Abstract
Mesenchymal stem cells (MSC) are adult-derived multipotent stem cells that have been derived from almost every tissue. They are classically defined as spindle-shaped, plastic-adherent cells capable of adipogenic, chondrogenic, and osteogenic differentiation. This capacity for trilineage differentiation has been the foundation for research into the use of MSC to regenerate damaged tissues. Recent studies have shown that MSC interact with cells of the immune system and modulate their function. Although many of the details underlying the mechanisms by which MSC modulate the immune system have been defined for human and rodent (mouse and rat) MSC, much less is known about MSC from other veterinary species. This knowledge gap is particularly important because the clinical use of MSC in veterinary medicine is increasing and far exceeds the use of MSC in human medicine. It is crucial to determine how MSC modulate the immune system for each animal species as well as for MSC derived from any given tissue source. A comparative approach provides a unique translational opportunity to bring novel cell-based therapies to the veterinary market as well as enhance the utility of animal models for human disorders. The current review covers what is currently known about MSC and their immunomodulatory functions in veterinary species, excluding laboratory rodents.
Abbreviations: AT, adipose tissue; BM, Bone marrow; CB, umbilical cord blood; CT, umbilical cord tissue; DC, dendritic cell; IDO, indoleamine 2;3-dioxygenase; MSC, mesenchymal stem cells; PGE2, prostaglandin E2; VEGF, vascular endothelial growth factor
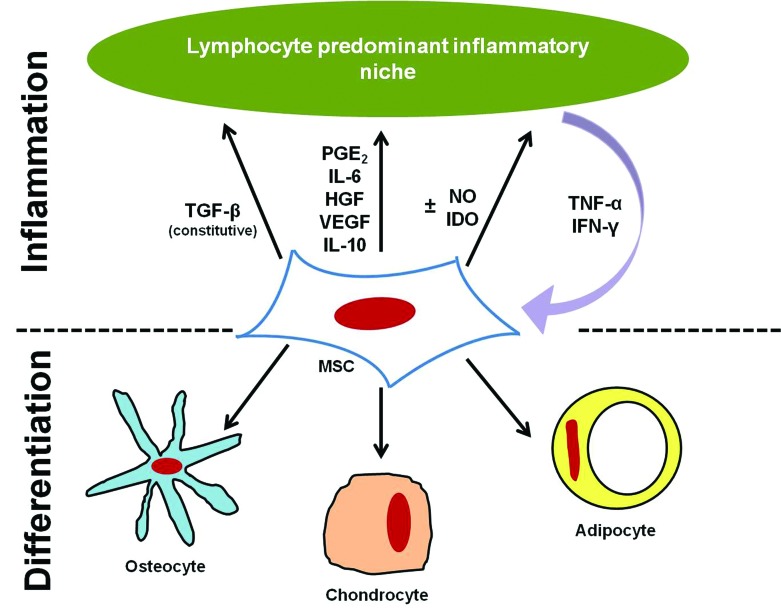
Mesenchymal stem cells (MSC, alternatively known as mesenchymal stromal cells) were first reported in the literature in 1968.39 MSC are thought to be of pericyte origin (cells that line the vasculature)21,22 and typically are isolated from highly vascular tissues. In humans and mice, MSC have been isolated from fat, placental tissues (placenta, Wharton jelly, umbilical cord, umbilical cord blood), hair follicles, tendon, synovial membrane, periodontal ligament, and every major organ (brain, spleen, liver, kidney, lung, bone marrow, muscle, thymus, pancreas, skin).23,121 For most current clinical applications, MSC are isolated from adipose tissue (AT), bone marrow (BM), umbilical cord blood (CB), and umbilical cord tissue (CT; Table 1). Both in human and veterinary medicine, MSC promote tissue regeneration and healing via modulation of the immune response, including decreasing the cells and cytokines associated with inflammation and increasing blood flow to promote normal healing rather than scarring.11,87,99 Clinical trials in human medicine focus on the use of MSC both for their antiinflammatory properties (graft-versus-host disease, irritable bowel syndrome) and their ability to aid in tissue and bone regeneration in combination with growth factors and bone scaffolds (clinicaltrials.gov).131 For tissue regeneration, the abilities of MSC to differentiate and to secrete mediators and interact with cells of the immune system likely contribute to tissue healing (Figure 1). The current review will not address the specific use of MSC for orthopedic applications and tissue regeneration, although the topic is covered widely in current literature for both human and veterinary medicine.57,62,90
Table 1.
Tissues from which MSC have been isolated
| Tissue source (reference no.) |
|||||
| Species | Fat | Bone marrow | Cord blood | Cord tissue | Other |
| Cat | 134 | 83 | 56 | ||
| Chicken | 63 | ||||
| Cow | 138 | 12 | 108 | ||
| Dog | 97 | 3, 59 | 78, 119 | 139 | Periodontal ligament65 |
| Goat | 66 | 96 | 4 | ||
| Horse | 26, 130 | 37, 40, 123 | 67 | 130 | Periodontal ligament and gingiva88 |
| Nonhuman primate | 28, 54 | 5 | |||
| Pig | 135 | 114 | 70 | 14, 20, 91 | |
| Rabbit | 128 | 80 | 32 | Fetal liver93 | |
| Sheep | 84 | 95 | 42, 55 | ||
Figure 1.
The dual roles of MSC: differentiation and modulation of inflammation.
Long-term studies in veterinary species have shown no adverse effects with the administration of MSC in a large number of animals.9,10,53 Smaller, controlled studies on veterinary species have shown few adverse effects, such as minor localized inflammation after MSC administration in vivo.7,15,17,45,86,92,98 Private companies, educational institutions, and private veterinary clinics (including Tufts University, Cummins School of Veterinary Medicine, University of California Davis School of Veterinary Medicine, VetStem, Celavet, Alamo Pintado Equine Medical Center, and Rood and Riddle Equine Hospital) offer MSC as a clinical treatment for veterinary species. Clinical uses include tendon and cartilage injuries, tendonitis, and osteoarthritis and, to a lesser extent, bone regeneration, spinal cord injuries, and liver disease in both large and small animals.38,41,113 Even with this broad clinical use, there have been no reports of severe adverse effects secondary to MSC administration in veterinary patients.
MSC Characterization
MSC are defined as highly proliferative, plastic-adherent, fibroblast-like cells capable of osteogenic, chondrogenic, and adipogenic differentiation.39,101 MSC are further characterized according to their surface protein expression by flow cytometry or immunophenotyping. Full characterization of MSC from veterinary species is hindered by the lack of available species-specific antibodies and reagents. The identification of crossreactive antibodies and the generation of species-specific reagents have been augmented recently by large-scale collaborations (http://www.umass.edu/vetimm/). In addition, several studies have screened antibodies for crossreactivity to MSC from veterinary species.25,118 Similar to those from humans and mice, MSC from veterinary species express MHC class I but not MHCII or the T-lymphocyte costimulatory molecules CD86 and CD80 (Table 2) .68,69 Because no single, specific MSC marker has been identified, a panel of antibodies is used. Human MSC are defined as positive for CD105, CD73, and CD90 and negative for CD45, CD34, CD14, CD11b, CD79a, and CD19.31 Table 2 provides a comprehensive list of antibodies used to identify MSC in veterinary species from different tissue sources.
Table 2.
MSC surface markers, as determined by flow cytometry, RT-PCR, or immunocytochemistry
| Species | MSC type | Positive | Negative | Reference | |
| Cat | AT | Reported | CD44, CD90, CD105 | CD4, MHCII | 134 |
| BM | Confirmed | MHCI, CD44, CD9 | CD4, CD8, CD13, CD14, CD18, CD41/61, CD45, MHCII | 83 | |
| Reported | CD90, CD105 | 134 | |||
| Chicken | BM | Confirmed | CD44, CD90, CD105 | CD45 | 63 |
| Dog | AT | Confirmed | MHCI, CD44, CD90 | MHCII, pan-lymphocyte, CD14, CD45, CD3, CD4, CD8, CD172a, CD11c | 61 |
| BM | Confirmed | CD29, CD90, CD44, CD73, CD106, CD10, CD13 | CD34, CD14, CD105, CD3, CD45 | 60, 77 | |
| Reported | MHCI | MHCII | 60 | ||
| CT | Reported | CD44, CD29, CD90 | CD34, CD45, CD14, CD117 | 139 | |
| CB | Reported | CD29, CD33, CD44, CD105, CD184 | CD4, CD8a, CD10, CD14, CD20, CD24, CD31, CD34, CD38, CD41a, MHCII, CD45, CD49b, CD41/61, CD62p, CD73, CD90, CD133 | 119 | |
| Goat | CT | Reported | CD44 | CD34 | 4 |
| Horse | AT | Confirmed | MHCI, CD29, CD90, CD44 | MHCII, CD86, F6B (pan-leukocyte) | 16 |
| Reported | CD73, CD105 | CD14, CD34, CD79a, CD45 | 13, 48, 107 | ||
| BM | Confirmed | MHCI, CD29, CD90, CD44 | MHCII, CD86, F6B (pan-leukocyte) | 16 | |
| Reported | CD73, CD105 | CD14, CD34, CD79a, CD45 | 13, 48, 107 | ||
| CT | Confirmed | MHCI, CD29, CD90, CD44, CD105, CD166 | MHCII, CD86, F6B (panleukocyte), CD34 | 15, 16 | |
| CB | Confirmed | MHCI, CD29, CD90, CD44 | MHCII, CD86, F6B (panleukocyte) | 16, 74 | |
| Reported | CD79a | 48 | |||
| Nonhuman primate | AT | Reported | CD90, MHCI, CD105, CD59, CD106, CD146, CD161, STRO1 | CD3, CD4, CD8, CD11b, CD13, CD31, CD164 | 54 |
| BM | Reported | CD29, CD90, CD44, MHCI, CD105, CD73, CD166, CD56, CD59, CD106, CD146, CD161, STRO1 | CD34, CD14, CD11c, CD45, CD56, CD31, MHCII, CD3, CD4, CD8, CD11b, CD13, CD164 | 8, 54 | |
| Pig | AT | Reported | CD44, CD34 | 136 | |
| BM | Confirmed | CD90, CD29, CD44, MHCI, CD46 | CD172a, CD106, CD56 | 94, 100 | |
| Reported | CD45, MHCII | 50 | |||
| CT | Confirmed | CD90, CD44, MHCI | CD31, CD45RA, MHCII | 20 | |
| CB | Reported | CD29, CD105, CD49b | CD45, CD133 | 70 | |
| Sheep | BM | Reported | CD44, CD105, CD29, CD166 | CD34, CD45, CD14, CD106, CD31, STRO1 | 85, 95 |
Markers listed as ‘confirmed’ were listed as such when the cited study included appropriate validation of antibody crossreactivity (for example through bioassay or Western blots) , documented gene sequences, or published appropriate positive and negative controls. Markers listed as ‘reported’ either could not be verified, usually because the original report did not include appropriate validation or controls, or have prompted controversy in the literature regarding whether the antibodies crossreact with the species tested.
Trilineage differentiation has been demonstrated for MSC from many species and tissues, including cow BM- and CB-MSC;12,108 dog AT-, BM-, CB-, and CT-MSC,59,97,119,139 goat AT- and BM-MSC;96,112 chicken BM-MSC;63 horse AT- BM-, CT-, and CB-MSC;37,67,107,130 sheep AT-, BM-, and CB-MSC;55,84,85,95 rabbit BM- and fetal-liver–MSC;80,93 pig AT- and BM-MSC;114,135 and nonhuman primate BM- and AT-MSC.8,54
Immunomodulatory Properties of MSC
A deeper understanding of the mechanisms by which MSC derived from veterinary species modulate inflammation and contribute to healing will benefit both humans and animals. Many veterinary species serve as models for human diseases for which cellular therapy is currently being investigated (for example, pigs for cardiovascular disease, goats for orthopedic lesions96,120). In addition, MSC therapy increasingly is used as a mainstay for a variety of companion animal disorders including tendon, bone, and cartilage injuries in horses and arthritis in dogs.9,123 MSC have been shown to interact with CD4 and CD8 lymphocytes and, once activated in the presence of pro-inflammatory mediators, secrete mediators that downregulate inflammation.122
Lymphocyte proliferation.
MSC derived from all tissue sources have potent immunomodulatory capabilities in vitro. Autologous and allogeneic MSC are nonimmunogenic, and completely unmatched MSC do not induce leukocyte proliferation in the absence of activation in vitro.16,95,103 MSC are also antiinflammatory. The ability of MSC to inhibit the proliferation of stimulated T lymphocytes in vitro has been well described for MSC from nonhuman primates,5,8 dogs,61,65,77 chickens,63 rabbits,80,93 pigs,20,103 sheep,95 and horses.16,99 Lymphocyte proliferation in vitro is maximally inhibited at a MSC:lymphocyte ratio of 1:1, 1:5 or 1:10.5,16,61,63,80,81 One proposed mechanism for this inhibition of lymphocyte proliferation is MSC-induced T-cell–cycle arrest in G0, which is thought to be regulated at a molecular level by decreases in lymphocytic cyclin D levels.44,65 MSC decrease the expression of activation markers (CD25, CD38, and CD69) on T cells, preventing their activation and proliferation.47,76 Pretreatment of MSC with IFNγ, a mediator largely present in inflammatory environments, further enhances the ability of MSC to decrease lymphocyte proliferation.80,103 Furthermore, in one in vitro experiment, xenogenic pig BM-MSC did not stimulate human lymphocyte proliferation; rather, they dose-dependently inhibited lymphocyte proliferation after stimulation.81
Both cell–cell contact and soluble factors are thought to play a role in MSC-induced inhibition of lymphocyte proliferation. Toll-like receptors, intracellular adhesion molecule 1, and vascular cell adhesion molecule 1 on the surface of MSC and FAS-ligand–dependent interactions are thought to play a part in cell–cell mediated immunosuppression, although this contribution has not been examined in veterinary species.2,27,79,111,127 MSC inhibit lymphocyte proliferation even in transwell assays where MSC are physically separated from lymphocytes, supporting the idea that MSC produce soluble factors involved in immunomodulatory activity.61,77,89 In addition, preconditioned media taken from cultures of activated MSC, defined as those MSC exposed to proinflammatory mediators, inhibits lymphocyte proliferation.61
MSC interactions with other immune cells have been studied widely in both humans and rodents, although this research has not yet been broadly extended to MSC from veterinary species. Human MSC decrease proliferation of both CD4+ and CD8+ T cells, cause a shift toward a Th2 phenotype, and inhibit Th17 differentiation and function.1,43 Human and rodent MSC modulate dendritic cell (DC) development from monocytes and impair DC function. Impaired DC function includes modulation in MHCII and T-cell costimulatory molecule expression, downregulation of cytokine production, and prevention of DC homing to lymph nodes.35,43,137 The downstream effect of these changes includes limitation of the ability of DC to stimulate a T cell response. Similar to their effect on T lymphocytes, human MSC inhibit B-cell proliferation in a dose-dependent manner, blocking progression of the cell cycle.46,125,137
Mediator production.
Soluble immunosuppressive factors demonstrated to be produced or expressed by MSC from veterinary species include TGFβ1,8,16,61,77,80,103 hepatocyte growth factor,61 PGE2,16,61,77 indoleamine 2,3-dioxygenase (IDO),61 nitric oxide (NO),16,63 vascular endothelial growth factor,8,77 and IL68,16,61 (Table 3). Mediator production by MSC in veterinary species has not been tested exhaustively, and the mediators reported in Table 3 are those that have been published in the literature. A mediator's absence from the list does not imply that MSC do not produce it but rather that its production has not yet been determined. Some mediators are produced constitutively, whereas others are secreted after MSC activation by cytokines or mediators found in inflammatory environments. Mediators produced by MSC downregulate inflammation and stimulate angiogenesis.33,64,136
Table 3.
Mediator production by MSC in veterinary species
| Mediators |
||||
| Species | MSC type | ELISA | Gene expression | Reference |
| Chicken | BM | NOa | 63 | |
| Dog | AT | TGFβ, HGF, PGE2, IDO | TGFβ, IL6, IL8, VEGF, HGF, COX2 | 61 |
| BM | TGFβ, VEGF, PGE2 | 77 | ||
| Horse | AT, CT | PGE2, IL6, TGFβ | 16 | |
| BM, CB | PGE2, IL6, TGFβ, NOa | 16 | ||
| Nonhuman primate | BM | IL6, VEGF, TGFβ, HGF | 8 | |
| Pig | BM | TGFβ, IL10 | 103 | |
| Rabbit | BM | IL10, TGFβ | 80 | |
HGF, hepatocyte growth factor; IDO, indoleamine 2;3-dioxygenase; IL, interleukin; PGE2, prostaglandin E2; TGF, transforming growth factor; VEGF, vascular endothelial growth factor
NO measured by using Greiss reagent.
The particular mediators produced by MSC can vary by species and by tissue source. Horse MSC differentially produce mediators depending on MSC tissue source: equine MSC derived from hemic sources (BM- and CB-MSC) produce NO, whereas those MSC derived from solid tissues (CT- and AT-MSC) do not.16 Whether these differences in mediator production in vitro confer any functional differences in vivo is unknown, although anecdotal evidence suggests decreased healing time for equine tendon lesions after the injection of equine CB-MSC compared with other tissue-derived MSC. We speculate that the production of NO by horse CB-MSC causes increased angiogenesis in vivo. Species- associated variability in the production of NO and IDO by MSC has been described. Human MSC produce high levels of IDO and do not produce NO, whereas mouse MSC produce high levels of NO and do not produce IDO.109 Human and mouse MSC are reported to induce T regulatory cells (CD4+CD25+ FoxP3+), a population of T cells that suppress the immune response and promote T-cell tolerance to antigens.29,36 This induction, or preferential production, of T-regulatory cells is dictated by the presence of TGFβ1 and IL10 in the microenvironment. Research is ongoing with regard to constitutive compared with induced production of TGFβ1 by human MSC.47,105
TGFβ1 production by veterinary species varies by species, tissue type, and MSC activation status. Dog BM-MSC, pig BM-MSC, and horse AT-, BM-, CT-, and CB-MSC do not increase TGFβ1 production after exposure to activated lymphocytes.16,77,103 Dog BM-MSC produce TGFβ1 at levels insufficient to inhibit lymphocyte proliferation.77 Conversely, dog AT-MSC increase TGFβ1 production after exposure to lymphocytes, and rabbit BM-MSC increase their TGFβ1 production after IFNγ pretreatment in vitro.61,80 To date, no studies in veterinary species have demonstrated that MSC production of TGFβ1 shifts T lymphocytes to a T-regulatory phenotype.
The role of specific mediators on lymphocyte proliferation or function can be evaluated via chemical blockade of individual mediators and measurement of corresponding lymphocyte proliferation in a MSC–lymphocyte coculture (mixed lymphocyte reaction). Although not yet documented in veterinary species, blocking TGFβ1 produced by human BM-MSC results in decreased production of T-regulatory cells36 and a significant reversal in the inhibition of T cell proliferation,30,47 indicating that TGFβ1 has an important role in MSC function. Blocking PGE261,77 or IDO61 produced by dog BM- or AT-MSC restores lymphocyte proliferation, indicating that both mediators have functional roles in modulating the MSC–lymphocyte interaction. In addition, according to our experience, PGE2 appears to be the primary mediator responsible for inhibition of lymphocyte proliferation by horse AT-, BM-, CT-, and CB-MSC because blocking PGE2 restores lymphocyte proliferation.
MSC immunomodulatory function is stimulated by proinflammatory mediators, namely IFNγ and TNFα34,103 (Figure 1). MSC derived from IFNγ-receptor knock-out mice are unable to inhibit a lymphocyte proliferative response.110 At baseline, neither unstimulated lymphocytes nor MSC produce these proinflammatory mediators.16,103 However, activated lymphocytes secrete IFNγ and TNFα and stimulate MSC. Dog AT-MSC decrease TNFα production and increase IFNγ production by lymphocytes,61 whereas horse BM-, AT-, CB-, and CT-MSC decrease both TNFα and IFNγ.16,99 Pig BM-MSC decrease IFNγ and IL2 production by lymphocytes. Whether the decrease in these mediators is related to a decrease in the total number of lymphocytes present in culture or due to a functional shift in lymphocyte phenotype and the induction of T-regulatory cells is unknown.
The relevance of these mediators in vivo is largely unknown in veterinary medicine. Few studies have measured mediators in fluids or tissues, and both the kinetics of inflammation and the redundant functions of many mediators make interpretation of mediator concentrations at isolated time points difficult. In an equine model of osteoarthritis, PGE2 levels were significantly decreased in synovial fluid after BM-MSC treatment compared with those in untreated affected joints.40 The authors attributed this difference to the decrease in swelling and inflammation noted 1 week after MSC administration.40 Human MSC exposed in vitro to joint fluids taken from patients with osteoarthritis and rheumatoid arthritis upregulated their mRNA expression of IL6 and IDO and suppressed lymphocyte proliferation.
There is still some disparity in the literature regarding species-specific identification of inflammatory mediators that are produced by or inhibited by MSC. Variability in results could stem from the methodology by which researchers measure cytokine production; test kits and reagents are not always specific for veterinary species and have been adapted from human or lab animal methodologies. Large-scale collaborations and increased communication through the veterinary stem cell research community will enable all researchers to ensure they are accessing the most appropriate tools for their research.
MSC In Vivo
In vitro compared with in vivo results.
MSC safety and function has been studied in vivo in healthy animals, in patients with naturally occurring disease, and in animals serving as models of human diseases. Although extensive in vitro studies have shown that MSC are effective at decreasing inflammation, in vivo results are variable. Factors that contribute to variable therapeutic outcomes include natural variation in disease processes and lack of standards for MSC dose, route of administration, tissue source, or measured endpoints. Table 4 outlines the species, MSC dose, MSC source, and route of administration for the in vivo studies discussed in the current review.
Table 4.
MSC sources, dose ranges, and routes of administration for in vivo studies
| Species | MSC type | Dose | Route of administration | Reference |
| Dog | BM | 1.1-1.8 × 106/kg | intravenous | 77 |
| BM | 1 × 106/kg | intravenous | 89 | |
| Horse | CT | 1 × 106 | intradermal | 15 |
| CT | 7.5 × 106 | intraarticular | 17 | |
| BM | 5.6-15 × 106 | intraarticular | 40 | |
| Nonhuman primate | BM | 20 × 106/kg | intravenous | 5 |
| BM | 4.3-6.4 × 108/kg | intraportal | 8 | |
| BM | 3.4-6.5 × 106/kg | intravenous | 8 | |
| Pig | BM | 15-18 × 106 | subcutaneous | 103 |
| BM | 10-40 × 106 | subpericardial | 103 | |
| BM | 1 × 107/dose | intravenous | 72 | |
| BM | 1 × 107/dose | intravenous | 71 |
An example of a strong correlation between in vitro and in vivo studies was a skin graft model in baboons. Skin grafts from MHC-mismatched donors were transplanted to adult baboons, after which a single BM-MSC dose was administered intravenously. Grafts in baboons that received MSC infusions were rejected more slowly (11 d) than were those in animals not treated with MSC (7 d).5 The delay in graft rejection attributed to MSC was comparable to the alleviation of graft rejection by antigraft rejection pharmaceuticals currently on the market.5 The authors of the study postulated that the MSC were sufficient to subdue the lymphocyte response but insufficient to inhibit recruitment of inflammatory cells to the graft.5
Other examples illustrate poor correlation between in vitro and in vivo studies. In vitro, pig BM-MSC decreased lymphocyte proliferation and inhibited production of pro-inflammatory mediators.103 In vivo, allogeneic pig BM-MSC elicited both a cellular and humoral response when injected either subcutaneously or intracardiac.103 Antidonor alloantibodies (IgM or IgG) were detected after a single or multiple subcutaneous doses of BM-MSC, although cytolytic activity was not detected after single doses of MSC.103 Intracardiac injection of single or multiple doses of BM-MSC elicited both alloantibody production and cytolytic activity of donor MSC;103 Limitations to the study included low sample number (n = 2 for subcutaneous, n = 3 for intracardiac) and variable numbers of injected MSC (range, 1.5 × 107 to 1.20 × 108 MSC). The results of the cited study suggest that allogeneic BM-MSC in this pig model are not anti-inflammatory and may not escape immune surveillance. The findings of this study103 are notable as a general exception to a body of literature in veterinary and human medicine suggesting that allogeneic MSC are well-tolerated and highlight the need for standardized methods of measuring the immune response to MSC both in vivo and in vitro.
Studies comparing autologous (self) and allogeneic (non-self) MSC are prominent in veterinary medicine, with a focus on healing of orthopedic injuries.49,58,102 A few studies in veterinary medicine have focused on the safety and efficacy of autologous and allogeneic MSC with regard to their immunomodulatory properties. Our study comparing intradermal injection of autologous and allogeneic equine CT-MSC revealed that 2 rounds of intradermal injections failed to induce a significant cell-mediated response, as measured in vivo by wheal formation and induration and ex vivo by histopathology on biopsied tissue and by mixed lymphocyte reactions.15 Wheal formation and induration indicated no difference between MSC injection and control (saline) injections.15 Results from mixed lymphocyte reactions indicated that neither autologous or allogeneic MSC stimulated nor suppressed baseline T-cell proliferation, even after multiple MSC injections. Taken together, the results indicate that CT-MSC could be administered in vivo multiple times without eliciting a cellular immune response.15 This in vivo study parallels the in vitro findings comparing immunomodulation by horse AT-, BM-, CT-, and CB-derived MSC.16 A similar study showed no difference in inflammatory response to horse autologous compared with allogeneic MSC after intraarticular administration.17
Graft-versus-host disease and T regulatory cells.
Clinical trials are underway to determine the efficacy of MSC in treating humans with graft-versus-host disease.52,73,75 However, results of studies using MSC to treat graft-versus-host disease in veterinary species have been mixed. Two studies in dogs showed no difference in the rate of graft rejection between dogs that received MSC after dog leukocyte antigen-identical bone marrow transplants compared with those that did not receive MSC treatment, even though in vitro data demonstrated diminished leukocyte proliferation in the presence of MSC.77,89 Two other studies found that pigs given composite tissue allografts and BM-MSC had prolonged graft survival when compared with animals that did not receive BM-MSC treatment.71,72 In the cited study,72 pigs given cyclosporine along with irradiation had marked evidence of graft-versus-host disease, whereas pigs given cyclosporine, irradiation, and MSC had no evidence of graft-versus-host disease and the least rejection of transplanted tissue.72 In a second set of experiments, T-cell phenotypes were investigated in peripheral blood and graft tissue.71 The authors found a significant increase in the percentages of CD4+CD25+ and CD4+FoxP3+ T cells (T regulatory cells) in both the blood and graft when pigs were given cyclosporine, irradiation, and MSC compared with pigs that did not receive MSC, indicating that T-regulatory cells were induced both globally and locally.71
T-regulatory cells increase after MSC infusion in a primate model of allogeneic islet cell engraftment. Nonhuman primates that received islet cells and BM-MSC had significantly enhanced islet function 1 month after transplantation, compared with those that had not received MSC. Rejection episodes in the animals that had not received MSC were reversed with additional infusions of allogeneic BM-MSC.8 The presence of T regulatory cells in peripheral blood was increased after episodes of rejection and additional MSC infusion when compared with levels before MSC infusion.8 Graft dysfunction was noted as T-regulatory cells decreased in peripheral blood.8
Benefits and Risks
Traditional drug therapy to downregulate the immune response (for example, cyclosporine, steroids) is associated with several undesirable side effects, including increased risk of secondary infections and other ‘bystander’ effects that limit the long-term use of these drugs. Although the associated research is in its infancy, modifying the immune response by using cellular therapy may reduce these bystander effects. The cells may be modified by the niche into which they are injected, with less prolonged immune suppression. MSC may be given as an adjunct therapy and thus lower the effective dose of immunosuppressive drugs. Several studies in humans and rodents have linked favorable outcomes in solid-organ transplant recipients with combined use of MSC and traditional immunosuppressive therapeutics.82,126 The dose and method of administration of cellular therapies can easily be adapted to suit the needs of the patient.
As with any new drug or procedure, risks related to the use of MSC as a clinical product are continually assessed and analyzed.51,104 Risks include the potential for tumor formation or inappropriate MSC differentiation, for transmission of infectious disease through contaminated product, and of trauma at the site of administration.51 Disease transmission via MSC has not been described in the literature, although in human medicine, stem cell donors are screened rigorously for infectious disease.115,124 Extensive screening of veterinary donors for infectious disease will be a necessary component of allogeneic MSC product use. MSC products are cultured routinely for microbial contamination prior to administration. Conceptually, given their ability for trilineage differentiation and their immunosuppressive features, MSC could either form tumors or impair tumor surveillance and thus increase the risks of tumor formation. Tumor promotion or formation after MSC administration has not been studied specifically in veterinary medicine, however it has been evaluated widely in rodents and human medicine.6,18,106,132 Most experimental data suggest that MSC do not form tumors in vivo. In addition, tumors in humans or veterinary patients that have received MSC have not been reported (many of our horse patients have been monitored for at least 6 y). Malignant transformation of MSC in vitro has been reported, although several studies have been retracted after the discovery that these MSC cell cultures were contaminated with cancer cell lines.24,117,129
Areas of Future Study
Several published reports have indicated that although MSC are largely anti-inflammatory, they also may have the capacity to function as antigen-presenting cells and further an inflammatory response.19,116 Human and mouse BM-MSC, exposed to low levels of IFNγ in vitro, showed increased MHCII expression and decreased ability to inhibit lymphocyte proliferation.19,116 It has been proposed that MSC are binary in nature, with the capability of sensing their environment through toll-like receptors (or yet other unidentified receptors) and responding as either pro-inflammatory antigen-presenting cells or as anti-inflammatory cells.133 This binary action may explain some of the discrepant results from in vivo studies and furthers the need for study of MSC in diverse species and disease conditions.
MSC from veterinary species have been well studied with regard to their ability to decrease lymphocyte proliferation and produce immunomodulatory mediators in vitro. However, many questions remain to be answered. For example, we do not know how MSC interact with and regulate B cells, natural killer cells, DCs, or neutrophils; whether MSC from veterinary species alter lymphocyte phenotype, induce the development of T regulatory cells in vitro as well as in vivo; or whether MSC are anti-inflammatory in vivo in all species. Additional studies undertaken by researchers and collaborative working groups, as well as information sharing though professional organizations such as the North American Veterinary Regenerative Medicine Association, will play a crucial role in answering many of these questions.
References
- 1.Aggarwal S, Pittenger MF. 2005. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815–1822 [DOI] [PubMed] [Google Scholar]
- 2.Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. 2012. Mesenchymal-stem–cell-induced immunoregulation involves FAS-ligand-, FAS-mediated T cell apoptosis. Cell Stem Cell 10:544–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arinzeh TL, Peter SJ, Archambault MP, van den Bos C, Gordon S, Kraus K, Smith A, Kadiyala S. 2003. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg Am 85-A:1927–1935 [DOI] [PubMed] [Google Scholar]
- 4.Azari O, Babaei H, Derakhshanfar A, Nematollahi-Mahani SN, Poursahebi R, Moshrefi M. 2011. Effects of transplanted mesenchymal stem cells isolated from Wharton's jelly of caprine umbilical cord on cutaneous wound healing; histopathological evaluation. Vet Res Commun 35:211–222 [DOI] [PubMed] [Google Scholar]
- 5.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. 2002. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30:42–48 [DOI] [PubMed] [Google Scholar]
- 6.Bauer G, Dao MA, Case SS, Meyerrose T, Wirthlin L, Zhou P, Wang X, Herrbrich P, Arevalo J, Csik S, Skelton DC, Walker J, Pepper K, Kohn DB, Nolta JA. 2008. In vivo biosafety model to assess the risk of adverse events from retroviral and lentiviral vectors. Mol Ther 16:1308–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beggs KJ, Lyubimov A, Borneman JN, Bartholomew A, Moseley A, Dodds R, Archambault MP, Smith AK, McIntosh KR. 2006. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant 15:711–721 [DOI] [PubMed] [Google Scholar]
- 8.Berman DM, Willman MA, Han D, Kleiner G, Kenyon NM, Cabrera O, Karl JA, Wiseman RW, O'Connor DH, Bartholomew AM, Kenyon NS. 2010. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes 59:2558–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black LL, Gaynor J, Adams C, Dhupa S, Sams AE, Taylor R, Harman S, Gingerich DA, Harman R. 2008. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther 9:192–200 [PubMed] [Google Scholar]
- 10.Black LL, Gaynor J, Gahring D, Adams C, Aron D, Harman S, Gingerich DA, Harman R. 2007. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther 8:272–284 [PubMed] [Google Scholar]
- 11.Borjesson DL, Peroni JF. 2011. The regenerative medicine laboratory: facilitating stem cell therapy for equine disease. Clin Lab Med 31:109–123 [DOI] [PubMed] [Google Scholar]
- 12.Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. 2005. Isolation and multilineage differentiation of bovine bone marrow mesenchymal stem cells. Cell Tissue Res 319:243–253 [DOI] [PubMed] [Google Scholar]
- 13.Braun J, Hack A, Weis-Klemm M, Conrad S, Treml S, Kohler K, Walliser U, Skutella T, Aicher WK. 2010. Evaluation of the osteogenic and chondrogenic differentiation capacities of equine adipose tissue-derived mesenchymal stem cells. Am J Vet Res 71:1228–1236 [DOI] [PubMed] [Google Scholar]
- 14.Carlin R, Davis D, Weiss M, Schultz B, Troyer D. 2006. Expression of early transcription factors Oct4, Sox2, and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod Biol Endocrinol 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrade DD, Affolter VK, Outerbridge CA, Watson JL, Galuppo LD, Buerchler S, Kumar V, Walker NJ, Borjesson DL. 2011. Intradermal injections of equine allogeneic umbilical cord-derived mesenchymal stem cells are well tolerated and do not elicit immediate or delayed hypersensitivity reactions. Cytotherapy 13:1180–1192 [DOI] [PubMed] [Google Scholar]
- 16.Carrade DD, Lame MW, Kent MS, Clark KC, Walker NJ, Borjesson DL. 2012. Comparative analysis of the immunomodulatory properties of equine adult-derived mesenchymal stem cells. Cell Med 4:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrade DD, Owens SD, Galuppo LD, Vidal MA, Ferraro GL, Librach F, Buerchler S, Friedman MS, Walker NJ, Borjesson DL. 2010. Clinicopathologic findings following intraarticular injection of autologous and allogeneic placentally derived equine mesenchymal stem cells in horses. Cytotherapy 13:419–430 [DOI] [PubMed] [Google Scholar]
- 18.Centeno CJ, Schultz JR, Cheever M, Robinson B, Freeman M, Marasco W. 2010. Safety and complications reporting on the reimplantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther 5:81–93 [DOI] [PubMed] [Google Scholar]
- 19.Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, Rameshwar P. 2006. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon γ. Blood 107:4817–4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho PS, Messina DJ, Hirsh EL, Chi N, Goldman SN, Lo DP, Harris IR, Popma SH, Sachs DH, Huang CA. 2008. Immunogenicity of umbilical cord tissue derived cells. Blood 111:430–438 [DOI] [PubMed] [Google Scholar]
- 21.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. 2008. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313 [DOI] [PubMed] [Google Scholar]
- 22.da Silva Meirelles L, Caplan AI, Nardi NB. 2008. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26:2287–2299 [DOI] [PubMed] [Google Scholar]
- 23.da Silva Meirelles L, Chagastelles PC, Nardi NB. 2006. Mesenchymal stem cells reside in virtually all postnatal organs and tissues. J Cell Sci 119:2204–2213 [DOI] [PubMed] [Google Scholar]
- 24.de la Fuente R, Bernad A, Garcia-Castro J, Martin MC, Cigudosa JC. 2010. Retraction: spontaneous human adult stem cell transformation. Cancer Res 70:6682. [DOI] [PubMed] [Google Scholar]
- 25.De Schauwer C, Piepers S, Van de Walle GR, Demeyere K, Hoogewijs MK, Govaere JL, Braeckmans K, Van Soom A, Meyer E. 2012. In search for crossreactivity to immunophenotype equine mesenchymal stromal cells by multicolor flow cytometry. Cytometry A 81:312–323 [DOI] [PubMed] [Google Scholar]
- 26.Del Bue M, Ricco S, Ramoni R, Conti V, Gnudi G, Grolli S. 2008. Equine adipose-tissue–derived mesenchymal stem cells and platelet concentrates: their association in vitro and in vivo. Vet Res Commun 32:S51–S55 [DOI] [PubMed] [Google Scholar]
- 27.DelaRosa O, Lombardo E. 2010. Modulation of adult mesenchymal stem cells activity by toll-like receptors: implications on therapeutic potential. Mediators Inflamm 2010:865601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devine SM, Bartholomew AM, Mahmud N, Nelson M, Patil S, Hardy W, Sturgeon C, Hewett T, Chung T, Stock W, Sher D, Weissman S, Ferrer K, Mosca J, Deans R, Moseley A, Hoffman R. 2001. Mesenchymal stem cells are capable of homing to the bone marrow of nonhuman primates following systemic infusion. Exp Hematol 29:244–255 [DOI] [PubMed] [Google Scholar]
- 29.Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, Sportoletti P, Falzetti F, Tabilio A. 2008. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol 36:309–318 [DOI] [PubMed] [Google Scholar]
- 30.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. 2002. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99:3838–3843 [DOI] [PubMed] [Google Scholar]
- 31.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317 [DOI] [PubMed] [Google Scholar]
- 32.Dormer NH, Singh M, Zhao L, Mohan N, Berkland CJ, Detamore MS. 2012. Osteochondral interface regeneration of the rabbit knee with macroscopic gradients of bioactive signals. J Biomed Mater Res A 100:162–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Menoufy H, Aly LA, Aziz MT, Atta HM, Roshdy NK, Rashed LA, Sabry D. 2010. The role of bone-marrow–derived mesenchymal stem cells in treating formocresol induced oral ulcers in dogs. J Oral Pathol Med 39:281–289 [DOI] [PubMed] [Google Scholar]
- 34.English K, Barry FP, Field-Corbett CP, Mahon BP. 2007. IFNγ and TNFα differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett 110:91–100 [DOI] [PubMed] [Google Scholar]
- 35.English K, Mahon BP. 2011. Allogeneic mesenchymal stem cells: agents of immune modulation. J Cell Biochem 112:1963–1968 [DOI] [PubMed] [Google Scholar]
- 36.English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. 2009. Cell contact, prostaglandin E(2) and transforming growth factor β 1 play nonredundant roles in human mesenchymal stem cell induction of CD4+CD25highforkhead box P3+ regulatory T cells. Clin Exp Immunol 156:149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortier LA, Nixon AJ, Williams J, Cable CS. 1998. Isolation and chondrocytic differentiation of equine bone marrow-derived mesenchymal stem cells. Am J Vet Res 59:1182–1187 [PubMed] [Google Scholar]
- 38.Fortier LA, Travis AJ. 2011. Stem cells in veterinary medicine. Stem Cell Res Ther 2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. 1968. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6:230–247 [PubMed] [Google Scholar]
- 40.Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. 2009. Evaluation of adipose-derived stromal vascular fraction or bone-marrow–derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res 27:1675–1680 [DOI] [PubMed] [Google Scholar]
- 41.Frisbie DD, Smith RK. 2010. Clinical update on the use of mesenchymal stem cells in equine orthopaedics. Equine Vet J 42:86–89 [DOI] [PubMed] [Google Scholar]
- 42.Fuchs JR, Hannouche D, Terada S, Zand S, Vacanti JP, Fauza DO. 2005. Cartilage engineering from ovine umbilical cord blood mesenchymal progenitor cells. Stem Cells 23:958–964 [DOI] [PubMed] [Google Scholar]
- 43.Ghannam S, Pene J, Torcy-Moquet G, Jorgensen C, Yssel H. 2010. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T-regulatory cell phenotype. J Immunol 185:302–312 [DOI] [PubMed] [Google Scholar]
- 44.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. 2005. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 105:2821–2827 [DOI] [PubMed] [Google Scholar]
- 45.Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RK. 2012. Implantation of bone-marrow–derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J 44:25–32 [DOI] [PubMed] [Google Scholar]
- 46.Griffin MD, Ritter T, Mahon BP. 2010. Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum Gene Ther 21:1641–1655 [DOI] [PubMed] [Google Scholar]
- 47.Groh ME, Maitra B, Szekely E, Koc ON. 2005. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol 33:928–934 [DOI] [PubMed] [Google Scholar]
- 48.Guest DJ, Ousey JC, Smith MRW. 2008. Defining the expression of marker genes in equine mesenchymal stromal cells. Stem Cells Cloning Adv Appl 2008:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guest DJ, Smith MR, Allen WR. 2008. Monitoring the fate of autologous and allogeneic mesenchymal progenitor cells injected into the superficial digital flexor tendon of horses: preliminary study. Equine Vet J 40:178–181 [DOI] [PubMed] [Google Scholar]
- 50.Guo KT, Schafer R, Paul A, Gerber A, Ziemer G, Wendel HP. 2006. A new technique for the isolation and surface immobilization of mesenchymal stem cells from whole bone marrow using high-specific DNA aptamers. Stem Cells 24:2220–2231 [DOI] [PubMed] [Google Scholar]
- 51.Herberts CA, Kwa MS, Hermsen HP. 2011. Risk factors in the development of stem cell therapy. J Transl Med 9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herrmann R, Sturm M, Shaw K, Purtill D, Cooney J, Wright M, Phillips M, Cannell P. 2012. Mesenchymal stromal cell therapy for steroid-refractory acute and chronic graft-versus-host disease: a phase 1 study. Int J Hematol 95:182–188 [DOI] [PubMed] [Google Scholar]
- 53.Herthel DJ. 2001. Enhanced suspensory ligament healing in 100 horses by stem cells and other bone marrow components. Proc Am Assoc Equine Practitioners 47:319–321 [Google Scholar]
- 54.Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. 2006. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem 99:1285–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jager M, Bachmann R, Scharfstadt A, Krauspe R. 2006. Ovine cord blood accommodates multipotent mesenchymal progenitor cells. In Vivo 20:205–214 [PubMed] [Google Scholar]
- 56.Jin GZ, Yin XJ, Yu XF, Cho SJ, Choi EG, Lee YS, Jeon JT, Yee ST, Kong IK. 2008. Generation of neuronal-like cells from umbilical cord blood-derived mesenchymal stem cells of a RFP-transgenic cloned cat. J Vet Med Sci 70:723–726 [DOI] [PubMed] [Google Scholar]
- 57.Jones E, Yang X. 2011. Mesenchymal stem cells and bone regeneration: current status. Injury 42:562–568 [DOI] [PubMed] [Google Scholar]
- 58.Jung DI, Ha J, Kang BT, Kim JW, Quan FS, Lee JH, Woo EJ, Park HM. 2009. A comparison of autologous and allogenic bone-marrow–derived mesenchymal stem cell transplantation in canine spinal cord injury. J Neurol Sci 285:67–77 [DOI] [PubMed] [Google Scholar]
- 59.Kadiyala S, Young RG, Thiede MA, Bruder SP. 1997. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant 6:125–134 [DOI] [PubMed] [Google Scholar]
- 60.Kamishina H, Deng J, Oji T, Cheeseman JA, Clemmons RM. 2006. Expression of neural markers on bone-marrow–derived canine mesenchymal stem cells. Am J Vet Res 67:1921–1928 [DOI] [PubMed] [Google Scholar]
- 61.Kang JW, Kang KS, Koo HC, Park JR, Choi EW, Park YH. 2008. Soluble factors mediated immunomodulatory effects of canine adipose-tissue–derived mesenchymal stem cells. Stem Cells Dev 17:681–693 [DOI] [PubMed] [Google Scholar]
- 62.Kanitkar M, Tailor HD, Khan WS. 2011. The use of growth factors and mesenchymal stem cells in orthopaedics. Open Orthop J 5 Suppl 2:271–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khatri M, O'Brien TD, Sharma JM. 2009. Isolation and differentiation of chicken mesenchymal stem cells from bone marrow. Stem Cells Dev 18:1485–1492 [DOI] [PubMed] [Google Scholar]
- 64.Kim CH, Lee JH, Won JH, Cho MK. 2011. Mesenchymal stem cells improve wound healing in vivo via early activation of matrix metalloproteinase-9 and vascular endothelial growth factor. J Korean Med Sci 26:726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim HS, Kim KH, Kim SH, Kim YS, Koo KT, Kim TI, Seol YJ, Ku Y, Rhyu IC, Chung CP, Lee YM. 2010. Immunomodulatory effect of canine periodontal ligament stem cells on allogenic and xenogenic peripheral blood mononuclear cells. J Periodontal Implant Sci 40:265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Knippenberg M, Helder MN, Zandieh Doulabi B, Wuisman PI, Klein-Nulend J. 2006. Osteogenesis versus chondrogenesis by BMP2 and BMP7 in adipose stem cells. Biochem Biophys Res Commun 342:902–908 [DOI] [PubMed] [Google Scholar]
- 67.Koch TG, Heerkens T, Thomsen PD, Betts DH. 2007. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol 7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krampera M, Franchini M, Pizzolo G, Aprili G. 2007. Mesenchymal stem cells: from biology to clinical use. Blood Transfus 5:120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krampera M, Pasini A, Pizzolo G, Cosmi L, Romagnani S, Annunziato F. 2006. Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr Opin Pharmacol 6:435–441 [DOI] [PubMed] [Google Scholar]
- 70.Kumar BM, Yoo JG, Ock SA, Kim JG, Song HJ, Kang EJ, Cho SK, Lee SL, Cho JH, Balasubramanian S, Rho GJ. 2007. In vitro differentiation of mesenchymal progenitor cells derived from porcine umbilical cord blood. Mol Cells 24:343–350 [PubMed] [Google Scholar]
- 71.Kuo YR, Chen CC, Shih HS, Goto S, Huang CW, Wang CT, Chen CL, Wei FC. 2011. Prolongation of composite tissue allotransplant survival by treatment with bone marrow mesenchymal stem cells is correlated with T-cell regulation in a swine hindlimb model. Plast Reconstr Surg 127:569–579 [DOI] [PubMed] [Google Scholar]
- 72.Kuo YR, Goto S, Shih HS, Wang FS, Lin CC, Wang CT, Huang EY, Chen CL, Wei FC, Zheng XX, Lee WP. 2009. Mesenchymal stem cells prolong composite tissue allotransplant survival in a swine model. Transplantation 87:1769–1777 [DOI] [PubMed] [Google Scholar]
- 73.Kuzmina LA, Petinati NA, Parovichnikova EN, Lubimova LS, Gribanova EO, Gaponova TV, Shipounova IN, Zhironkina OA, Bigildeev AE, Svinareva DA, Drize NJ, Savchenko VG. 2012. Multipotent mesenchymal stromal cells for the prophylaxis of acute graft-versus-host disease-a phase ii study. Stem Cells Int 2012:968213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lange Consiglio A, Corradetti B, Rutigliano L, Cremonesi F, Bizzaro D. 2011. In vitro studies of horse umbilical cord matrix-derived cells: from characterization to labeling for magnetic resonance imaging. Open Tissue Eng Regen Med J 4:120–133 [Google Scholar]
- 75.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O. 2008. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371:1579–1586 [DOI] [PubMed] [Google Scholar]
- 76.Le Blanc K, Rasmusson I, Gotherstrom C, Seidel C, Sundberg B, Sundin M, Rosendahl K, Tammik C, Ringden O. 2004. Mesenchymal stem cells inhibit the expression of CD25 (interleukin 2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol 60:307–315 [DOI] [PubMed] [Google Scholar]
- 77.Lee WS, Suzuki Y, Graves SS, Iwata M, Venkataraman GM, Mielcarek M, Peterson LJ, Ikehara S, Torok-Storb B, Storb R. 2011. Canine bone-marrow–derived mesenchymal stromal cells suppress alloreactive lymphocyte proliferation in vitro but fail to enhance engraftment in canine bone marrow transplantation. Biol Blood Marrow Transplant 17:465–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lim JH, Byeon YE, Ryu HH, Jeong YH, Lee YW, Kim WH, Kang KS, Kweon OK. 2007. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci 8:275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liotta F, Angeli R, Cosmi L, Fili L, Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, Santarlasci V, Consoloni L, Angelotti ML, Romagnani P, Parronchi P, Krampera M, Maggi E, Romagnani S, Annunziato F. 2008. Toll-like receptors 3 and 4 are expressed by human bone-marrow–derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells 26:279–289 [DOI] [PubMed] [Google Scholar]
- 80.Liu H, Kemeny DM, Heng BC, Ouyang HW, Melendez AJ, Cao T. 2006. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. J Immunol 176:2864–2871 [DOI] [PubMed] [Google Scholar]
- 81.Liu J, Lu XF, Wan L, Li YP, Li SF, Zeng LY, Zeng YZ, Cheng LH, Lu YR, Cheng JQ. 2004. Suppression of human peripheral blood lymphocyte proliferation by immortalized mesenchymal stem cells derived from bone marrow of Banna minipig inbred line. Transplant Proc 36:3272–3275 [DOI] [PubMed] [Google Scholar]
- 82.Longoni B, Szilagyi E, Puviani L, Mazzanti B, Paoli GT, Urbani S, Quaranta P, Antonini S, Tripodi S, Cintorino M, Saccardi R, Nardo B, Mosca F. 2012. Mesenchymal stem-cell–based immunomodulation in allogeneic heterotopic heart-lung transplantation. J Transplant Technol Res 2:107 [Google Scholar]
- 83.Martin DR, Cox NR, Hathcock TL, Niemeyer GP, Baker HJ. 2002. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp Hematol 30:879–886 [DOI] [PubMed] [Google Scholar]
- 84.Martinez-Lorenzo MJ, Royo-Canas M, Alegre-Aguaron E, Desportes P, Castiella T, Garcia-Alvarez F, Larrad L. 2009. Phenotype and chondrogenic differentiation of mesenchymal cells from adipose tissue of different species. J Orthop Res 27:1499–1507 [DOI] [PubMed] [Google Scholar]
- 85.McCarty RC, Gronthos S, Zannettino AC, Foster BK, Xian CJ. 2009. Characterisation and developmental potential of ovine bone marrow derived mesenchymal stem cells. J Cell Physiol 219:324–333 [DOI] [PubMed] [Google Scholar]
- 86.McIlwraith CW, Frisbie DD, Rodkey WG, Kisiday JD, Werpy NM, Kawcak CE, Steadman JR. 2011. Evaluation of intraarticular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy 27:1552–1561 [DOI] [PubMed] [Google Scholar]
- 87.Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. 2009. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 20:419–427 [DOI] [PubMed] [Google Scholar]
- 88.Mensing N, Gasse H, Hambruch N, Haeger JD, Pfarrer C, Staszyk C. 2011. Isolation and characterization of multipotent mesenchymal stromal cells from the gingiva and the periodontal ligament of the horse. BMC Vet Res 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mielcarek M, Storb R, Georges GE, Golubev L, Nikitine A, Hwang B, Nash RA, Torok-Storb B. 2011. Mesenchymal stromal cells fail to prevent acute graft-versus-host disease and graft rejection after dog leukocyte antigen-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant 17:214–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Milner PI, Clegg PD, Stewart MC. 2011. Stem cell-based therapies for bone repair. Vet Clin North Am Equine Pract 27:299–314 [DOI] [PubMed] [Google Scholar]
- 91.Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D, Medicetty S. 2003. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells 21:50–60 [DOI] [PubMed] [Google Scholar]
- 92.Mokbel A, El-Tookhy O, Shamaa AA, Sabry D, Rashed L, Mostafa A. 2011. Homing and efficacy of intraarticular injection of autologous mesenchymal stem cells in experimental chondral defects in dogs. Clin Exp Rheumatol 29:275–284 [PubMed] [Google Scholar]
- 93.Moreno R, Martinez-Gonzalez I, Rosal M, Farwati A, Gratacos E, Aran JM. 2010. Characterization of mesenchymal stem cells isolated from the rabbit fetal liver. Stem Cells Dev 19:1579–1588 [DOI] [PubMed] [Google Scholar]
- 94.Moscoso I, Rodriguez-Barbosa JI, Barallobre-Barreiro J, Anon P, Domenech N. 2012. Immortalization of bone-marrow–derived porcine mesenchymal stem cells and their differentiation into cells expressing cardiac phenotypic markers. J Tissue Eng Regen Med 6:655–665 [DOI] [PubMed] [Google Scholar]
- 95.Mrugala D, Bony C, Neves N, Caillot L, Fabre S, Moukoko D, Jorgensen C, Noel D. 2008. Phenotypic and functional characterisation of ovine mesenchymal stem cells: application to a cartilage defect model. Ann Rheum Dis 67:288–295 [DOI] [PubMed] [Google Scholar]
- 96.Murphy JM, Fink DJ, Hunziker EB, Barry FP. 2003. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum 48:3464–3474 [DOI] [PubMed] [Google Scholar]
- 97.Neupane M, Chang CC, Kiupel M, Yuzbasiyan-Gurkan V. 2008. Isolation and characterization of canine adipose-derived mesenchymal stem cells. Tissue Eng Part A 14:1007–1015 [DOI] [PubMed] [Google Scholar]
- 98.Park SS, Lee YJ, Lee SH, Lee D, Choi K, Kim WH, Kweon OK, Han HJ. 2012. Functional recovery after spinal cord injury in dogs treated with a combination of Matrigel and neural-induced adipose-derived mesenchymal stem cells. Cytotherapy 14:584–597 [DOI] [PubMed] [Google Scholar]
- 99.Peroni JF, Borjesson DL. 2011. Anti-inflammatory and immunomodulatory activities of stem cells. Vet Clin North Am Equine Pract 27:351–362 [DOI] [PubMed] [Google Scholar]
- 100.Peterbauer-Scherb A, van Griensven M, Meinl A, Gabriel C, Redl H, Wolbank S. 2010. Isolation of pig bone marrow mesenchymal stem cells suitable for 1-step procedures in chondrogenic regeneration. J Tissue Eng Regen Med 4:485–490 [DOI] [PubMed] [Google Scholar]
- 101.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. 1999. Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed] [Google Scholar]
- 102.Planka L, Gal P, Kecova H, Klima J, Hlucilova J, Filova E, Amler E, Krupa P, Kren L, Srnec R, Urbanova L, Lorenzova J, Necas A. 2008. Allogeneic and autogenous transplantations of MSC in treatment of the physeal bone bridge in rabbits. BMC Biotechnol 8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Poncelet AJ, Vercruysse J, Saliez A, Gianello P. 2007. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation 83:783–790 [DOI] [PubMed] [Google Scholar]
- 104.Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Le Blanc K, Phinney DG, Simmons PJ, Sensebe L, Keating A. 2010. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy 12:576–578 [DOI] [PubMed] [Google Scholar]
- 105.Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. 2005. Immunomodulatory effect of human adipose-tissue–derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol 129:118–129 [DOI] [PubMed] [Google Scholar]
- 106.Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, Kim YJ, Jo JY, Yoon EJ, Choi HJ, Kwon E. 2011. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev 20:1297–1308 [DOI] [PubMed] [Google Scholar]
- 107.Ranera B, Lyahyai J, Romero A, Vazquez FJ, Remacha AR, Bernal ML, Zaragoza P, Rodellar C, Martin-Burriel I. 2011. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet Immunol Immunopathol 144:147–154 [DOI] [PubMed] [Google Scholar]
- 108.Raoufi MF, Tajik P, Dehghan MM, Eini F, Barin A. 2011. Isolation and differentiation of mesenchymal stem cells from bovine umbilical cord blood. Reprod Domest Anim 46:95–99 [DOI] [PubMed] [Google Scholar]
- 109.Ren G, Su J, Zhang L, Zhao X, Ling W, L'Huillie A, Zhang J, Lu Y, Roberts AI, Ji W, Zhang H, Rabson AB, Shi Y. 2009. Species variation in the mechanisms of mesenchymal stem-cell–mediated immunosuppression. Stem Cells 27:1954–1962 [DOI] [PubMed] [Google Scholar]
- 110.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. 2008. Mesenchymal stem-cell–mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2:141–150 [DOI] [PubMed] [Google Scholar]
- 111.Ren G, Zhao X, Zhang L, Zhang J, L'Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, Shi Y. 2010. Inflammatory cytokine-induced intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 in mesenchymal stem cells are critical for immunosuppression. J Immunol 184:2321–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ren Y, Wu H, Zhou X, Wen J, Jin M, Cang M, Guo X, Wang Q, Liu D, Ma Y. 2012. Isolation, expansion, and differentiation of goat adipose-derived stem cells. Res Vet Sci 93:404–411 [DOI] [PubMed] [Google Scholar]
- 113.Ribitsch I, Burk J, Delling U, Geissler C, Gittel C, Julke H, Brehm W. 2010. Basic science and clinical application of stem cells in veterinary medicine. Adv Biochem Eng Biotechnol 123:219–263 [DOI] [PubMed] [Google Scholar]
- 114.Ringe J, Kaps C, Schmitt B, Buscher K, Bartel J, Smolian H, Schultz O, Burmester GR, Haupl T, Sittinger M. 2002. Porcine mesenchymal stem cells. Induction of distinct mesenchymal cell lineages. Cell Tissue Res 307:321–327 [DOI] [PubMed] [Google Scholar]
- 115.Rollin R, Alvarez-Lafuente R, Marco F, Jover JA, Hernandez-Garcia C, Rodriguez-Navas C, Lopez-Duran L, Fernandez-Gutierrez B. 2007. Human parvovirus B19, varicella zoster virus, and human herpesvirus 6 in mesenchymal stem cells of patients with osteoarthritis: analysis with quantitative real-time polymerase chain reaction. Osteoarthritis Cartilage 15:475–478 [DOI] [PubMed] [Google Scholar]
- 116.Romieu-Mourez R, Francois M, Boivin MN, Stagg J, Galipeau J. 2007. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFNγ, TGFβ, and cell density. J Immunol 179:1549–1558 [DOI] [PubMed] [Google Scholar]
- 117.Rosland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lonning PE, Bjerkvig R, Schichor C. 2009. Long-term cultures of bone-marrow–derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res 69:5331–5339 [DOI] [PubMed] [Google Scholar]
- 118.Rozemuller H, Prins HJ, Naaijkens B, Staal J, Buhring HJ, Martens AC. 2010. Prospective isolation of mesenchymal stem cells from multiple mammalian species using crossreacting antihuman monoclonal antibodies. Stem Cells Dev 19:1911–1921 [DOI] [PubMed] [Google Scholar]
- 119.Seo MS, Jeong YH, Park JR, Park SB, Rho KH, Kim HS, Yu KR, Lee SH, Jung JW, Lee YS, Kang KS. 2009. Isolation and characterization of canine umbilical cord blood-derived mesenchymal stem cells. J Vet Sci 10:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. 2002. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg 73:1919–1925 [DOI] [PubMed] [Google Scholar]
- 121.Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, Xu C, Chen X, Huang Y, Zhu Z, Huang X, Han X, Xie N, Ren G. 2010. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res 20:510–518 [DOI] [PubMed] [Google Scholar]
- 122.Singer NG, Caplan AI. 2011. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol 6:457–478 [DOI] [PubMed] [Google Scholar]
- 123.Smith RK, Korda M, Blunn GW, Goodship AE. 2003. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet J 35:99–102 [DOI] [PubMed] [Google Scholar]
- 124.Sundin M, Orvell C, Rasmusson I, Sundberg B, Ringden O, Le Blanc K. 2006. Mesenchymal stem cells are susceptible to human herpesviruses, but viral DNA cannot be detected in the healthy seropositive individual. Bone Marrow Transplant 37:1051–1059 [DOI] [PubMed] [Google Scholar]
- 125.Tabera S, Perez-Simon JA, Diez-Campelo M, Sanchez-Abarca LI, Blanco B, Lopez A, Benito A, Ocio E, Sanchez-Guijo FM, Canizo C, San Miguel JF. 2008. The effect of mesenchymal stem cells on the viability, proliferation, and differentiation of B lymphocytes. Haematologica 93:1301–1309 [DOI] [PubMed] [Google Scholar]
- 126.Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J, Gao X, Pileggi A, Ricordi C. 2012. Induction therapy with autologous mesenchymal stem cells in living related kidney transplants: a randomized controlled trial. JAMA 307:1169–1177 [DOI] [PubMed] [Google Scholar]
- 127.Tomchuck SL, Zwezdaryk KJ, Coffelt SB, Waterman RS, Danka ES, Scandurro AB. 2008. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells 26:99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Torres FC, Rodrigues CJ, Stocchero IN, Ferreira MC. 2007. Stem cells from the fat tissue of rabbits: an easy-to-find experimental source. Aesthetic Plast Surg 31:574–578 [DOI] [PubMed] [Google Scholar]
- 129.Torsvik A, Rosland GV, Svendsen A, Molven A, Immervoll H, McCormack E, Lonning PE, Primon M, Sobala E, Tonn JC, Goldbrunner R, Schichor C, Mysliwietz J, Lah TT, Motaln H, Knappskog S, Bjerkvig R. 2010. Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: putting the research field on track—letter. Cancer Res 70:6393–6396 [DOI] [PubMed] [Google Scholar]
- 130.Toupadakis CA, Wong A, Genetos DC, Cheung WK, Borjesson DL, Ferraro GL, Galuppo LD, Leach JK, Owens SD, Yellowley CE. 2010. Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am J Vet Res 71:1237–1245 [DOI] [PubMed] [Google Scholar]
- 131.Trounson A, Thakar RG, Lomax G, Gibbons D. 2011. Clinical trials for stem cell therapies. BMC Med 9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Y, Han ZB, Song YP, Han ZC. 2012. Safety of mesenchymal stem cells for clinical application. Stem Cells Int 2012:652034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. 2010. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS ONE 5:e10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Webb TL, Quimby JM, Dow SW. 2012. In vitro comparison of feline bone-marrow–derived and adipose-tissue–derived mesenchymal stem cells. J Feline Med Surg 14:165–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Williams KJ, Picou AA, Kish SL, Giraldo AM, Godke RA, Bondioli KR. 2008. Isolation and characterization of porcine adipose-tissue–derived adult stem cells. Cells Tissues Organs 188:251–258 [DOI] [PubMed] [Google Scholar]
- 136.Wu Y, Chen L, Scott PG, Tredget EE. 2007. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25:2648–2659 [DOI] [PubMed] [Google Scholar]
- 137.Yi T, Song SU. 2012. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res 35:213–221 [DOI] [PubMed] [Google Scholar]
- 138.Zhao Y, Waldman SD, Flynn LE. 2012. The effect of serial passaging on the proliferation and differentiation of bovine adipose-derived stem cells. Cells Tissues Organs 195:414–427 [DOI] [PubMed] [Google Scholar]
- 139.Zucconi E, Vieira NM, Bueno DF, Secco M, Jazedje T, Ambrosio CE, Passos-Bueno MR, Miglino MA, Zatz M. 2010. Mesenchymal stem cells derived from canine umbilical cord vein—a novel source for cell therapy studies. Stem Cells Dev 19:395–402 [DOI] [PubMed] [Google Scholar]