Abstract
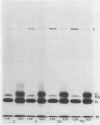
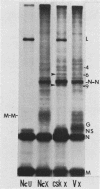
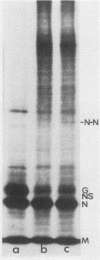
The pathway of vesicular stomatitis virus N protein from synthesis to assembly into capsids was studied by use of detergent extraction of infected HeLa cells together with protein cross-linking. One half of the newly synthesized N protein was extracted with the soluble cell proteins and, when cross-linked, never formed the N-N dimer characteristic of mature nucleocapsids. In contrast, the cytoskeleton-bound N protein first showed a diffuse spectrum of protein-protein cross-links but, after a lag of 40 min, assumed the cross-link pattern of N protein in nucleocapsids. The efficiency of forming N-N cross-linked dimers is the same for N protein on the skeleton as in nucleocapsids derived from mature virus, suggesting very similar configurations. However, the N protein bound on the skeletal framework formed several additional cross-links that were not found in mature virus and were apparently formed to cellular proteins estimated to be ca. approximately 46,000 and 60,000 in molecular weight.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson P. H., Moyer S. A., Summers D. F. Assembly of vesicular stomatitis virus glycoprotein and matrix protein into HeLa cell plasma membranes. J Mol Biol. 1976 Apr 15;102(3):613–631. doi: 10.1016/0022-2836(76)90338-7. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Horowitz M., Skolnik H., Abulafia R., Laub O., Aloni Y. The metabolism of SV40 RNA is associated with the cytoskeletal framework. Virology. 1981 Jun;111(2):475–487. doi: 10.1016/0042-6822(81)90350-0. [DOI] [PubMed] [Google Scholar]
- Blikstad I., Lazarides E. Synthesis of spectrin in avian erythroid cells: association of nascent polypeptide chains with the cytoskeleton. Proc Natl Acad Sci U S A. 1983 May;80(9):2637–2641. doi: 10.1073/pnas.80.9.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S., Levinson W., Spudich J. A. Cytoskeletal elements of chick embryo fibroblasts revealed by detergent extraction. J Supramol Struct. 1976;5(2):119–130. doi: 10.1002/jss.400050203. [DOI] [PubMed] [Google Scholar]
- Capco D. G., Krochmalnic G., Penman S. A new method of preparing embeddment-free sections for transmission electron microscopy: applications to the cytoskeletal framework and other three-dimensional networks. J Cell Biol. 1984 May;98(5):1878–1885. doi: 10.1083/jcb.98.5.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Dubovi E. J., Wagner R. R. Spatial relationships of the proteins of vesicular stomatitis virus: induction of reversible oligomers by cleavable protein cross-linkers and oxidation. J Virol. 1977 May;22(2):500–509. doi: 10.1128/jvi.22.2.500-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. H., Kingsbury D. W., Murti K. G. Assembly of vesicular stomatitis virus nucleocapsids in vivo: a kinetic analysis. J Virol. 1979 Oct;32(1):304–313. doi: 10.1128/jvi.32.1.304-313.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Separate pathways of maturation of the major structural proteins of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1128–1139. doi: 10.1128/jvi.21.3.1128-1139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk R., Penman S. The cytoskeletal framework and poliovirus metabolism. Cell. 1979 Feb;16(2):289–301. doi: 10.1016/0092-8674(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Lenk R., Ransom L., Kaufmann Y., Penman S. A cytoskeletal structure with associated polyribosomes obtained from HeLa cells. Cell. 1977 Jan;10(1):67–78. doi: 10.1016/0092-8674(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Lomant A. J., Fairbanks G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J Mol Biol. 1976 Jun 14;104(1):243–261. doi: 10.1016/0022-2836(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Murti K. G., Goorha R. Interaction of frog virus-3 with the cytoskeleton. I. Altered organization of microtubules, intermediate filaments, and microfilaments. J Cell Biol. 1983 May;96(5):1248–1257. doi: 10.1083/jcb.96.5.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. The detertent-resistant cytoskeleton of tissue culture cells includes the nucleus and the microfilament bundles. Exp Cell Res. 1977 May;106(2):339–349. doi: 10.1016/0014-4827(77)90179-3. [DOI] [PubMed] [Google Scholar]
- Penman S., Fulton A., Capco D., Ben Ze'ev A., Wittelsberger S., Tse C. F. Cytoplasmic and nuclear architecture in cells and tissue: form, functions, and mode of assembly. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):1013–1028. doi: 10.1101/sqb.1982.046.01.094. [DOI] [PubMed] [Google Scholar]
- Quinlan M. P., Knipe D. M. Nuclear localization of herpesvirus proteins: potential role for the cellular framework. Mol Cell Biol. 1983 Mar;3(3):315–324. doi: 10.1128/mcb.3.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]