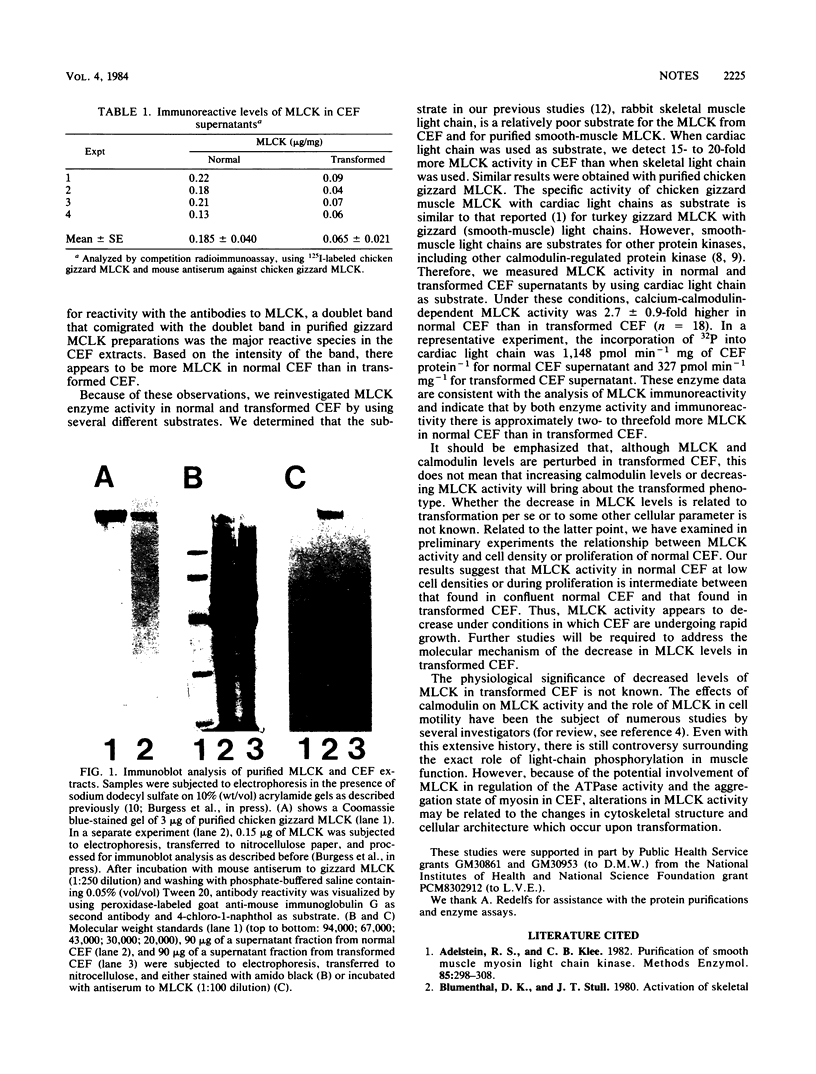
Abstract
Calmodulin, a calcium-modulated effector protein, is an important mediator of the intracellular actions of calcium through its interaction with calmodulin-binding proteins. We report here that the immunoreactive levels of a calmodulin-binding protein, myosin light-chain kinase, are decreased in transformed chicken embryo fibroblasts.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Klee C. B. Purification of smooth muscle myosin light-chain kinase. Methods Enzymol. 1982;85(Pt B):298–308. doi: 10.1016/0076-6879(82)85029-5. [DOI] [PubMed] [Google Scholar]
- Dabrowska R., Aromatorio D., Sherry J. M., Hartshorne D. J. Composition of the myosin light chain kinase from chicken gizzard. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1263–1272. doi: 10.1016/0006-291x(77)91429-2. [DOI] [PubMed] [Google Scholar]
- Flockhart D. A., Corbin J. D. Regulatory mechanisms in the control of protein kinases. CRC Crit Rev Biochem. 1982 Feb;12(2):133–186. doi: 10.3109/10409238209108705. [DOI] [PubMed] [Google Scholar]
- Hathaway D. R., Adelstein R. S. Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1653–1657. doi: 10.1073/pnas.76.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schworer C. M., Soderling T. R. Substrate specificity of liver calmodulin-dependent glycogen synthase kinase. Biochem Biophys Res Commun. 1983 Oct 31;116(2):412–416. doi: 10.1016/0006-291x(83)90538-7. [DOI] [PubMed] [Google Scholar]
- Singh T. J., Akatsuka A., Huang K. P. Phosphorylation of smooth muscle myosin light chain by five different kinases. FEBS Lett. 1983 Aug 8;159(1-2):217–220. doi: 10.1016/0014-5793(83)80449-9. [DOI] [PubMed] [Google Scholar]
- Van Eldik L. J., Burgess W. H. Analytical subcellular distribution of calmodulin and calmodulin-binding proteins in normal and virus-transformed fibroblasts. J Biol Chem. 1983 Apr 10;258(7):4539–4547. [PubMed] [Google Scholar]
- Van Eldik L. J., Watterson D. M. Reproducible production of antiserum against vertebrate calmodulin and determination of the immunoreactive site. J Biol Chem. 1981 May 10;256(9):4205–4210. [PubMed] [Google Scholar]
- Watterson D. M., Van Eldik L. J., Smith R. E., Vanaman T. C. Calcium-dependent regulatory protein of cyclic nucleotide metabolism in normal and transformed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2711–2715. doi: 10.1073/pnas.73.8.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zendegui J. G., Zielinski R. E., Watterson D. M., Van Eldik L. J. Biosynthesis of calmodulin in normal and virus-transformed chicken embryo fibroblasts. Mol Cell Biol. 1984 May;4(5):883–889. doi: 10.1128/mcb.4.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]