Abstract
Tariquidar, a potent, nontoxic, third-generation P-glycoprotein (P-gp) inhibitor, is a possible reversal agent for central nervous system drug resistance. In animal studies, tariquidar has been shown to increase delivery of P-gp substrates into brain by several-fold. The aim of this study was to measure P-gp function at the human blood-brain barrier (BBB) after tariquidar administration using PET and the model P-gp substrate (R)–11C-verapamil. Methods: 5 healthy volunteers underwent paired (R)–11C-verapamil PET scans and arterial blood sampling, before and at 2 h 50 min after i.v. administration of tariquidar (2 mg/kg body weight). Inhibition of P-gp on CD56+ peripheral lymphocytes of each volunteer was determined by means of the rhodamine-123 efflux assay. Tariquidar concentrations in venous plasma were quantified using liquid chromatography/mass spectrometry. Results: Tariquidar administration resulted in significant increases (Wilcoxon test for paired samples) in the distribution volume (DV, +24±15%) and influx rate constant (K1, +49±36%) of (R)–11C-verapamil across the BBB (DV=0.65±0.13 and 0.80±0.07, p=0.043, K1=0.034±0.009 and 0.049±0.009, p=0.043, before and after tariquidar, respectively). A strong correlation was observed between change in brain DV after administration of tariquidar and tariquidar exposure in plasma (r=0.90, p=0.037). The mean plasma concentration of tariquidar achieved during the second PET scan (490±166 ng/mL) corresponded to 100% inhibition of P-gp function in peripheral lymphocytes. Conclusion: Tariquidar significantly increased brain penetration of (R)–11C-verapamil-derived activity, due to increased influx. As opposed to peripheral P-gp function, central P-gp inhibition appeared to be far from complete after the administered tariquidar dose.
Keywords: PET, (R)-11C-verapamil, tariquidar, P-glycoprotein, blood-brain barrier
Introduction
The multidrug efflux transporter P-glycoprotein (P-gp) is highly expressed at the luminal endothelium of the human blood-brain barrier (BBB). Due to its high transport capacity and abundance, P-gp limits entry into the central nervous system (CNS) for a large number of CNS-active drugs and is believed to contribute to patient-to-patient variability in response to CNS pharmacotherapy (1). Limited brain entry of therapeutic drugs mediated by active efflux transport is believed to contribute to the phenomenon of drug resistance in neurological disorders, such as epilepsy and depression (“transporter hypothesis” of drug resistance) (1). A promising strategy to enhance drug penetration across the BBB and thereby overcome drug resistance is the administration of new generation P-gp inhibitors, which were originally developed as an adjunctive therapy for drug-resistant cancers and are characterized by a high potency in the nanomolar range, lack of toxicity and lack of cytochrome P450 interactions (2).
Animal studies with the third-generation P-gp inhibitors zosuquidar, elacridar and tariquidar demonstrated substantially increased brain levels of cytostatics, antiepileptics, virostatics and other CNS drugs (3-5). However, in most animal studies higher than clinically used doses of P-gp inhibitors were given, resulting in chemical knockout of P-gp and thereby largely overestimating the extent of clinically relevant P-gp inhibition in humans. In addition, considerable species differences in cerebral P-gp functionality have been described (5, 6). Very few studies have so far investigated the magnitude of P-gp-inhibition at the human BBB. In a proof-of-concept study in 8 healthy volunteers, Sadeque et al. showed that administration of loperamide caused respiratory depression, a CNS pharmacologic effect, when the P-gp inhibitor quinidine was co-administered (7). A follow-up clinical study using reduction in pupil diameter as a surrogate marker for central opioid effect, on the other hand, suggested that CNS activity of loperamide in humans is not affected by administration of tariquidar at 2 mg/kg body weight (BW) (8). However, the employed approach might suffer from lack of sensitivity to measure moderate levels of P-gp inhibition as expected after administration of clinically used doses of P-gp modulators. Positron emission tomography (PET) with the radiolabelled P-gp substrate (R)-11C-verapamil is a validated method to measure P-gp function at the human BBB (9). It has been shown that the distribution volume (DV) of (R)-11C-verapamil is inversely related to cerebral P-gp activity (10). A study in non-human primates showed that brain uptake of racemic 11C-verapamil was increased by 4.61-fold after infusion of the second-generation P-gp inhibitor valspodar at a dose of 20 mg/kg/2 hr (11). A similarly conducted PET study in healthy human volunteers revealed a significant but less pronounced (~90%) increase of brain activity uptake, after cerebral P-gp inhibition with cyclosporine A, although higher than clinically recommended doses of cyclosporine A were given (12).
Tariquidar is one of the most potent and selective third-generation P-gp inhibitors known to date, which has been developed up to clinical phase 3 (13). It is a noncompetitive inhibitor of P-gp and not a substrate of it (14). Tariquidar also inhibits breast cancer resistance protein (BCRP), but at higher concentrations than it inhibits P-gp. However, unlike first- and second-generation P-gp inhibitors tariquidar does not inhibit multidrug resistance-associated proteins (MRPs), such as MRP1 (15). Preclinical studies in monkey have shown that tariquidar is rapidly excreted via the hepatobiliary pathway with an elimination half-life of 5 h. In monkey plasma, most of the tariquidar-related material (>95%) was found to exist as unchanged parent compound (16). Tariquidar has been tested up to a dose of 2 mg/kg BW in a total of 48 healthy subjects and more than 500 cancer patients in the course of 9 clinical trials (13). In some of these studies, besides the clinical response of cancer patients, inhibition of rhodamine-123 (Rh-123) efflux from CD56+ lymphocytes and reduction in clearance of technetium-99m sestamibi from the liver, were assessed as surrogate efficacy markers of tariquidar-induced inhibition of P-gp in the periphery (17, 18). Interestingly, greater resistance of P-gp at the BBB to inhibition, compared with other tissues, has been suggested (3, 8).
For translating the concept of P-gp modulation for improving CNS delivery of drugs to the clinic, studies measuring surrogate markers of transport activity and inhibition at the human BBB are needed to validate P-gp modulation at the BBB, to define the magnitude and time frame of inhibition, and in an ultimate step, to assess the clinical effect of P-gp modulation at the human BBB on CNS pharmacotherapy (19). In an ideal setting, a selective P-gp modulator would only transiently enhance BBB penetration of a targeted therapeutic drug.
We have previously performed a (R)-11C-verapamil microPET study in rats showing that tariquidar given at a dose of 15 mg/kg resulted in a 12-fold increase of the (R)-11C-verapamil DV (10). In this study, we translate the set-up used in the previous microPET study to human subjects to investigate, for the first time, the effect of tariquidar on the functional activity of P-gp at the human BBB.
Material and Methods
The study was performed at the Department of Clinical Pharmacology at the Medical University of Vienna. It was carried out as a single-dose pilot clinical study in 5 healthy male subjects with a mean age of 32±8 years and a mean body weight (BW) (mean±standard deviation, SD) of 74.2±5.4 kg. Healthy volunteers were free of any medication known to interfere with cytochrome P450 enzymes or P-gp function.
The study protocol was approved by the local Ethics Committee and was performed in accordance with the Declaration of Helsinki (1964) in the revised version of 2000 (Edinburgh), the Guidelines of the International Conference of Harmonization, the Good Clinical Practice Guidelines and the Austrian drug law (Arzneimittelgesetz). All subjects were given a detailed description of the study and their written consent was obtained prior to the enrollment in the study.
PET imaging and experimental procedures
On study day, each study participant underwent two consecutive PET scans of 120 and 40 min duration, respectively, with an interval of 2 h between the 2 scans (Fig 1). During PET imaging, each subject was positioned supine on the imaging bed of the PET camera with the head in a fixing device in order to avoid movement artifacts. PET images were acquired with an Advance PET scanner (General Electrics Medical Systems, Wukesha, WI, USA) run in 3D mode. In order to correct for tissue attenuation of photons a transmission scan of 5-min duration using two 400 MBq 68Ge pin sources was recorded prior to radiotracer injection. One venous and one arterial catheter were placed on one arm for drug infusion and a second venous catheter was placed on the other arm to obtain blood samples for quantification of tariquidar concentrations in plasma. At the start of each PET scan, 384±13 MBq of (R)-11C-verapamil (5.2±0.36 MBq/kg), synthesized from (R)-norverapamil (ABX advanced biochemical compounds, Radeberg, Germany) and [11C]methyl triflate and dissolved in a volume of 10 mL of physiological saline solution/ethanol (9/1, v/v) was intravenously injected over 20 s. Dynamic PET imaging and arterial blood sampling were started at the time of radiotracer injection. The following frame sequence was used for PET imaging: 1×15 s, 3×5 s, 3×10 s, 2×30 s, 3×60 s, 2×150 s, 2×300 s, 10×600 s (scan 1) and 1×15 s, 3×5 s, 3×10 s, 2×30 s, 3×60 s, 2×150 s, 2×300 s, 2×600 s (scan 2). During both scan 1 and 2, arterial blood samples were drawn at intervals of 7 s during the first 3 min after radiotracer injection and subsequently at 3.5, 5, 10, 20, 30, 55, 70, and 100 min after radiotracer injection (scan 1) and at 3.5, 5, 10, 20, 30 and 40 min after radiotracer injection (scan 2).
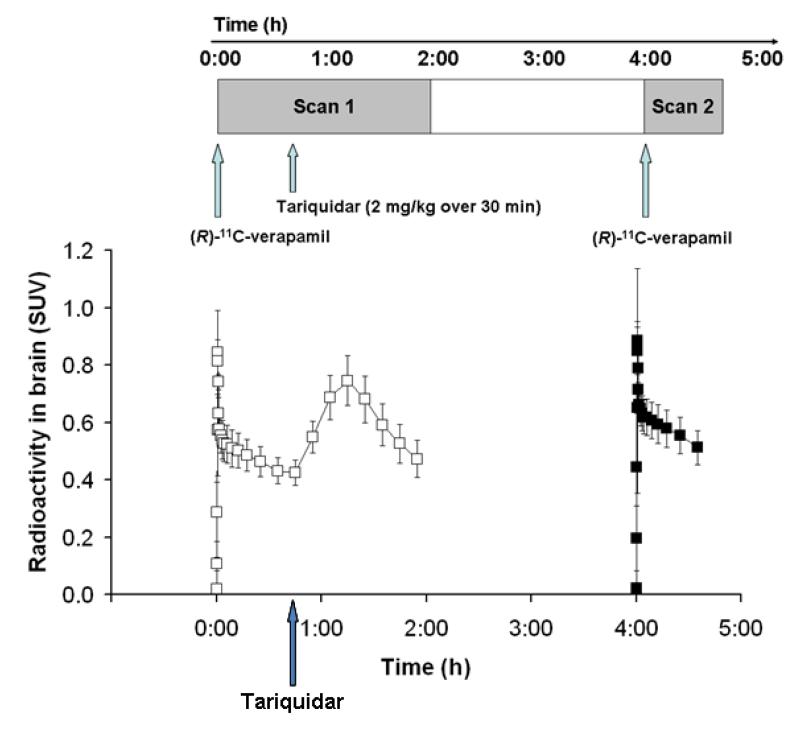
FIGURE 1.
Time-activity curves (mean SUV±SD) of (R)-11C-verapamil in whole brain grey matter for the paired PET scans (scan 1: open squares; scan 2: closed squares). Tariquidar (2 mg/kg BW) was administered as an i.v. infusion over 30 min at 40 min after start of PET scan 1 (see timeline above).
During scan 1 (i.e. at 40 min after tracer injection) tariquidar was administered at a dose of 2 mg/kg BW as an i.v. infusion over 30 min. Scan 2 was performed at 2 h 50 min after the end of tariquidar infusion (Fig 1). 4-mL venous blood samples were drawn before (predose) as well as at time points of 5, 15, 30, 60, 120, 200, 220, 240, 360, 480, 600, 720 and 1440 min after start of infusion of tariquidar.
Study medication
Tariquidar was administered at a dose of 2 mg/kg BW. 10 mL vials of tariquidar for i.v. infusion containing 7.5 mg/mL of tariquidar free base in 20% ethanol/80% propylene glycol were provided by AzaTrius Pharmaceuticals Pvt Ltd (London, UK). The volume (mL) of concentrate for injection was calculated as BW (kg) × 2/7.5. Aqueous dextrose solution (5%, g/v) was added to yield a final volume of 250 mL, which was administered over 30 min.
PET data analysis
Reconstruction of the PET data was performed by means of iterative reconstruction using the ordered subsets-expectation maximization method with 28 subsets and 2 iterations. The loop filter (Gaussian) was set to a full-width at half-maximum (FWHM) of 4.3 mm, and a post-filtering algorithm of 6.00 mm FWHM was applied. Attenuation correction was performed using the manufacturer’s segmentation algorithm for transmission data. T1-weighted magnetic resonance imaging (MRI) scans had been recorded within one month before the PET scan. MRI and PET data were processed with Analyze 8.0 (Biomedical Imaging Resource, Mayo Foundation, Rochester, MN, USA) and SPM5 (SPM5, Wellcome Department of Imaging Neuroscience, UCL, London, UK) software as published before (20). A whole brain grey matter region of interest was defined by using the Hammersmith n30r83 three-dimensional maximum probability atlas of the human brain (21). Radioactivity concentrations (kiloBecquerels per gram tissue, kBq/g) were normalized to the injected radiotracer amount and expressed as standardized uptake values (SUV). The radioactivity concentration data were combined to provide time-activity curves (TACs) for the whole observation period.
Blood and metabolite analysis
Radioactivity counts in aliquots of arterial blood and plasma were measured with a gamma counter. Plasma samples were analyzed for radiolabelled metabolites of (R)-11C-verapamil by employing a previously described combined solid-phase extraction (SPE)/high-performance liquid chromatography (HPLC) assay (22, 23). The polar radiolabelled metabolites of (R)-11C-verapamil (11C-formaldehyde and related species) were determined by SPE, whereas the lipophilic radiolabelled metabolites of (R)-11C-verapamil (i.e. the 11C-labelled N-dealkylation products D-617 and D-717), which are also P-gp substrates, were measured with HPLC. The 10, 20, 30 and 40 min plasma samples of both scan 1 and 2 were analyzed for radiolabelled metabolites of (R)-11C-verapamil using the SPE/HPLC assay. Due to time constraints because of the short half-life of 11C, the 3.5, 5, 55, 70 and 100 min plasma samples were analyzed by SPE only. Plasma protein binding of (R)-11C-verapamil was determined by incubating plasma samples obtained before and after administration of tariquidar with (R)-11C-verapamil during 30 min at 37°C, followed by ultrafiltration using Amicon Microcon YM-10 centrifugal filter devices (Millipore Corporation, USA). The total concentration of radioactivity in whole blood was used for vascular correction of the PET data, assuming that blood constitutes 5% of brain volume.
Kinetic modeling of (R)-11C-verapamil
PET data sets (uncorrected for blood activity) from 0-40 min for scan 1 and scan 2 were used for data analysis. A standard 1-tissue 2-rate-constant (1T2K) or 2-tissue 4-rate-constant (2T4K) compartment model was fitted to the (R)-11C-verapamil time-activity curves (TACs) in whole brain grey matter (9, 20) to estimate the influx and efflux rate constants of activity across the BBB (K1 and k2, respectively) as well as the distribution volume (DV). The arterial input function of (R)-11C-verapamil was constructed by correcting total decay-corrected activity concentrations in arterial plasma (kBq/mL) for the fraction of polar radiolabelled metabolites of (R)-11C-verapamil, as determined by SPE, and by subsequent interpolation of the activity data. Fits were performed by the method of weighted nonlinear least squares as implemented in the Optimization Toolbox of MATLAB (Mathworks, Natick, MA, USA). Goodness-of-fit was assessed by visual inspection of observed and predicted concentrations versus time, by the correlation between observed and predicted concentrations, by the randomness of the residuals (runs test), and by estimating parameter uncertainties (variances) from the inverse of the appropriate Fisher information matrix. In order to obtain a model-independent estimate of DV, Logan graphical analysis was applied to the PET and arterial plasma data using MATLAB (24). To analyze the time course of the effect of tariquidar administration on brain activity during scan 1, an indirect response pharmacokinetic-pharmacodynamic (PK-PD) model was used (25) (see Supplemental Data).
Statistical analysis
All statistical analysis was performed using SPSS (SPSS Version 16 for Windows). Model parameters of (R)-11C-verapamil in brain (K1, k2, DV) before and after administration of tariquidar were compared using the Wilcoxon test for paired samples. Correlations between pharmacokinetic parameters of tariquidar in plasma and model parameters of verapamil in brain were calculated using Spearman‘s rank coefficient.
Analysis of tariquidar concentration in plasma
Tariquidar was assayed in human plasma by means of combined liquid chromatography tandem mass-spectrometry (LC/MS). Plasma samples (100 μL) were spiked with elacridar as an internal standard. Drug was extracted from plasma samples using XAD4 polymeric adsorbent beads. The analytes were eluted from the beads with ethyl acetate/methanol (1/1, v/v). The eluate was concentrated to dryness, redissolved in methanol/water (1/9, v/v) and injected into the LC/MS system. LC/MS analysis was performed using a Thermo Scientific Surveyor HPLC system coupled to a Thermo Finnigan TSQ Quantum Discovery MAX mass spectrometer with the flow rate maintained at 200 μL/min. Separation was performed using an Agilent Zorbax XDB C18 Eclipse column (2.1 × 50 mm; 3.5 μm). A gradient method using a mixture of methanol containing 0.1% aqueous formic acid (solvent A) and 0.2% aqueous formic acid (solvent B) was used. From the tariquidar concentration-time profiles in plasma pharmacokinetic parameters were estimated as described in the Supplemental Data.
Measurement of tariquidar-induced inhibition of P-gp activity in peripheral white blood cells by the rhodamine-123 efflux assay
Native fresh whole blood samples (18 mL) were drawn from each volunteer before administration of tariquidar. The assay for inhibition of P-gp in CD56+ lymphocytes was carried out with slight modifications according to previously published methods (17). Suspensions of isolated white blood cells (1 mL) were incubated at 37°C with rhodamine-123 (Rh-123, 0.2 μg/mL) and 10 different dilutions of tariquidar in dimethlysulfoxide (10 μL) resulting in tariquidar concentrations from 0.01 nM–1 μM (0.0084–839 ng/mL). All data points were measured in duplicate. Accumulation levels of Rh-123 were measured by fluorescence activated cell sorting (FACS) analysis using a FACS-Calibur system (Becton Dickinson). Mean fluorescence units per cell directly correlate with the amount of cell-associated Rh-123. Hyperbolic dose response curves were fitted through the data points to estimate half-maximum effect concentration (EC50) values for tariquidar.
Results
At the administered dose of 2mg/kg BW, tariquidar was well tolerated without occurrence of severe or serious adverse events. Mild adverse events, possibly related to administration of the study medication were mild hypotension, headache and nausea, each in one patient and dizziness in 2 patients.
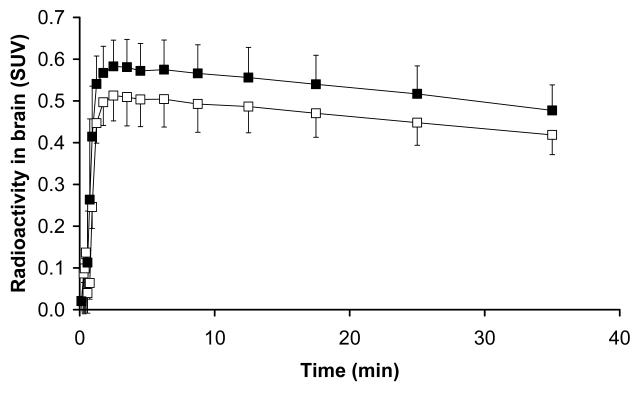
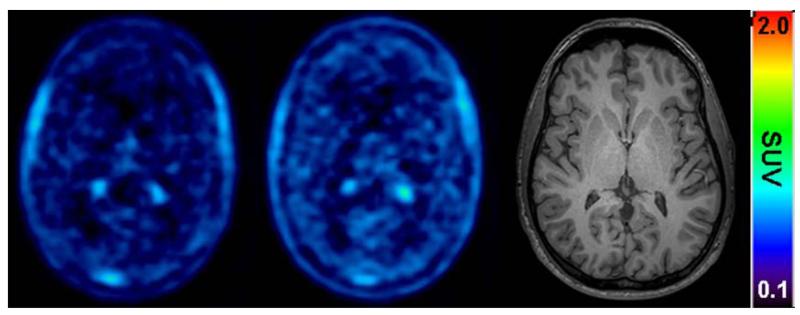
Fig 1 shows mean TACs for the paired PET scans. The time points of (R)-11C-verapamil injection and tariquidar infusion are indicated on the timeline above. As a response to the start of tariquidar infusion during scan 1, brain radioactivity started to rise. A maximum increase of +74% was reached at 5 min after end of tariquidar infusion (t=75 min), as compared to baseline values (i.e. before the start of tariquidar infusion (t=40 min). At 35 min after end of tariquidar infusion (t=115 min), this effect had decreased to +10% on average. An indirect response PK-PD model was fitted to the time course of brain activity following tariquidar infusion. Model fits and estimated outcome parameters are summarized in the Supplemental Data (Fig S2, S3, table S2). Fig 2 directly compares brain TACs (corrected for blood activity) during the first 40 min of scan 1 and scan 2 (i.e. before and after tariquidar administration). Fig 3 shows PET summation images (scan 1 and 2) from the subject with the greatest increase in brain radioactivity after administration of tariquidar (42% increase in DV during scan 2, when compared to the first 40 min of scan 1).
FIGURE 2.
Time-activity curves (mean SUV±SD), corrected for radioactivity in the vasculature, of (R)-11C-verapamil in whole brain grey matter from time 0-40 min for PET scan 1 (before administration of tariquidar, open squares) and PET scan 2 (after administration of tariquidar, closed squares).
FIGURE 3.
Transaxial PET summation pictures (10-40 min) of the volunteer (subject 2) with the greatest increase in (R)-11C-verapamil-derived brain activity, before (left picture) and after (middle picture) administration of tariquidar (2 mg/kg BW). Activity concentration is expressed as the SUV and the radiation scale is set from 0.2 to 2.0. The right picture shows a transaxial T1-weighted MRI of the same subject. The intense foci in the PET images represent activity in the choroid plexus and venous sinus.
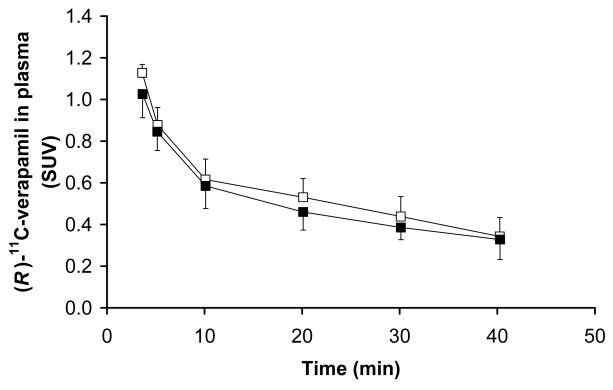
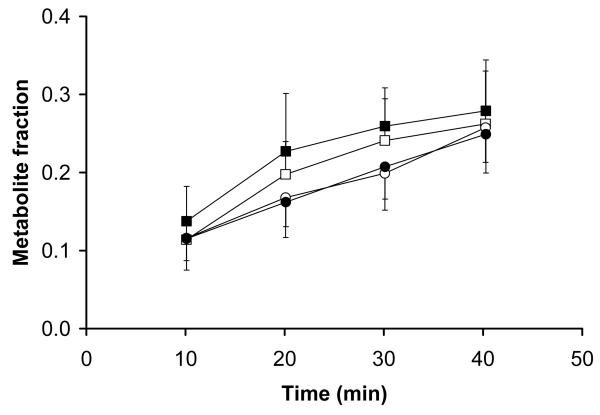
Fig 4 displays mean TACs of unchanged (R)-11C-verapamil in plasma during scan 1 and 2. (R)-11C-verapamil concentrations were slightly decreased after administration of tariquidar (AUC0-40=38.3±5.2 min and 34.3±3.5 min, before and after administration of tariquidar, respectively, p=0.043). Administration of tariquidar did not have significant influence on the metabolism of (R)-11C-verapamil. Fig 5 displays the time course of the fractions of lipophilic and polar metabolites of (R)-11C-verapamil during scan 1 and 2. The AUC0-40 values for the fractions of lipophilic and polar metabolites of (R)-11C-verapamil in plasma were 7.3±1.4 and 8.4±2 min (p=0.14) and 6.1±1.3 and 6.1±1.3 min (p=0.89) before and after administration of tariquidar, respectively. At time points >40 min in scan 1, the combined fraction of unchanged (R)-11C-verapamil and its lipophilic metabolites remained almost constant (0.74±0.04 at t=40 min, 0.72±0.03 at t=70 min and 0.66±0.01 at t=100 min) (data not shown in Fig 5). Total plasma activity levels also remained almost unchanged from t=40 to t=100 min of scan 1 (mean SUVs: 0.71±0.07 at t=40 min, 0.67±0.06 at t=70 min and 0.66±0.06 at t=100 min).
FIGURE 4.
Time-activity curves (mean SUV±SD) of unchanged (R)-[11C]verapamil in arterial plasma (3-40 min) before (open squares) and after (closed squares) administration of tariquidar (2 mg/kg BW).
FIGURE 5.
Fractions of total plasma radioactivity (mean±SD) of lipophilic (squares) and polar (circles) radiolabelled metabolites of (R)-[11C]verapamil over time (10-40 min) before (open symbols) and after (filled symbols) administration of tariquidar (mean±SD).
The plasma protein-bound percentage of (R)-11C-verapamil was not significantly altered by administration of tariquidar (93±2% versus 94.±2% before and after administration of tariquidar, respectively, p=0.07). The plasma-to-blood ratio of activity was slightly decreased after administration of tariquidar (1.41±0.03 versus 1.35±0.05 before and after tariquidar, respectively, p=0.04).
As previously described for (R)–11C-verapamil distribution in rat brain following P-gp modulation (10), the 2T4K model provided better fits of the PET data in scan 2 than the 1T2K model (mean Akaike Information Criterion, AIC, −28.6 versus −36.5 for 1T2K versus 2T4K model). For PET scan 1 (i.e. before P-gp modulation) both models provided fits of comparable quality (AIC, −22.2 versus −20.3 for 1T2K versus 2T4K model). Parameter estimates obtained from the 2T4K model are displayed in table 1. The DV of (R)–11C-verapamil as well as the influx rate constant of activity (K1) across the BBB increased significantly after administration of tariquidar (DV: +24±15%, p=0.043; K1: +49±36%, p=0.043). There was no significant difference in efflux of activity across the BBB. Compartmental-model derived DV values were in good agreement with DVs estimated by Logan graphical analysis.
TABLE 1.
Outcome Parameters of the 2T4K Model
| Parameter | Before tariquidar | After tariquidar* |
|---|---|---|
| K1 (mL·mL−1·min−1) | 0.034±0.009 (15) † | 0.049±0.009 (9) † |
| k2 (min−1) | 0.091±0.007 (111) | 0.128±0.070 (59) |
| k3 (min−1) | 0.127±0.037 (241) | 0.162±0.104 (119) |
| k4 (min−1) | 0.181±0.082 (94) | 0.177±0.062 (52) |
| DV (mL·mL−1) | 0.65±0.13 (6) † | 0.80±0.07 (2) † |
| DV (Logan) (mL·mL−1) | 0.64±0.12 (1) † | 0.79±0.07 (1) † |
Tariquidar was administered i.v. at a dose of 2 mg/kg over 30 min at 3 h 20 min before start of the second PET scan.
A statistically significant difference was observed between scan 1 and 2 (Wilcoxon test for paired samples, p<0.05).
Outcome parameters are given as mean±SD averaged over the 5 study subjects. The value in parentheses represents the precision of parameter estimates (expressed as their coefficient of variation in percent), averaged over the 5 study subjects.
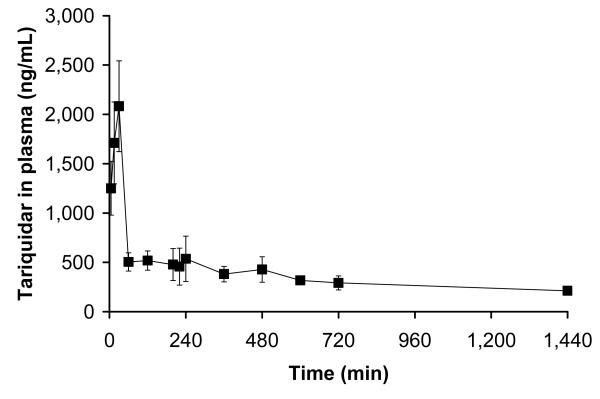
Fig 6 shows mean concentration-time profiles of tariquidar in venous plasma. Pharmacokinetic parameters of tariquidar are summarized in table S1 (Supplemental Data). Table 2 shows area under the curve from time zero to 4 h (i.e. end of scan 2, AUC0-4) values of tariquidar in plasma of individual study subjects along with (R)–11C-verapamil DVs before and after tariquidar administration. Positive correlation was observed between the percent change in (R)-11C-verapamil DV after administration of tariquidar and AUC0-4 (r=0.74, p=0.16) as well as the area under the curve from time zero to 24 h (AUC0-24, r=0.90, p=0.037).
FIGURE 6.
Concentration-time profile (ng/mL) of tariquidar (mean±SD) in venous plasma.
TABLE 2.
Plasma AUCs of Tariquidar and (R)-11C-verapamil DVs (2T4K Model) before and after Tariquidar Administration in Individual Subjects
| Subject |
AUC0-4 (μg·h·mL−1) |
DV before tariquidar (mL·mL−1) |
DV after tariquidar (mL·mL−1) |
Change (%) |
|---|---|---|---|---|
| 1 | 2.95 | 0.60 (2) | 0.78 (2) | +30 |
| 2 | 3.42 | 0.53 (18) | 0.75 (2) | +42 |
| 3 | 2.71 | 0.86 (3) | 0.92 (3) | +7 |
| 4 | 2.61 | 0.68 (3) | 0.75 (2) | +10 |
| 5 | 2.65 | 0.60 (4) | 0.79 (4) | +32 |
The precision of the parameter estimate in percent is given in parentheses (see table 1).
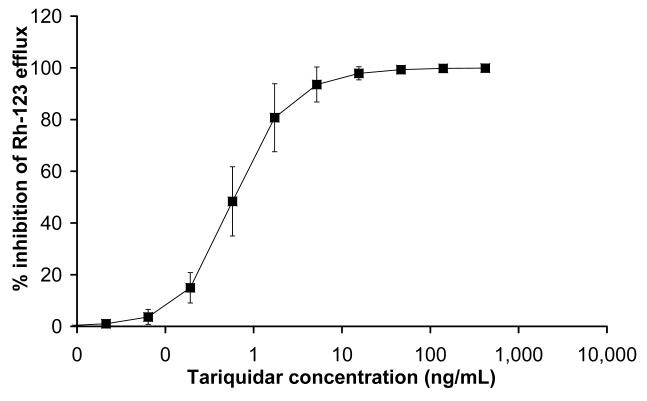
In peripheral white blood cells, ex vivo addition of different tariquidar concentrations led to a dose-dependent increase in cellular Rh-123 accumulation. Fig 7 shows mean concentration-effect curves of tariquidar. Half-maximum inhibition of Rh-123 efflux from white blood cells was observed at an EC50 of 0.5±0.2 ng/mL.
FIGURE 7.
Inhibition of P-gp-mediated rhodamine-123 efflux from peripheral white blood cells by different tariquidar concentrations added ex vivo. Note that the x-axis is in a logarithmic scale. The data present the mean±SD of the results obtained with whole blood from the same 5 volunteers who underwent the paired PET scans. The mean EC50 was 0.5±0.2 ng/mL.
Discussion
(R)-11C-verapamil, in combination with PET has previously proved suitable for studying P-gp activity at the BBB (9, 10). In this pilot PET study we investigated, for the first time, the ability of a potent, third-generation P-gp modulator, tariquidar, to inhibit P-gp function at the human BBB, at a dose which has been shown to decrease transporter activity in peripheral tissues (17, 18). The percent increase in the DV of (R)-11C-verapamil in brain, after administration of tariquidar, was used as surrogate parameter of changes in P-gp function in the BBB (9). The paired scan paradigm that we used in this study was in fact identical to a set-up that we had previously used in a microPET study in rats (10). A previous study in healthy volunteers had shown excellent test-retest variability of paired (R)-11C-verapamil PET scans as were used in this study (~4% test-retest variability for DV values) (9).
The mean increase in DV of (R)-11C-verapamil in whole brain grey matter following tariquidar administration was +24±15% (table 2), almost 10-fold less than the increase determined in the previous rat microPET study (10). However, in the microPET study, rats were administered 15 mg/kg of tariquidar (resulting in plasma tariquidar concentrations of about 1,400 ng/mL at the time of the PET scan), i.e. 7-fold higher doses than we administered to the volunteers in the present study. In rats, comparable plasma tariquidar levels as achieved in the present PET study in humans (about 500 ng/mL) resulted in 3-4-fold increases of (R)-11C-verapamil DVs (26) suggesting species differences in tariquidar-induced cerebral P-gp inhibition or differences in P-gp expression and function between rats and humans (6).
The modest increase in brain DV which we observed was strongly correlated with individual plasma exposure of tariquidar and could therefore most likely be attributed to tariquidar-induced P-gp inhibition. Moreover, tariquidar neither affected plasma protein binding nor metabolism of (R)-11C-verapamil (Fig 5), thus ruling out the possibility that these factors confounded the increase in brain DV of (R)-11C-verapamil. However, a previous study had shown that at 60 min after injection of (R)-11C-verapamil into rats about 50% of brain activity was composed of polar radiolabelled metabolites, which presumably crossed the BBB independent of P-gp function (23). While the polar fraction of radioactivity in plasma is about 3-fold lower in humans than in rats (23), it still cannot be excluded that part of the brain PET signal measured in this study was attributable to polar radiolabelled metabolites, which might have masked to some extent the P-gp inhibitory effect of tariquidar. Besides the formation of polar metabolites, (R)-11C-verapamil is also metabolized to lipophilic radiolabelled metabolites, which are supposed to be substrates of P-gp and were therefore included into the arterial input function (9). As it cannot be excluded that the lipophilic metabolites of (R)-11C-verapamil behaved differently to parent tracer, their inclusion into the input function might lead to inaccurate measures of P-gp function with this radiotracer. Moreover, verapamil has been shown to be also a substrate of MRP1 (27), which is also expressed at the BBB. However, as tariquidar does not inhibit MRP1 function (15), it is unlikely that the change in brain PET signal observed after tariquidar administration was related to changes in MRP1 transport of the radiotracer.
Distribution of unbound drug across the BBB is determined by influx versus efflux. As previously described for P-gp inhibition at the BBB by tariquidar (10) and other P-gp inhibitors (28, 29), the increase in DV after administration of tariquidar in our study, was associated with a significant increase (+49±36%) in the influx rate constant K1 (table 1), suggesting that, in the presence of an inhibitor, this balance is shifted in favor of drug influx and underlining the gate keeper function of P-gp at the BBB.
By administering tariquidar during scan 1 rather than in between scan 1 and 2 (see study outline in Fig 1) we attempted to better define the time window of P-gp modulation, in order to optimize a possible time point of therapeutic drug administration relative to administration of the inhibitor drug. The TACs shown in Fig 1 describe the time course of tariquidar-induced P-gp inhibition at the BBB. Surprisingly, brain activity started to rise significantly in immediate response to tariquidar infusion. Plasma analysis had shown that total activity counts and the fraction of (R)-11C-verapamil and its lipophilic metabolites, which are also P-gp substrates, were almost constant during the time course of tariquidar infusion thereby suggesting that tariquidar-induced P-gp blockade facilitated brain entry of activity that had remained in the circulation. On the other hand, as perfusion was not determined in the present study and as the effect of tariquidar on cerebral blood flow is unknown at present, it cannot be excluded that at least part of the immediate rise in activity following tariquidar infusion can be attributed to increased perfusion rather than decreased P-gp activity. However, (R)-11C-verapamil is a low-extraction radiotracer (K1=0.049 after P-gp modulation in scan 2 corresponding to an extraction fraction (E) of 0.098 when assuming an absolute blood flow (F) of 0.5 mL·g−1·min−1 according to the relationship K1=F·E) (28). Therefore, brain uptake of (R)-11C-verapamil can be considered to be insensitive to changes in cerebral blood flow under conditions of moderate P-gp inhibition, as employed in this study. The mean maximum increase in activity concentrations in brain was observed at 5 min after the end of tariquidar infusion. 40 min later, activity concentrations in brain had significantly declined, which suggested that the effect of tariquidar was reversible. However, during scan 2, which was performed at about 3 h after tariquidar administration, moderate P-gp inhibition was still evident. Brain activity influx following tariquidar infusion was analyzed by simple PK-PD models (see Supplemental Data), which suggested that during the time course of tariquidar action the modulation of P-gp function entails both influx enhancement as well as efflux inhibition of activity at the BBB.
Our findings are quite in opposition to previous data showing that in vitro the P-gp inhibitory effect of tariquidar lasts for up to 22 h after removal of tariquidar from the incubation medium (15) and that in vivo tariquidar fully inhibits P-gp mediated Rh-123 efflux from peripheral lymphocytes for over 24 h (3, 8, 17), at similar plasma concentrations as were used in this study. The concentrations of tariquidar in plasma during the second PET scan (200-240 min after the start of tariquidar infusion) and at 24 h after tariquidar infusion were 490±166 ng/mL and 212±24 ng/mL (Fig 6), i.e. 980 and 424-fold higher than the EC50 for tariquidar-induced inhibition of Rh-123 efflux from peripheral white blood cells (Fig 7), respectively. In humans, tariquidar is bound to 99.5% to plasma proteins (Ulrich Elben, written communication, 2008). When correcting plasma concentrations for protein binding, the concentration values for unbound tariquidar at the time of the second scan (2.45±0.83 ng/mL), were still about 5-fold higher than the in vitro EC50. The fact that P-gp at the BBB exhibits greater resistance to inhibition than P-gp in lymphocytes has previously been pointed out by others (3, 8). For instance, Choo and colleagues found that the EC50 of tariquidar to enhance brain penetration of loperamide in mice was 25-fold higher than the EC50 for inhibition of Rh-123 efflux from lymphocytes (3). Although the mechanisms underlying this differential sensitivity have not yet been elucidated, a higher transporter density of P-gp at the BBB as well as structural distinctions in transporter proteins expressed in brain and lymphocytes have been suggested (3, 30, 31).
Conclusion
Using the P-gp substrate (R)-11C-verapamil as a PET tracer, we could show that tariquidar, administered at a well-tolerated dose of 2 mg/kg BW, induced a modest, but significant increase in brain DV, most likely due to inhibition of P-gp activity in the BBB. This finding is in contrast to a previous study (8) that had failed to demonstrate a significant effect of the same dose of tariquidar on central opioid effects of the P-gp substrate loperamide in humans, thus underlining the greater sensitivity of direct measurement of CNS drug concentrations by PET as compared to surrogate pharmacologic markers of CNS drug penetration. Tariquidar-induced P-gp modulation at the human BBB appeared to be transient and its magnitude was directly proportionate to serum drug exposure. P-gp in the BBB appeared to be more resistant to inhibition than in peripheral white blood cells. Whether higher drug plasma concentrations can safely be obtained in the clinic, in order to overcome this resistance and achieve higher levels of cerebral P-gp inhibition, needs to be investigated.
Supplementary Material
Acknowledgments
The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement number 201380 (“Euripides”) and from the Austrian Science Fund (FWF) project “Transmembrane Transporters in Health and Disease” (SFB F35). The authors wish to thank Divya Maheshwari from AzaTrius Pharmaceuticals Pvt Ltd (London, UK) for providing them with vials of tariquidar for i.v. infusion. This study would not have been possible without the excellent technical support of Rainer Bartosch (Department of Nuclear Medicine), research nurse Edith Lackner, Johann Stanek and Aiman Abrahim (Department of Clinical Pharmacology). Rebecca Brauner from the Department of Chemical Analytics at Austrian Research Centers GmbH-ARC is gratefully acknowledged for performing the LC/MS analysis of tariquidar plasma concentrations.
The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement number 201380 (“Euripides”) and from the Austrian Science Fund (FWF) project “Transmembrane Transporters in Health and Disease” (SFB F35).
References
- 1.Löscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol. 2005;76:22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 3.Choo EF, Kurnik D, Muszkat M, et al. Differential in vivo sensitivity to inhibition of P-glycoprotein located in lymphocytes, testes, and the blood-brain barrier. J Pharmacol Exp Ther. 2006;317:1012–1018. doi: 10.1124/jpet.105.099648. [DOI] [PubMed] [Google Scholar]
- 4.Choo EF, Leake B, Wandel C, et al. Pharmacological inhibition of P-glycoprotein transport enhances the distribution of HIV-1 protease inhibitors into brain and testes. Drug Metab Dispos. 2000;28:655–660. [PubMed] [Google Scholar]
- 5.Cutler L, Howes C, Deeks NJ, Buck TL, Jeffrey P. Development of a P-glycoprotein knockout model in rodents to define species differences in its functional effect at the blood-brain barrier. J Pharm Sci. 2006;95:1944–1953. doi: 10.1002/jps.20658. [DOI] [PubMed] [Google Scholar]
- 6.Syvänen S, Lindhe O, Palner M, et al. Species differences in blood-brain barrier transport of three PET radioligands with emphasis on P-glycoprotein transport. Drug Metab Dispos. 2009;37:635–643. doi: 10.1124/dmd.108.024745. [DOI] [PubMed] [Google Scholar]
- 7.Sadeque AJ, Wandel C, He H, Shah S, Wood AJ. Increased drug delivery to the brain by P-glycoprotein inhibition. Clin Pharmacol Ther. 2000;68:231–237. doi: 10.1067/mcp.2000.109156. [DOI] [PubMed] [Google Scholar]
- 8.Kurnik D, Sofowora GG, Donahue JP, et al. Tariquidar, a selective P-glycoprotein inhibitor, does not potentiate loperamide’s opioid brain effects in humans despite full inhibition of lymphocyte P-glycoprotein. Anesthesiology. 2008;109:1092–1099. doi: 10.1097/ALN.0b013e31818d8f28. [DOI] [PubMed] [Google Scholar]
- 9.Lubberink M, Luurtsema G, van Berckel BN, et al. Evaluation of tracer kinetic models for quantification of P-glycoprotein function using (R)-[(11)C]verapamil and PET. J Cereb Blood Flow Metab. 2007;27:424–433. doi: 10.1038/sj.jcbfm.9600349. [DOI] [PubMed] [Google Scholar]
- 10.Bankstahl JP, Kuntner C, Abrahim A, et al. Tariquidar-induced P-glycoprotein inhibition at the rat blood-brain barrier studied with (R)-11C-verapamil and PET. J Nucl Med. 2008;49:1328–1335. doi: 10.2967/jnumed.108.051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YJ, Maeda J, Kusuhara H, et al. In vivo evaluation of P-glycoprotein function at the blood-brain barrier in nonhuman primates using [11C]verapamil. J Pharmacol Exp Ther. 2006;316:647–653. doi: 10.1124/jpet.105.088328. [DOI] [PubMed] [Google Scholar]
- 12.Sasongko L, Link JM, Muzi M, et al. Imaging P-glycoprotein transport activity at the human blood-brain barrier with positron emission tomography. Clin Pharmacol Ther. 2005;77:503–514. doi: 10.1016/j.clpt.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Fox E, Bates SE. Tariquidar (XR9576): a P-glycoprotein drug efflux pump inhibitor. Expert Rev Anticancer Ther. 2007;7:447–459. doi: 10.1586/14737140.7.4.447. [DOI] [PubMed] [Google Scholar]
- 14.Martin C, Berridge G, Mistry P, Higgins C, Charlton P, Callaghan R. The molecular interaction of the high affinity reversal agent XR9576 with P-glycoprotein. Br J Pharmacol. 1999;128:403–411. doi: 10.1038/sj.bjp.0702807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mistry P, Stewart AJ, Dangerfield W, et al. In vitro and in vivo reversal of P-glycoprotein-mediated multidrug resistance by a novel potent modulator, XR9576. Cancer Res. 2001;61:749–758. [PubMed] [Google Scholar]
- 16.Investigator Brochure . Tariquidar (XR9576). P-Glycoprotein Pump Inhibitor. Version Number: 11 Aväant Pharmaceuticals Inc.; May 28, 2007. [Google Scholar]
- 17.Stewart A, Steiner J, Mellows G, Laguda B, Norris D, Bevan P. Phase I trial of XR9576 in healthy volunteers demonstrates modulation of P-glycoprotein in CD56+ lymphocytes after oral and intravenous administration. Clin Cancer Res. 2000;6:4186–4191. [PubMed] [Google Scholar]
- 18.Agrawal M, Abraham J, Balis FM, et al. Increased 99mTc-sestamibi accumulation in normal liver and drug-resistant tumors after the administration of the glycoprotein inhibitor, XR9576. Clin Cancer Res. 2003;9:650–656. [PubMed] [Google Scholar]
- 19.Sisodiya SM, Bates SE. Treatment of drug resistance in epilepsy: one step at a time. Lancet Neurol. 2006;5:380–381. doi: 10.1016/S1474-4422(06)70422-7. [DOI] [PubMed] [Google Scholar]
- 20.Langer O, Bauer M, Hammers A, et al. Pharmacoresistance in epilepsy: a pilot PET study with the P-glycoprotein substrate R-[11C]verapamil. Epilepsia. 2007;48:1774–1784. doi: 10.1111/j.1528-1167.2007.01116.x. [DOI] [PubMed] [Google Scholar]
- 21.Hammers A, Allom R, Koepp MJ, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abrahim A, Luurtsema G, Bauer M, et al. Peripheral metabolism of (R)-[(11)C]verapamil in epilepsy patients. Eur J Nucl Med Mol Imaging. 2008;35:116–123. doi: 10.1007/s00259-007-0556-5. [DOI] [PubMed] [Google Scholar]
- 23.Luurtsema G, Molthoff CF, Schuit RC, Windhorst AD, Lammertsma AA, Franssen EJ. Evaluation of (R)-[11C]verapamil as PET tracer of P-glycoprotein function in the blood-brain barrier: kinetics and metabolism in the rat. Nucl Med Biol. 2005;32:87–93. doi: 10.1016/j.nucmedbio.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Logan J, Fowler JS, Volkow ND, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 25.Syvänen S, Blomquist G, Sprycha M, et al. Duration and degree of cyclosporin induced P-glycoprotein inhibition in the rat blood-brain barrier can be studied with PET. Neuroimage. 2006;32:1134–1141. doi: 10.1016/j.neuroimage.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 26.Langer O, Kuntner C, Bankstahl JP, et al. Mapping regional P-gp function in normal and epileptic rat brain with (R)–[11C]verapamil PET [symposium abstract] J Nucl Med. 2009;50:158P. [Google Scholar]
- 27.Germann UA, Ford PJ, Shlyakhter D, Mason VS, Harding MW. Chemosensitization and drug accumulation effects of VX-710, verapamil, cyclosporin A, MS-209 and GF120918 in multidrug resistant HL60/ADR cells expressing the multidrug resistance-associated protein MRP. Anticancer Drugs. 1997;8:141–155. doi: 10.1097/00001813-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Liow JS, Kreisl W, Zoghbi SS, et al. P-Glycoprotein Function at the Blood-Brain Barrier Imaged Using 11C-N-Desmethyl-Loperamide in Monkeys. J Nucl Med. 2009;50:108–115. doi: 10.2967/jnumed.108.056226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muzi M, Mankoff DA, Link JM, et al. Imaging of Cyclosporine Inhibition of P-Glycoprotein Activity Using 11C-Verapamil in the Brain: Studies of Healthy Humans. J Nucl Med. 2009;50:1267–1275. doi: 10.2967/jnumed.108.059162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 31.Trambas C, Wang Z, Cianfriglia M, Woods G. Evidence that natural killer cells express mini P-glycoproteins but not classic 170 kDa P-glycoprotein. Br J Haematol. 2001;114:177–184. doi: 10.1046/j.1365-2141.2001.02885.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.