Abstract
Alzheimer's disease (AD) is a devastating neurodegenerative disease causing irreversible cognitive decline in the elderly. There is no disease-modifying therapy for this condition and the mechanisms underpinning neuronal dysfunction and neurodegeneration are unclear. Compromised cytoskeletal integrity within neurons is reported in AD. This is believed to result from loss-of-function of the microtubule-associated protein tau, which becomes hyper-phosphorylated and deposits into neurofibrillary tangles in AD. We have developed a Drosophila model of tauopathy in which abnormal human tau mediates neuronal dysfunction characterised by microtubule destabilisation, axonal transport disruption, synaptic defects and behavioural impairments. Here we show that a microtubule-stabilising drug, NAPVSIPQ (NAP), prevents as well as reverses these phenotypes even after they have become established. Moreover, it does not alter abnormal tau levels indicating that it by-passes toxic tau altogether. Thus, microtubule stabilisation is a disease-modifying therapeutic strategy protecting against tau-mediated neuronal dysfunction, which holds great promise for tauopathies like AD.
Keywords: Alzheimer's disease, axonal transport, Drosophila, microtubules, NAP (davunetide)
Introduction
Although Alzheimer's disease (AD) was first described over 100 years ago, the neurodegenerative mechanisms underpinning this disease remain unclear. Thus, disease-modifying therapies still elude us and treatment is restricted to provision of symptomatic relief.1 One avenue of research investigates how hyper-phosphorylated tau, a microtubule-associated protein forming the tangle pathology in AD, causes neuronal dysfunction and degeneration. Hyper-phosphorylated tau cannot effectively bind and stabilise microtubules. It is hypothesised that this compromises cytoskeletal integrity within affected neurons in AD,2, 3, 4 disrupting axonal transport and synaptic function5, 6 and manifesting in clinical symptoms.7 Compromised and reduced numbers of microtubules are reported in post-mortem AD brains.8 Furthermore, a direct correlation between hyper-phosphorylated tau-mediated axonal transport disruption and behavioural impairment is demonstrated in Drosophila (htau0N3R) and rodent (tau-T44 tg mice) models of AD.6, 9 Supporting this, tangle pathology closely correlates with cognitive decline in AD patients.10 Hence, tau-based therapies are being considered for AD.11, 12 Current strategies include drugs to reduce tau phosphorylation and aggregation. Recently, the use of microtubule-stabilising drugs to compensate for tau loss-of-function has been explored.13 Paclitaxel, a microtubule-stabilising drug, reduces axonopathy and improves behaviour in a rodent model of tauopathy.14 Although this supports the idea of by-passing pathogenic tau by directly stabilising microtubules, the high toxicity and poor blood–brain barrier (BBB) permeability of such drugs make them unsuitable for treating patients. This prompted the development of a new generation of microtubule-stabilising agents exhibiting low toxicity and high BBB permeability.15, 16
The small octapeptide NAPVSIPQ (NAP), derived from activity-dependent neuroprotective protein,15 interacts with and stabilises neuron-specific βIII-tubulin in vitro.17 Importantly, NAP penetrates the BBB and is non-toxic.18, 19 The neuroprotective activities of NAP are documented in many in vivo and in vitro models.16 Studies in rodent models of AD, suggest that NAP's protective capacity is mediated by its ability to stabilise the microtubule cytoskeleton.20, 21, 22 However, this is yet to be demonstrated in vivo.
Here we show that NAP by-passes abnormal tau and compensates for tau loss-of-function thus protecting against neuronal dysfunction in a Drosophila model of tauopathy. This model is ideally suited for this investigation because the phenotypes arise as a consequence of abnormal tau-mediated cytoskeletal destabilisation in the absence of tau aggregation.4, 5, 6
Materials and methods
Stocks and drug treatment
Expression of htau0N3R (Bloomington Stock Centre, Bloomington, IN, USA; stock no. 181), htau0N4R (provided by Dr E Skoulakis; Alexander Fleming Institute, Athens, Greece) and amyloid beta-42 (Aβ42):Aβ42;htau0N4R (provided by Dr D Crowther; University of Cambridge, Cambridge, UK) was directed to Drosophila melanogaster motor neurons using either the motor neuron-specific drivers D42-Gal4, or the D42-Gal4 driver recombined with green fluorescent protein (GFP)-tagged neuropeptide-Y (D42-GAL4.UAS-NPY:GFP)6 (UAS-NPY:GFP provided by Dr I Robinson, University of Cambridge). Pan-neural expression was achieved with the Elav-Gal4 driver (Bloomington Stock Centre; stock no. 458). NAP (Peptide Protein Research, Fareham, UK) was delivered to basic fly food at concentrations of 50 ng ml–1, 500 ng ml–1, 2.5 μg ml–1 or 25 μg ml–1.
Larval locomotion and electron microscopy analysis were conducted as previously described.4, 23
In vivo axonal transport analysis
Treatment groups were subjected to timed lays on apple juice agar plates. F1 eggs were transferred to basic or NAP food. Htau0N3R larvae were assessed at L3 wandering stage (day 5) at 23 °C. Htau0N4R and Aβ42;htau0N4R larvae were reared at 29 °C. For the NAP rescue experiment, early L3 larvae were removed from food plates at day 4 and either subjected to axonal transport analysis or moved onto a basic food plate or a NAP food plate for 24 h. Axonal transport was analysed as previously described.6, 24
Immunohistochemistry
Dissected L3 larvae were immunostained as previously described.5 Body walls were incubated in goat anti-horseradish peroxidase conjugated to fluorescein isothiocyanate (1:1000; ICN/Cappel, Solon, OH, USA) and mouse anti-tubulin (1:500; E7-Developmental Hybridoma Bank, Iowa City, IA, USA) antibodies and with Alexa Fluor 594 donkey anti-mouse (1:200; Invitrogen, Paisley, UK) secondary antibody. Muscle 4 from abdominal segments A3–A7 was imaged.5
Microtubule-binding assay
Three heads of 1 to 3-day-old flies, or 10 larval (L3) ventral cords were pooled and homogenised in 30 and 40 μl, respectively, of microtubule-binding assay buffer as previously described.4
Immunopreciptiation assay
Ten heads of 1 to 3-day-old flies were homogenised in 500 μl of microtubule-binding assay buffer and interactions between htau0N3R and dtau were assessed as described previously.4
Western blotting
Adult heads or dissected L3 larval ventral cords were homogenised, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Blots were probed with anti-human tau (1:15,000, Dako, Cambridge, UK), anti-dtau (1:500, Professor St Johnston, University of Cambridge), anti-phospho-tau: PHF-1 (1:2000, Dr Peter Davies, Albert Einstein College of Medicine, Bronx, NY, USA), AT180 (1:100, Biosciences, Nottingham, UK), AT8 (1:800, Biosciences), tau-1 (1:2000, Millipore, Watford Herts, UK), pS262 (1:1000, Invitrogen), anti-tubulin (1:200) and anti-kinesin (1:5000, Cytoskeleton, Denver, CO, USA). Signal was detected using fluorescently conjugated secondary antibodies.
Statistics
Statistical analysis was carried out using Prism 5.0 (GraphPad, University of Southampton, Southampton, UK). T-test or one-way analysis of variance, with Bonferroni's Multiple comparison post-hoc analysis.
Results
NAP prevents htau0N3R-mediated disruption of locomotor behaviour
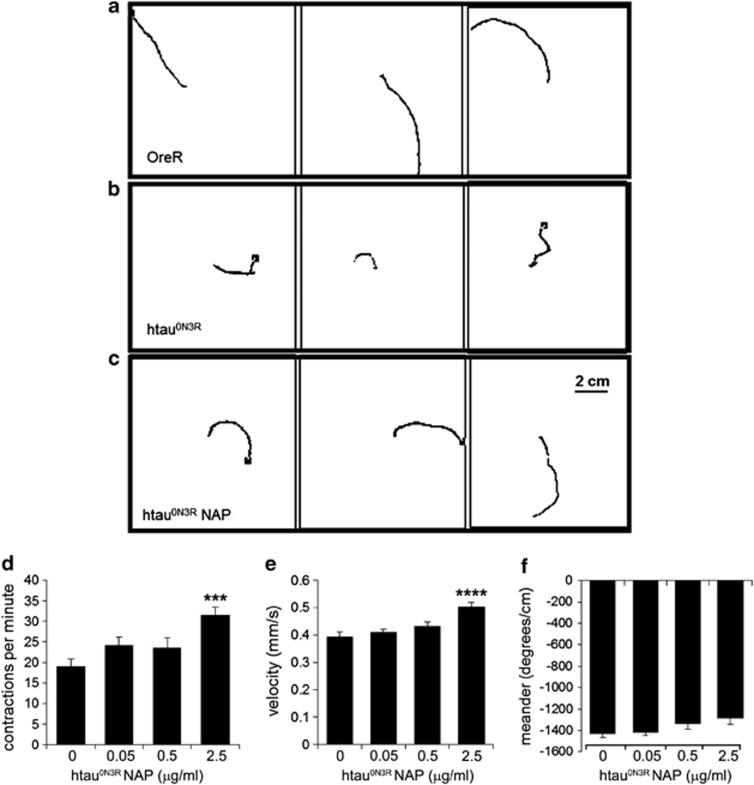
Expression of the highly phosphorylated 0N3R human tau isoform (htau0N3R) in motor neurons of Drosophila manifests in a number of distinct phenotypes including crawling defects in larvae.6 We first tested whether this phenotype could be prevented by NAP. Untreated htau0N3R larvae exhibited a restricted and non-continuous crawling behaviour (Figure 1b; Supplementary Video 1) compared with controls (Figure 1a; Supplementary Video 2). This defective crawling behaviour improved significantly following NAP treatment leading to smoother, more directed movement over a greater distance like controls (Figure 1c; Supplementary Video 3). Using the image-tracking software Ethovision, crawling parameters were quantified including body wall contractions, velocity and meander (turning rate per distance travelled). In all, 2.5 μg ml–1 NAP significantly improved body wall contractions and velocity compared with un-treated htau0N3R larvae (Figures 1d and e). Meander was also improved, but did not reach significance (P=0.06) (Figure 1f). Crawling behaviour of controls treated with NAP was no different to untreated controls (Supplementary Figure 1).
Figure 1.
NAPVSIPQ (NAP) improves the locomotor phenotype in htau0N3R-expressing Drosophila. Oral administration of 2.5 μg ml–1 NAP improves the locomotor performance of htau0N3R wandering third instar larvae. (a–c) Traces of paths taken by L3 larvae in 2-min free crawling on 100 cm2 plates are shown for OreR controls (a), htau0N3R untreated larvae (b) and htau0N3R larvae treated with NAP (c). Crawling performance was quantified using a tracking-software Ethovision. In all, 2.5 μg ml–1 NAP improved crawling performance on three different measures: body wall contractions per minute (d), velocity (e) and meander (f). Scale bar: 2 cm. Error bars represent mean±s.e.m., (d–f); one-way analysis of variance, post-hoc Bonferroni's multiple comparison test, ***P<0.001 (body wall contractions); ****P<0.0001 (velocity); P=0.06 (meander). n=16–20.
NAP stabilises microtubules and improves axonal transport and neuromuscular junction (NMJ) morphology
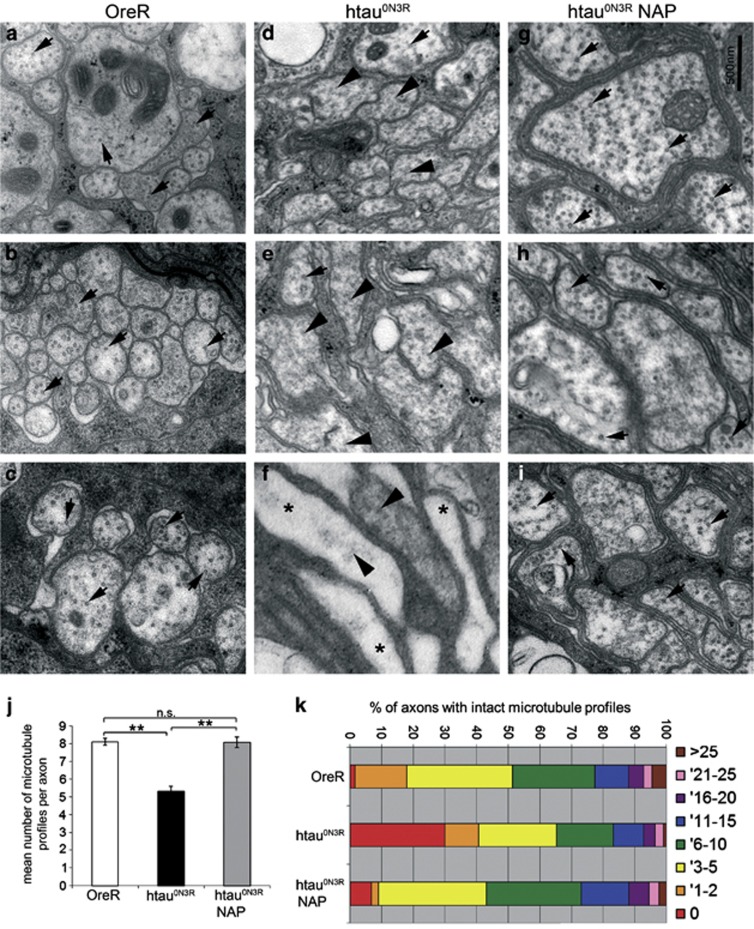
As disruption of the microtubule cytoskeleton causes behavioural defects in this model, we investigated whether microtubule stabilisation was the mechanism by which NAP rescued the locomotor phenotype of htau0N3R larvae. The ultrastructural organisation of the microtubule cytoskeleton was examined in transverse sections of peripheral nerves by transmission electron microscopy. Controls displayed characteristic, 25 nm circular transverse microtubule profiles (black arrows Figures 2a–c). In contrast, htau0N3R larvae had noticeably fewer such microtubule profiles and displayed many disorganised and mis-aligned microtubules (black arrowheads Figures 2d–f). A number of axons relatively devoid of microtubules were also evident in htau0N3R larvae (asterisks Figure 2f). NAP re-stabilised the disrupted and disorganised microtubules as evidenced by the appearance of more intact transverse microtubule profiles in treated htau0N3R larvae (black arrows Figures 2g–i). Quantification of the number of intact microtubule profiles per axon confirmed that there were significantly fewer microtubule profiles in htau0N3R axons (5.32±0.28) compared with controls (8.10±0.20). NAP increased this number back to control levels (8.07±0.30; Figure 2j). The range of intact microtubules per individual axon in each condition is illustrated in Figure 2k. Almost half the axons from controls contained large numbers of microtubules (>5), with very few axons containing no microtubules (1%). However, the majority (∼65%) of htau0N3R axons contained either none (30%) or very few (<5) microtubules (35%). In contrast, the peripheral nerves of NAP-treated htau0N3R larvae had a similar microtubule profile to controls. There was an increase in the number of axons containing large numbers (>5) of intact transverse microtubules (58%) compared with untreated htau0N3R (35%), and a reduction in the number of axons with no microtubules (7% Figure 2k). NAP treatment did not change the number of microtubule profiles in controls (untreated controls had 9.22±0.42 intact microtubule (MT) profiles whereas NAP-treated controls had 9.98±0.45 intact MT profiles; Supplementary Figure 2).
Figure 2.
NAPVSIPQ (NAP) protects against microtubule destabilisation in htau0N3R Drosophila. Representative electron micrographs showing transverse sections of peripheral nerves in L3 larvae. Axon profiles in control larvae show regularly spaced correctly aligned transverse microtubules (black arrows in a–c). In axons of htau0N3R larvae, the microtubules were disrupted, with fewer correctly aligned transverse microtubules (black arrows in d–f) and more disorganised microtubules visible in the same axon profiles (black arrowheads in d–f). Axons relatively devoid of microtubules were also observed (asterisks in f). However, in htau0N3R larvae fed 2.5 μg ml–1 NAP, there was a complete rescue of microtubule integrity (g–i). Scale bar 500 nm. Control axons contained on average 8.10±0.20 correctly aligned microtubule profiles per cross-section; larvae-expressing htau0N3R contained only 5.32±0.28, and those expressing htau0N3R but reared on NAP contained 8.07±0.30 profiles (j). Average numbers of correctly aligned visible microtubule profiles in axons of control and htau0N3R NAP treated animals were significantly greater than those of untreated htau0N3R animals. Axons exhibited a range of intact correctly aligned transverse microtubules (k): the majority of control axons contained large numbers of microtubules (>5), and very few axons contained no microtubules. However, the majority of htau0N3R axons contain either none or very few microtubules (<5). Htau0N3R larvae reared on NAP had a greater number of axons containing large numbers of intact transverse microtubules (>5) and fewer axons with no microtubules (k). (Error bars represent mean±s.e.m., **P<0.01, as determined by unpaired Student's two-tailed t-test, n=5). NS, not significant.
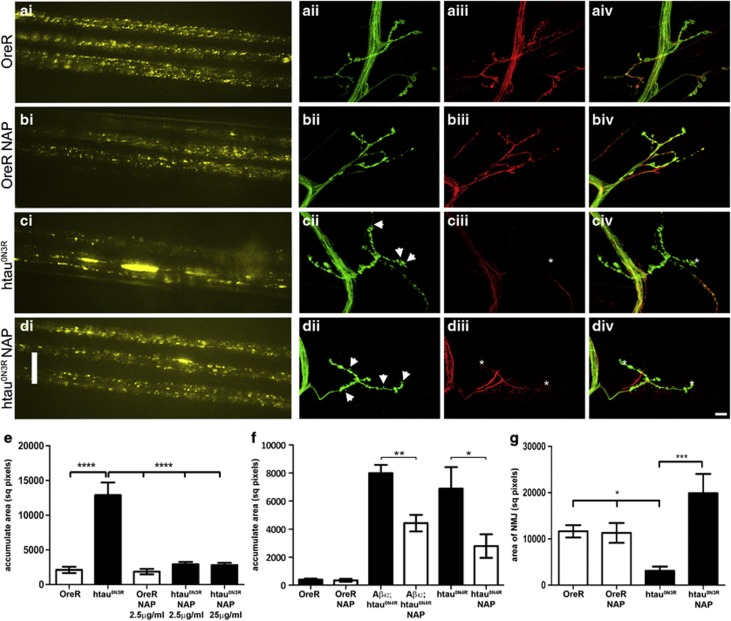
We next investigated whether the restoration of microtubule integrity in NAP-treated htau0N3R larvae improves axonal transport as we know that axonal transport is disrupted in this model as a consequence of microtubule breakdown.6 Expression of vesicular neuropeptide-Y-GFP (vGFP) in motor neurons of larvae allows this htau0N3R-mediated phenotype to be visualised in vivo, in real time, in living intact larvae. A homogeneous distribution of vGFP indicating efficient axonal transport was evident in motor neurons of NAP-treated and untreated controls (Figures 3b.i and a.i). As expected, in htau0N3R larvae the distribution of vGFP was dramatically different, with the appearance of large vGFP accumulates within axons, illustrating profound axonal transport disruptions (Figure 3c.i). However, in NAP-treated htau0N3R larvae axonal transport appeared normal, with very few vGFP accumulates (Figure 3d.i). Quantification of the total area occupied by vGFP accumulates confirmed this result (Figure 3e). A 10-fold higher dose of NAP (25 μg ml–1) did not lead to any further improvement (Figure 3e). NAP also significantly reduced axonal transport defects in animals expressing comparable levels of another tau isoform (htau0N4R alone or htau0N4R and Aβ42 (Aβ42;htau0N4R)), illustrating that NAP can rescue defects induced by both 3 and 4 repeat tau isoforms (Figure 3f and Supplementary Figure 3).
Figure 3.
NAPVSIPQ (NAP) prevents axonal transport deficits and improves neuromuscular junction (NMJ) morphology. Control NAP-treated and untreated larvae showed a homogeneous distribution of vesicular neuropeptide-Y-GFP (vGFP) fluorescence in peripheral nerves (b.i, a.i). Htau0N3R larvae showed accumulation of vGFP, indicative of disrupted axonal transport (c.i). 2.5 μg ml–1 NAP prevented accumulation of vGFP in htau0N3R larvae (d.i). Larvae were reared at 23 °C. Scale bars:10 μm. The total area encompassed by vGFP accumulates was greater in htau0N3R larvae compared with control. 2.5 μg ml–1 (low) and 25 μg ml–1 (high) NAP reduced the area covered by vesicular accumulates back to control levels (e). Disruption of axonal transport was also evident in Aβ42;htau0N4R and htau0N4R-expressing larvae when reared at 29 °C. NAP treatment significantly reduced the area covered by vGFP accumulates in both lines (f). NMJs on muscle 4 were revealed by anti-horseradish peroxidase (HRP) staining green (ii) and the tubulin cytoskeleton by anti-tubulin staining red (iii), overlay (iv). Control NAP-treated and untreated larvae exhibited a characteristic bead-like distribution of presynaptic boutons (b.ii, a.ii) and the tubulin cytoskeleton extended into the distal ends of the NMJ (b.iii, a.iii). Morphological aberrations (thinning of inter-bouton axon bundles and ‘mini' satellite boutons) were seen in htau0N3R NMJ's (arrowheads in c.ii). Anti-tubulin staining showed disruption of the cytoskeleton, with complete absence in the most distal regions of the NMJ (asterisk in c.iii). The NMJs of htau0N3R NAP-treated animals contained a greater number of satellite boutons (arrowheads in d.ii) compared with untreated animals. However, restoration and extension of the cytoskeleton into the most distal ends of the NMJ was observed (asterisk in d.iii). Quantification of the area encompassed by NMJ's shows a significantly smaller NMJ area in htau0N3R-expressing larvae when compared with controls. NAP treatment significantly increases the NMJ area in htau0N3R-expressing larvae (g). Error bars represent mean±s.e.m., (e, g; one-way analysis of variance, post-hoc Bonferroni's multiple comparison test, *P<0.05; ***P<0.001; ****P<0.0001), (f; unpaired Student's two-tailed t-test, *P<0.05; **P<0.01). n=10 (e), n=5 (f), n=3–5 (g).
Synaptic function and NMJ morphology are also compromised in our model.5 To ascertain whether the protective effect of NAP extends to the synapse, the NMJ on muscle 4 was visualised using an antibody against a neuronal surface antigen (anti-horseradish peroxidase). Anti-tubulin antibody was also used to visualise the microtubule cytoskeleton within the NMJ terminals. The NMJ morphology in controls was characterised by a bead-like distribution of pre-synaptic boutons (Figures 3a.ii–iv). The tubulin cytoskeleton extended into the distal ends of the NMJ (Figure 3a.iii). Neither the morphology nor the tubulin cytoskeleton of these controls was altered by NAP (Figure 3b.ii-iv). In contrast, htau0N3R larvae NMJs exhibited profound morphological aberrations, including thinning of inter-bouton axon segments and appearance of ‘mini' satellite boutons (arrowheads Figure 3c.ii). The cytoskeleton was also disrupted, with a complete absence of tubulin immunoreactivity in the most distal regions (asterisk Figure 3c.iii). NMJs of NAP-treated htau0N3R larvae also exhibited morphological aberrations similar to those of untreated htau0N3R larvae, but with a greater number of ‘mini' satellite boutons (arrowheads Figure 3d.ii). However, the tubulin cytoskeleton in these animals extended into the most distal ends of the NMJ similar to that of the controls (asterisks Figure 3d.iii). The area encompassed by this NMJ was significantly smaller in htau0N3R larvae compared with controls. NAP treatment significantly increased this (Figure 3g).
Tau phosphorylation is not altered by NAP
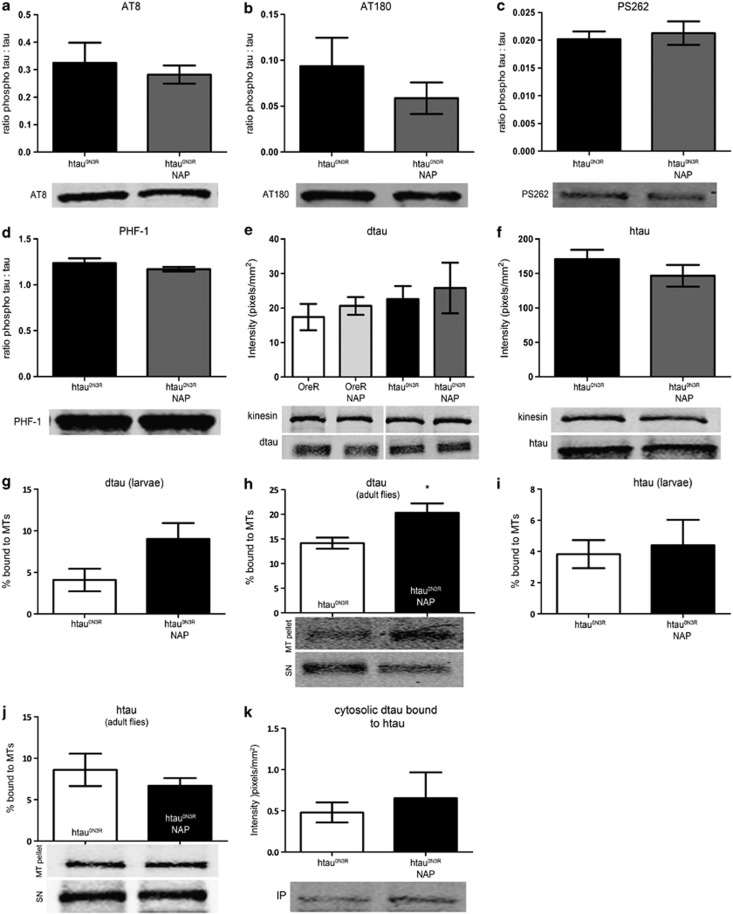
Hyper-phosphorylation of htau in our model reduces its (and the endogenous Drosophila tau (dtau)'s) microtubule-binding ability thus compromising cytoskeletal integrity.4 We therefore asked whether NAP was preventing the emergence of the htau0N3R-mediated phenotypes by reducing tau phosphorylation. We examined a number of phospho-tau epitopes associated with AD in both NAP-treated and untreated htau0N3R larvae and adults. NAP did not alter tau phosphorylation at any of the sites examined (Figures 4a–d and Supplementary Figures 4A–E). Although there was a trend for reduced tau phosphorylation at thr231/235 (AT180) following NAP treatment, this was not statistically significant (P=0.3409; Figure 4b). As phospho-tau levels were unaltered, we next explored the possibility that NAP was protecting the htau0N3R larvae by increasing endogenous dtau and/or by reducing toxic htau. Neither the total htau or dtau levels were altered by NAP in both larvae and adults, suggesting that NAP does not block or enhance degradation of these proteins (Figures 4e and f; Supplementary Figures 4F and G). Interestingly, although dtau levels were unaltered by NAP, the percentage of dtau bound to MTs increased following NAP treatment in both htau0N3R-expressing larvae and adults (Figures 4g and h). However, htau binding to MTs was not similarly increased (Figures 4i and j). This subcellular redistribution of dtau to the MTs was not attributable to the reduction of pathological htau0N3R/dtau interaction that we have previously reported4 (Figure 4k).
Figure 4.
NAPVSIPQ (NAP) does not alter tau phosphorylation at a number of sites relevant to Alzheimer's disease (AD); however, it increases dtau binding to the microtubules without altering the dtau/htau interaction. For each phospho-tau antigen, intensity of signal (pixels mm–2) was normalised to total tau. There was no significant change in the levels of the phospho-tau epitopes detected by AT8 (a), AT180 (b), PS262 (c) and PHF-1 (d) after treatment with NAP. Total dtau and htau levels were not altered by NAP treatment (e, f). Representative blots are shown below relevant graphs (a–f). Microtubule-binding assays were performed using larvae (g, i) or 1- to 3-day-old adult fly heads (h, j) expressing htau0N3R treated with or without 2.5 μg ml–1 NAP. Brain extracts were fractionated to yield a microtubule pellet (MT) and a soluble supernatant (SN). Both fractions were probed with anti-htau and anti-dtau antibodies. Treatment with NAP increased binding of endogenous dtau to the microtubules (g, h) but did not alter binding of htau to microtubules (i, j). Soluble human tau binds to Drosophila tau in a phosphorylation-dependent manner. An anti-htau antibody was used to immunoprecipitate (IP) out htau from 1- to 3-day-old adult fly heads expressing htau0N3R, treated with or without 2.5 μg ml–1 NAP. Dtau co-immunoprecipitated with htau and NAP-treatment did not alter this interaction (k). All lanes were run on the same gel; however, (e) was non-contiguous. (Error bars represent mean±s.e.m., *P<0.05, unpaired Student's two-tailed t test, n=5–7 (e–f), n=3–5 (g–k)).
NAP not only prevents the emergence of tau phenotype, it also rescues it once established
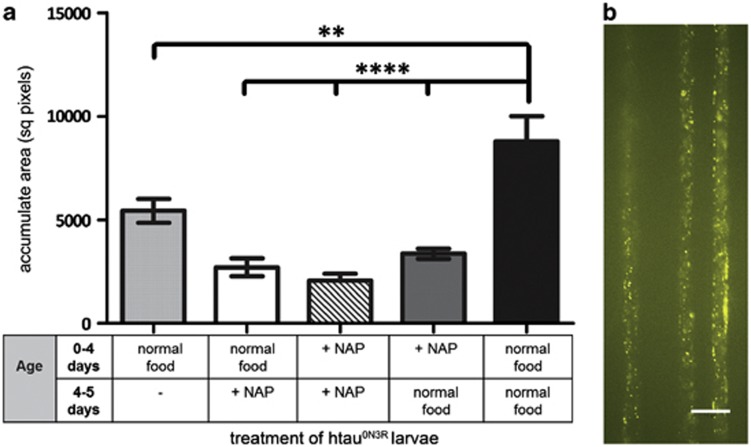
The results thus far show that NAP prevents the emergence of hyper-phosphorylated tau-mediated neuronal and behavioural phenotypes. To investigate whether NAP can alter tau phenotypes if administered after they are already established, we devised a ‘rescue protocol'. In this protocol, htau0N3R larvae were reared on normal food for 4 days and then transferred to food containing NAP for 1 day. Axonal transport was assessed at day 4 before NAP treatment was begun and then again at day 5, after only 1 day of NAP treatment. Axonal transport was disrupted in untreated htau0N3R larvae even at day 4 (light grey bar Figure 5a). However, NAP treatment for 1 day significantly reduced this axonal transport disruption (white bar Figure 5a). Importantly, this rescue of axonal transport deficit following only 1 day of NAP treatment was the same as if it were given continuously for 5 days (striped bar Figure 5a). Furthermore, the rescue persisted even if htau0N3R larvae reared on NAP for 4 days were taken off the drug and reared on normal food for a further 1 day (dark grey bar Figure 5a). Overall, the deficits observed at day 5 in all htau0N3R larvae reared on these variations of NAP treatment was significantly less than that observed in untreated htau0N3R larvae reared on normal food for 5 days (black bar Figure 5a). Interestingly, if NAP treatment was not begun at any point, the axonal transport disruption became progressively worse as the animals aged from day 4 to day 5 (first grey bar vs black bar in Figure 5a).
Figure 5.
A 24 h NAPVSIPQ (NAP) treatment rescues the tau-mediated axonal transport phenotype. The total area encompassed by vesicular neuropeptide-Y-GFP (vGFP) accumulates was quantified over a pre-determined region of peripheral nerves in L3 larvae. A disruption of axonal transport was evident in untreated htau0N3R larvae at day 4 (early L3) (light grey bar, far left) and became progressively worse by day 5 (black bar). However, NAP treatment for 24 h (white bar) significantly reduced the area covered by vesicular accumulates to the same level as that seen in larvae treated with NAP for 5 days (striped bar), thus rescuing the axonal transport deficit once established. NAP treatment for 4 days followed by transfer of larvae to normal food for 24 h was also sufficient to maintain protection at day 5 (dark grey bar) (a). Representative vGFP-tagged vesicle distribution in htau0N3R, L3 larval peripheral nerves treated for 24 h with 2.5 μg ml–1 NAP (b). Scale bar: 10 μm. (Error bars represent mean±s.e.m., **P<0.01; ****P<0.0001, one-way analysis of variance, post-hoc Bonferroni's multiple comparison test, n=10).
To confirm the low toxicity of NAP,16, 19, 22 we assessed pupariation/ecclosion rates of control and htau0N3R larvae with and without NAP treatment. NAP did not affect pupariation or ecclosion of either lines (Supplementary Figure 5).
Discussion
We demonstrate that compensating for tau loss-of-function by stabilising microtubules is a disease-modifying therapeutic strategy that should be considered for the treatment of tauopathies. We show that treatment with the microtubule-stabilising peptide NAP significantly improves all phenotypes caused by expression of highly phosphorylated tau in vivo. This includes reinstatement of cytoskeletal integrity, prevention of axonal transport and synaptic dysfunction and improvement of behavioural defects. Intriguingly, although hyper-phosphorylated tau is considered to be the toxic species in tauopathies, NAP rescued all phospho-tau phenotypes without altering phosphorylation at key disease-associated epitopes. Furthermore, NAP treatment is sufficient to rescue neuronal dysfunction even after it has already become established. Thus, NAP prevents, but more importantly, rescues phospho-tau-mediated neuronal dysfunction in vivo.
NAP stabilises microtubules
Microtubule integrity is critical for intracellular transport of materials and cytoskeletal dysfunction contributes to degeneration in many neurodegenerative conditions. Our previous findings illustrate that, microtubule destabilisation caused by tau loss-of-function is a key mechanism by which highly phosphorylated tau mediates neuronal dysfunction.4, 6 In other models too, tau loss-of-function and subsequent trafficking impairments cause neurodegeneration.25 The data presented here further underlines this and demonstrates that microtubule stabilisation can effectively protect against this.
Taxol-based microtubule-stabilising drugs are considered for treating AD11, 13, 26, 27 but their widespread toxicity and poor BBB penetrability precludes their use. To overcome this, a newer generation of drugs like small peptides (NAP) and small molecules (Epothilones, for example, EpoD) have been formulated.28, 29, 30 Both NAP and EpoD interact with MTs in vitro, reduce axonopathy and improve behaviour with minimal toxicity in rodent models of tauopathy.29, 31 In agreement with these studies, we also report an improvement in behavioural deficits of htau0N3R-expressing larvae following treatment with NAP. However, we further extend these findings and demonstrate for the first time that re-instatement of the structure and function of microtubules is the mechanism by which NAP confers neuroprotection in vivo. Furthermore, our study pioneers evaluations of live axonal transport directly visualised in a living intact animal treated with NAP, or indeed any other microtubule-stabilising agent. Corroborating studies in rodents using Mn++ enhanced magnetic resonance imaging are underway.32
We also observed that NAP restored the tubulin cytoskeleton within NMJs, and the resultant impact on synaptic function will have contributed to the behavioural improvements seen following NAP treatment. Furthermore, NAP has been shown to increase expression of the synaptic protein synaptophysin in neuronal cultures33 and in vivo,34 which is associated with neuroprotection.35 However, the NMJs of NAP-treated htau0N3R-expressing animals had a greater number of ‘mini' satellite boutons, which may reflect compensatory sprouting to maintain neuronal function. Indeed NAP has been shown to induce neurite outgrowth in vitro,17 and this would support our observations.
The molecular mechanism by which NAP stabilises MTs is not known. We hypothesise that NAP directly associates with MTs and modulates their dynamics. In support for this, NAP directly colocalises with tubulin17, 36 and alters MT dynamics in cell culture.37 Our data also suggest that disorientated/misaligned MTs (such as those present in the htau-expressing larvae4) are NAP's most likely substrate, which is why it does not alter cytoskeletal integrity (and therefore neuronal function or behaviour) in controls. This is in complete agreement with recent studies showing a more robust effect on MT polymerisation in the compromised state.37, 38
NAP by-passes hyper-phosphorylated tau
Clinical research in AD has largely focused on anti-amyloid therapies, but the growing consensus now is that truly effective disease-modifying therapies will need to target tau as well. Thus, tau-based therapeutic targets are eagerly anticipated. Agents that reduce tau phosphorylation and aggregation are currently being tested in clinical trials.1, 39 Interestingly, we find that microtubule stabilisation rescues neuronal dysfunction by by-passing hyper-phosphorylated tau altogether and directly compensating for its detrimental effects. Similar results were reported in a rodent model of tauopathy in which EpoD improves cognitive performance without altering tau phosphorylation in young mice.29 However, in older mice with pre-existing tau pathology, EpoD reduces hyper-phosphorylated tau.40 Consistent with this, intranasal administration of NAP for 3 months reduces tau phosphorylated at AT8 and AT180 sites in triple transgenic AD mice.21 In contrast to these findings, we did not observe any significant changes in levels of either total tau or in phosphorylation of tau at the sites we examined following NAP treatment. This suggests that NAP is not affecting tau degradation or phosphorylation pathways. It is possible that in our model, where very early pre-tangle stages of disease are modelled and neuronal dysfunction before death is assessed, NAP-mediated protection occurs primarily due to microtubule stabilisation. However, it is conceivable that after prolonged periods of NAP treatment, as is the case in the studies described above,21, 29, 40 the restoration of microtubule integrity reinstates some of the homeostatic mechanisms (including some which impact tau-phosphorylation) that may be lost in the presence of hyper-phosphorylated tau. Our finding that NAP increases the percentage of dtau bound to MTs, which we previously showed is compromised by hyper-phosphorylated htau,4 supports this argument. We speculate that this occurs because stabilisation of MTs by NAP increases the MT surface area available for dtau binding, thus further improving MT integrity. As the level of dtau and the pathological dtau/htau interaction is unaltered (this is not surprising given that this interaction is dependent on the phosphorylation state of htau,4 which is unchanged by NAP), it is likely that dtau from the soluble cytosolic pool is recruited back to the MTs. Indeed dtau levels were reciprocally reduced in the supernatant fraction of NAP-treated animals.
NAP rescues as well as prevents tau-mediated dysfunction
An important consideration for therapies aiming to target dysfunctional cellular processes is the extent of damage that will have already occurred by the time clinical symptoms manifest. We therefore examined NAP's capacity to reinstate microtubule integrity after it had already become compromised and found that treatment after onset of dysfunction was just as efficacious as prophylactic treatment before onset. This is supported by the findings of others that NAP is protective when administered immediately after injury18, 41, 42 and also when given chronically after the onset of dysfunction in rodent models of AD.20, 22, 43 However, these studies have not investigated the effect of NAP treatment on MT integrity and function. Ours is the first study to investigate NAP's effects on both MT integrity (electron microscopy) and function (axonal transport) after the onset of dysfunction in vivo.
Furthermore NAP-mediated neuroprotection was long lasting, both in our model and in other models of neuronal injury.18, 44 These results and ours are intriguing and suggest that, as speculated by Sudo and Bass,38 NAP may stabilise the microtubule lattice in such a way that its protective effect is retained, possibly like an ‘imprint memory', even after its removal.
Conclusion
The mechanism(s) by which abnormally hyper-phosphorylated and aggregated tau cause neuronal dysfunction and neurodegeneration in tauopathies like AD is not entirely clear. The general consensus is that disease-associated aberrations (such as hyper-phosphorylation in AD and mutation in familial tauopathies), which reduce tau binding to microtubules cause cytoskeletal destabilisation leading to disruption of axonal transport before tau aggregation. Thus, microtubule stabilisation and restoration of axonal transport is probably the most promising disease-modifying therapeutic target to counter abnormal tau-mediated neuronal dysfunction. Our results support this view by demonstrating that a microtubule-stabilising drug can effectively protect against all hyper-phosphorylated tau-mediated phenotypes spanning from cellular and molecular dysfunction to behavioural defects in vivo. Microtubule-stabilising drugs such as NAP that penetrate the BBB and are non-toxic therefore hold great promise for the treatment of all tauopathies. Encouragingly phase II clinical trials showed promising results with improvements in cognitive scores, evidence of BBB permeability and good tolerability in patients with amnestic mild cognitive impairment.45 Therefore, NAP has been investigated in phase II/III clinical trials for the treatment of Progressive Supranuclear Palsy, a ‘pure' severe tauopathy45 and the results of this are currently being evaluated. Understanding the mode of action of NAP will pave the way for similar disease-modifying therapies in AD and related tauopathies that have thus far been untreatable.
Acknowledgments
This work was funded by the Alzheimer's Society with support from Henry Smith Charity and the Rosetrees Trust. We would like to thank Professor Illana Gozes (Tel Aviv University, Israel) for constructive comments.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- Bonda DJ, Lee HP, Lee HG, Friedlich AL, Perry G, Zhu X, et al. Novel therapeutics for Alzheimer's disease: an update. Curr Opin Drug Discov Dev. 2010;13:235–246. [PMC free article] [PubMed] [Google Scholar]
- Alonso AC, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovestone S, Reynolds CH. The phosphorylation of tau: a critical stage in neurodevelopment and neurodegenerative processes. Neuroscience. 1997;78:309–324. doi: 10.1016/s0306-4522(96)00577-5. [DOI] [PubMed] [Google Scholar]
- Cowan CM, Bossing T, Page A, Shepherd D, Mudher A. Soluble hyper-phosphorylated tau causes microtubule breakdown and functionally compromises normal tau in vivo. Acta Neuropathol. 2010;120:593–604. doi: 10.1007/s00401-010-0716-8. [DOI] [PubMed] [Google Scholar]
- Chee FC, Mudher A, Cuttle MF, Newman TA, MacKay D, Lovestone S, et al. Over-expression of tau results in defective synaptic transmission in Drosophila neuromuscular junctions. Neurobiol Dis. 2005;20:918–928. doi: 10.1016/j.nbd.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Mudher A, Shepherd D, Newman TA, Mildren P, Jukes JP, Squire A, et al. GSK-3beta inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Mol Psychiatry. 2004;9:522–530. doi: 10.1038/sj.mp.4001483. [DOI] [PubMed] [Google Scholar]
- Terry RD. The pathogenesis of Alzheimer disease: an alternative to the amyloid hypothesis. J Neuropathol Exp Neurol. 1996;55:1023–1025. [PubMed] [Google Scholar]
- Cash AD, Aliev G, Siedlak SL, Nunomura A, Fujioka H, Zhu X, et al. Microtubule reduction in Alzheimer's disease and aging is independent of tau filament formation. Am J Pathol. 2003;162:1623–1627. doi: 10.1016/s0002-9440(10)64296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, et al. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24:751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42 (3 Pt 1:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Ballatore C, Brunden KR, Trojanowski JQ, Lee VM, Smith AB, 3rd, Huryn DM. Modulation of protein-protein interactions as a therapeutic strategy for the treatment of neurodegenerative tauopathies. Curr Top Medicinal Chem. 2011;11:317–330. doi: 10.2174/156802611794072605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I. Tau pathology and future therapeutics. Curr Alzheimer Res. 2010;7:685–696. doi: 10.2174/156720510793611628. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Smith AB, Huryn D, Lee VM. Microtubule-stabilising drugs for therapy of Alzheimer's disease and other neurodegenerative disorders with axonal transport impairments. Exp Opin Pharmacother. 2005;6:683–686. doi: 10.1517/14656566.6.5.683. [DOI] [PubMed] [Google Scholar]
- Zhang B, Maiti A, Shively S, Lakhani F, McDonald-Jones G, Bruce J, et al. Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc Natl Acad Sci USA. 2005;102:227–231. doi: 10.1073/pnas.0406361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem. 1999;72:1283–1293. doi: 10.1046/j.1471-4159.1999.0721283.x. [DOI] [PubMed] [Google Scholar]
- Gozes I, Morimoto BH, Tiong J, Fox A, Sutherland K, Dangoor D, et al. NAP: research and development of a peptide derived from activity-dependent neuroprotective protein (ADNP) CNS Drug Rev. 2005;11:353–368. doi: 10.1111/j.1527-3458.2005.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divinski I, Holtser-Cochav M, Vulih-Schultzman I, Steingart RA, Gozes I. Peptide neuroprotection through specific interaction with brain tubulin. J Neurochem. 2006;98:973–984. doi: 10.1111/j.1471-4159.2006.03936.x. [DOI] [PubMed] [Google Scholar]
- Leker RR, Teichner A, Grigoriadis N, Ovadia H, Brenneman DE, Fridkin M, et al. NAP, a femtomolar-acting peptide, protects the brain against ischemic injury by reducing apoptotic death. Stroke; J Cerebl Circ. 2002;33:1085–1092. doi: 10.1161/01.str.0000014207.05597.d7. [DOI] [PubMed] [Google Scholar]
- Gozes I, Steingart RA, Spier AD. NAP mechanisms of neuroprotection. J Mol Neurosci. 2004;24:67–72. doi: 10.1385/JMN:24:1:067. [DOI] [PubMed] [Google Scholar]
- Shiryaev N, Jouroukhin Y, Giladi E, Polyzoidou E, Grigoriadis NC, Rosenmann H, et al. NAP protects memory, increases soluble tau and reduces tau hyperphosphorylation in a tauopathy model. Neurobiol Dis. 2009;34:381–388. doi: 10.1016/j.nbd.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Gray AJ, Hirata-Fukae C, Minami SS, Waterhouse EG, Mattson MP, et al. Intranasal NAP administration reduces accumulation of amyloid peptide and tau hyperphosphorylation in a transgenic mouse model of Alzheimer's disease at early pathological stage. J Mol Neurosci. 2007;31:165–170. doi: 10.1385/jmn/31:02:165. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Jouroukhin Y, Gray AJ, Ma L, Hirata-Fukae C, Li HF, et al. A neuronal microtubule-interacting agent, NAPVSIPQ, reduces tau pathology and enhances cognitive function in a mouse model of Alzheimer's disease. J Pharmacol Exp Ther. 2008;325:146–153. doi: 10.1124/jpet.107.130526. [DOI] [PubMed] [Google Scholar]
- Sinadinos C, Cowan CM, Wyttenbach A, Mudher A. Increased throughput assays of locomotor dysfunction in Drosophila larvae. J Neurosci Methods. 2012;203:325–334. doi: 10.1016/j.jneumeth.2011.08.037. [DOI] [PubMed] [Google Scholar]
- Sinadinos C, Burbidge-King T, Soh D, Thompson LM, Marsh JL, Wyttenbach A, et al. Live axonal transport disruption by mutant huntingtin fragments in Drosophila motor neuron axons. Neurobiol Dis. 2009;34:389–395. doi: 10.1016/j.nbd.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med. 2012;18:291–295. doi: 10.1038/nm.2613. [DOI] [PubMed] [Google Scholar]
- Michaelis ML, Dobrowsky RT, Li G. Tau neurofibrillary pathology and microtubule stability. J Mol Neurosci. 2002;19:289–293. doi: 10.1385/JMN:19:3:289. [DOI] [PubMed] [Google Scholar]
- Michaelis ML, Chen Y, Hill S, Reiff E, Georg G, Rice A, et al. Amyloid peptide toxicity and microtubule-stabilizing drugs. J Mol Neurosci. 2002;19:101–105. doi: 10.1007/s12031-002-0018-2. [DOI] [PubMed] [Google Scholar]
- Brunden KR, Yao Y, Potuzak JS, Ferrer NI, Ballatore C, James MJ, et al. The characterization of microtubule-stabilizing drugs as possible therapeutic agents for Alzheimer's disease and related tauopathies. Pharmacol Res. 2011;63:341–351. doi: 10.1016/j.phrs.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden KR, Zhang B, Carroll J, Yao Y, Potuzak JS, Hogan AM, et al. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J Neurosci. 2010;30:13861–13866. doi: 10.1523/JNEUROSCI.3059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I, Divinski I. NAP, a neuroprotective drug candidate in clinical trials, stimulates microtubule assembly in the living cell. Curr Alzheimer Res. 2007;4:507–509. doi: 10.2174/156720507783018208. [DOI] [PubMed] [Google Scholar]
- Barten DM, Fanara P, Andorfer C, Hoque N, Wong PY, Husted KH, et al. Hyperdynamic microtubules, cognitive deficits, and pathology are improved in tau transgenic mice with low doses of the microtubule-stabilizing agent BMS-241027. J Neurosci. 2012;32:7137–7145. doi: 10.1523/JNEUROSCI.0188-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davunetide (NAP) and D-NAP (AL-408) protect against tau hyperphosphorylation in a model of ALS: implications for axonal transport Neuroscience Meeting Planner Society for Neuroscience: New Orleans, LA, USA; 2012. abstract 242.05/E23. [Google Scholar]
- Smith-Swintosky VL, Gozes I, Brenneman DE, D'Andrea MR, Plata-Salaman CR. Activity-dependent neurotrophic factor-9 and NAP promote neurite outgrowth in rat hippocampal and cortical cultures. J Mol Neurosci. 2005;25:225–238. doi: 10.1385/JMN:25:3:225. [DOI] [PubMed] [Google Scholar]
- Idan-Feldman A, Schirer Y, Polyzoidou E, Touloumi O, Lagoudaki R, Grigoriadis NC, et al. Davunetide (NAP) as a preventative treatment for central nervous system complications in a diabetes rat model. Neurobiol Dis. 2011;44:327–339. doi: 10.1016/j.nbd.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Pascual M, Guerri C. The peptide NAP promotes neuronal growth and differentiation through extracellular signal-regulated protein kinase and Akt pathways, and protects neurons co-cultured with astrocytes damaged by ethanol. J Neurochem. 2007;103:557–568. doi: 10.1111/j.1471-4159.2007.04761.x. [DOI] [PubMed] [Google Scholar]
- Divinski I, Mittelman L, Gozes I. A femtomolar acting octapeptide interacts with tubulin and protects astrocytes against zinc intoxication. J Biol Chem. 2004;279:28531–28538. doi: 10.1074/jbc.M403197200. [DOI] [PubMed] [Google Scholar]
- Oz S, Ivashko-Pachima Y, Gozes I. The ADNP derived peptide, NAP modulates the tubulin pool: implication for neurotrophic and neuroprotective activities. PloS One. 2012;7:e51458. doi: 10.1371/journal.pone.0051458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo H, Baas PW. Strategies for diminishing katanin-based loss of microtubules in tauopathic neurodegenerative diseases. Hum Mol Genet. 2011;20:763–778. doi: 10.1093/hmg/ddq521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer's disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- Zhang B, Carroll J, Trojanowski JQ, Yao Y, Iba M, Potuzak JS, et al. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J Neurosci. 2012;32:3601–3611. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beni-Adani L, Gozes I, Cohen Y, Assaf Y, Steingart RA, Brenneman DE, et al. A peptide derived from activity-dependent neuroprotective protein (ADNP) ameliorates injury response in closed head injury in mice. J Pharmacol Exp Ther. 2001;296:57–63. [PubMed] [Google Scholar]
- Rotstein M, Bassan H, Kariv N, Speiser Z, Harel S, Gozes I. NAP enhances neurodevelopment of newborn apolipoprotein E-deficient mice subjected to hypoxia. J Pharmacol Exp Ther. 2006;319:332–339. doi: 10.1124/jpet.106.106898. [DOI] [PubMed] [Google Scholar]
- Vulih-Shultzman I, Pinhasov A, Mandel S, Grigoriadis N, Touloumi O, Pittel Z, et al. Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J Pharmacol Exp Ther. 2007;323:438–449. doi: 10.1124/jpet.107.129551. [DOI] [PubMed] [Google Scholar]
- Jehle T, Dimitriu C, Auer S, Knoth R, Vidal-Sanz M, Gozes I, et al. The neuropeptide NAP provides neuroprotection against retinal ganglion cell damage after retinal ischemia and optic nerve crush. Graefe's archive for clinical and experimental ophthalmology=Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2008;246:1255–1263. doi: 10.1007/s00417-007-0746-7. [DOI] [PubMed] [Google Scholar]
- Gold M, Lorenzl S, Stewart AJ, Morimoto BH, Williams DR, Gozes I. Critical appraisal of the role of davunetide in the treatment of progressive supranuclear palsy. Neuropsychiatric Disease Treatment. 2012;8:85–93. doi: 10.2147/NDT.S12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.