i.e. means coding, to be associated with posterior lesions, in particular within the IPL. On the contrary, we assumed deficits at the higher representational level, i.e., outcome coding, to be related to anterior lesions, especially in the IFG. These hypotheses were tested in a large sample of left-hemisphere stroke participants in a behavioral experiment designed after Hamilton and Grafton (2008)’s repetition suppression study.
2. Material and methods
2.1. Participants
Forty-four participants completed the study. Twenty-three were left hemisphere stroke participants (15 male; 22 right-handed), and 21 were healthy adults (9 male, 18 right-handed). Stroke and healthy participants were matched for age, t(1,42) = 1.26, p = .21, and education, t(1,42) = 0.76, p = .45. Participants had no history of traumatic brain injury, neurologic disorders, alcohol or drug abuse, or history of psychosis, and all completed language comprehension, visual, and attention screening tests (Comprehension subtest of Western Aphasia Battery (Kertesz, 1982), Bells Cancellation Test (Gauthier, Dehaut, & Joanette, 1989), and visual field cut and extinction testing according to the NIH Stroke Scale). All 23 stroke participants were chronic patients, on average 72 months post-onset (SD 80 months), and had lesions including the cortex identifiable by MRI (n = 15) or CT (n = 8) scan. Scans were collected between 2 and 321 months after stroke (mean=44, SD = 69).
All participants were recruited from the Moss Rehabilitation Research Institute Research Registry (Schwartz, Brecher, Whyte, & Klein, 2005), gave informed consent according to guidelines of the Institutional Review Boards of Albert Einstein Healthcare Network and the University of Pennsylvania, and were paid for their participation.
Of the 31 stroke patients who consented to the study, 2 were excluded due to reported history of traumatic brain injury unrelated to the stroke, 2 for failure to understand experimental instructions, 2 for performance at or below chance accuracy in Baseline or Identical experimental conditions (see below), and 2 because their brain scans did not reveal cortical hemispheric lesions. The final stroke group thus included 23 participants (Table 1).
Table 1.
Demographic data and behavioral scores of the 23 stroke participants. Scores below cut-off values (control group mean minus 2 standard deviations) are marked with an asterisk.
| Patient | Gender | Age | Handedness | Education | Lesion volume (voxels) |
Means detection |
Outcome detection |
Baseline | Identical | WAB comp. (/10) |
Object-Related gesture production (%) |
Meaningless gesture imitation (%) |
NAT (/18) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT01 | M | 73 | R | 12 | 96377 | 0.094* | 0.781 | 0.813* | 0.948 | 8.5* | 90.0 | 80.0* | 18 |
| PT02 | F | 42 | R | 13 | 48305 | 0.844 | 0.844 | 1.000 | 0.677 | 9.2* | 95.0 | 87.5 | 18 |
| PT03 | F | 61 | R | 16 | 33183 | 0.188* | 0.656 | 0.719* | 0.927 | 8.7* | 84.0 | 72.5* | 18 |
| PT04 | M | 73 | R | 20 | 35133 | 0.688 | 0.875 | 0.969 | 0.896 | 8.9* | 92.0 | 90.0 | 18 |
| PT05 | M | 57 | R | 16 | 99980 | 0.586* | 0.710 | 0.793* | 0.802 | 8.5* | 82.5 | 62.5* | 15 |
| PT06 | M | 64 | R | 19 | 131222 | 0.844 | 0.813 | 1.000 | 0.969 | 9.6* | 77.5* | 65.5* | 15 |
| PT07 | M | 66 | R | 18 | 82800 | 0.500* | 0.750 | 0.750* | 0.875 | 9.4* | 80.0 | 92.5 | 15 |
| PT08 | M | 61 | R | 12 | 109969 | 0.594* | 0.719 | 0.938 | 0.917 | 9.9* | 90.0 | 85.0 | 15 |
| PT09 | M | 54 | R | 14 | 55174 | 0.500* | 0.688 | 0.844* | 0.896 | 8.7* | 92.5 | 67.5* | 15 |
| PT10 | F | 61 | R | 19 | 44512 | 0.063* | 0.625* | 0.813* | 0.906 | 10.0 | 87.5 | 65.0* | 18 |
| PT11 | M | 58 | R | 15 | 185937 | 0.781 | 0.656 | 1.000 | 0.802 | 9.0* | 80.0 | 82.5* | 13* |
| PT12 | M | 49 | R | 14 | 5350 | 0.250* | 0.750 | 0.875* | 0.906 | 9.5* | 83.3 | 45.0* | 15 |
| PT13 | M | 65 | L | 16 | 225021 | 0.125* | 0.688 | 0.719* | 0.865 | 4.8* | 71.4* | 80.0* | 10* |
| PT14 | M | 75 | R | 13 | 68247 | 0.313* | 0.656 | 0.844* | 0.927 | 10.0 | 87.5 | 87.5 | 15 |
| PT15 | M | 53 | R | 13 | 156486 | 0.781 | 0.719 | 0.906 | 0.885 | 9.9* | 97.5 | 85.0 | 17 |
| PT16 | F | 60 | R | 16 | 71022 | 0.594* | 0.781 | 0.750* | 0.792* | 9.6* | 72.5* | 62.5* | 18 |
| PT17 | F | 60 | R | 14 | 7425 | 0.656 | 0.813 | 0.969 | 0.906 | 10.0 | 95.0 | 67.5* | 18 |
| PT18 | M | 71 | R | 16 | 51998 | 0.375* | 0.625* | 0.938 | 0.688* | 7.9* | 92.5 | 70.0* | 8* |
| PT19 | F | 33 | R | 19 | 62852 | 0.063* | 0.406* | 0.563* | 0.896 | 6.6* | 72.5* | 67.5* | 12* |
| PT20 | M | 67 | R | 14 | 64793 | 0.469* | 0.844 | 0.938 | 0.865 | 4.6* | 77.5* | 65.0* | 8* |
| PT21 | F | 68 | R | 12 | 13563 | 0.406* | 0.500* | 0.750* | 0.896 | 9.5* | 85.0 | 70.0* | 16 |
| PT22 | F | 48 | R | 13 | 80020 | 0.781 | 0.594* | 0.969 | 0.885 | 9.2* | 90.0 | 70.0* | 16 |
| PT23 | M | 49 | R | 14 | 52416 | 0.594* | 0.719 | 0.969 | 0.979 | 8.5* | 72.2* | 80.0* | 13* |
2.2. Apraxia Testing
2.2.1. Object-related gesture production (Buxbaum, Kyle, & Menon, 2005)
Patients were shown 10 objects on a table-top and asked to pantomime object use (without touching the objects) with the left hand. Gestures were scored from videotape by an experienced, reliable coder (see Buxbaum, Giovannetti, & Libon, 2000; Buxbaum et al., 2007, 2005 for reliability methods) on semantic content, hand posture, arm posture, amplitude, and timing components. Mean combined scores over the latter 4 measures were calculated, excluding objects on which a semantic content error was performed. The 23 stroke participants performed on average at 84.7% correct (SD=8.1%).
2.2.2. Meaningless gesture imitation (Buxbaum et al., 2005)
Patients viewed 10 videos of an experimenter performing a meaningless hand/arm gesture and imitated the gesture with the left hand; they were permitted to begin at any time. Gestures were scored from the videos by a reliable coder on hand posture, arm posture, amplitude, and timing components according to Buxbaum et al. (2005). Mean scores over the 4 measures were calculated. The 23 stroke participants performed on average at 73.9% correct (SD=11.5%).
2.2.3. Naturalistic Action Test (NAT; Buxbaum, Schwartz, & Montgomery, 1998; Schwartz, Segal, Veramonti, Ferraro, & Buxbaum, 2002)
Patients performed the Naturalistic Action Test (NAT) comprising 3 naturalistic action tasks (make coffee and toast, wrap a gift, pack a lunchbox). Performance was scored from the videos by experienced, reliable coders based on accomplishment of tasks and sub-tasks (e. g. making coffee, adding sugar to coffee), and errors (e.g. task omission, tool substitution, perseveration, step mis-sequencing). Maximum score was 18 points. The 23 stroke participants received an average score of 15 points (SD=3).
2.3. Neuroanatomical Analyses
2.3.1. Scanning and lesion segmentation
All patients underwent a structural, high-resolution, 3D magnetic resonance imaging (MRI) (n=15) or CT (n=8) scan. The lesions of patients who received an MRI were segmented manually upon the digital MRI image file using MRIcron software (Rorden, Karnath, & Bonilha, 2007), and then registered (Avants, Schoenemann, & Gee, 2006) to a common 1 × 1 × 1 mm template (Montreal Neurological Institute space “Colin27”; Holmes et al., 1998), and inspected by an experienced neurologist who was naïve to the behavioral data. The lesions of patients who received a CT were drawn by the same neurologist directly onto the Colin27 template using MRIcron software. All lesions were then thresholded and quantized on the criteria that if more than 50% of a voxel was lesioned, it was assigned a value of 1, all other partially damaged or spared voxels were assigned a value of 0. For a more detailed description of this procedure, please see Kalenine et al., 2010.
Percent damage to four regions of interest (Brodmann’s Areas 39 and 40 in the IPL, and 44 and 45 in the IFG) was calculated using the MRIcron image analysis program (www.mricro.com/mricron).
2.3.2. Regression analyses
To assess the degree to which lesions in our regions of interest predicted performance on the Means and Outcome Detection tasks, we performed multiple regressions in which percent damage to BAs 39, 40, 44, and 45 were entered separately as predictors of Means and Outcome Ratio scores. These BAs each sustained at least 10% damage in at least 30% of the participants. To control for overall severity, lesion volume was also entered into the regressions as independent variables. Normality of residuals was verified. Outliers in the regression models were identified using Cook’s distance, studentized residuals and leverage values.
2.3.3. Voxel-based lesion-symptom mapping (VLSM)
VLSM analyses were conducted using the Non-Parametric Mapping (NPM) module of MRIcron (http://www.sph.sc.edu/comd/rorden/mricron/stats.html). Voxels lesioned in fewer than 5 participants were excluded from analysis. At each voxel, a pairwise t-test was conducted to test for differences between behavioral scores of participants with and without lesion in that voxel. False Discovery Rate corrections, which control the expected rate of false positives among all significant voxels (see Benjamini & Hochberg, 1995), were used to control for Type I errors.
2.4. Means and Outcomes Detection Task
2.4.1. Materials
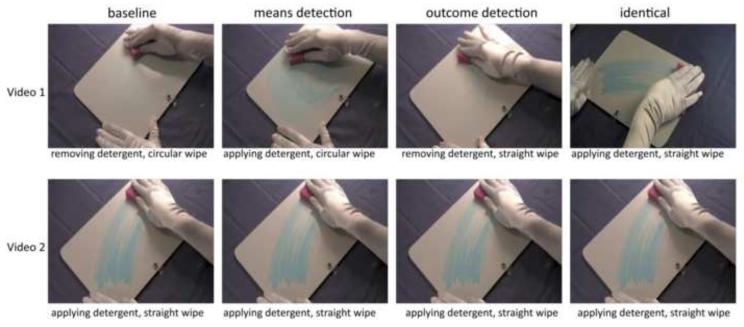
The experimental task required participants to judge whether the actions depicted in pairs of videos were the same or different. Stimuli were 3-second video clips depicting a right hand manipulating an object. The Baseline condition comprised video pairs whose actions on a target object (e.g., sponge) differed in both outcome and means. In the Means Detection condition the videos in each pair differed in means but not outcomes (e.g., straight versus circular movement to remove detergent). In the Outcome Detection condition, the videos in a pair differed only in outcome (e.g., straight movement to apply versus remove detergent). In the Identity condition means and outcomes were the same. To prevent matching of stimuli based on visual features, half of the Identity pairs were displayed from different perspectives. Outcome (e.g., erasing a board) and means (e.g, circular wipe) were simultaneously visible in each stimulus. Examples of action pairs for the four different conditions are presented in Figure 1.
Figure 1.
Example of video pairs used in the Baseline, Means Detection, Outcome Detection, and Identical conditions for a given target object (sponge).
For each of 16 target objects there were 12 pairs of action videos: 2 pairs each in Baseline, Outcome, and Means Detection conditions, and 6 pairs in the Identity condition, for a total of 192 pairs. Forty-eight additional video pairs involving 4 additional target objects not used in the main experiment were used for practice trials.
Trials in the Baseline, Means Detection, and Outcome Detection conditions were critical trials in which the correct response was always “different”. Trials in the Identity condition served as filler trials and required a “same” response. Thus, there were 16 (target objects) × 2 (“different” trials) × 3 conditions = 96 critical trials and 16 (target objects) × 6 (“same” trials) = 96 filler trials. A complete list of the target objects and their related actions is provided in the Appendix.
2.4.2. Procedure
The experiment was programmed using E-prime 2.0 software. Trial types were presented in random order. Trials were triggered by the experimenter when participants appeared ready. Each trial began with a fixation cross in the center of the screen. The two videos on each trial were presented in succession, each displayed for 3 seconds with a 500 msec inter-stimulus interval (black screen). After the second video, a blank screen was displayed for a maximum of 3 seconds or until the participant responded.
Participants were instructed to indicate by means of a button press whether the two videos in a trial were the same or different, and to attend both to what the actor was doing and/or how the actor was doing it. Participants were also informed that the same action might be presented from different perspectives. Participants responded with one of two fingers of the same hand using two keys of a vertically-oriented E-prime SR box (top vs. bottom key). All patients responded with their ipsilesional (left) hand. Eleven healthy participants responded with the right hand and 10 responded with the left hand. The mapping of yes-no responses and top-bottom keys was counterbalanced across subjects. Accuracy and reaction times were recorded.
Prior to experimental trials, participants performed 48 practice trials in which they received feedback from the experimenter as to accuracy.
3. Results
3.1. Behavioral data
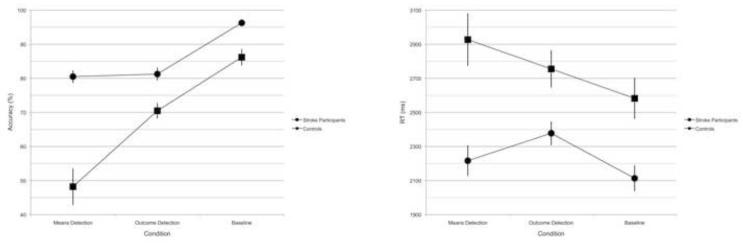
Accuracy and reaction time measures of stroke and control participants were normally distributed in the Mean Detection, Outcome Detection and Baseline conditions. Healthy participants who responded with the left versus right hand did not differ in accuracy (t(1, 19) = .34, p = .73) or reaction times (t(1, 19) = ..82, p = .42); thus these two groups were combined in subsequent analyses. Healthy participants performed more accurately in the Baseline condition (Mn. 96.3%, SD 3.2%) than both the Means Detection (Mn. 80.5%, SD 8.7%) and Outcome Detection (Mn. 81.3%, SD 8.7%) conditions (t(1,20) = 9.11, p < .001 and t(1,20) = 7.91, p < .001, respectively); the latter two conditions did not differ (t(1, 20) = 0.37, p = .71). Healthy participants’ responses were also faster in the Baseline condition (Mn. = 2156 msec, SD 350 msec) than in both the Means (Mn. = 2279 msec, SD 413 msec) and Outcome Detection (Mn. 2436 msec, SD 326 msec) conditions (t1, 20) = 4.13, p < .001 and t(1,20) = 8.97, p < .001, respectively). Furthermore, they were faster in the Means condition than the Outcome condition (t(1,20) = 3.34, p < .005).
Stroke participants, like healthy participants, performed more accurately in the Baseline condition (Mn. 86.2%, SD 11.7%) than the Means (Mn. 48.2%, SD 25.8%) or Outcome Detection conditions (Mn. 70.5%, SD 11.1%) (t(1,22) = 9.54, p < .001 and t(1,22) = 7.12, p < .001, respectively). Unlike healthy participants, stroke participants performed more accurately in the Outcome than Means condition (t(1,22) = 4.62, p < .001). In stroke participants, there was no significant reaction time differences between conditions (all p’s > .14), likely due to the variability of response times of these participants: Means Detection (Mn. 2903 msec, SD 574 msec), Outcome Detection (Mn. 2860 msec, SD 470 msec), and Baseline (Mn. 2656 msec, SD 537 msec). Therefore, behavioral and lesion analyses were conducted on accuracy measures only.
Mean accuracy and correct reaction times in the Baseline, Means Detection and Outcome Detection conditions for each participant group are presented in Figure 2. Pairwise comparisons between groups confirmed that stroke participants significantly differed from controls in all conditions (all p’s < .001).
Figure 2.
Mean percentage accuracy (left) and correct reaction times (right) in the Means Detection, Outcome Detection and Baseline conditions for the Control (circle) and Stroke (square) participant groups. Error bars represent ± 1 standard error (SE) of the group’s mean accuracy (left) and reaction time (right).
Data from the experimental Means-Outcome Detection task were considered together with neuropsychological data in subsequent factor and regression analyses. To use Means and Outcome measures that control for overall level of performance in the Means-Outcome Detection task, we transformed Means and Outcome scores into ratio scores controlling for performance on the Baseline condition: Means Ratio = (Means-Baseline)/(Means+Baseline) and Outcome Ratio = (Outcome-Baseline)/(Outcome+Baseline). Thus, there were two experimental measures, Means Ratio and Outcome Ratio, with more negative ratios indicating greater relative impairment, and 4 neuropsychological measures including performance on gesture to sight, meaningless imitation, NAT, and WAB.
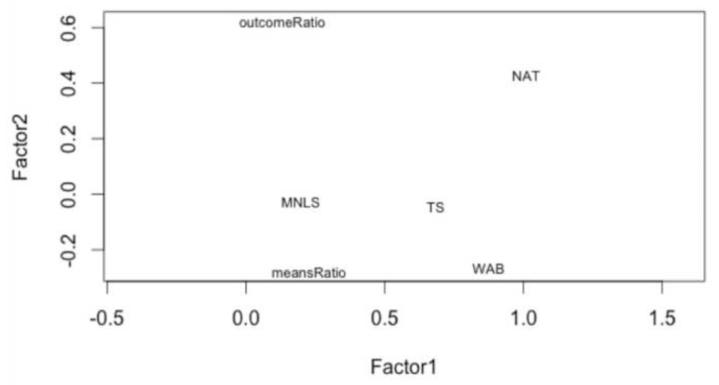
Factor analysis on the 6 measures identified two main factors (Kaiser criterion, eigenvalues > 1), which explained 39% and 12% of variance, respectively. WAB, gesture to sight and NAT loaded most heavily on the first factor, while NAT and Outcome Ratio loaded most heavily on the second factor (Figure 3). While no relationship between Means Ratio and neuropsychological measures was revealed in this preliminary analysis, it suggested that Outcome Ratio and NAT shared some underlying processes.
Figure 3.
Factor loading plot from maximum likelihood factor analysis (oblique rotation) of behavioral data.
Then, to assess the ability of Means and Outcome Ratios to predict scores from the three Apraxia Battery measures (Gesture to sight, meaningless imitation, NAT), we performed regression analyses with the former as independent variables and the latter as dependent variables. As an additional control upon more general impairment severity, we also entered WAB comprehension scores as independent variables in the regressions along with the ratio scores. Normality of residuals was verified. Outliers in the regression models were identified using Cook’s distance, studentized residuals and leverage values.
After controlling for WAB, Outcome Ratio significantly predicted NAT performance (R2 = 0.30 p < .01), but not Gesture Production or Meaningless Gesture Imitation (p’s > .4). Means Ratio scores were not predictive of any of the Apraxia Battery scores either before or after controlling for WAB (all p’s > .2).
3.2. Neuroanatomical Data
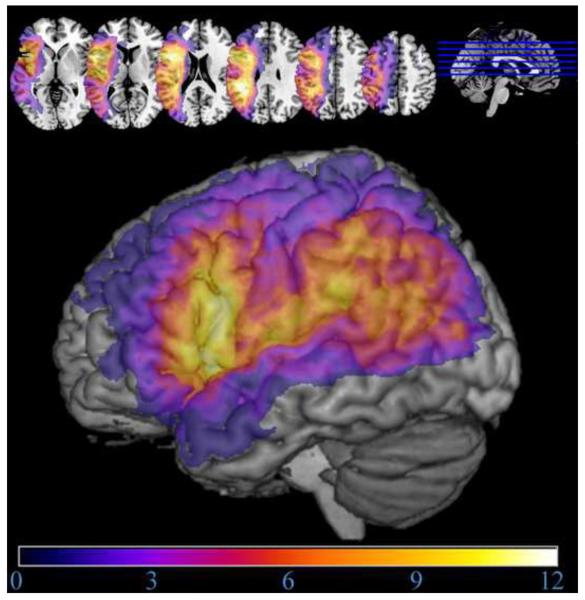
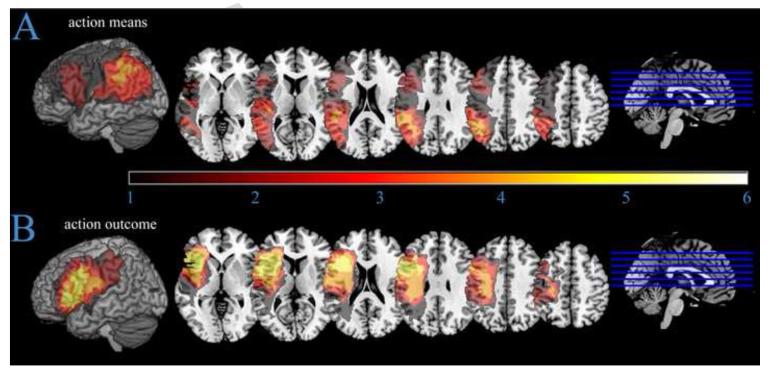
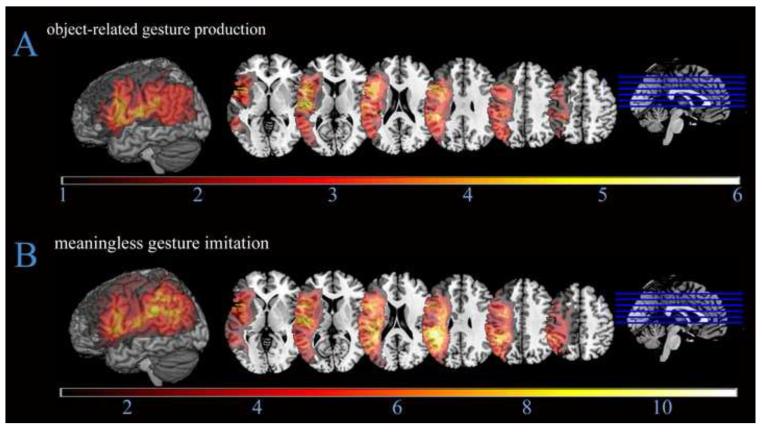
The overlapping lesions of the 23 stroke participants are presented in Figure 4. The figure shows good coverage in our regions of interest in the IFG and IPL. Figure 5 shows differences in lesions of stroke participants relatively impaired in Means Ratios versus Outcome Ratios. Figure 6 shows an overlap of the lesions of stroke participants below cut-off on object-related gesture production and meaningless gesture imitation tests.
Figure 4.
Map depicting lesion overlap of the 23 stroke participants. The regions of maximum overlap, rendered in the lightest colours (light orange and yellow), are lesioned in more than one-third (7) of the stroke participants.
Figure 5.
Lesion overlap maps of the stroke participants with the smallest (n = 5) and largest (n =5) disparity between means and outcome ratio scores. Four of the 5 participants with the greatest means–outcome differences (relatively poor on means detection) showed lesion overlap in the IP (A). Conversely, all 5 participants with the smallest difference in means-outcome ratios (relatively poor on outcome detection) had a lesion involving the IFG (B).
Figure 6.
Lesion overlap maps of the stroke participants below cut-off on object-related gesture production (n = 6) and meaningless gesture imitation (n =17). Maximum overlap is observed in the inferior frontal cortex for object-related gesture production (A), and in the inferior frontal and parietal cortices for meaningless gesture imitation (B).
In the regression analyses in which overall lesion volume and percentage damage to BAs 39, 40, 44 and 45 were used as predictors, one patient (PT13) was an outlier by Cook’s distance value and was excluded. As hypothesized, results showed that percentage damage to BA 39 (R2 = 0.360, p< .005) and BA 40 (R2 = 0.470, p< .001) significantly predicted Means Ratio scores after controlling for overall lesion volume. None of the other BAs were predictors of either Means Ratio or Outcome Ratio after controlling for lesion volume (all R2 values < 0.2, all p’s > .12).
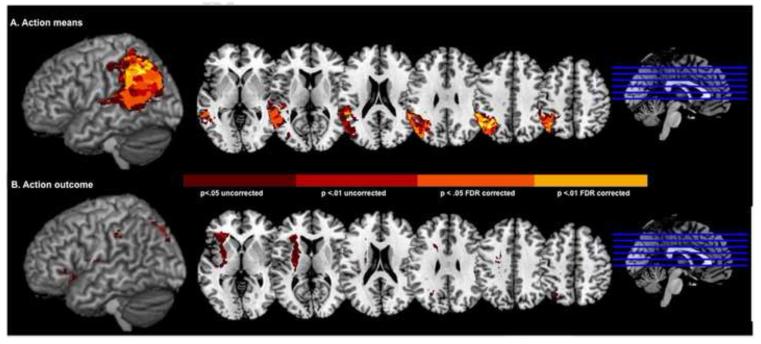
VLSM analyses indicated that lesions to voxels in a large swath of posterior temporal cortex and IPL were significantly associated with poor Means Detection scores (Figure 7A, Z-scores > 2.73, p< .05 FDR corrected). More than 3000 of these significant voxels fell in the IPL (i.e., combined areas 39 and 40). For Outcome Ratio scores, no voxels overcame the statistical threshold corrected for multiple comparisons. Even at lower, uncorrected thresholds, no cluster related to Outcome Ratio scores could be identified (Figure 7B).
Figure 7.
Maps of the reliability (Z-scores) of the difference in means (A) and outcome (B) ratio scores between stroke participants with and without lesions in each voxel (rendered on the Montreal Neurological Institute–space ch2bet volume). Voxels rendered in dark red, light red, orange and yellow correspond to Z-scores > 1.65 (p <0.05 uncorrected), Z-scores >2.33 (p < 2.33 uncorrected), Z-scores > 2.73 (p < 0.05 FDR corrected) and Z-scores > 3.96 (p < 0.01 FDR corrected), respectively.
Note that complementary VLSM analyses of apraxia scores (i.e. scores on object-related gesture production and meaningless gesture imitation tests) did not reveal any voxels significantly associated with gesture production deficits in this patient group.
4. Discussion
The present study demonstrates three major findings. First, action means and outcome representations are independently processed during action observation in both healthy and brain-lesioned participants. Second, means and outcome representations do not equally contribute to naturalistic action performance since outcome detection, but not means detection, was an independent predictor of NAT scores. Third, means detection selectively relies on the integrity of the left inferior parietal cortex, whereas no selective critical brain region could be identified for outcome detection.
4.1. Independent coding of action means and outcome
Behavioral data confirm a separate coding of action means and outcome during action observation in both healthy and stroke participants. Indeed, differences between two videos in only outcome or only means were more difficult to detect than differences in both, indicating that each representational level is involved during action processing independently of the other. These results mirror those of previous behavioral studies assessing means and outcome representation during action execution or observation in healthy subjects and extend them to participants with neurological and cognitive impairments. Despite overall lower performance, stroke participants as a group showed a similar pattern of performance, consistent with independent and complementary representations of action means and outcome.
Interestingly, stroke participants were globally more accurate in detecting action outcomes than means, suggesting that outcome representations may be overall more resistant to brain damage. This is consistent with our failure to find strong evidence of specific neuroanatomic substrates for outcome detection deficits. These data are also potentially consistent with the proposal that action representations are primarily organized in terms of higher-level goals (van Elk, van Schie, & Bekkering, 2008). Primacy of higher-level action goal representations has been reported in young children during action imitation (Bekkering, Wohlschlag & Gattis, 2000) and in primates (but not humans) during simple self-monitored tasks (Kaneko & Tomonaga, 2012). We can speculate that the developmentally (and perhaps, evolutionarily) earlier involvement of higher-level goal representations in action monitoring plays a role in their relatively broad neural distribution, and thus, in their resistance to brain damage.
4.2. Parallel between means and outcome representational deficits in perception and production
Results from behavioral regression analyses revealed that outcome detection scores, but not means detection scores, predicted naturalistic action performance beyond overall cognitive impairment severity. This finding provides further evidence in favor of distinct means and outcome representations and shows that the ability to perform everyday life actions is governed in part by the capacity to access and maintain higher-level goals throughout the activity. This result is consistent with the existence of a common action representation structure in perception and production, with high-level representational deficits during action perception being associated with high-level representational difficulties during action execution.
A number of investigators have observed that patients with apraxia perform better with tools in hand (e.g., in single object use or multiple objects tasks) than in pantomime tasks (e.g., Laimgruber, Goldenberg, and Hermsdörfer, 2005; Randerath, Goldenberg, Spijkers, Li, and Hermsdörfer, 2011). One possibility is that relatively intact outcome processing plays a role in this advantage.
The selective relation between high-level goal representations and everyday action achievement may have interesting implications for rehabilitation research. One study tested the effect of goal cues on naturalistic action performance in dementia and found some facilitative effect of the cues on certain aspects of the action task (Brennan et al., 2009). However, effects were not systematic enough to enable conclusions about the clinical utility of the method. The present finding suggests that facilitating the robustness or accessibility of action outcome representations may improve autonomy in everyday life activities in stroke patients.
We expected means detection ability to selectively predict performance on apraxia tests (i.e. object-related gesture production and/or meaningless gesture imitation), but did not observe any specific relation between means or outcome detection performance and apraxia scores, even when we did not control for general impairment severity. Reasons for this null effect remain unclear. A possible explanation is related to the general praxis level of this participant sample. As a group, stroke participants were only mildly impaired on apraxia tasks, especially on gesture to sight. The overall high gesture to sight scores might be responsible for the difficulty in identifying the expected relationship between gesture to sight and the experimental measures. A second possible explanation for the absence of relation between means detection and apraxia measures may lie in the definition of means representations used. Following Grafton and Hamilton (2008), means variations could include differences in movement trajectory (e.g., removing detergent with straight vs. circular wipe, hand posture (e.g., opening automatic door with palm or poke), grasp location (e.g., writing holding pencil from above or below) or a combination of these different dimensions (see complete list of stimuli in the Appendix). Patients may not equally rely on these different dimensions when performing transitive or meaningless gestures in apraxia tests. It has been shown for instance that when the quality of gesture production is coded on different dimensions such as hand posture, arm posture, amplitude and timing, apraxic patients following left inferior parietal lesions tend to be more strongly impaired on the hand posture dimension (Buxbaum et al., 2007). Thus, we can imagine that the parallel between means representational deficits in perception and production occurs at the level of a particular dimension of action kinematics (e.g. hand posture), but that such an effect is washed out by mixing multiple dimensions during means detection assessment. Although the present design does not allow testing for this hypothesis a posteriori (most trials in the Means Detection condition combined more than one dimension), further studies on action representations should try to assess the different dimensions of means representations separately. Finally, another possibility is that distinct means representations are recruited in perception (experimental task) and production (apraxia tests), in contrast to outcome representations that are recruited for both perception and production. However, this third account is relatively unlikely given the results of the lesion analyses, to be discussed next.
4.3. Critical involvement of left fronto-parietal cortex in means and outcome representations
Regression analyses at the region level and VLSM analyses at the voxel level provided a similar pattern of results: the left IPL was critically and selectively associated with means representations. This finding is consistent with findings from gesture production tasks in patients (e.g., Buxbaum et al., 2007; Jax et al., 2006) and shows a parallel between means perception and production at the neural level, if not in the behavioral data. Moreover, this study demonstrates the critical relationship between the left IPL and the means level of action representations. Previous functional neuroimaging studies either did not obtain reliable IPL activation during means detection and/or test whether IPL recruitment is critical to this level of action representation (Hamilton & Grafton, 2008; Hesse et al., 2009; Ogawa & Inui, 2012). The present findings revealed that left IPL is significantly involved in some aspects of object-related action processing during action observation. Its essential role appears to be restricted to the analysis of gesture kinematics and does not include higher-goal representations such as the representation of action outcome.
Lesions of the stroke participants with relatively poor performance on outcome overlapped in the IFG, suggesting the possibility that this region is particularly important for outcome representation. Nevertheless, statistical analyses of lesion data failed to find a selective relationship between action outcome coding and IFG. Even at more lenient statistical thresholds, outcome coding performance was associated neither with left IPL integrity nor with other cortical regions of the left hemisphere. The fact that no critical region could be localized for outcome coding is surprising considering that previous neuroimaging data on the means versus outcome distinction showed a strong activation of the fronto-parietal cortex during action outcome planning and perception (De Baene et al., 2012; de Lange et al., 2008; Hamilton & Grafton, 2008; Ortigue et al., 2010; van Schie & Bekkering, 2007).
One possibility is that the critical neural substrates of action outcome representations involve the right hemisphere. This hypothesis is consistent with conclusions from previous neuroimaging studies, which attributed a greater role to the right than the left fronto-parietal cortex in higher-level action representations such as action intention or outcome (Hamilton & Grafton, 2008; Iacoboni et al., 2005; Ortigue et al., 2010). If this hypothesis were correct, then we would expect patients with right-hemisphere lesions – especially in the frontal lobe – to be selectively impaired at representing actions at the outcome level. Given that right hemisphere patients may sometimes be difficult to test due to deficits in arousal and spatial attention, future research may consider “virtual lesion” tools to assess the critical right-hemisphere regions involved in outcome versus mean levels of action representations. Recently, Cattaneo, Sandrini, and Schwarzbach (2010) used Transcranial Magnetic Stimulation (TMS) in an adaptation paradigm to highlight the fronto-parietal regions that coded observed actions dependent upon or independent of the effector used. A similar paradigm might be well suited to test critical recruitment of left and right cortical regions in means versus outcome coding.
A second non-mutually exclusive explanation for the absence of selective association between outcome coding and left frontal regions entails a largely distributed network underlying outcome representation, extending beyond MNS-related regions. The privileged relation between naturalistic action performance and the frontal lobes has been questioned (Goldenberg, Hartmann-Schmid, Sürer, Daumüller, & Hermsdörfer, 2007; Schwartz, 2006), in part based on evidence that lesion volume, and not location in the left or right hemisphere, is a significant predictor of performance. Since our behavioral results showed that outcome detection and naturalistic action performance are closely tied, the absence of selective frontal involvement during outcome processing may reflect the same lack of focal organization. Moreover, neuroimaging data from Spunt and colleagues (Spunt, Falk, & Lieberman, 2010; Spunt & Lieberman, 2012) suggest that representing high level action representations recruits areas outside of the fronto-parietal network. Encoding the “why” versus the “how” of an observed action activates a neural network referred to as the mentalizing network, which includes the dorsomedial prefrontal cortex, the posterior cingulate cortex, the left temporo-parietal junction and the anterior temporal lobes. Interestingly, this mentalizing network showed strong functional connectivity with the fronto-parietal MNS regions involved in the “how” aspect of observed actions, indicating important interactions between the two networks. These findings provide interesting directions for further research and suggest testing the criticality of brain regions within and outside the left MNS as well as their functional connections to better understand the neural substrates of action outcome representation.
5. Conclusion
The present study highlights cognitive and neural dissociations between the means and outcome levels of action representations in left-hemisphere stroke participants. Outcome – but not means – processing during observation of object-directed actions predicts naturalistic action performance. Means – but not outcome – coding relies on the integrity of the inferior parietal lobe. These findings confirm the existence of separate levels of action representations, which, at least for the lower level, overlap between perception and execution. In addition, they demonstrate the selective and essential role of the left inferior parietal cortex in the representation of action means.
Highlights.
Means and outcome action representations are dissociable at behavior and brain levels
Outcome detection abilities predict naturalistic action performance
Means detection selectively relies on left inferior parietal cortex integrity
9. Appendix.
| Object | Action means 1 | Action means 2 | Action outcome 1 | Action outcome 2 |
|---|---|---|---|---|
| Automatic door | poke button | palm button | door open | door close |
| Cabinet | pinch handle | clench handle | slide open | slide closed |
| Computer Monitor | button poke | button pinch | screen on | screen off |
| Desk Lamp | pinch chain bottom | pinch chain top | lamp on | lamp off |
| Door knob | pinch key | clench handle | door open | door close |
| Measuring tape reel | pinch tape | pinch handle | unroll tape | retract tape |
| Paint brush | grasp brush bottom | grasp brush top | apply color | remove color |
| Pencil | grasp pencil top | grasp pencil bottom | write | erase |
| Rope knot | pinch rope | clench rope | knot tighten | knot release |
| Slide top box | pinch knob | clench edge | box open | box close |
| Soda | pinch straw | clench glass | drink | blow bubbles |
| Sponge | straight wipe | circular wipe | apply detergent | remove detergent |
| Umbrella | poke button | pinch runner | umbrella open | umbrella close |
| Wheel-operated light | grip wheel bottom | grip wheel top | wheel left + light on | wheel right + light off |
| Wheelchair | palm wheel | clench wheel | roll forward | roll backward |
| Window blinds | hand at bottom of rod | hand at top of rod | blinds open | blinds close |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. References
- Avants BB, Schoenemann PT, Gee JC. Lagrangian frame diffeomorphic image registration: Morphometric comparison of human and chimpanzee cortex. Medical Image Analysis. 2006;10:397–412. doi: 10.1016/j.media.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Bekkering H, Wohlschla A, Gattis M. Imitation of Gestures in Children is Goal-directed. The Quarterly journal of experimental psychology. 2000;53A:153–165. doi: 10.1080/713755872. [DOI] [PubMed] [Google Scholar]
- Bekkering H, Wohlschläger A, Gattis M. Imitation of gestures in children is goal-directed. The Quarterly journal of experimental psychology. A, Human experimental psychology. 2000;53(1):153–164. doi: 10.1080/713755872. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Bernstein NA. On dexterity and its development. In: Latash ML, Turvey MT, editors. Dexterity and its development. Lawrence Erlbaum Associates; Mahwah, NJ: 1996. [Google Scholar]
- Brennan L, Giovannetti T, Libon DJ, Bettcher BM, Duey K. The impact of goal cues on everyday action performance in dementia. Neuropsychological rehabilitation. 2009;19(4):562–582. doi: 10.1080/09602010802405623. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ. Ideomotor apraxia: a call to action. Neurocase. 2001;7(6):445–458. doi: 10.1093/neucas/7.6.445. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Giovannetti T, Libon D. The role of the dynamic body schema in praxis: evidence from primary progressive apraxia. Brain and cognition. 2000;44(2):166–191. doi: 10.1006/brcg.2000.1227. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle K, Grossman M, Coslett HB. Left inferior parietal representations for skilled hand-object interactions: evidence from stroke and corticobasal degeneration. Cortex. 2007;43:411–423. doi: 10.1016/s0010-9452(08)70466-0. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Menon R. On beyond mirror neurons: internal representations subserving imitation and recognition of skilled object-related actions in humans. Brain research. Cognitive brain research. 2005;25(1):226–239. doi: 10.1016/j.cogbrainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Schwartz MF, Montgomery MW. Ideational apraxia and naturalistic action. Cognitive Neuropsychology. 1998;15(6/7/8):617–643. doi: 10.1080/026432998381032. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Sandrini M, Schwarzbach J. State-dependent TMS reveals a hierarchical representation of observed acts in the temporal, parietal, and premotor cortices. Cerebral cortex. 2010;20(9):2252–2258. doi: 10.1093/cercor/bhp291. [DOI] [PubMed] [Google Scholar]
- Cooper RP, Schwartz MF, Yule P, Shallice T. The simulation of action disorganisation in complex activities of daily living. Cognitive Neuropsychology. 2005;22(8):959–1004. doi: 10.1080/02643290442000419. [DOI] [PubMed] [Google Scholar]
- Cooper RP, Shallice T. Contention scheduling and the control of routine activities. Cognitive neuropsychology. 2000;17(4):297–338. doi: 10.1080/026432900380427. [DOI] [PubMed] [Google Scholar]
- Cooper RP, Shallice T. Hierarchical schemas and goals in the control of sequential behavior. Psychological review. 2006;113(4):887–916. doi: 10.1037/0033-295X.113.4.887. discussion 917–931. [DOI] [PubMed] [Google Scholar]
- De Baene W, Albers AM, Brass M. The what and how components of cognitive control. NeuroImage. 2012;63(1):203–211. doi: 10.1016/j.neuroimage.2012.06.050. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Spronk M, Willems RM, Toni I, Bekkering H. Complementary systems for understanding action intentions. Current biology. 2008;18(6):454–457. doi: 10.1016/j.cub.2008.02.057. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP. A hierarchical neuronal network for planning behavior. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(24):13293–13298. doi: 10.1073/pnas.94.24.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag C, Troiani V, Bonner M, Powers C, Avants B, Gee J, Grossman M. Hierarchical organization of scripts: converging evidence from FMRI and frontotemporal degeneration. Cerebral cortex (New York, N.Y. : 1991) 2010;20(10):2453–2463. doi: 10.1093/cercor/bhp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier L, Dehaut F, Joanette Y. The bells test, a quantitative and qualitative test for visual neglect. International Journal of Clinical Neuropsychology. 1989;11:1149–1154. [Google Scholar]
- Goldenberg G, Hartmann-Schmid K, Sürer F, Daumüller M, Hermsdörfer J. The impact of dysexecutive syndrome on use of tools and technical devices. Cortex. 2007;43(3):424–435. doi: 10.1016/s0010-9452(08)70467-2. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hamilton AFDC. Evidence for a distributed hierarchy of action representation in the brain. Human movement science. 2007;26(4):590–616. doi: 10.1016/j.humov.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AFDC, Grafton ST. Goal representation in human anterior intraparietal sulcus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(4):1133–1137. doi: 10.1523/JNEUROSCI.4551-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AFDC, Grafton ST. Action outcomes are represented in human inferior frontoparietal cortex. Cerebral cortex (New York, N.Y. : 1991) 2008;18(5):1160–1168. doi: 10.1093/cercor/bhm150. [DOI] [PubMed] [Google Scholar]
- Hartmann K, Goldenberg G, Daumüller M, Hermsdörfer J. It takes the whole brain to make a cup of coffee: the neuropsychology of naturalistic actions involving technical devices. Neuropsychologia. 2005;43(4):625–637. doi: 10.1016/j.neuropsychologia.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Hesse MD, Sparing R, Fink GR. End or means--the “what” and “how” of observed intentional actions. Journal of cognitive neuroscience. 2009;21(4):776–790. doi: 10.1162/jocn.2009.21058. [DOI] [PubMed] [Google Scholar]
- Hickok G, Hauser M. Mis)understanding mirror neurons. Current biology : CB. 2010;20(14):593–594. doi: 10.1016/j.cub.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one’s own mirror neuron system. PLoS biology. 2005;3(3):529–535. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jax SA, Buxbaum LJ, Moll AD. Deficits in movement planning and intrinsic coordinate control in ideomotor apraxia. Journal of cognitive neuroscience. 2006;18(12):2063–2076. doi: 10.1162/jocn.2006.18.12.2063. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH, Newman-Norlund R, Grafton ST. A distributed left hemisphere network active during planning of everyday tool use skills. Cerebral cortex (New York, N.Y. : 1991) 2005;15(6):681–695. doi: 10.1093/cercor/bhh169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalénine S, Buxbaum LJ, Coslett HB. Critical brain regions for action recognition: lesion symptom mapping in left hemisphere stroke. Brain : a journal of neurology. 2010;133(11):3269–3280. doi: 10.1093/brain/awq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Tomonaga M. Relative contributions of goal representation and kinematic information to self-monitoring by chimpanzees and humans. Cognition. 2012;125(2):168–178. doi: 10.1016/j.cognition.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Kertesz A. The western aphasia battery. Grune & Stratton; New York: 1982. [Google Scholar]
- Kilner JM. More than one pathway to action understanding. Trends in cognitive sciences. 2011;15(8):352–357. doi: 10.1016/j.tics.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimgruber K, Goldenberg G, Hermsdörfer J. Manual and hemispheric asymmetries in the execution of actual and pantomimed prehension. Neuropsychologia. 2005;43(5):682–692. doi: 10.1016/j.neuropsychologia.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. Journal of physiology, Paris. 2008;102(1-3):59–70. doi: 10.1016/j.jphysparis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual review of neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Norman W, Shallice T. Attention to action. In: Davidson R, Schwartz G, Shapiro D, editors. Consciousness and self regulation: Advances in research and theory. vol. 4. Plenum; New York: 1986. pp. 1–18. [Google Scholar]
- Ocampo B, Painter DR, Kritikos A. Event coding and motor priming: how attentional modulation may influence binding across action properties. Experimental brain research. Experimentelle Hirnforschung. Expérimentation cérébrale. 2012;219(1):139–150. doi: 10.1007/s00221-012-3073-0. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Inui T. Multiple neural representations of object-directed action in an imitative context. Experimental brain research. Experimentelle Hirnforschung. Expérimentation cérébrale. 2012;216(1):61–69. doi: 10.1007/s00221-011-2908-4. [DOI] [PubMed] [Google Scholar]
- Ortigue S, Sinigaglia C, Rizzolatti G, Grafton ST. Understanding actions of others: the electrodynamics of the left and right hemispheres. A high-density EEG neuroimaging study. PloS one. 2010;5(8):1–13. doi: 10.1371/journal.pone.0012160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath J, Goldenberg G, Spijkers W, Li Y, Hermsdörfer J. From pantomime to actual use: how affordances can facilitate actual tool-use. Neuropsychologia. 2011;49(9):2410–2416. doi: 10.1016/j.neuropsychologia.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual review of neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O, Bonilha L. Improving lesion-symptom mapping. Journal of cognitive neuroscience. 2007;19(7):1081–8. doi: 10.1162/jocn.2007.19.7.1081. doi:10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Schneider DW, Logan GD. Retrieving information from a hierarchical plan. Journal of experimental psychology. Learning, memory, and cognition. 2007;33(6):1076–1091. doi: 10.1037/0278-7393.33.6.1076. [DOI] [PubMed] [Google Scholar]
- Schwartz MF. The cognitive neuropsychology of everyday action and planning. Cognitive neuropsychology. 2006;23(1):202–221. doi: 10.1080/02643290500202623. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Brecher AR, Whyte J, Klein MG. A patient registry for cognitive rehabilitation research: a strategy for balancing patients’ privacy rights with researchers’ need for access. Archives of physical medicine and rehabilitation. 2005;86(9):1807–1814. doi: 10.1016/j.apmr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Segal ME, Veramonti T, Ferraro M, Buxbaum LJ. The Naturalistic Action Test: A standardised assessment for everyday-action impairment. Neuropsychological rehabilitation. 2002;12:311–339. [Google Scholar]
- Shallice T, Burgess P. The domain of supervisory processes and temporal organization of behaviour. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 1996;351(1346):1405–1411. doi: 10.1098/rstb.1996.0124. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Zalla T, Pillon B, Grafman J, Agid Y, Dubois B. Encoding of sequence and boundaries of scripts following prefrontal lesions. Cortex. 1996;32(2):297–310. doi: 10.1016/s0010-9452(96)80052-9. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Falk EB, Lieberman MD. Dissociable neural systems support retrieval of how and why action knowledge. Psychological science. 2010;21(11):1593–1598. doi: 10.1177/0956797610386618. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Lieberman MD. Dissociating modality-specific and supramodal neural systems for action understanding. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(10):3575–3583. doi: 10.1523/JNEUROSCI.5715-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton NA. Hierarchical task analysis: developments, applications, and extensions. Applied ergonomics. 2006;37(1):55–79. doi: 10.1016/j.apergo.2005.06.003. [DOI] [PubMed] [Google Scholar]
- van Elk M, van Schie HT, Bekkering H. Conceptual knowledge for understanding other’s actions is organized primarily around action goals. Experimental brain research. 2008;189(1):99–107. doi: 10.1007/s00221-008-1408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schie HT, Bekkering H. Neural mechanisms underlying immediate and final action goals in object use reflected by slow wave brain potentials. Brain research. 2007;1148:183–197. doi: 10.1016/j.brainres.2007.02.085. [DOI] [PubMed] [Google Scholar]
- Wild KS, Poliakoff E, Jerrison A, Gowen E. The influence of goals on movement kinematics during imitation. Experimental brain research. 2010;204(3):353–360. doi: 10.1007/s00221-009-2034-8. [DOI] [PubMed] [Google Scholar]
- Zalla T, Plassiart C, Pillon B, Grafman J, Sirigu A. Action planning in a virtual context after prefrontal cortex damage. Neuropsychologia. 2001;39(8):759–770. doi: 10.1016/s0028-3932(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Zalla T, Pradat-Diehl P, Sirigu A. Perception of action boundaries in patients with frontal lobe damage. Neuropsychologia. 2003;41(12):1619–1627. doi: 10.1016/s0028-3932(03)00098-8. [DOI] [PubMed] [Google Scholar]
- Zanini S. Generalised script sequencing deficits following frontal lobe lesions. Cortex. 2008;44(2):140–149. doi: 10.1016/j.cortex.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Zanini S, Rumiati RI, Shallice T. Action sequencing deficit following frontal lobe lesion. Neurocase. 2002;8(1-2):88–99. doi: 10.1093/neucas/8.1.88. [DOI] [PubMed] [Google Scholar]