Abstract
Amyloidosis is a progressive and ultimately fatal disease in which amyloid, an insoluble fibrillar protein, is deposited inappropriately in multiple organs, eventually leading to organ dysfunction. Although this condition commonly affects macaques, there is currently no reliable method of early diagnosis. Changes in clinical pathology parameters have been associated with amyloidosis but occur in late stages of disease, are nonspecific, and resemble those seen in chronic, idiopathic enterocolitis. A review of animal records revealed that amyloidosis was almost always diagnosed postmortem, with prevalences of 15% and 25% in our rhesus and pig-tailed macaque colonies, respectively. As a noninvasive, high-throughput diagnostic approach to improve antemortem diagnosis of amyloidosis in macaques, we evaluated serum amyloid A (SAA), an acute-phase protein and the precursor to amyloid. Using necropsy records and ELISA analysis of banked serum, we found that SAA is significantly elevated in both rhesus and pig-tailed macaques with amyloid compared with those with chronic enterocolitis and healthy controls. At necropsy, 92% of rhesus and 83% of pig-tailed had amyloid deposition in either the intestines or liver. Minimally invasive biopsy techniques including endoscopy of the small intestine, mucosal biopsy of the colon, and ultrasound-guided trucut biopsy of the liver were used to differentiate macaques in our colonies with similar clinical presentations as either having amyloidosis or chronic, idiopathic enterocolitis. Our data suggest that SAA can serve as an effective noninvasive screening tool for amyloidosis and that minimally invasive biopsies can be used to confirm this diagnosis.
Abbreviations: SAA, serum amyloid A
Amyloidosis is a pathologic condition that occurs spontaneously in humans, mammals, birds, and reptiles.47 Secondary systemic amyloidosis, also referred to as reactive amyloidosis, is the most common form described in domestic animals.46 It is a progressive disease in which an insoluble fibrillar protein consisting of β pleated sheets, amyloid, is deposited inappropriately in multiple organs, eventually leading to dysfunction.40,46 Secondary amyloidosis is most often the result of chronic infections or inflammatory disease. In humans, it occurs with a wide variety of conditions including inflammatory bowel disease,3 osteoarthritis including rheumatoid and juvenile forms,20,25 chronic infections such as tuberculosis, and hereditary disease such as familial Mediterranean fever.43 Similarly, in nonhuman primates, the disease has been described with several conditions of chronic infection or inflammation including bacterial enterocolitis,4,19,30,37 chronic indwelling catheters,9 parasitism,2,4 respiratory disease,30,37 trauma,37 and rheumatoid arthritis.6
Despite reported prevalences as high as 30% in rhesus (Macaca mulatta)4 and 47% in pig-tailed macaques (Macaca nemestrina),19 amyloidosis remains a challenge to diagnose. The current diagnostic ‘gold standard’ in macaques is histopathology of the affected organ;19 however, amyloid can be deposited in tissues for as long as 3 y before the development of clinical signs.16 Histologic diagnoses of amyloidosis typically are confirmed with Congo red staining, in which amyloid proteins appear apple-green and birefringent under polarized light. In addition, electron microscopy can detect the fibrillar amyloid proteins in tissues, and other histologic stains including methyl violet, sulphonated Alcian blue, and thioflavin S and T can be used but are less specific than is Congo red.33 Although changes in clinical pathology parameters such as decreases in serum albumin and total protein have been associated with amyloidosis,19,29 they are often nonspecific and resemble those seen in the frequently comorbid conditions chronic anorexia and chronic, idiopathic enterocolitis. Furthermore, imaging techniques such as abdominal X-ray and ultrasonography have been shown to be nondiagnostic in macaques with amyloidosis.19 Consequently, at our institution and in other macaque colonies, diagnosis of amyloidosis is often made at necropsy.
The current standard of diagnosis in humans is biopsy with histopathology of affected organs, but unlike in nonhuman primates, minimally invasive tissue sampling has been extensively explored.17 Aspiration or biopsy of the subcutaneous abdominal fat pad has currently replaced many biopsy techniques as the preliminary diagnostic, with reported sensitivities ranging from 66% to 92%.5,24,28,39,44 Rectal biopsy was previously the preferred minimally invasive approach and is now often used adjunctively when subcutaneous abdominal fat is negative for amyloid but the clinical suspicion for amyloidosis remains high.5,17 Additional tissue biopsy sites with limited morbidity such as skin, gingiva, and stomach have been reported with lesser sensitivities.5,34,39,44 In contrast, limited information is published on the usefulness of minimally invasive biopsy techniques for diagnosing amyloidosis in macaques. One report found endoscopic biopsy of the stomach and colon to be of limited utility in diagnosing amyloidosis in a colony of pig-tailed macaques.19 Similarly, a single publication reported colonoscopy to be noninformative and labor-intensive in a colony of rhesus macaques.15 Retrospective studies of macaque colonies have shown a predilection for amyloid deposition in the intestines and liver,4,30,38 suggesting that endoscopic or percutaneous biopsy of these tissues may reliably provide definitive antemortem diagnosis for amyloidosis.
In addition to biopsy, identification of the relevant amyloid precursor protein within the blood is an integral part of the diagnosis of amyloidosis in human patients17 and holds promise as a screening tool in macaque colonies because of its high throughput potential in comparison to biopsy. Serum amyloid A (SAA), an acute-phase protein, can be found circulating in the blood and is the precursor for amyloid formation and deposition in secondary systemic amyloidosis. Specifically, when elevated SAA persists in the bloodstream, it ultimately progresses to amyloid deposition in tissues.13,45 Profound elevations in SAA occur in the bloodstream as a result of acute inflammation, but these elevations are transient as SAA then is rapidly degraded and removed from the peripheral circulation.7,45 Although the exact role of chronic inflammation and SAA in the pathogenesis of secondary, systemic amyloidosis is not well understood, SAA is pathologically persistently elevated in human patients with chronic inflammatory disease that develop secondary systemic amyloidosis. In contrast, serum SAA remains at normal lower levels in human patients without amyloidosis but ongoing chronic inflammatory disease.13,14,26 Furthermore, quantification of SAA is more effective than are organ function tests as a prognostic measure of amyloid disease and is routinely used to monitor disease progression and response to treatment in humans.14 In rhesus and pig-tailed macaques, SAA is elevated in subjects with amyloidosis as compared with those that are clinically normal.8,19 The ability to distinguish between healthy animals and those with subclinical amyloidosis would be clinically useful. Human studies indicate that establishing a diagnosis of secondary amyloidosis in its early stages followed by prompt treatment of the inciting chronic inflammatory process can arrest the progression of amyloidosis and can even result in disease remission in some cases.21,23,31,32,36 Of equal interest would be the ability to distinguish amyloidosis from chronic, idiopathic enterocolitis, a common disease among macaque colonies12,35 that has considerable clinical overlap with the late stages of amyloidosis but different therapeutic options and prognosis than does systemic amyloidosis. Although there is no definitive treatment for amyloidosis in humans or macaques, recent human case reports suggest that antiinflammatory therapy with newer targeted monocolonal antibody medications, such as IL6 receptor antagonists, can successfully reverse the disease. This outcome has been demonstrated in several cases by both the reduction of circulating SAA to normal levels and by the histologic disappearance of amyloid proteins in biopsies of affected tissues.21,23,31,32,36 Accurate antemortem diagnosis of amyloidosis in macaques potentially would support further investigations into the novel application of these drugs for the treatment of amyloidosis in both human and macaque patients.
We hypothesize that SAA, in addition to being a useful screening method for identifying animals with amyloidosis, can be used to distinguish between macaques with this disease and those with chronic, idiopathic enterocolitis. We further hypothesize that, in agreement with retrospective studies from macaques at other institutions, the intestines and liver will be commonly affected in amyloidotic macaques in our own colonies and that minimally invasive biopsy of these tissues can provide definitive, antemortem diagnosis of amyloidosis.
Materials and Methods
Animals.
All macaques were housed in large indoor–outdoor enclosures at the Johns Hopkins University Research Farm. Animals at this facility are socially housed in matriarchal harem breeding groups of varying age, sex, and number compositions. Pig-tailed and rhesus macaques are housed separately. In some cases for which more extensive veterinary care is required, animals are brought to the Johns Hopkins School of Medicine campus and singly housed for evaluation and treatment. All macaques are provided ad libitum water and a standard commercial diet (rhesus macaques: 2050, Harlan Teklad, Madison, WI; pig-tailed macaques: Purina LabDiet 5038, PMI, St Louis, MO) supplemented with fresh fruits, vegetables, and forage daily. Animals are screened annually and are negative for SIV, simian retrovirus, simian T-cell leukemia virus, and herpesvirus B. Necropsy with tissue and serum banking are standard procedures immediately after any macaque death at the Johns Hopkins Research Farm. For the current study, all necropsy reports from January 2002 to January 2012 for rhesus (n = 79) and pig-tailed (n = 71) macaques older than 1 y were reviewed. All cases of amyloidosis identified in this review; 12 rhesus (n = 11 female, n = 1 male) and 18 pig-tailed (n = 14 female, n = 4 male) were included for description and comparison in the ‘necropsy review’ section of this study. SAA analysis was conducted on banked serum from macaques diagnosed at necropsy during this 10-y period with either amyloidosis or chronic, idiopathic enterocolitis without amyloidosis. Banked frozen serum was available and analyzed for 21 cases of macaques with amyloidosis in one or more organs (female rhesus, n = 7; female pig-tailed, n = 10; male pig-tailed, n = 4) and for 16 cases of macaques with chronic, idiopathic enterocolitis and no evidence of amyloidosis (female rhesus, n = 8; female pig-tailed, n = 7; male pig-tailed, n = 1). Frozen banked serum from clinically healthy adult macaques in our colony with no history of ongoing or chronic disease and with normal hematologic and serum chemical profiles were included for SAA analysis as healthy controls (female rhesus , n = 16; male rhesus, n = 3; female pig-tailed, n = 5; male pig-tailed, n = 5).
For the evaluation of minimally invasive tissue biopsy techniques, biopsies were conducted on live macaques from the Johns Hopkins University Research Farm that presented over a period of approximately 1 y with clinical syndromes suggestive of amyloidosis or chronic, idiopathic enterocolitis. Animals were included when they presented with poor body condition, a history of recurrent diarrhea, negative fecal culture, negative fecal float, and no other specific hematologic or serum chemical findings. The ages of biopsied macaques ranged from 3.6 y to 19.3 y, with an average age of 8.6 y. Intestinal biopsies were performed on 12 macaques (female rhesus, n = 5; female pig-tailed, n = 5; male pig-tailed, n = 2), and liver biopsies were performed on 6 macaques (female rhesus, n = 2; female pig-tailed, n = 2; male pig-tailed, n = 2). One rhesus macaque that was diagnosed with amyloidosis by minimally invasive tissue biopsy was euthanized due to poor prognosis prior to the end of the necropsy review and was included in the necropsy review. All procedures were approved by the Johns Hopkins University IACUC and conducted within an AAALAC-accredited facility. All research was performed in compliance with the Animal Welfare Act1 and subsequent amendments and the recommendations set forth in the Guide for the Care and Use of Laboratory Animals.22
Anesthesia.
Most biopsy procedures were performed under general anesthesia. Macaques were sedated with ketamine HCl (10 mg/kg; KetaVed, Vedco, St Joseph, MO) administered intramuscularly. They subsequently were intubated and placed on isoflurane gas anesthesia (Forane, Baxter Healthcare, Deerfield, IL). Multiparameter anesthetic monitoring (PM-9000Vet, Mindray, Mahwah, NJ) included pulse oximetry, capnography, temperature, and electrocardiography. In cases where colon biopsy was the sole procedure, macaques were sedated with ketamine (10 mg/kg IM) only.
Blood collection and storage.
Macaques were sedated with ketamine HCl intramuscularly (10 mg/kg; KetaVed, Vedco). Blood was collected from the femoral vein and placed in serum-separator phlebotomy tubes. Aliquots of serum were frozen and stored at −20 °C.
Tissue biopsy.
Endoscopy-guided small intestinal biopsies were obtained by using a flexible fiber gastroscope (diameter, 7.9 mm; channel, 2.0 mm; field of view, 100°; GIF XP20, Olympus, Center Valley, PA). Prior to recovery, all macaques received a single, subcutaneous dose of famotidine (0.5 mg/kg, Baxter Healthcare). Colon biopsy was performed blindly by using the endoscopy biopsy tool (Radial Jaw 4, Boston Scientific, Natick, MA). The colon was prepared by manual removal of feces from the rectum at the time of biopsy; because visualization via colonoscopy was not conducted, further colon preparations such as oral bowel cleansing or enema were not performed. The biopsy tool was inserted into the colon to a depth approximately 10 cm from the anorectal junction. Digital pressure was used to guide the biopsy tool to the mucosal surface of the colon for sampling. The biopsy tool was directed in different directions for each sample to ensure that repeat biopsies were not conducted at the same site. This blind mucosal colonic sampling technique has been used routinely in macaques without complication at our facility over the last 5 y as part of an unrelated Johns Hopkins University IACUC-approved protocol.
For intestinal biopsies, 3 to 5 pinch biopsies of each tissue were obtained. Minimally invasive biopsy of the liver (n = 6) was accomplished by using digital ultrasound diagnostic imaging (DP-6600Vet, Mindray). After an appropriate biopsy site was selected via ultrasonography, the skin was prepared aseptically, and an 18-gauge needle was used to create a superficial puncture through the skin. A trucut biopsy needle (14 gauge; length, 15 cm; CareFusion, McGraw Park, IL) was used according to manufacturer directions to obtain a liver biopsy. Digital pressure was applied, and ultrasonography confirmed adequate hemostasis. Liver biopsy was attempted only when easy access and clinical safety could be assured.
Necropsy and histopathology.
Tissues from biopsies and necropsies were placed in 10% neutral buffered formalin, embedded in paraffin, cut, and stained with hematoxylin and eosin according to standard tissue processing techniques. Light microscopy was used to evaluate tissues. Sections with a histologic appearance suggestive of amyloid deposition were stained with Congo Red to confirm the presence of amyloid, as needed for diagnosis.46 Histopathology was reviewed by board-certified pathologists in the Department of Molecular and Comparative Pathobiology at Johns Hopkins. Necropsy reports for macaques diagnosed with amyloidosis over the last 10 y were evaluated to determine the prevalence of amyloidosis in our colonies and the prevalence of affected organs in individual macaques.
SAA ELISA.
SAA analysis was conducted on frozen banked serum from adult macaques with a necropsy diagnosis of amyloidosis or chronic, idiopathic enterocolitis. SAA analysis was not available for macaques that underwent minimally invasive tissue biopsy. Serum Amyloid A Multispecies ELISA kits (Invitrogen, Carlsbad, CA) used with frozen (−20 °C) serum samples that were diluted 1:2000 with PBS, as recommended by the manufacturer.
Statistical analysis.
All results were evaluated by using a commercial statistical software package (Prism, GraphPad Software, San Diego, CA). A P value less than 0.05 was considered significant. The SAA values compiled from all macaques failed a D'Agostino–Pearson test for normality, so subsequent analysis of SAA levels was performed by using nonparametric tests. To compare SAA levels among different disease categories, macaques were grouped according to disease status and analyzed by using a Kruskal–Wallis test, with Dunn multiple-comparison posttests where indicated. Spearman r analysis was used to test for correlation between SAA levels and number of affected tissues. The relationship between organ distribution and SAA levels was made by sorting subjects into 2 groups for each organ according to whether that organ was affected or not on necropsy and then comparing SAA levels between these groups by using a Mann–Whitney test. χ2 analysis and Fisher exact tests were used to determine the sensitivity and specificity of SAA values in determining disease status and to compare the organ distributions of amyloid in rhesus compared with pig-tailed macaques and male compared with female macaques. For determining the sensitivity and specificity of SAA values in detecting disease, a cut-off value was chosen that was based on a combination of published normal ranges and manual evaluation of our data set.8,14
Results
Necropsy review.
A total of 79 rhesus and 71 pig-tailed macaques older than 1 y were necropsied during the 10 y (January 2002 to January 2012) of records reviewed. Among the animals, 12 rhesus (11 female, 1 male) and 18 pig-tailed (14 female, 4 male) macaques were diagnosed with amyloidosis in one or more organs. These data suggest an apparent prevalence of 15% in our rhesus colony and 25% in our pig-tailed colony. Because prevalence was calculated by using cases of amyloid detected at necropsy, these numbers may actually underestimate the true prevalence. The ages of rhesus macaques diagnosed with amyloidosis at necropsy ranged from 3.2 y to 19.8 y, with an average age of 11.6 y (n = 12). Similarly, the ages of pig-tailed macaques ranged from 2.8 y to 18.3 y with an average age of 10.5 y (n = 18). The most commonly affected organs differed between the 2 macaque species. In rhesus macaques with amyloidosis, the small intestines were affected most commonly (83%), followed by the spleen (73%), and large intestine (58%; Table 1). In pig-tailed macaques, the small intestines, spleen, and lymph nodes were most commonly affected and demonstrated amyloid deposition with similar frequencies 50%, 53%, and 53%, respectively (Table 2). Rhesus macaques had a significantly higher prevalence (4 of 12 animals; Fisher exact, P = 0.0181) of kidney amyloidosis than did pig-tailed macaques, in which kidney amyloidosis was not diagnosed in any of the 18 necropsies. In addition, male macaques were more likely (Fisher exact, P = 0.009; relative risk, 3.125) to have hepatic amyloidosis than were female macaques.
Table 1.
Organs with amyloidosis at necropsy of rhesus macaques
| ID | Sex | Small intestine | Spleen | Lymph node | Liver | Large intestine | Stomach | Adrenal | Kidney | Ovary/testes |
| 1 | Female | + | + | – | + | + | + | + | + | + |
| 2 | Female | + | + | + | + | + | – | – | + | – |
| 3 | Female | + | + | – | + | + | – | + | + | no tissue |
| 4 | Female | + | no tissue | no tissue | + | + | no tissue | – | – | + |
| 5 | Female | + | – | – | – | + | + | – | – | – |
| 6 | Female | + | + | + | – | – | – | – | – | no tissue |
| 7 | Female | + | + | – | – | + | no tissue | – | – | – |
| 8 | Female | + | + | no tissue | – | – | – | – | + | – |
| 9 | Female | – | + | + | – | – | – | – | – | – |
| 10 | Female | + | – | – | – | – | – | – | – | no tissue |
| 11 | Female | – | – | – | + | – | – | – | – | – |
| 12 | Male | + | + | + | + | + | + | – | – | – |
| Prevalence | 83% | 73% | 40% | 50% | 58% | 30% | 17% | 33% | 22% |
+, amyloid proteins present; –, no amyloid protein present.
Table 2.
Organs with amyloidosis at necropsy of pig-tailed macaques
| ID | Sex | Small intestine | Spleen | Lymph node | Liver | Large intestine | Stomach | Adrenal | Kidney | Ovary/testes |
| 13 | Female | + | + | + | no tissue | + | + | – | – | – |
| 14 | Female | + | + | + | – | + | + | – | – | – |
| 15 | Female | + | + | + | – | – | + | – | – | no tissue |
| 16 | Female | + | – | + | – | – | – | – | – | – |
| 17 | Female | + | + | + | – | – | – | – | – | no tissue |
| 18 | Female | + | + | + | – | – | – | – | – | – |
| 19 | Female | + | + | – | – | + | – | – | – | – |
| 20 | Female | + | + | – | – | – | + | – | – | – |
| 21 | Female | – | – | + | – | – | – | – | – | – |
| 22 | Female | – | – | + | – | – | – | – | – | no tissue |
| 23 | Female | – | – | no tissue | + | – | – | – | – | no tissue |
| 24 | Female | – | – | – | + | – | – | – | – | – |
| 25 | Female | – | – | – | – | + | no tissue | – | – | – |
| 26 | Female | – | + | – | – | – | no tissue | – | – | – |
| 27 | Male | + | no tissue | + | + | – | no tissue | + | – | – |
| 28 | Male | – | – | – | + | – | – | + | – | – |
| 29 | Male | – | + | – | + | – | – | – | – | – |
| 30 | Male | – | – | – | + | – | – | – | – | – |
| Prevalence | 50% | 53% | 53% | 35% | 22% | 27% | 11% | 0% | 0% |
+, amyloid proteins present; –, no amyloid protein present.
In the necropsy reports of amyloidotic macaques, histologic amyloid deposition was noted only once in the heart among the 28 cases evaluated, once in the tongue among 25 cases, once in the thyroid among 22 cases, and once in the bone marrow among 19 cases. However, each of these animals had amyloid protein deposits in at least 2 other more commonly affected tissues (intestines, spleen, liver). Lymph nodes positive for amyloidosis were from one or more locations, including axillary, bronchial, colonic, inguinal, mesenteric, and submandibular nodes. The number of tissues affected varied from a single organ in 9 (7 pig-tailed, 2 rhesus) macaques to as many as 8 different tissues in a rhesus macaque. In the 9 macaques in which only a single organ was identified at necropsy as showing amyloidosis, the liver was most common (4 of 9 cases) site, followed by lymph nodes (2 of 9 cases) and small intestine, large intestine, and spleen (1 case each). Contrary to a report where amyloid was detected in biopsies of grossly normal skin in 42% of human patients with secondary systemic amyloidosis,34 amyloid was not detected in skin samples from amyloidotic macaques (n = 7).
Tissue biopsy.
Review of necropsy records indicated that the small intestine, colon, and liver would be the most clinically rewarding biopsy sites, given that 92% of rhesus and 83% of pig-tailed macaques had amyloid deposition in at least one of these locations. Of the 12 macaques that underwent minimally invasive tissue biopsy, 3 unrelated animals were diagnosed with amyloidosis (Table 3). In the 3 animals with amyloidosis, 2 were positive for amyloid in all 3 tissues sampled (liver, small intestine, colon). In both of these cases, amyloid deposition was defined as diffuse and moderate to severe in the liver and small intestine. Hepatomegaly was noted on physical exam in just one of these animals. Colonic biopsies from these 2 macaques revealed one animal with severe and diffuse colon amyloidosis and the other with minimal colon amyloid deposition. The third macaque diagnosed with amyloidosis via minimally invasive biopsy had histologic evidence of amyloid deposition only in the colon. In this case, amyloid deposition was described as multifocal and minimal. This diagnosis was confirmed on subsequent biopsies over the next year, with similar amyloid deposition developing in the small intestine and liver.
Table 3.
Histologic diagnosis after minimally invasive biopsy techniques of small intestine (endoscopic), large intestine (blind mucosal pinch), and liver (ultrasound-guided trucut)
| ID | Species | Sex | Age (y) | Small intestine | Large intestine | Liver |
| 31 | Rhesus | Female | 9.3 | Amyloidosis and enteritis | Amyloidosis and colitis | Amyloidosis |
| 32 | Rhesus | Female | 8.5 | Enteritis | Colitis | Hepatitis |
| 33 | Rhesus | Female | 19.3 | Enteritis | Colitis | No tissue |
| 34 | Rhesus | Female | 7.8 | Enteritis | Colitis | No tissue |
| 35 | Rhesus | Female | 3.6 | Enteritis | Colitis | No tissue |
| 36 | Pigtail | Female | 6.5 | Insufficient quantity or quality of tissue for diagnosis | Amyloidosis and colitis | Normal |
| 37 | Pigtail | Female | 4 | Enteritis | Colitis | Normal |
| 38 | Pigtail | Female | 9.8 | Enteritis | Colitis and Balantidium sp. | No tissue |
| 39 | Pigtail | Female | 3.8 | Enteritis | Colitis and Balantidium sp. | No tissue |
| 40 | Pigtail | Female | 11.1 | Enteritis | Colitis | No tissue |
| 41 | Pigtail | Male | 13.7 | Amyloidosis and enteritis | Amyloidosis and colitis | Amyloidosis |
| 42 | Pigtail | Male | 5.3 | Enteritis | Colitis | Normal |
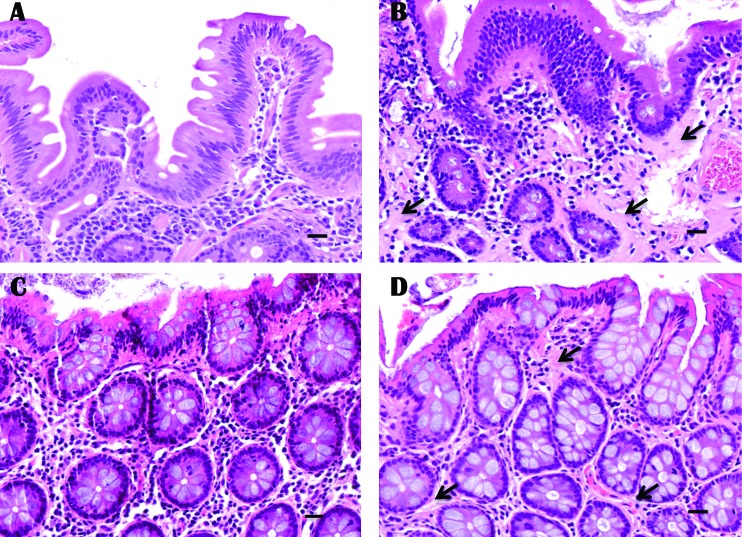
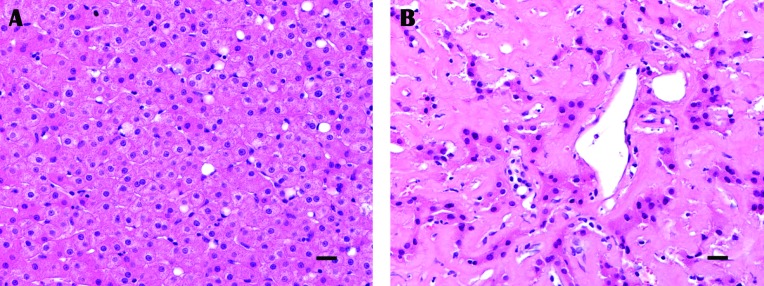
Nine macaques were diagnosed with chronic, idiopathic enterocolitis from biopsies of the small and large intestines. Two pig-tailed macaques diagnosed with chronic, idiopathic enterocolitis were siblings from the same sire and housed in the same social group. Two rhesus macaques diagnosed with chronic, idiopathic enterocolitis were unrelated but housed in the same social group. The most common finding on intestinal biopsy was multifocal lymphoplasmacytic inflammation that ranged from mild to moderate in both the small and large bowel (Figure 1). This pattern was true for both species of macaques regardless of the presence of amyloid within the intestines. With the exception of one set of small intestinal biopsies in which the quantity and quality of tissue was insufficient for histopathologic diagnosis, all intestinal mucosal pinch biopsies obtained in the current study were considered diagnostic. In addition, mucosal pinch biopsies extended into or beyond the lamina propria, the layer of the small and large intestine where amyloid is found typically (Figure 1). Consistent with previous studies in macaques,19,38 hepatic amyloid expanded the sinusoids and perisinusoidal spaces (Figure 2). Mild hemorrhage consistent with biopsy techniques was noted in some samples but did not impede interpretation. The rhesus macaque diagnosed with amyloidosis was euthanized due to poor prognosis. Two other rhesus macaques were sold, and the remaining 9 macaques, 2 with amyloidosis, were returned to the macaque colonies under veterinary supervision.
Figure 1.
Histopathology of intestinal mucosal pinch biopsies of (A) small intestine with chronic, idiopathic enterocolitis, (B) small intestine with amyloidosis, (C) colon with chronic, idiopathic entercolitis, and (D) colon with amyloidosis. Amyloid protein is homogenous, amorphous, eosinophilic, and indicated by black arrows. Hematoxylin and eosin stain; bar, 20 µm.
Figure 2.
Histopathology of trucut liver biopsy from (A) normal liver tissue from a macaque diagnosed with chronic, idiopathic enterocolitis and (B) liver tissue from a macaque with extensive amyloidosis and sparse remaining hepatocytes. Amyloid protein is homogenous, amorphous, and eosinophilic. Hematoxylin and eosin stain; bar, 20 µm.
SAA analysis.
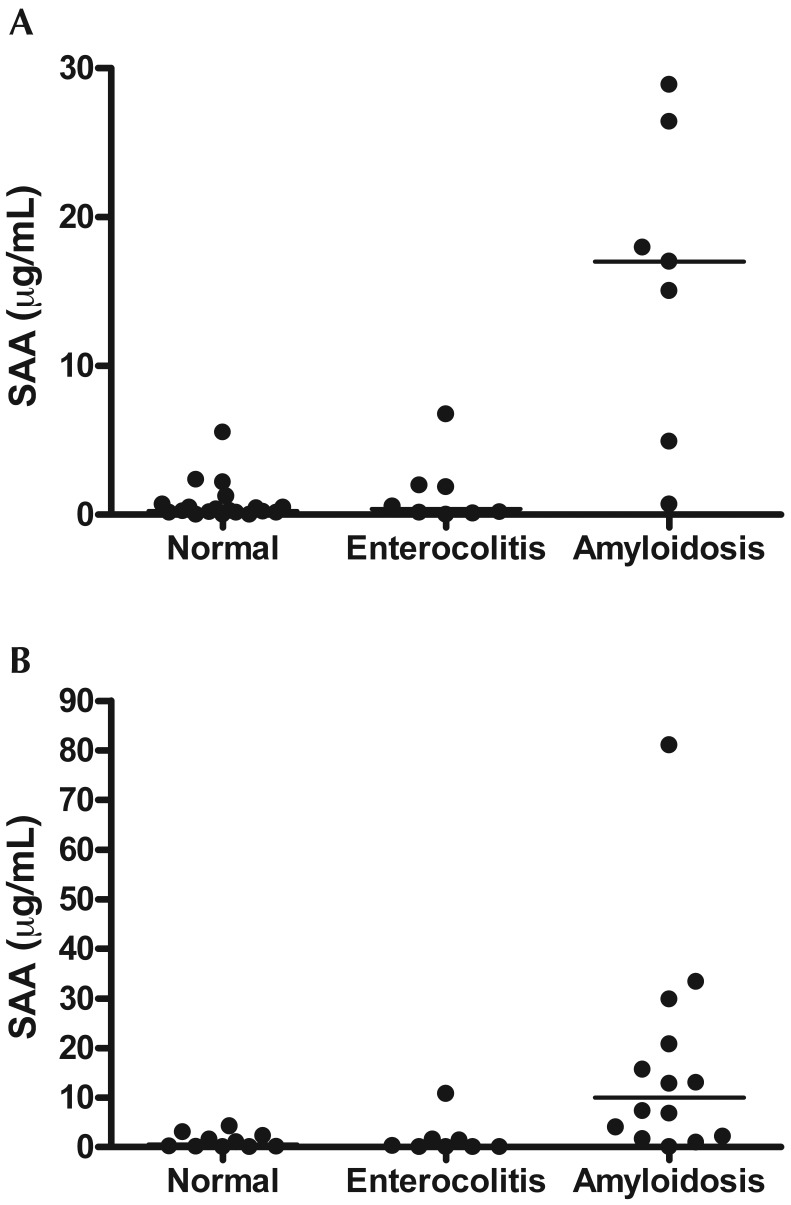
Serum amyloid A varied significantly by disease status in both rhesus (Kruskal–Wallis, P = 0.0015, n = 24) and pig-tailed (Kruskal–Wallis, P = 0.0014, n = 31; Figure 3) macaques. In rhesus, macaques SAA was significantly (Dunn multiple-comparison test, P < 0.05) elevated in animals with amyloidosis compared with normal macaques and those with chronic, idiopathic enterocolitis. This association also was true for SAA analysis of similar groups of pig-tailed macaques (Dunn multiple-comparison test, P < 0.05). There was no correlation between the number of tissues with amyloid deposition and the quantity of SAA in individual animals (Spearman: r = 0.08017, P = 0.7369, n = 55). In macaques with amyloidosis, elevated SAA was not dependent on the presence of amyloid in any particular tissue, including the intestines and liver. However, macaques with amyloid deposition in the stomach (n = 3) were had significantly higher (Mann–Whitney, P = 0.0314) SAA than did those with no amyloid in the stomach (n = 13). A cut-off value of 4 μg/mL was selected on the basis of a combination of previous publications, which used cut-off values of 3 to 6 μg/mL,8,14,21 and manual evaluation of our data. In this regard, 6 of the 7 rhesus macaques and 9 of the 14 pig-tailed macaques with amyloidosis had a serum SAA value that exceeded 4 μg/mL. When clinically healthy pig-tailed macaques, pig-tailed macaques with amyloidosis, and those with chronic, idiopathic enterocolitis were compared, a SAA level above 4 μg/mL was 71.4% sensitive and 88.9% specific and had an 83.3% positive predictive value (Fisher exact, P = 0.0008) for distinguishing amyloidotic macaques from those that were clinically normal or had chronic, idiopathic enterocolitis. When pig-tailed macaques with amyloidosis or enterocolitis were compared, an SAA level above 4 μg/mL was 71.4% sensitive and 87.5% specific and had a 90.9% positive predictive value (Fisher exact, P = 0.0237). Similar results were obtained when we compared amyloidotic rhesus macaques with those that were clinically normal or had chronic, idiopathic enterocolitis. In these animals, an SAA level above 4 μg/mL was 85.7% sensitive and 92.6% specific and had a 75.0% positive predictive value (Fisher exact, P = 0.0001). The positive predictive value improved to 85.7%, and an SAA level above 4 μg/mL was 85.7% sensitive and 87.5% specific (Fisher exact P = 0.0101) when amyloidotic rhesus macaques were compared only with rhesus macaques with chronic, idiopathic enterocolitis.
Figure 3.
ELISA results of serum amyloid A (SAA) serum concentration compared with diagnosis at necropsy for (A) rhesus and (B) pig-tailed macaques; bars represent median values. Serum amyloid A varied significantly by disease status in both rhesus (Kruskal–Wallis, P = 0.0015, n = 24) and pig-tailed macaques (Kruskal–Wallis, P = 0.0014, n = 31).
Discussion
SAA has the potential to be an informative and accurate diagnostic screening tool for amyloidosis in colonies of captive rhesus and pig-tailed macaques. The prognosis for secondary systemic amyloidosis is poor for animals including nonhuman primates,17 with limited survival times after diagnosis as a result of subsequent organ failure.18 This disadvantage can be attributed in part to the late stage at which clinical signs of amyloidosis become apparent; however, accurate diagnosis of amyloidosis is further complicated in macaques due to the similar clinical presentations of animals with chronic, idiopathic enterocolitis. We have shown that SAA not only identifies both rhesus and pig-tailed macaques with amyloidosis as compared with healthy animals but also, more importantly, can differentiate them from macaques with chronic, idiopathic enterocolitis. Both conditions commonly cause morbidity and mortality in macaque colonies, making SAA an invaluable tool for the clinician attempting to institute appropriate therapy, pursue additional diagnostics, or determine an accurate prognosis. Macaques with amyloidosis or chronic, idiopathic enterocolitis often yield inconclusive diagnostic results, and recurrent clinical signs and can be poor surgical candidates. Therefore, SAA analysis holds promise as a noninvasive diagnostic indicator of amyloid disease.
As a screening tool, SAA serves to identify animals with a high index of suspicion for amyloid disease. Follow-up biopsy by minimally invasive techniques can definitively diagnose animals as amyloidotic, but a negative biopsy cannot be considered proof of the absence of amyloid. In our colony, 8% of rhesus and 16% of pig-tailed macaques with systemic amyloidosis diagnosed at necropsy did not have amyloid in the intestines or liver. Similarly, 21% of pig-tailed macaques with amyloidosis had no amyloid deposition in the intestines or liver in a previous study.19 In these cases of systemic amyloidosis at our institution in which the intestines and liver were unaffected, tissues that are more difficult to biopsy, such as the adrenals, visceral lymph nodes, spleen, and kidney, were affected. We have found that SAA is elevated in macaques with amyloidosis regardless of what tissue is affected by amyloid deposition. Notably, at least one animal among the limited number of macaques with amyloidosis that underwent SAA analysis was identified as having high SAA despite the lack of detectable amyloid in any of the commonly biopsied tissues (intestines and liver). Therefore, SAA may be a more sensitive and practical diagnostic tool than is multiple biopsies, particularly in cases where the intestines and liver are not affected. Because SAA can be elevated during diverse acute inflammatory responses,7 a single elevated SAA value should not be interpreted as a definitive diagnosis and should be reviewed on a case-by-case basis. In the absence of clinical signs, persistently elevated SAA should raise the clinical suspicion for amyloidosis. Elevated SAA in macaques with clinical signs consistent with amyloidosis, such as hepatomegaly, cachexia, and chronic diarrhea,4,19 should be evaluated further via repeat SAA analysis or tissue biopsy.
Retrospective analyses of amyloidotic macaques in our colony indicated that the small intestine is the preferred tissue for minimally invasive biopsy, with 83% of rhesus and 50% of pig-tailed macaques affected at this location. However, endoscopy may be considered to be impractical in some settings, given that it requires general anesthesia of subjects, expensive equipment, and technical skill. Gastric biopsy may be accomplished easily, but amyloid deposition in the stomach was noted in fewer than 30% of macaques with amyloidosis at necropsy, and all of these animals also had amyloid deposits in the small intestine. These necropsy findings support the limited success of gastric biopsies in detecting amyloidosis in a previous study involving pig-tailed macaques, in which small intestinal biopsies were not attempted.19 Consistent with previous human reports, intestinal mucosal samples obtained in the previous study included adequate sections of lamina propria to definitively diagnose amyloidosis, supporting our hypothesis that full-thickness biopsies and laparotomy are unnecessary.41,42 In 2 macaques from which tissues were taken both clinically via exploratory laparotomy and the minimally invasive techniques previously described, there was 100% congruence between methodologies in the diagnosis of amyloidosis. Although beyond the scope of the current study, the degree of amyloid deposition in endoscopic gastric and duodenal biopsies is a negative prognostic indicator in human patients with amyloidosis and refractory chronic inflammatory disease;25 this association may prove to be true in nonhuman primate species as well.
Similar to the potential limitations for endoscopy in macaque colonies, a previous report found that the labor-intensity of colonoscopy outweighed its potential benefits in the clinical management of a rhesus macaque colony.15 Although colonoscopy can aid in the selection of optimal biopsy sites, direct visualization of the mucosa does not in itself provide a diagnostic advantage in cases of intestinal amyloidosis because the mucosal surface appears grossly normal despite amyloid disease.19,27 We therefore suggest that extensive colonic preparation and visualization of the mucosal surface via colonoscopy may not be necessary for the diagnosis of amyloidosis. We have found that diagnostic tissue samples can be obtained by using blind mucosal pinch biopsy of the colon. This technique requires minimal preparation beyond routine feed limitations prior to sedation and can be performed with injectable intramuscular sedation alone, thus eliminating much of the labor described in the previous report. Although a histopathologically normal biopsy does not rule out systemic amyloidosis, this technique can provide definitive diagnosis when amyloid is present in the colon, which was true for 58% of rhesus and 22% of pig-tailed macaques with amyloidosis in our necropsy records. Consequently, blind colon biopsy can provide an alternative approach when endoscopy is not available or practical.
In the absence of intestinal amyloidosis, the liver was most commonly affected in our animals. Hepatomegaly has been associated with hepatic amyloidosis in both rhesus and pig-tailed macaques, however, this clinical finding is generally limited to cases of moderate to severe hepatic amyloid deposition.4,19 Of the 2 animals we identified by tissue biopsy with moderate to severe hepatic amyloidosis, only one had clinically apparent hepatomegaly. Whether liver biopsy in the presence of amyloidosis is safe has prompted discussion, given the friability of the tissue and the potential for bleeding.11 Consequently, we recommend reserving liver biopsy for cases when intestinal biopsies are inconclusive and when amyloidosis of the liver is suspected in light of elevated SAA, hepatomegaly, or other abnormalities in serum chemistry profiles consistent with liver amyloidosis, such as increased ALP, AST, or GGT.29 In addition, male macaques should be considered for liver biopsy, because in our current study male macaques more often had hepatic amyloidosis than did female macaques. Although the underlying cause for this result is unclear, it is consistent with a study of amyloidosis in chimpanzees.18 We further recommend that percutaneous liver biopsy be performed with ultrasonography to both guide the biopsy and to ensure adequate postprocedural hemostasis. Although no complications were noted as a result of trucut biopsy in macaques diagnosed with hepatic amyloidosis in the current study, macaque 15 (Table 1) was euthanized after liver fracture and extensive hemorrhage during routine abdominal palpation and ultrasonography unassociated with liver biopsy.
Due to the complications associated with biopsy of pathologic internal organs, great strides have been made regarding minimally invasive diagnostic techniques for amyloidosis in humans, most notably the routine use of serum SAA evaluation.17 Persistently elevated SAA has been well described in human patients with secondary systemic amyloidosis as compared with human patients with nonamyloid chronic inflammatory disease.13,14,26 Although some patients with refractory chronic inflammatory conditions do not develop secondary, systemic amyloidosis, others go on to develop potentially life-threatening amyloid disease. In these patients, SAA is not only evaluated for elevations (indicating the development of secondary systemic amyloidosis) but also is used to monitor response to treatment.21,23,32,36 Our current study suggests that similar application of SAA evaluation would be appropriate in macaque colonies, in which some animals with chronic, idiopathic enterocolitis ultimately develop secondary systemic amyloidosis and others do not. This pattern is supported by previous studies in pig-tailed macaques, in which animals with histopathologic evidence of enterocolitis or clinical histories of diarrheal disease were at increased risk of developing amyloidosis.37,38
Biopsy or aspiration of the subcutaneous fat pad has replaced more invasive biopsies in human patients with amyloidosis.5,10 Gross necropsy records from our macaque colonies indicate that the subcutaneous abdominal fat often is not sufficiently massive to support aspiration or biopsy during the terminal stages of amyloidosis or chronic, idiopathic enterocolitis. These findings are consistent with a previous report that described amyloidosis in rhesus macaques4 and were true for macaques selected for minimally invasive tissue biopsy in the current study as a result of chronic, recurrent clinical illness. Although beyond the scope of our report, the evaluation of the subcutaneous fat pad may be diagnostic during the subclinical stages of amyloidosis, when body condition has not yet been affected by the disease. Interestingly, a previous study in rhesus macaques showed that SAA elevated more highly in asymptomatic animals with amyloidosis than in those with high amyloid burden and clinical signs of disease.29 A larger prospective study is necessary to determine the operating characteristics of serum SAA measurements as a screening test for amyloidosis in asymptomatic animals and potentially could be conducted in combination with investigations into the diagnostic value of subcutaneous fat pad biopsy.
We further suggest that SAA is a convenient surveillance tool in macaque colonies because it can be evaluated in a high-throughput fashion by using serum samples that often are otherwise obtained routinely for viral screening and health monitoring. Although commercial laboratories currently do not offer macaque SAA analysis, the SAA ELISA kit that we used in the current study is available for US$441.20 and can be used to test as many as 80 animals at a cost of US$5.52 per animal. This cost is comparable to the current commercial rates for routine macaque viral screening, at approximately US$5 per virus per animal. Moreover, although endoscopy and intestinal biopsy was well tolerated by all macaques in the current study regardless of clinical illness, the value of such procedures with regard to their potential complications and labor intensity still needs to be considered. SAA analysis is noninvasive, requiring only a serum sample, and can be completed for as many as 80 animals in as few as 2 h, whereas minimally invasive biopsies require approximately 1 h of anesthesia time per animal.
In summary, we have confirmed our hypothesis that SAA can be used to differentiate macaques with amyloidosis from those with chronic, idiopathic enterocolitis. We have shown that SAA is not only elevated in macaques with amyloidosis as compared with normal macaques but also in amyloidotic macaques compared with macaques with chronic, idiopathic enterocolitis. In addition, we have confirmed that systemic amyloidosis remains a common pathologic finding in our macaque colonies, with an apparent prevalence of 15% in rhesus macaques and 25% in pig-tailed macaques. Retrospective analysis of necropsy reports from macaques with systemic amyloidosis confirmed that the intestines and liver are among the organs typically affected by amyloid deposition. We then were able to demonstrate that minimally invasive biopsy of the intestines and liver can be used to provide a definitive antemortem diagnosis in clinically diseased animals when these tissues are affected. A negative biopsy, however, does not exclude a diagnosis of amyloidosis. Furthermore, multiple minimally invasive biopsies may be associated with complications and require technical skill, time, and equipment that may be considered impractical in some settings. Therefore, we propose that SAA analysis can be used as a less invasive, cost-effective, and accurate tool for the diagnosis of amyloidosis in macaques. The availability and utility of this biomarker have the potential to close the gap between advances in the diagnosis and treatment of secondary systemic amyloidosis in humans, where SAA analysis is used routinely for diagnosis and therapeutic monitoring, and the current management (or lack thereof) of the disease in macaque colonies. Although there currently is no definitive treatment for amyloidosis in humans or animals, routine use of serum SAA analysis in macaque colonies may allow for early diagnosis and subsequent investigations into disease progression and novel therapeutic options.
Acknowledgments
This work was supported by the National Center for Research Resources, the Office of Research Infrastructure Programs (ORIP) through grant no. P40-OD013117. We thank Dr Joseph Mankowski for his invaluable mentorship with the direction of this project and Dr David Huso for his guidance with histopathology. In addition, we acknowledge the contributions and support of the veterinary and animal care staff at Johns Hopkins University.
References
- 1.Animal Welfare Act as Amended. 2007.7 USC §2131–2159.
- 2.Bacciarini LN, Gottstein B, Pagan O, Rehmann P, Grone A. 2004. Hepatic alveolar echinococcosis in cynomolgus monkeys (Macaca fascicularis). Vet Pathol 41:229–234 [DOI] [PubMed] [Google Scholar]
- 3.Basturk T, Ozagari A, Ozturk T, Kusaslan R, Unsal A. 2009. Crohn's disease and secondary amyloidosis: early complication? A case report and review of the literature. J Ren Care 35:147–150 [DOI] [PubMed] [Google Scholar]
- 4.Blanchard JL, Baskin GB, Watson EA. 1986. Generalized amyloidosis in rhesus monkeys. Vet Pathol 23:425–430 [DOI] [PubMed] [Google Scholar]
- 5.Bogov B, Lubomirova M, Kiperova B. 2008. Biopsy of subcutaneus fatty tissue for diagnosis of systemic amyloidosis. Hippokratia 12:236–239 [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman WL, Jr, Crowell WA. 1977. Amyloidosis in rhesus monkeys with rheumatoid arthritis and enterocolitis. J Am Vet Med Assoc 171:855–858 [PubMed] [Google Scholar]
- 7.Cray C, Zaias J, Altman NH. 2009. Acute-phase response in animals: a review. Comp Med 59:517–526 [PMC free article] [PubMed] [Google Scholar]
- 8.Doepel FM, Glorioso JC, Newcomer CE, Skinner M, Abrams GD. 1981. Enzyme-linked immunosorbent assay of serum protein SAA in rhesus monkeys with secondary amyloidosis. Lab Invest 45:7–13 [PubMed] [Google Scholar]
- 9.Doepel FM, Ringler DH, Petkus AR. 1984. Secondary amyloidosis in rhesus monkeys with chronic indwelling venous catheters. Lab Anim Sci 34:494–496 [PubMed] [Google Scholar]
- 10.Duston MA, Skinner M, Meenan RF, Cohen AS. 1989. Sensitivity, specificity, and predictive value of abdominal fat aspiration for the diagnosis of amyloidosis. Arthritis Rheum 32:82–85 [DOI] [PubMed] [Google Scholar]
- 11.Ebert EC, Nagar M. 2008. Gastrointestinal manifestations of amyloidosis. Am J Gastroenterol 103:776–787 [DOI] [PubMed] [Google Scholar]
- 12.Elmore DB, Anderson JH, Hird DW, Sanders KD, Lerche NW. 1992. Diarrhea rates and risk factors for developing chronic diarrhea in infant and juvenile rhesus monkeys. Lab Anim Sci 42:356–359 [PubMed] [Google Scholar]
- 13.Falck HM, Maury CP, Teppo AM, Wegelius O. 1983. Correlation of persistently high serum amyloid A protein and C-reactive protein concentrations with rapid progression of secondary amyloidosis. Br Med J (Clin Res Ed) 286:1391–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillmore JD, Lovat LB, Persey MR, Pepys MB, Hawkins PN. 2001. Amyloid load and clinical outcome in AA amyloidosis in relation to circulating concentration of serum amyloid A protein. Lancet 358:24–29 [DOI] [PubMed] [Google Scholar]
- 15.Gullett PA, Tarara R, Markovits JF. 1996. Colon biopsy in the management of chronic diarrhea in rhesus macaques. Contemp Top Lab Anim Sci 35:74–75 [PubMed] [Google Scholar]
- 16.Hawkins PN, Wootton R, Pepys MB. 1990. Metabolic studies of radioiodinated serum amyloid P component in normal subjects and patients with systemic amyloidosis. J Clin Invest 86:1862–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazenberg BP, van Gameren, Bijzet J, Jager PL, van Rijswijk MH. 2004. Diagnostic and therapeutic approach of systemic amyloidosis. Neth J Med 62:121–128 [PubMed] [Google Scholar]
- 18.Hubbard GB, Lee DR, Steele KE, Lee S, Binhazim AA, Brasky KM. 2001. Spontaneous amyloidosis in 12 chimpanzees, Pan troglodytes. J Med Primatol 30:260–267 [DOI] [PubMed] [Google Scholar]
- 19.Hukkanen RR, Liggitt HD, Anderson DM, Kelley ST. 2006. Detection of systemic amyloidosis in the pig-tailed macaque (Macaca nemestrina). Comp Med 56:119–127 [PubMed] [Google Scholar]
- 20.Hull RG. 1988. Outcome in juvenile arthritis. Br J Rheumatol 27 Suppl 1:66–71 [PubMed] [Google Scholar]
- 21.Inoue D, Arima H, Kawanami C, Takiuchi Y, Nagano S, Kimura T, Shimoji S, Mori M, Tabata S, Yanagita S, Matsushita A, Nagai K, Imai Y, Takahashi T. 2010. Excellent therapeutic effect of tocilizumab on intestinal amyloid a deposition secondary to active rheumatoid arthritis. Clin Rheumatol 29:1195–1197 [DOI] [PubMed] [Google Scholar]
- 22.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press.
- 23.Kishida D, Okuda Y, Onishi M, Takebayashi M, Matoba K, Jouyama K, Yamada A, Sawada N, Mokuda S, Takasugi K. 2011. Successful tocilizumab treatment in a patient with adult-onset Still's disease complicated by chronic active hepatitis B and amyloid A amyloidosis. Mod Rheumatol 21:215–218 [DOI] [PubMed] [Google Scholar]
- 24.Klemi PJ, Sorsa S, Happonen RP. 1987. Fine-needle aspiration biopsy from subcutaneous fat. An easy way to diagnose secondary amyloidosis. Scand J Rheumatol 16:429–431 [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi H, Tada S, Fuchigami T, Okuda Y, Takasugi K, Matsumoto T, Iida M, Aoyagi K, Iwashita A. 1996. Secondary amyloidosis in patients with rheumatoid arthritis: diagnostic and prognostic value of gastroduodenal biopsy. Br J Rheumatol 35:44–49 [DOI] [PubMed] [Google Scholar]
- 26.Lachmann HJ, Goodman HJ, Gilbertson JA, Gallimore JR, Sabin CA, Gillmore JD, Hawkins PN. 2007. Natural history and outcome in systemic AA amyloidosis. N Engl J Med 356:2361–2371 [DOI] [PubMed] [Google Scholar]
- 27.Lee JG, Wilson JA, Gottfried MR. 1994. Gastrointestinal manifestations of amyloidosis. South Med J 87:243–247 [DOI] [PubMed] [Google Scholar]
- 28.Libbey CA, Skinner M, Cohen AS. 1983. Use of abdominal fat tissue aspirate in the diagnosis of systemic amyloidosis. Arch Intern Med 143:1549–1552 [PubMed] [Google Scholar]
- 29.MacGuire JG, Christe KL, Yee JL, Kalman-Bowlus AL, Lerche NW. 2009. Serologic evaluation of clinical and subclinical secondary hepatic amyloidosis in rhesus macaques (Macaca mulatta). Comp Med 59:168–173 [PMC free article] [PubMed] [Google Scholar]
- 30.Naumenko ES, Krylova RI. 2003. Amyloidosis in macaques in Adler Primatological Center. Bull Exp Biol Med 136:80–83 [DOI] [PubMed] [Google Scholar]
- 31.Nishida S, Hagihara K, Shima Y, Kawai M, Kuwahara Y, Arimitsu J, Hirano T, Narazaki M, Ogata A, Yoshizaki K, Kawase I, Kishimoto T, Tanaka T. 2009. Rapid improvement of AA amyloidosis with humanised antiinterleukin 6 receptor antibody treatment. Ann Rheum Dis 68:1235–1236 [DOI] [PubMed] [Google Scholar]
- 32.Okuda Y, Takasugi K. 2006. Successful use of a humanized antiinterleukin-6 receptor antibody, tocilizumab, to treat amyloid A amyloidosis complicating juvenile idiopathic arthritis. Arthritis Rheum 54:2997–3000 [DOI] [PubMed] [Google Scholar]
- 33.Picken MM. 2010. Amyloidosis—where are we now and where are we heading? Arch Pathol Lab Med 134:545–551 [DOI] [PubMed] [Google Scholar]
- 34.Rubinow A, Cohen AS. 1978. Skin involvement in generalized amyloidosis. A study of clinically involved and uninvolved skin in 50 patients with primary and secondary amyloidosis. Ann Intern Med 88:781–785 [DOI] [PubMed] [Google Scholar]
- 35.Russell RG, Rosenkranz SL, Lee LA, Howard H, DiGiacomo RF, Bronsdon MA, Blakley GA, Tsai CC, Morton WR. 1987. Epidemiology and etiology of diarrhea in colony-born Macaca nemestrina. Lab Anim Sci 37:309–316 [PubMed] [Google Scholar]
- 36.Sato H, Sakai T, Sugaya T, Otaki Y, Aoki K, Ishii K, Horizono H, Otani H, Abe A, Yamada N, Ishikawa H, Nakazono K, Murasawa A, Gejyo F. 2009. Tocilizumab dramatically ameliorated life-threatening diarrhea due to secondary amyloidosis associated with rheumatoid arthritis. Clin Rheumatol 28:1113–1116 [DOI] [PubMed] [Google Scholar]
- 37.Slattum MM, Rosenkranz SL, DiGiacomo RF, Tsai CC, Giddens WE., Jr 1989. Amyloidosis in pigtailed macaques (Macaca nemestrina): epidemiologic aspects. Lab Anim Sci 39:560–566 [PubMed] [Google Scholar]
- 38.Slattum MM, Tsai CC, DiGiacomo RF, Giddens WE., Jr 1989. Amyloidosis in pigtailed macaques (Macaca nemestrina): pathologic aspects. Lab Anim Sci 39:567–570 [PubMed] [Google Scholar]
- 39.Sorsa S, Happonen RP, Klemi P. 1988. Oral biopsy and fine-needle aspiration biopsy from subcutaneous fat in diagnosis of secondary amyloidosis. Int J Oral Maxillofac Surg 17:14–16 [DOI] [PubMed] [Google Scholar]
- 40.Stone MJ. 1990. Amyloidosis: a final common pathway for protein deposition in tissues. Blood 75:531–545 [PubMed] [Google Scholar]
- 41.Tada S, Iida M, Iwashita A, Matsui T, Fuchigami T, Yamamoto T, Yao T, Fujishima M. 1990. Endoscopic and biopsy findings of the upper digestive tract in patients with amyloidosis. Gastrointest Endosc 36:10–14 [DOI] [PubMed] [Google Scholar]
- 42.Tada S, Iida M, Yao T, Kawakubo K, Okada M, Fujishima M. 1994. Endoscopic features in amyloidosis of the small intestine: clinical and morphologic differences between chemical types of amyloid protein. Gastrointest Endosc 40:45–50 [DOI] [PubMed] [Google Scholar]
- 43.Urban BA, Fishman EK, Goldman SM, Scott WW, Jr, Jones B, Humphrey RL, Hruban RH. 1993. CT evaluation of amyloidosis: spectrum of disease. Radiographics 13:1295–1308 [DOI] [PubMed] [Google Scholar]
- 44.van Gameren, Hazenberg BP, Bijzet J, van Rijswijk MH. 2006. Diagnostic accuracy of subcutaneous abdominal fat tissue aspiration for detecting systemic amyloidosis and its utility in clinical practice. Arthritis Rheum 54:2015–2021 [DOI] [PubMed] [Google Scholar]
- 45.Wegelius O, Teppo AM, Maury CP. 1982. Reduced amyloid-A-degrading activity in serum in amyloidosis associated with rheumatoid arthritis. Br Med J (Clin Res Ed) 284:617–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woldemeskel M. 2012. A concise review of amyloidosis in animals. Vet Med Int 2012:427296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zschiesche W, Jakob W. 1989. Pathology of animal amyloidoses. Pharmacol Ther 41:49–83 [DOI] [PubMed] [Google Scholar]