Abstract
Cardiac hypertrophy is a common postmortem finding in owl monkeys. In most cases the animals do not exhibit clinical signs until the disease is advanced, making antemortem diagnosis of subclinical disease difficult and treatment unrewarding. We obtained echocardiograms, electrocardiograms, and thoracic radiographs from members of a colony of owl monkeys that previously was identified as showing a 40% incidence of gross myocardial hypertrophy at necropsy, to assess the usefulness of these modalities for antemortem diagnosis. No single modality was sufficiently sensitive and specific to detect all monkeys with cardiac hypertrophy. Electrocardiography was the least sensitive method for detecting owl monkeys with hypertrophic cardiomyopathy. Thoracic radiographs were more sensitive than was electrocardiography in this context but cannot detect animals with concentric hypertrophy without an enlarged cardiac silhouette. Echocardiography was the most sensitive method for identifying cardiac hypertrophy in owl monkeys. The most useful parameters suggestive of left ventricular hypertrophy in our owl monkeys were an increased average left ventricular wall thickness to chamber radius ratio and an increased calculated left ventricular myocardial mass. Parameters suggestive of dilative cardiomyopathy were an increased average left ventricular myocardial mass and a decreased average ratio of left ventricular free wall thickness to left ventricular chamber radius. When all 4 noninvasive diagnostic modalities (physical examination, echocardiography, electrocardiography, and thoracic radiography) were used concurrently, the probability of detecting hypertrophic cardiomyopathy in owl monkeys was increased greatly.
Abbreviations: LVFWTd, left ventricular free wall thickness at end-diastole; STd, interventricular septal thickness at end-diastole; EDD, left ventricular chamber diameter at end-diastole; ESD, left ventricular chamber diameter at end-systole; VHS, vertebral heart scale
Owl monkeys (Aotus spp.) are maintained and used primarily as a nonhuman primate model for the study of malaria.17,38,45 These neotropical monkeys have also been shown to be useful for the study of visceral leishmaniasis,5 various viruses,1,20-22,27 streptotrichosis,19 campylobacteriosis,16 and toxoplasmosis.34 Owl monkeys are naturally susceptible to a variety of internal parasites,37,39 hemolytic anemia, and glomerulonephritis.8,13,40
Approximately 40% of Aotus monkeys dying from all causes in a colony maintained for dispersement to investigators for the study of malaria have gross evidence of myocardial hypertrophy at necropsy.40 This incidence is similar to the 41% mortality ascribed to cardiovascular disease in captive adult lowland gorillas,25 and the report that, in humans, cardiovascular disease represents 42% of all deaths in the United States.9 The hypertrophy in owl monkeys almost obliterates the left ventricular chamber and causes marked thickening of the left ventricular free wall and interventricular septum. A similar incidence of myocardial hypertrophy has previously been reported in other owl monkey colonies.13,31,32
Most of the deaths in the owl monkey colony at our institution can be attributed to various identifiable causes typical for this species.40 However, several monkeys in this colony had gross evidence of myocardial hypertrophy at death but had shown no clinical signs of disease and displayed no gross or microscopic etiology for death. A prominent feature of the spontaneous deaths within this particular colony of Aotus monkeys was ‘sudden death,’ particularly during periods of high physical and psychologic stress.40 Ventricular arrhythmias have been hypothesized as possible cause of sudden death in chimpanzees with cardiomyopathy,10 and a similar mechanism might be responsible for sudden death in captive owl monkeys.
Gross hypertrophy of the left ventricle has many causes in humans and animals. In the absence of gross evidence of resistive lesions (that is, coarctation of the aorta, aortic stenosis) or shunting lesions (that is, ventricular septal defect, patent ductus arteriosus, atrial septal defect), hypertrophic cardiomyopathy and hypertension are the 2 most likely causes of or stimulus for the left ventricular hypertrophy observed in our colony.4,18 Because no gross lesions that contribute to myocardial hypertrophy have been found at necropsy, we presumed that the hypertrophic changes in our monkeys are due to hypertrophic cardiomyopathy or to increased afterload secondary to hypertension. Spontaneous hypertension has been reported as causing dilated cardiomyopathy in wooly monkeys,11 and vitamin E deficiency is a cause of cardiomyopathy (primarily dilative) in gelada baboons as well as other primates.23,24 Cardiac changes secondary to experimental trypanosomiasis have occurred in vervet28 and squirrel monkeys29 and after group A streptococcal infection in rhesus monkeys.26 However, only a few reports detail spontaneous myocardial hypertrophic changes in nonhuman primates.3,10,13,32 Because of the high incidence of cardiac hypertrophy in clinically normal owl monkeys, it is difficult to establish ‘normal’ reference values for the species. One group of authors31 was reluctant to suggest reference intervals for normal echocardiographic variables in owl monkeys due to inability to confirm that monkeys classified as normal were free of cardiac disease.
The left ventricular hypertrophy in the owl monkey colony we present has been speculated to be the result of renal-induced hypertension. Many of these monkeys have evidence of glomerular lesions at necropsy. Spontaneous primary systemic hypertension has been suggested as the etiology of the cardiomyopathy and renal disease in owl monkeys,13 however, no relationship between antemortem blood pressure and the presence or absence of renal lesions and myocardial hypertrophy in these monkeys had been established until recently. A recent study35 using chronically implanted pressure transducers in A. nancymae found that 30% of the monkeys had resting mean arterial pressures in the hypertensive range (>110 mm Hg), and all animals had exaggerated pressor responses in response to routine husbandry procedures (mean arterial pressure, 125 to 196 mm Hg). These same authors concluded that the hypertension observed was consistent with a neural-based essential hypertension that possibly was engendered by the frequent hyperreactive responses of the sympathetic nervous system of these animals to environmental events.35
This study was undertaken to identify individual owl monkeys with left ventricular hypertrophy by using noninvasive diagnostic techniques antemortem. Physical examination, echocardiography, electrocardiography, and thoracic radiography were selected as potential diagnostic modalities with the greatest probability of yielding sensitive and specific information regarding left ventricular hypertrophy in individual monkeys prior to postmortem examination.
Materials and Methods
Animals.
The owl monkey colony at Pacific Northwest National Laboratory (Richland, WA) includes both colony-born and wild-caught animals. Most of the owl monkeys belong to the species Aotus nancymae, but a few A. vociferans and A. lemurinus griseimembra are maintained also. The monkeys were housed and cared for according to the Guide for the Care and Use of Laboratory Animals.14 The monkeys were housed as pairs or singly in stainless steel cages with a reverse light cycle, were fed standard New World primate biscuit, and received at least one enrichment food daily. Housing and husbandry details for this owl monkey colony have been previously described.2,41 Because of the movement of individual healthy monkeys into and out of the colony, it was not possible to obtain similar data on all monkeys or to monitor individual animals for progression of disease over time. Adult owl monkeys from both sexes were examined antemortem by using physical examination and 3 noninvasive diagnostic modalities (echocardiography, electrocardiography, and thoracic radiography) to ascertain the presence of myocardial disease. Each monkey was judged to be either clinically normal or abnormal on the basis of physical examination by the attending veterinarian and daily observations by animal care specialists. Signs used to classify an individual monkey as abnormal included the presence of heart murmur, arrhythmia, or other abnormality on thoracic auscultation; prolonged lethargy and depression; weight loss (recent or sustained); anorexia; mouth breathing; fatigue; reduced exercise tolerance; dyspnea; ascites; and subcutaneous edema. All of these signs were considered to be suggestive of cardiac insufficiency.40 Colony animals with clinical signs of congestive heart failure were treated symptomatically by using diuretics (furosemide, 2 mg/kg PO or IM once or twice daily) and low-sodium diets. Monkeys that did not respond to treatment or with severe clinical signs of heart failure were euthanized by using sodium pentobarbital overdose.
All monkeys were sedated with ketamine hydrochloride (10 mg/kg IM in the posterior thigh muscle) approximately 5 to 10 min prior to each of the diagnostic procedures. Because of the short duration of restraint produced by ketamine and the duration of some of the diagnostic procedures, a separate sedation was used for each procedure. Physical examinations, echocardiography, and electrocardiography were performed on the same day; thoracic radiographs were completed within 30 d of the other procedures.
Echocardiography.
We examined 45 Aotus monkeys for antemortem evidence of cardiomyopathy. All echocardiograms were obtained and evaluated by the same person (GGK) to reduce the possibility of interoperator and interreader variability. Each monkey was examined for evidence of myocardial changes by using 2-dimensional and M-mode–guided technique with a 7.5-MHz mechanical sector scanner (model XL, Interspec, Ambler, PA). Of the 45 monkeys examined with echocardiography, 24 were considered to be clinically normal; the remaining 21 were classified as abnormal as defined earlier. All images were obtained from a right or left parasternal position with the monkey in dorsal recumbency. A 2-dimensional short-axis view of the left ventricular chamber was used to place the cursor to bisect the short axis of the left ventricle between the 2 papillary muscles. The cursor was placed just ventral to the plane of the mitral valve to maximize the dimensional measurements of the left ventricular chamber. Pulsed-wave Doppler echocardiography was performed in 8 monkeys, but none of the images were of sufficient quality to obtain deceleration time or the ratio between early and late ventricular filling.
All echocardiograms were analyzed according to criteria established by the American Society of Echocardiography.33 Measured variables included left ventricular free wall thickness at end-diastole (LVFWTd), interventricular septal thickness at end-diastole (STd), left ventricular chamber diameter at end-diastole (EDD), and left ventricular chamber diameter at end-systole (ESD). Values calculated from the echocardiogram included percentage fractional shortening,
Fractional shortening = (EDD - ESD) / EDD,
the ratio of average left ventricular wall thickness in diastole to left ventricular chamber radius in diastole,
and left ventricular myocardial mass,
A simultaneous lead II electrocardiogram was recorded with each echocardiogram to obtain simultaneous heart rate.
Electrocardiography.
Standard 6-lead electrocardiography was performed on 33 of the 45 Aotus monkeys screened by echocardiography. Of the 33 monkeys, 15 were considered to be clinically normal; the remaining 18 were judged to be abnormal as described earlier. All electrocardiograms were obtained with the monkey in dorsal recumbency and the leads attached at the wrists and ankles. All electrocardiograms were interpreted by the same person (GGK) to reduce interreader variability.
The electrocardiographic leads recorded included leads I, II, III, aVr, aVl, and aVf. The lead II electrocardiogram was used for all electrocardiographic variables measured, except for the measurements necessary for determination of the mean electrical axis in the frontal plane. Measured variables on the lead II electrocardiograms included the duration of the P wave; the duration of the PQ interval (from the onset of the P wave to the onset of the Q wave); the duration of the QRS complex; the duration of the QT interval (from the onset of the Q wave to the end of the T wave); the voltage of the P wave; the voltage of the R wave in leads I, II, and aVf; and the voltages of the Q and S waves in leads I and aVf.
Heart rate was determined by counting the number of QRS complexes in 6 s and multiplying by 10. The mean electrical axis in the frontal plane was determined by finding the angle from lead I of the resultant vector determined from a rectangle, 2 sides of which were defined by the sum of the Q, R, and S wave voltages in leads I and aVf.
Radiography.
Standard dorsoventral and lateral thoracic radiographs were obtained in 21 of the 33 Aotus monkeys screened by echocardiography and electrocardiography; these images were obtained at a constant 40-inch focal film distance, and radiographs taken on full inspiration. Exposure factors for an average size Aotus (900 to 1100 g) were set at 200 mA, 1/60 s (3.33 mAs), and 50 kVp. Intensifying screens (Quantum III, Sigma, St Louis, MO) and medical X-ray film (Cronex 7, Sigma) was used for each exposure. Of the 2l monkeys examined radiographically, 5 were judged to be clinically normal, and the 16 remaining monkeys radiographed were considered to be abnormal as described earlier. A single reader (RLP) interpreted all radiographs without prior knowledge of the clinical status of the monkeys or other diagnostic findings in any of the monkeys. In addition, the vertebral heart scale (VHS) index was calculated to provide objective criteria when defining normal compared with abnormal heart radiographic images.6 Because there are no established parameters for VHS indices in owl monkeys, the radiographs were used to screen for signs of cardiovascular disease. Radiographs were considered normal when there were 1) no signs of pulmonary disease, 2) no cardiac enlargement, and 3) a VHS index of 9.9 or less. Radiographs were considered abnormal with evidence of 1) pulmonary disease suggestive of pulmonary edema, 2) cardiac enlargement, 3) enlarged pulmonary vessels, 4) hepatomegaly, or (5) a VHS index of 10.0 or greater. Distinct evidence of cardiovascular disease was determined by the compromise of the animal's physical status.
Statistics.
The Student t test for unpaired data was used to compare groups (male compared with female; clinically normal compared with abnormal).36 Mean values for quantitated variables were considered significantly different when a P value of less than 0.05 was present.
Results
Echocardiography.
Results from the echocardiographic examination of 45 Aotus monkeys (Table 1) revealed that clinically normal male monkeys had a significantly (P < 0.05) smaller calculated myocardial mass than did clinically abnormal male monkeys. Clinically normal female monkeys differed from abnormal female monkeys in the thickness of the left ventricular free wall, interventricular septal thickness, and the ratio of the average left ventricular wall thickness to left ventricular chamber radius. Compared with their abnormal counterparts, normal female monkeys had a significantly (P < 0.05) smaller mean values for left ventricular free wall thickness, interventricular septal thickness, and average wall thickness to chamber radius ratio.
Table 1.
Results of echocardiographic examinations of 45 owl monkeys stratified according to sex and clinical condition
| Male monkeys |
Female monkeys |
|||
| Normal (n = 12) | Abnormal (n = 11) | Normal (n = 12) | Abnormal (n = 10) | |
| Body weight (g) | 939.00 ± 128.30 | 947.64 ± 97.69 | 883.42 ± 79.60 | 883.60 ± 113.32 |
| End-diastolic diameter (mm) | 12.17 ± 1.70 | 12.73 ± 2.65 | 12.67 ± 2.02 | 11.30 ± 2.21 |
| End-systolic diameter (mm) | 8.50 ± 2.07 | 8.91 ± 3.24 | 9.08 ± 2.35 | 7.60 ± 2.50 |
| LV Free wall thickness (mm) | 3.33 ± 0.98 | 3.91 ± 0.94 | 3.00 ± 0.74a | 3.90 ± 1.10a |
| Septal thickness (mm) | 2.58 ± 0.79 | 3.27 ± 0.90 | 2.25 ± 0.45a | 3.30 ± 0.82a |
| Shortening fraction (%) | 30.21 ± 12.78 | 32.03 ± 12.02 | 28.87 ± 10.12 | 33.21 ± 15.14 |
| Wall thickness:chamber radius | 0.50 ± 0.14 | 0.61 ± 0.27 | 0.43 ± 0.12a | 0.68 ± 0.25a |
| Myocardial mass (g) | 2.21 ± 0.78a | 3.06 ± 0.92a | 1.97 ± 0.56 | 2.58 ± 0.83 |
Value significantly (P < 0.05) different between normal and abnormal monkeys of the same sex.
There were no significant echocardiographic differences between male and female monkeys (Table 2). Mean values for left ventricular free wall thickness and interventricular septal thickness were significantly (P < 0.05) smaller in the clinically normal monkeys than in abnormal monkeys. Mean average wall thickness to chamber radius ratio and calculated myocardial mass were smaller in clinically normal than in abnormal monkeys (Figure 1 A through D).
Table 2.
Results of echocardiographic examination of 45 owl monkeys stratified according to clinical condition
| Normal monkeys (n = 24) | Abnormal monkeys (n = 21) | P | |
| End-diastolic diameter (mm) | 12.42 ± 1.84 | 12.05 ± 2.50 | 0.5807 |
| End-systolic diameter (mm) | 8.79 ± 2.19 | 8.29 ± 2.92 | 0.5193 |
| LV free wall thickness (mm) | 3.17 ± 0.87 | 3.90 ± 0.99 | 0.0120 |
| Septal thickness (mm) | 2.42 ± 0.65 | 3.29 ± 0. 85 | 0.0005 |
| Septal + LV wall thickness (mm) | 5.58 ± 1.25 | 7.19 ± 1.63 | 0.0008 |
| Shortening fraction (%) | 29.54 ± 11.29 | 32.59 ± 13.26 | 0.4138 |
| Wall thickness:chamber radius | 0.46 ± 0.14 | 0.64 ± 0.26 | 0.0084 |
| Myocardial mass (g) | 2.09 ± 0.68 | 2.83 ± 0.90 | 0.0036 |
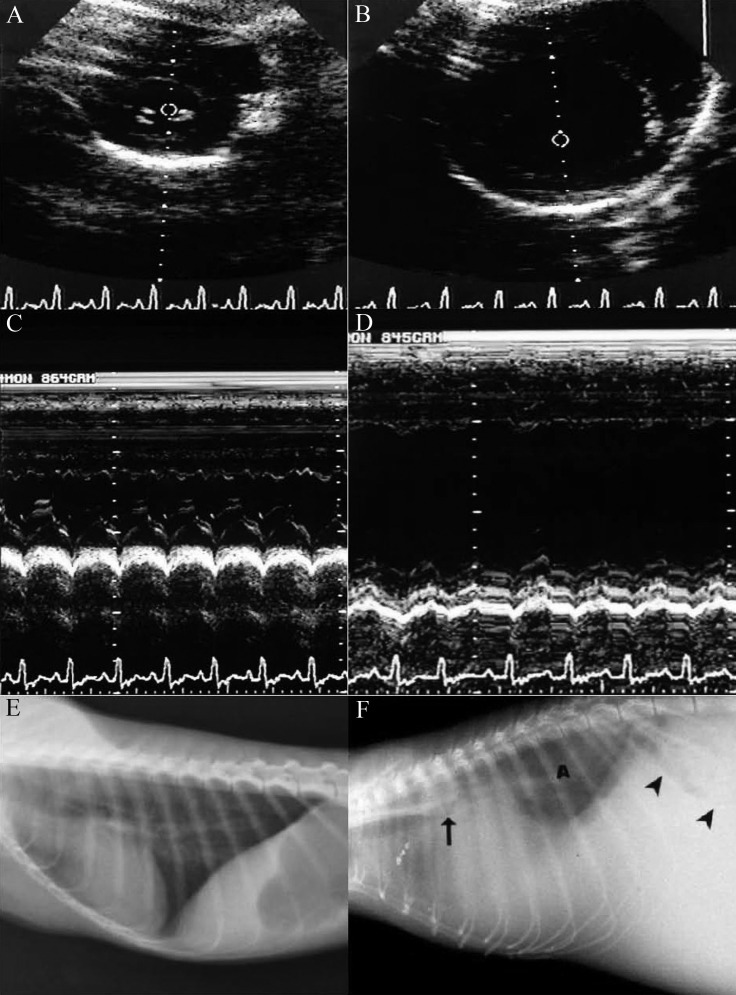
Figure 1.
A) 2-Dimensional echocardiogram image of left ventricle on cross-section at level of papillary muscles during end-diastole in a normal owl monkey. B) 2-Dimensional echocardiogram image of left ventricle on cross section at level of papillary muscles during end diastole in an owl monkey with marked myocardial hypertrophy and dilatation. C) M-mode echocardiogram image of left ventricle on cross section of the same normal animal. D) M-mode echocardiogram image of left ventricle on cross section of the same animal with marked hypertrophy and dilatation. Note the poor ventricular contraction. E) Lateral thoracic radiograph of a normal owl monkey. F) Lateral thoracic radiograph of the owl monkey depicted in panels B and D. Note the severe hypertrophic cardiomyopathy, with elevation of the carina (arrow) secondary to left heart enlargement. The heart and the diaphragm are obscured by extensive pleural effusion. Note the pulmonary radiodensity (marked A in the image) due to pulmonary edema, stomach with caudal shift to the axis (arrowheads) from hepatomegaly, and loss of abdominal detail from peritoneal fluid resulting in marked abdominal distention. This monkey had a vertebral heart score of 13.
Electrocardiography.
Electrocardiographic examination of 33 Aotus monkeys revealed no significant differences in the mean values of the electrocardiographic variables between clinically normal monkeys and those with clinical signs of heart disease (Table 3). When the group of clinically normal owl monkeys is considered to represent a sample from a normal population and 2 standard deviation ranges are determined around the mean for each of the measured electrocardiographic variables, individual monkeys in the clinically abnormal group have electrocardiographic values that are outside the normal ranges. Specifically, 2 monkeys had abnormally tall R waves in lead II, 2 monkeys showed left axis deviation in the frontal plane, and one monkey had an abnormally tall P wave in lead II. Tall R waves in lead II and left axis deviation in the frontal plane were interpreted to indicate the presence of left ventricular enlargement. Tall P waves in lead II indicated right atrial enlargement.
Table 3.
Results of electrocardiographic examinations of 33 owl monkeys stratified according to clinical classification
| Normal monkeys (n = 15) | Abnormal monkeys (n = 18) | |
| P wave (s) | 0.036 ± 0.005 | 0.037 ± 0.004 |
| PQ interval (s) | 0.075 ± 0.015 | 0.073 ± 0.008 |
| QRS duration (s) | 0.044 ± 0.005 | 0.042 ± 0.004 |
| QT interval (s) | 0.136 ± 0.036 | 0.135 ± 0.028 |
| P wave (mv) | 0.29 ± 0.074 | 0.308 ± 0.096 |
| R wave, lead II (mv) | 1.15 ± 0.694 | 1.375 ± 0.68 |
| Heart rate (bpm) | 258 ± 63 | 261 ± 45 |
| R wave, lead I (mv) | 0.487 ± 0.357 | 0.636 ± 0.485 |
| R-wave, lead aVf (mv) | 0.823 ± 0.71 | 1.011 ± 0.822 |
| Mean frontal axis (°) | 62.41 ± 21.4 | 56.28 ± 25.27 |
Radiography.
Anterior–posterior and lateral thoracic radiographs of clinically normal and abnormal owl monkeys revealed that some monkeys observed to be clinically normal (2 of 5, 40%) were classified as demonstrating moderate to severe (VHS index greater than 11) evidence of cardiovascular disease on thoracic radiography. This classification of normal monkeys as having moderate to severe changes was based on enlargement of the cardiac silhouette in both radiographic projections of the thorax and on the VHS index. However, these 2 animals were removed from the colony, thereby preventing follow up to determine whether they eventually developed clinical signs of heart disease. The VHS index (mean ± SE) for the clinically normal group (n = 5) was 10.7 ± 0.69 (range, 9.0 to 13.0).
Some of the monkeys previously identified as having clinical signs of disease did not demonstrate radiographic signs of cardiovascular disease. These animals might have concentric hypertrophy of the left ventricle; this condition causes a decreased chamber size with low cardiac output but does not necessarily yield an enlarged cardiac silhouette. However, 11 of 16 (68.7%) monkeys with clinical evidence of disease showed radiographic evidence of cardiovascular disease (enlargement; with a VHS index of 10 or higher) on thoracic radiographs (Figure 1 E, F). The VHS index for the clinically abnormal group (n = 16) was 10.7 ± 0.25 (range, 9.1 to 12.7).
Discussion
The current data demonstrate that clear-cut identification of Aotus monkeys with myocardial hypertrophy is difficult. We agree with previous authors,31 who were reluctant to suggest normal reference cardiac echocardiographic parameters in owl monkeys due to the difficulty of confirming that normal subjects were free of heart disease. A recent study30 reported an unexpected large number of clinically normal owl monkeys with cardiomyopathy. Establishing standard echocardiographic reference standards in a species with such a high incidence of cardiac disease is difficult. Many apparently clinically healthy animals would have to be imaged and then immediately euthanized for gross and histologic examination to validate that the animals were free of cardiac disease. However, this scenario is not an option with these valuable animals. In addition, comparing and validating echocardiographic measurements obtained during systole and diastole with in situ cardiac measurements taken postmortem is extremely difficult, if not impossible. No animals were euthanized during the current study; historically, animals with severe decompensated cardiac failure showed concentric hypertrophy of the left ventricle and interventricular septum with marked dilatation at necropsy. Microscopically, the myocardium showed interstitial fibrosis, separation of muscle bundles, cytoplasmic vacuolation, wavy fibers, contraction-band necrosis, and loss of myofibers.13 In addition, in some cases, the muscular layer of myocardial coronary arteriolae showed hypertrophy and hyperplasia of the smooth muscle fibers with cytoplasmic vacuolation, which is a common lesion in people with sustained arterial hypertension.12
Because cardiomyopathy is a common disease in most owl monkey colonies in the United States and overseas, we believe it is not a familial condition but one that affects this particular genus. As previously reported, the cardiomyopathy observed in Aotus spp. is most likely a compensatory hypertrophy result of neural-based essential hypertension that possibly was generated as a consequence of frequent hyperreactive responses of the sympathetic nervous system to environmental events.35 This hypothesis is supported by the fact that hypertrophic cardiomyopathy in owl monkeys is associated with time in captivity.13
Classic clinical signs of left ventricular hypertrophy are related to diastolic dysfunction of the left ventricle.4 These signs are manifest primarily as a result of low cardiac output that is unable to meet the oxygen demand of the body (forward heart failure). However, signs of left ventricular diastolic dysfunction also result in increased pulmonary capillary hydrostatic pressure and clinical signs of pulmonary edema (backward heart failure). If the myocardial hypertrophy is uniform and the right ventricle is affected, signs of backward failure also will include hepatomegaly, ascites, and jugular pulses. General signs of exercise intolerance, fatigue, depression, anorexia, and lethargy can be part of the constellation of signs in heart failure.4 One study31 found that the most useful parameters for discriminating between healthy owl monkeys and those with cardiac dilatation were an increase in end-systolic dimensions with consequent reduction in the corresponding fractional dimensional change, without an increase in end-diastole dimensions or left atrial enlargement. No differences between male and female monkeys were found.31 Another study30 found that left ventricular diameter and ejection fraction appeared to be the best parameters for distinguishing normal and cardiomyopathic owl monkeys; no comparison between male and female monkeys was reported. These studies did not find differences in electrocardiograms between groups.30,31
In our current study, the most useful parameters suggestive of left ventricular hypertrophy were an increased average left ventricular wall thickness to chamber radius ratio with an increased calculated left ventricular myocardial mass. Parameters suggestive of dilatory cardiomyopathy were increased average left ventricular myocardial mass with decreased average ratio of left ventricular free wall thickness to left ventricular chamber radius. In contrast to previous reports,30,31 we found differences between male and female owl monkeys. Calculated myocardial mass appeared to be a more important indicator of hypertrophic cardiomyopathy in male monkeys, whereas left ventricular free wall thickness, interventricular septal thickness, and average left ventricular wall thickness to left ventricular chamber radius seemed to be more important in females. Shortening fraction did not differ between normal and abnormal owl monkeys in our study. This apparent difference from previous studies30,31 might be due to the fact that the abnormal groups in the previous studies primarily comprised animals with marked dilated cardiomyopathy (6 animals died during the course of one study) and were more likely to exhibit severe clinical signs of cardiac failure when compared with monkeys with hypertrophic cardiomyopathy alone. Dilated cardiomyopathy in owl monkeys is likely an end-stage manifestation of cardiac hypertrophy, similar to what has been observed in humans with chronic hypertension and should be viewed similarly.
We did not evaluate blood pressure in our current study. To obtain these data, owl monkeys must be chronically implanted with pressure transducers and blood pressure measured by telemetry. This assessment was not attempted on any of the animals included in this report because they were destined for use malaria vaccine development studies at other facilities. However, blood pressure measurements obtained by direct femoral arterial puncture on 54 owl monkeys under ketamine anesthesia at our facility revealed extremely high blood pressures compared with those in all other known primate species, including humans.35 Because the effects of capturing, restraining, and subsequently injecting the monkeys with the anesthetic agent likely had an immediate effect on blood pressure, one group performed a pilot study in which 2 owl monkeys were chronically implanted with pressure transducers in the aorta and blood pressures recorded on fully awake animals. In this pilot study, systolic blood pressures at rest were 120 to 150 mm Hg; when the animals were disturbed by a technician walking into the room, pressures increased to 220 to 250 mm Hg.35
Ketamine produces a decrease in left ventricular ejection fraction in swine42 and reduces left ventricular systolic and diastolic function in human patients with ischemic heart disease.15 Because all animals on the current study were sedated with ketamine, the possible effect of the drug on heart function is expected to occur in both groups and does not significantly alter one group over the other for the purposes of data comparison. Not using sedation for physical restraining for the purposes of this study would have been extremely stressful for the monkeys, particularly in those with cardiomyopathy.
The echocardiographic findings in our study are consistent with left ventricular hypertrophy secondary to pressure overload or spontaneous hypertrophic cardiomyopathy. Because no gross lesions that would contribute to myocardial hypertrophy (that is, coarctation of the aorta, aortic stenosis) have been found at necropsy, we presume that the hypertrophic changes present in these Aotus monkeys are due to hypertrophic cardiomyopathy or are the result of increased afterload secondary to hypertension (with or without an identifiable etiology), as previously suggested13 and recently confirmed by using telemetry.35
Selecting objective echocardiographic criteria for the identification of monkeys with myocardial hypertrophy from the reported echocardiographic data is not straightforward. Those monkeys with thick left ventricular walls and small left ventricular chamber diameters would be a logical choice. However, a monkey that is moderately to severely dehydrated for any of a number of reasons will have a reduced intravascular volume and decreased filling of the heart.7 Such a monkey will appear subjectively to have a small-diameter left ventricular chamber with relatively thick left ventricular walls and interventricular septum. Because of the small size of the heart in Aotus monkeys, even a small change (reduction) in left ventricular volume will produce a subjective perception of a thick-walled heart with a small left ventricular chamber.
The calculation of myocardial mass would obviate the problem of the increased wall thickness to chamber radius ratio that would be found in a dehydrated monkey with a normal heart. However, gross assumptions (simplification) about the geometry of the left ventricular shape are necessary to calculate myocardial mass from the quantitative variables of septal thickness, left ventricular free wall thickness, and left ventricular chamber diameter determined by echocardiography. In dogs, assumptions of a spherical shape for the left ventricular chamber, with the mass evenly distributed about this sphere, have shown good correlation with mass determined by angiography and at necropsy.43,44
With small hearts, such as in Aotus monkeys, our experience has been that subjective impressions obtained from real-time images are not nearly as useful in identifying forms of hypertrophy as compared with those of larger species. Therefore, both an increase in calculated left ventricular myocardial mass and an increase in the average left ventricular wall thickness to chamber radius ratio should probably be present before a monkey is classified as having definitive evidence of myocardial hypertrophy. Selecting 2 SD above the mean average left ventricular wall thickness to chamber radius ratio for clinically normal monkeys (from this sample, 0.74) as being an acceptable upper limit allows the identification of 6 monkeys in this sample group as having evidence for an altered (increased) average wall thickness to chamber radius ratio. Selecting 2 SD above the mean calculated left ventricular myocardial mass for normal monkeys (from this sample, 3.45 g) as being an acceptable upper limit allows the identification of 5 monkeys in this sample group as having evidence of an altered (increased) calculated myocardial mass. When both criteria (average left ventricular wall thickness to chamber radius ratio greater than 2 SD above the mean and calculated myocardial mass greater than 2 SD above the mean) are used to identify monkeys with hypertrophic changes, only one monkey (4.1% of the population) meets both criteria.
When the upper limit of normal is chosen to be 1 SD above the mean for both calculated variables, 13 monkeys exceed the upper limit (greater than 0.60) for an increased ratio of left ventricular wall thickness to chamber radius ratio, and 11 monkeys exceed the upper limit (greater than 2.77 g) for an increased calculated left ventricular myocardial mass. Simultaneous application of both upper limits at 1 SD identifies 7 of the 24 (29.1%) clinically normal monkeys as meeting the objective criteria for left ventricular myocardial hypertrophy.
An argument can be made that because of the limited sample size of monkeys on which echocardiographic variables were determined (45 total), establishing the upper limit of normal should parallel the occurrence of observed cardiac hypertrophy at necropsy, that is, the upper 40% of monkeys should be considered abnormal. Accordingly, if the sample of monkeys selected were a true random sample from the population of all Aotus monkeys and if the incidence of myocardial hypertrophy were actually 40% in all Aotus monkeys, 18 of the 45 monkeys in this sample likely have myocardial hypertrophic changes.
When we select the 18 monkeys with the largest left ventricular average wall thickness to chamber radius ratios and the 18 monkeys with the largest calculated left ventricular myocardial mass, the resultant combined list includes all of the monkeys with myocardial hypertrophy. The monkeys that appear on both lists are quite likely to have myocardial hypertrophy of a concentric (that is, due to hypertrophic cardiomyopathy or hypertension) nature rather than myocardial hypertrophy of an eccentric nature. This selection process identifies 11 monkeys (24% of the entire sample) as meeting both criteria for hypertrophy.
These findings are consistent with subjective interpretations of the 2-dimensional echocardiographic views obtained during the initial examination of each monkey. However, some additional monkeys were judged as being hypertrophic but do not appear on the list of 11 monkeys described in the previous paragraph. Similarly, other monkeys appreciated subjectively to have dilated left ventricular chambers (thin left ventricular free wall, thin interventricular septum, relatively large left ventricular chamber, and depressed shortening fraction) do not appear on either list. Furthermore, at least one monkey deemed to have dilative disease appears very high on the list of monkeys with increased left ventricular myocardial mass but very low on the listing of the ratio of average left ventricular free wall thickness to left ventricular chamber radius, contrary to expectation. However, this observation emphasizes the importance of using multiple echocardiographic criteria to ascertain the presence of myocardial hypertrophy of a concentric nature. Of the 11 monkeys that met 2 criteria for the presence of left ventricular myocardial hypertrophy, only one displayed evidence of electrocardiographic changes consistent with left ventricular myocardial hypertrophy. That monkey displayed left axis deviation on the electrocardiogram and was clinically abnormal prior to the study.
Two of the 11 monkeys that met both echocardiographic criteria for left ventricular myocardial hypertrophy were judged to have a normal radiographic appearance and were felt to be clinically abnormal prior to the study. Three monkeys that met both echocardiographic criteria for left ventricular hypertrophy had moderate to severe cardiac enlargement radiographically; 2 of these animals were clinically normal prior to the study whereas the third was abnormal. Another 2 monkeys that met both echocardiographic criteria showed mild cardiac enlargement radiographically and were clinically abnormal prior to the study. It should be noted that monkeys with cardiac concentric hypertrophy of the left ventricle do not necessarily show an enlarged cardiac silhouette radiographically. However, these animals will have a decreased cardiac chamber size and, if decompensated, will display clinical signs suggestive of cardiac insufficiency. Because of the constant movement of animals into and out of the colony, only 33 of the 45 animals that were screened initially by echocardiography were available for electrocardiography and only 21 were available for thoracic radiography, thereby limiting the usefulness of the radiographic data.
In conclusion, electrocardiography appears to be the least sensitive method to identify owl monkeys with hypertrophic cardiomyopathy. Thoracic radiographs were more sensitive than was electrocardiography for detecting animals that have hypertrophic cardiomyopathy, but radiography will not detect animals with concentric hypertrophy without an enlarged cardiac silhouette. Echocardiography appears to be the most sensitive modality for identifying owl monkeys with hypertrophic cardiomyopathy, allowing the detection of statistically significant differences between clinically normal and abnormal animals. The most useful parameters suggestive of left ventricular hypertrophy in owl monkeys in our colony were an increased average left ventricular wall thickness to chamber radius ratio with an increased calculated left ventricular myocardial mass. Parameters suggestive of dilatory cardiomyopathy were increased average left ventricular myocardial mass with decreased average ratio of left ventricular free wall thickness to left ventricular chamber radius.
This study emphasizes the importance of using multiple diagnostic modalities and multiple criteria when attempting to select monkeys with myocardial hypertrophy antemortem. Because of the importance and utility of a naturally occurring nonhuman primate animal model of either hypertrophic cardiomyopathy or myocardial changes secondary to hypertension, methods should be developed and validated to accurately identify and track the progression of either disease in the live monkey. We recommend the combined use of all 4 of the diagnostic modalities assessed in the current study (physical examination, echocardiography, electrocardiography, and thoracic radiography) for identifying and following the progress of Aotus monkeys suspected of having hypertrophic myocardial changes.
Acknowledgments
We extend a note of appreciation to Interspec Corporation for the loan of equipment used to obtain the echocardiograms reported in the current article. This study was supported by the US Agency for International Development (contract DPE-0453-C-00-6061-00) and the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIAID), Comparative Medicine Branch, and the Office of Research Support.
References
- 1.Asher LV, Binn LN, Mensing TL, Marchwicki RH, Vassell RA, Young GD. 1995. Pathogenesis of hepatitis A in orally inoculated owl monkeys (Aotus trivirgatus). J Med Virol 47:260–268 [DOI] [PubMed] [Google Scholar]
- 2.Baer JF. 1994. Husbandry and medical management of the owl monkey, p353–374. In: Baer JF, Weller RE, Kakoma I. Aotus: the owl monkey. San Diego (CA): Academic Press. [Google Scholar]
- 3.Brady AG, Watford JW, Massey CV, Rodning KJ, Gibson SV, Williams LE, Abee CR. 2003. Studies of heart disease and failure in aged female squirrel monkeys (Saimiri spp.). Comp Med 53:657–662 [PubMed] [Google Scholar]
- 4.Braundwald E. 1980. Pathophysiology of heart failure, p 456–460. In: Braunwald E, editor. Heart disease: a textbook of cardiovascular medicine. Philadelphia (PA): WB Saunders. [Google Scholar]
- 5.Broderson JR, Chapman WL, Jr, Hanson WL. 1986. Experimental visceral leishmaniasis in the owl monkey. Vet Pathol 23:293–302 [DOI] [PubMed] [Google Scholar]
- 6.Buchanan JW. 2000. Vertebral scale system to measure heart size in radiographs. Vet Clin North Am Small Anim Pract 30:379–393 [PubMed] [Google Scholar]
- 7.Campbell FE, Kittleson MD. 2007. The effect of hydration status on the echocardiographic measurements of normal cats. J Vet Intern Med 21:1008–1015 [DOI] [PubMed] [Google Scholar]
- 8.Chalifoux LV, Bronson RT, Sehgal P, Blake BJ, King NW. 1981. Nephritis and hemolytic anemia in owl monkeys (Aotus trivirgatus). Vet Pathol 18:23–37 [DOI] [PubMed] [Google Scholar]
- 9.Criqui MH. 2000. Epidemiology of cardiovascular disease, p 167–170. In: Goldman L, Bennett JC. Cecil textbook of medicine. Philadelphia (PA): WB Saunders. [Google Scholar]
- 10.Doane CJ, Lee DR, Sleeper MM. 2006. Electrocardiogram abnormalities in captive chimpanzees (Pan troglodytes). Comp Med 56:512–518 [PubMed] [Google Scholar]
- 11.Giddens WE, Combs CA, Smith OA, Klein EC. 1987. Spontaneous hypertension and its sequelae in wooly monkeys (Lagothrix lagotricha). Lab Anim Sci 37:750–756 [PubMed] [Google Scholar]
- 12.Gozalo AS, Chavera A, Montoya EJ, Takano J, Weller RE. 2008. Relationship of creatine kinase, aspartate aminotransferase, lactate dehydrogenase, and proteinuria to cardiomyopathy in the owl monkey (Aotus vociferans). J Med Primatol 37 Suppl 1:29–38 [DOI] [PubMed] [Google Scholar]
- 13.Gozalo AS, Dagle G, Montoya E, Weller R, Málaga C. 1992. Spontaneous cardiomyopathy and nephropathy in the owl monkey (Aotus spp.) in captivity. J Med Primatol 21:279–284 [PubMed] [Google Scholar]
- 14.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 15.Jakobsen CJ, Torp P, Vester AE, Folkersen L, Thougaard A, Sloth E. 2010. Ketamine reduce left ventricular systolic and diastolic function in patients with ischaemic heart disease. Acta Anaesthesiol Scand 54:1137–1144 [DOI] [PubMed] [Google Scholar]
- 16.Jones FR, Baqar S, Gozalo A, Nunez G, Espinoza N, Reyes SM, Salazar M, Meza R, Porter CK, Walz SE. 2006. New World monkey Aotus nancymae as a model for Campylobacter jejuni infection and immunity. Infect Immun 74:790–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones T, Narum D, Gozalo A, Aguiar J, Fuhrmann S, Liang H, Haynes D, Moch JK, Lucas C, Luu T, Magill A, Hoffman S, Sim K. 2001. Protection of Aotus monkeys by Plasmodium falciparum EBA175 region II DNA prime–protein boost immunization regimen. J Infect Dis 183:303–312 [DOI] [PubMed] [Google Scholar]
- 18.Kashgarian M. 1990. Hypertensive disease and kidney structure, p 389–398. In: Laragh JH, Brenner BM. Hypertension: pathophysiology, diagnosis, and management. New York (NY): Raven Press. [Google Scholar]
- 19.King NW, Fraser CE, Wolf LA, Garcia FG, Williamson ME. 1971. Cutaneous streptotrichosis (dermatophiliasis) in owl monkeys. Lab Anim Sci 21:67–74 [PubMed] [Google Scholar]
- 20.Kochel T, Raviprakash K, Hayes C, Watts D, Russell K, Gozalo A, Phillips I, Ewing D, Murphy G, Porter K. 2000. A dengue virus serotype 1 DNA vaccine induces virus neutralizing antibodies and provides partial protection from viral challenge in Aotus monkeys. Vaccine 18:3166–3173 [DOI] [PubMed] [Google Scholar]
- 21.Kochel T, Watts D, Gozalo A, Ewing D, Porter K, Russell K. 2005. Cross-serotype neutralization of dengue virus in Aotus nancymae monkeys. J Infect Dis 191:1000–1004 [DOI] [PubMed] [Google Scholar]
- 22.LeDuc JW, Lemon SM, Keenan CM, Graham RR, Marchwicki RH, Binn LN. 1983. Experimental infection of new world monkey (Aotus trivirgatus) with hepatitis A virus. Infect Immun 40:766–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu SK, Dolensek EP, Tappe JP. 1985. Cardiomyopathy and vitamin E deficiency in zoo animals and birds. Heart Vessels 1:288–293 [DOI] [PubMed] [Google Scholar]
- 24.Liu SK, Dolensek EP, Tappe JP, Stover J, Adams CR. 1984. Cardiomyopathy associated with vitamin E deficiency in 7 gelada baboons. J Am Vet Med Assoc 185:1347–1350 [PubMed] [Google Scholar]
- 25.Meehan TE, Lowenstein LJ. 1994. Causes of mortality in captive lowland gorillas: a survey of the SSP population, p 216–218. In: Proceedings of the Annual Conference of American Association of Zoo Veterinarians. Yulee (FL): Annual Conference of American Association of Zoo Veterinarians. [Google Scholar]
- 26.Mohan C, Ganguly NK, Chakravarti RN. 1987. Experimental production of cardiac injury in rhesus monkeys by L forms of group A streptococci. Indian J Med Res 86:361–371 [PubMed] [Google Scholar]
- 27.Mohr FC, Bronson RT, Hunt RD. 1983. Failure of Herpesvirus saimiri to enhance atherogenesis in owl monkeys (Aotus trivirgatus). Atherosclerosis 46:173–179 [DOI] [PubMed] [Google Scholar]
- 28.Poltera AA, Sayer PD. 1983. Cardiac lymph drainage in experimental African trypanosomiasis in vervet monkeys. Bull Soc Pathol Exot 76:614–621 [PubMed] [Google Scholar]
- 29.Pung OJ, Hulsebos LH, Kuhn RE. 1988. Experimental Chagas disease (Trypanosoma cruzi) in the Brazilian squirrel monkey (Saimiri sciureus): hematology, cardiology, and cellular and humoral immune responses. Int J Parasitol 18:115–120 [DOI] [PubMed] [Google Scholar]
- 30.Rajendra RS, Brady AG, Parks VL, Massey CV, Gibson SV, Abee CR. 2010. The normal and abnormal owl monkey (Aotus sp.) heart: looking at cardiomyopathy changes with echocardiography and electrocardiography. J Med Primatol 39:143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rishniw M, Schiavetta AM, Johnson TO, Erb HN. 2005. Cardiomyopathy in captive owl monkeys (Aotus nancymae). Comp Med 55:162–168 [PubMed] [Google Scholar]
- 32.Rodger RF, Hartley LH, Ringler DJ, Nicolosi RJ. 1986. Hypertrophic cardiomyopathy in owl monkeys (Aotus trivirgatus): clinical diagnosis and clinicopathologic correlations. Lab Anim Sci 36:561 [Google Scholar]
- 33.Sahn DJ, DeMaria A, Kisslo J, Weyman A. 1978. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 58:1072–1083 [DOI] [PubMed] [Google Scholar]
- 34.Seibold AR, Wolf RH. 1971. Toxoplasmosis in Aotus trivirgatus and Callicebus moloch. Lab Anim Sci 21:118–120 [PubMed] [Google Scholar]
- 35.Smith OA, Astley CA. 2007. Naturally occurring hypertension in New World nonhuman primates: potential role of the perifornical hypothalamus. Am J Physiol Regul Integr Comp Physiol 292:R937–R945 [DOI] [PubMed] [Google Scholar]
- 36.Steel RGD, Torrie JH. 1980. Principles and procedures of statistics: a biometrical approach. New York (NY): McGraw Hill [Google Scholar]
- 37.Tantaleán M, Gozalo A. 1994. Parasites of the Aotus monkey, p 353–374. In: Baer JF, Weller RE, Kakoma I. Aotus: the owl monkey. San Diego (CA): Academic Press. [Google Scholar]
- 38.Voller A, Hawkey CM, Richards WH, Ridley DS. 1969. Human malaria (Plasmodium falciparum) in owl monkeys (Aotus trivirgatus). J Trop Med Hyg 72:153–160 [PubMed] [Google Scholar]
- 39.Wellde BT, Johnson AJ, Williams JS, Langbehn HR, Sadun EH. 1971. Hematologic, biochemical, and parasitologic parameters of the night monkey (Aotus trivirgatus). Lab Anim Sci 21:575–580 [PubMed] [Google Scholar]
- 40.Weller RE. 1994. Infectious and noninfectious diseases of owl monkeys, p 177–215. In: Baer JF, Weller RE, Kakoma I. Aotus: the owl monkey. San Diego (CA): Academic Press. [Google Scholar]
- 41.Weller RE, Wierman EL, Malaga CA, Baer JF, LeMieux TP. 1991. Battelle primate facility. J Med Primatol 20:133–137 [PubMed] [Google Scholar]
- 42.Wessler B, Madias C, Pandian N, Link MS. 2011. Short-term effects of ketamine and isoflurane on left ventricular ejection fraction in an experimental swine model. ISRN Cardiol 2011:582658 Epub 2011 Jun 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wyatt HL, Heng MK, Meerbaum S, Gueret P, Hestenes J, Dula E, Corday E. 1980. Cross-sectional echocardiography: II. Analysis of mathematic models for quantifying volume of the formalin-fixed left ventricle. Circulation 61:1119–1125 [DOI] [PubMed] [Google Scholar]
- 44.Wyatt HL, Heng MK, Meerbaum S, Hestenes JD, Cobo JM, Davidson RM, Corday E. 1979. Cross-sectional echocardiography: I. Analysis of mathematic models for quantifying mass of the left ventricle in dogs. Circulation 60:1104–1113 [DOI] [PubMed] [Google Scholar]
- 45.Young MD, Baerg DG, Rossan RN. 1976. Studies with induced malarias in Aotus monkeys. Lab Anim Sci 26:1131–1137 [PubMed] [Google Scholar]