Abstract
Water deprivation and restriction are common features of many physiologic and behavioral studies; however, there are no data-driven humane standards regarding mice on water deprivation or restriction studies to guide IACUC, investigators, and veterinarians. Here we acutely deprived outbred CD1 mice of water for as long as 48 h or restricted them to a 75% or 50% water ration; physical and physiologic indicators of dehydration were measured. With acute water deprivation, the appearance and attitude of mice deteriorated after 24 h, and weight loss exceeded 15%. Plasma osmolality was increased, and plasma volume decreased with each time interval. Plasma corticosterone concentration increased with duration of deprivation. There were no differences in any dehydration measures between mice housed in conventional static cages or ventilated racks. Chronic water restriction induced no significant changes compared with ad libitum availability. We conclude that acute water deprivation of as long as 24 h produces robust physiologic changes; however, deprivation in excess of 24 h is not recommended in light of apparent animal distress. Although clearly thirsty, mice adapt to chronic water restriction of as much as 50% of the ad libitum daily ration that is imposed over an interval of as long as 8 d.
Water deprivation and restriction are common features of biomedical and behavioral research. Although IACUC are charged with ensuring the humane use of animals, little information is available regarding humane guidelines for mice subjected to water deprivation or restriction. Water deprivation studies are designed to produce a behavioral or physiologic effect by withholding water from animals for various periods of time. Investigators, IACUC, and veterinary staff must determine the optimal time point that produces the desired experimental effect but that minimizes animal distress. Unlike deprivation (the complete withholding of water), restriction studies are often chronic studies in which water is reduced to either a specific daily ration or provided for only a specified period of time during each day. Most studies use the water restriction paradigm to produce a consistent state of physiologic need that can be used to study fluid homeostasis or to induce a motivational stimulus to perform a behavioral task.8 The chronic nature of this type of study requires close monitoring of the animals’ overall health and behavior throughout the experiment.19,24
Currently, mice are the laboratory animal model of choice for many physiologic and behavioral studies. Benchmarks for water deprivation or restriction in larger rodents, particularly rats, have been reported. For example, plasma osmolality in rats increased only 4% after 48 h of water deprivation, with a modest weight loss of approximately 11% after 72 h of water deprivation.2,10,17,19 Because body size is much smaller in mice than rats, variations in body weight, appearance, and physiology in the face of dehydration likely differ as well. Therefore, dehydration data in rats cannot be applied to smaller rodents, such as mice.19,24
The present study investigated various regimens of water deprivation and restriction and their effects on the appearance, attitude, and select key physiologic indicators of dehydration in outbred CD1 mice (Mus musculus). In addition, conventional static and ventilated housing were compared to determine whether the higher airflow in individually ventilated cages leads to dehydration more rapidly.
In the first experiment, mice housed in either static or ventilated racks were acutely deprived of water for 12, 24, or 48 h, and body weight, food intake, plasma volume and osmolality, corticosterone and plasma renin activity (PRA) were measured. The second experiment evaluated similar parameters after 7 d of chronic water restriction to either 75% or 50% of the normal water intake. Because both static and ventilated racks yielded similar results in the first experiment, only static housing was used in the second.
Using this study, we seek to establish guidelines for investigators involved in biomedical and behavioral research by determining the optimal period of water deprivation and restriction to achieve physiologic changes yet balance animal welfare concerns.
Materials and Methods
Animals.
Male CD1 mice (30 to 40 g; age, 8 to 10 wk) were obtained from Harlan Laboratories (Indianapolis, IN). All mice were housed individually in polycarbonate cages on corncob bedding (7092, Harlan Teklad, Madison, WI) with free access to standard rodent chow (7912, Harlan Teklad). The vivarium was programmed with a 12:12-h light:dark cycle (lights on, 0700 to 1900) and was maintained at 22 ± 2 °C with approximately 15 air changes hourly. Cages were either placed on shelves under standard static conditions with microfilter lids or on a ventilated rack at 60 air changes hourly (Allentown Caging Equipment, Allentown, NJ). Mice were allowed 1 wk of acclimation to these conditions before experimental manipulation was initiated. During acclimation, mice had free access to water bottles with reverse-osmosis water, but access was prevented or restricted during the experimental phase. All experiments were conducted in accordance with institutional guidelines and approved by the University of Florida IACUC.
Acute water deprivation.
A total of 192 mice were used, and each mouse was randomly assigned to 1 of 8 experimental groups of approximately 24 mice each: ad lib control (static and ventilated caging), 12-h water deprivation (static and ventilated), 24-h water deprivation (static and ventilated), and 48-h water deprivation (static and ventilated). Deprivation of water for the 12-h groups occurred during the active dark cycle (2100 to 0900). All deprivation periods ended at the same time (0900).
Prior to the deprivation period, both experimental and control mice were evaluated by one of the authors (CB) for appearance and attitude according to a 5-point scale (Figure 1) and then placed on clean, dry bedding and deprived of water for the specified duration. A specific amount of rodent chow (approximately 50 g) was placed in each cage. At the end of the deprivation period, mice were weighed to determine weight loss and were reevaluated for appearance and attitude. The remaining food was weighed to determine food intake; a few animals shredded large amounts of food, and their intake data were discarded. For the purposes of water intake, approximately one third of the animals in the control groups were randomly assigned to the 12-, 24-, or 48-h subgroups and euthanized at those time points to determine normal water intakes for those time intervals. Mice then were anesthetized by isoflurane inhalation and exsanguinated by cardiocentesis (25-gauge needle). A capillary tube was filled from the hub of the needle for the determination of PCV and total protein levels, as described later. The remaining blood sample was placed into tubes containing EDTA or no additives (for serum collection) for other assays. Urine was collected either by sampling any urine voided after euthanasia, or urine was aspirated from the bladder at the postmortem examination. Major organs (brain, lungs, heart, liver, and kidneys) were collected from both experimental and control mice for histopathology.
Figure 1.

Appearance and attitude scales.
Chronic water restriction.
A total of 40 mice were used. Because individually ventilated cages and static housing yielded similar results during the acute deprivation experiment, only static housing was used in the present experiment. Each mouse was randomly assigned to 1 of 3 groups: ad lib control (n = 12), 75% water ration (n = 14), or 50% water ration (n = 14). Normal water intake for a 24-h period (100% ration) was determined during the acclimation period by measuring the daily water intake of each mouse and calculating a group mean (approximately 7.2 mL). As for the acute deprivation experiment, mice were weighed, evaluated for appearance and attitude according to the same 5-point scale, and were placed on the same type of clean, dry bedding. Mice then were offered ad libitum (control) access to water or 75% or 50% of the normal water ration daily at the same time (0900) for 7 d. The ration for each mouse was measured into a 10-mL glass beaker that was presented inside the cage secured with a metal stirrup. A measured amount of rodent chow was placed in cage at the beginning of the restriction period. Food intake was calculated, as described earlier, after 4 and 8 d, with fresh food added at the end of day 4. On the eighth day, just before the ration would normally have been given, both control and experimental mice were euthanized by isoflurane for blood and tissue collection.
Hematology and urinary analyses.
Capillary tubes were processed on a hematocrit centrifuge (MB, International Equipment Company, Nashville, TN), and PCV was measured by using a hematocrit capillary tube reader. The tubes were scored and snapped, and the plasma was extruded for measurement of total protein by using a hand refractometer (Brix, Atago, Bellavue, WA). A full CBC profile was performed on EDTA-treated blood by using an automated analyzer (Hemavet 1700, Drew Scientific, Oxford, CT). Whole blood samples were centrifuged, and aliquots of plasma samples were stored at −40 °C until assay. Plasma and urine osmolality were measured by using a vapor pressure microosmometer (Vapro 5520, Wescor Biomedical Systems, Logan, UT), both plasma and urine sodium and potassium concentrations by flame photometer (IL 943 flame photometer, Instrumentation Laboratories, Bedford, MA), and plasma BUN and creatinine by automated chemistry analyzer (VetAce, ALFAWasserman, West Caudwell, NJ).
Immunoassays of corticosterone and PRA.
Corticosterone concentrations were measured by radioimmunoassay on whole blood aliquots according to directions (Coat-A-Count TKRC1, Siemens Health Care Diagnostics, Deerfield, IL). PRA was measured as the difference in angiotensin I generated between samples incubated at 37 °C and 4 °C by using a small-sample adaptation of a radioimmunoassay kit (Gamma Coat CA1533, DiaSorin, Stillwater, MN).
Histologic assessment.
Formalin-fixed organs (brain, heart, lungs, liver, and kidneys) were embedded in paraffin, sectioned at 5 to 7 µm, and stained with hematoxylin and eosin for assessment by a board-certified veterinary pathologist (MKR).
Statistical analysis.
The data were analyzed by using 1- and 2-way ANOVA, with posthoc contrasts according to the Newman–Kuels test and with an α level of 0.05 (SPSS, IBM, New York, NY). Data from the acute deprivation experiment were analyzed for the effects of caging type and duration of dehydration. No significant effects of caging type were evident, so results were reanalyzed after data from combined ventilated and static caging were combined. In a few cases, insufficient sample was obtained for a particular assay; therefore, the number of data points is not the same for all measures. Data from the restriction experiment were analyzed for differences among restriction levels by one-way ANOVA.
Results
Acute water deprivation.
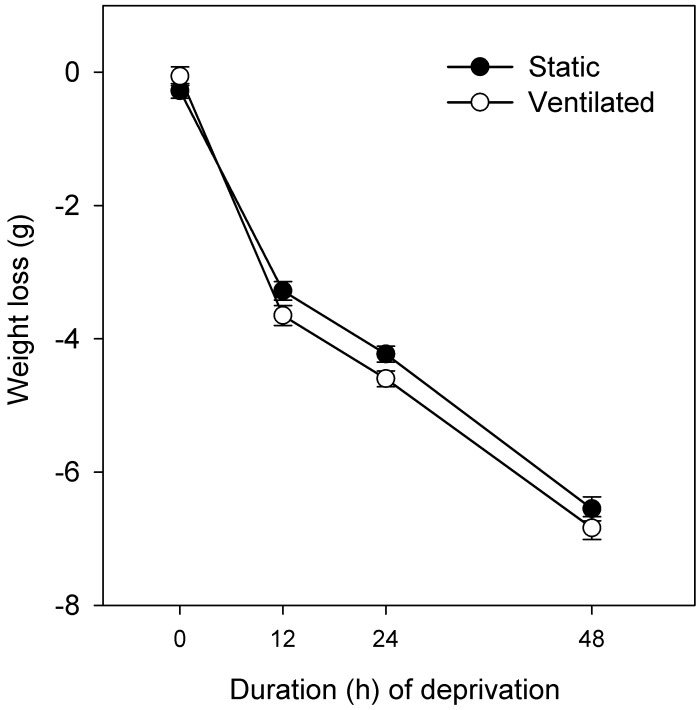
All groups of mice had similar body weights at the start of the deprivation period (group means, 35.1 to 37.0 g). Weight loss during water deprivation was analyzed as absolute change from the initial weight. The changes in body weight are shown in Figure 2. A 2-way ANOVA revealed that body weight declined significantly with the duration of deprivation (F3,184 = 675.2, P < 0.001) but did not differ as a function of caging type (F1,184 = 3.62, P = 0.059). In addition, there was no significant interaction between duration of deprivation and caging type (F3,184 = 1.78, P > 0.05). Subsequent one-way ANOVA for each caging type revealed that the body weight declined with the duration of water deprivation (static: F3,86 = 308.6, P < 0.001; ventilated: F3,91 = 368.8, P < 0.001), and Newman–Kuels comparisons revealed that each deprivation group differed significantly (P < 0.05) from each other.
Figure 2.
Weight loss (g; mean ± SE, n = 24) in mice housed on either static or ventilated racks and acutely deprived of water for as long as 48 h: experiment 1. Weight loss was determined as absolute change from initial weight. There was no statistical difference between static and ventilated racks. After 24 and 48 h of acute water deprivation, mice had lost approximately 12% and 18%, respectively, of their baseline body weight; 15% weight loss corresponds to approximately 5.4 g.
Food intakes are shown in Table 1. Mice in the water-deprived groups ate less than did the ad libitum controls (F1,169 = 26.05, P < 0.001), with a significant interaction between each duration of deprivation (F2,169 = 5.63, P < 0.001). Comparisons between water-deprived groups and the matched ad libitum subgroups showed that intake was not significantly reduced at the 24-h time point, with a mean reduction of 11%, but was significantly reduced after 48 h by an average of 25% (P < 0.02). Therefore, food intake between hours 24 to 48 in the 48-h water-deprived group was approximately 65% of that of the controls. There was no difference between caging conditions at 24 h. However, food intake was significantly (P < 0.05) lower at 12 h in control mice housed in the static cages as compared with control mice housed in ventilated cages; in the ventilated cages, 75% of the 24-h intake occurred during the first 12 h (that is, mostly at night) as compared with only 50% in the static cages.
Table 1.
Food intake (g; means ± SE) of control (n = 6 to 8) and water-deprived (n = 18 to 22) mice after various durations of water deprivation
| Housing | Condition | 12 h | 24 h | 48 h |
| Static | Control | 2.7 ± 0.4 | 5.5 ± 0.5 | 11.9 ± 1.1 |
| Water-deprived | 2.2 ± 0.2 | 4.9 ± 0.3 | 9.2 ± 0.5b | |
| Ventilated | Control | 3.6 ± 0.6 | 4.8 ± 0.3 | 10.9 ± 0.7 |
| Water-deprived | 1.9 ± 0.2a | 4.3 ± 0.2 | 8.0 ± 0.4c |
P < 0.05 compared with value for control (that is, ad libitum water intake) group.
P < 0.02 compared with value for control group.
P < 0.01 compared with value for control group.
Scores for attitude and appearance were not different between static and ventilated caging conditions. At times 0 and 12 h, most of the mice scored 5, whereas at 24 and 48 h, scores of 4 and 3 became more prevalent, with no score of less than 3. The mean scores differed significantly (P < 0.001) as a function of duration of deprivation, with 24- and 48-h scores differing (P < 0.05, Newman–Kuels test) from those at 0 or 12 h and from each other.
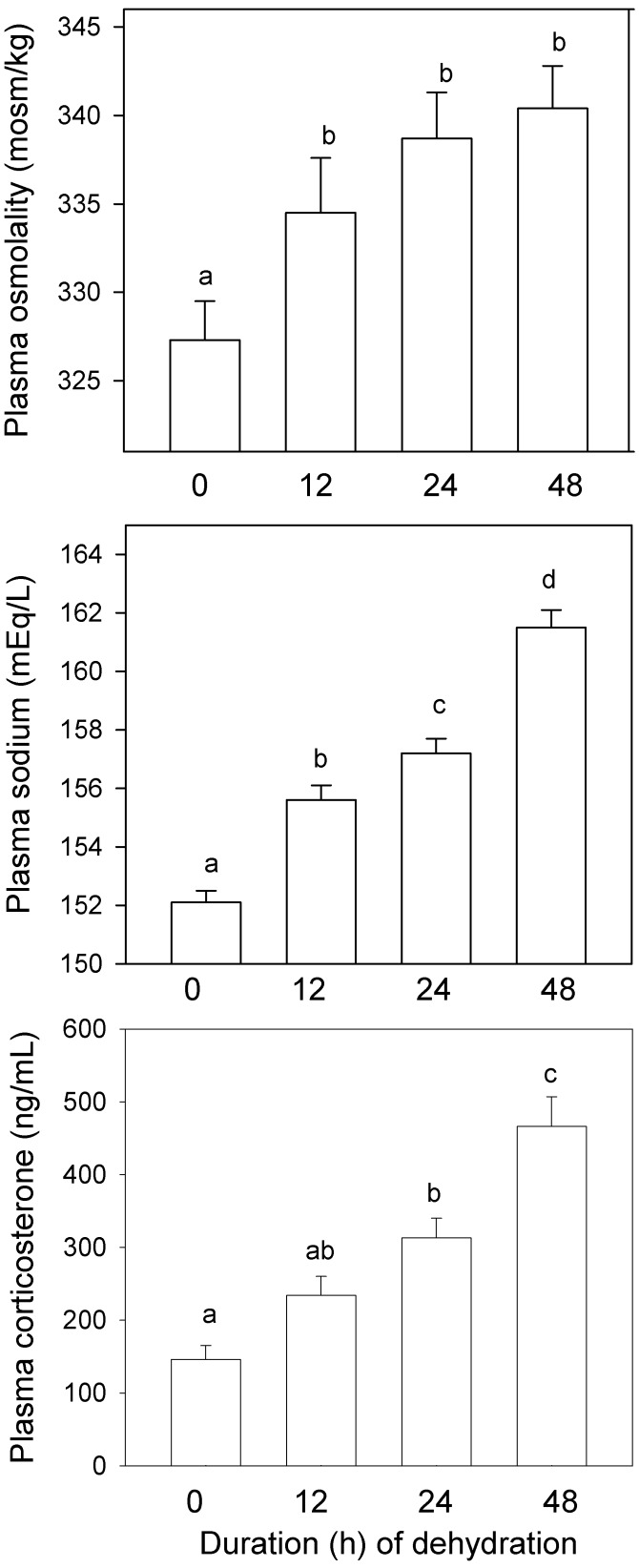
Plasma measures showed no significant differences as a function of caging. To simplify presentation, data from the 2 caging types have been combined. Compared with ad libitum controls, water-deprived mice showed higher plasma osmolality (F3,172 = 5.23, P < 0.01) and plasma sodium concentration (F3,174 = 57.53, P < 0.001) (Figure 3), whereas plasma potassium did not change (F3,173 = 2.11; data not shown). Plasma sodium increased significantly (P < 0.05) at each successive time interval. Plasma osmolality did not change after 12 h, but values from deprived mice at all time points were elevated (P < 0.05) from control values. After 12, 24, and 48 h, the mean percentage elevations were 2.3%, 3.4%, and 6.2% (sodium) and 2.2%, 3.5%, and 4.0% (osmolality), respectively. Corresponding values of urinary osmolality and sodium and potassium concentrations taken from the bladder at euthanasia are shown in Table 2. These measures were higher (P < 0.05) than control data but showed no further increase after 12 h of water deprivation.
Figure 3.
Plasma osmolality (top panel), sodium concentration (middle panel), and corticosterone level (bottom panel) of mice deprived of water as long as 48 h: experiment 1. Caging types have been combined; data are given as mean ± SE (n = 42 to 45). Within a panel, bars marked with different letters differ significantly (P < 0.05); values that are marked with the same letter are not significantly different.
Table 2.
Urinary parameters after various durations of water deprivation
| Duration (h) | Sodium concentration (mEq/l) | Potassium concentration (mEq/l) | Osmolality (mOsm/kg) |
| 0 | 191 ± 12a | 301 ± 16a | 1721 ± 90a |
| 12 | 365 ± 19b | 432 ± 16b | 2861 ± 107b |
| 24 | 380 ± 22b | 474 ± 27b | 2867 ± 118b |
| 48 | 231 ± 10c | 466 ± 22b | 2622 ± 111b |
Data are expressed as mean ± SE for control and deprived animals (n = 37 to 47). Within columns, values marked with different letters differ significantly (P < 0.05); values marked with the same letter are not significantly different.
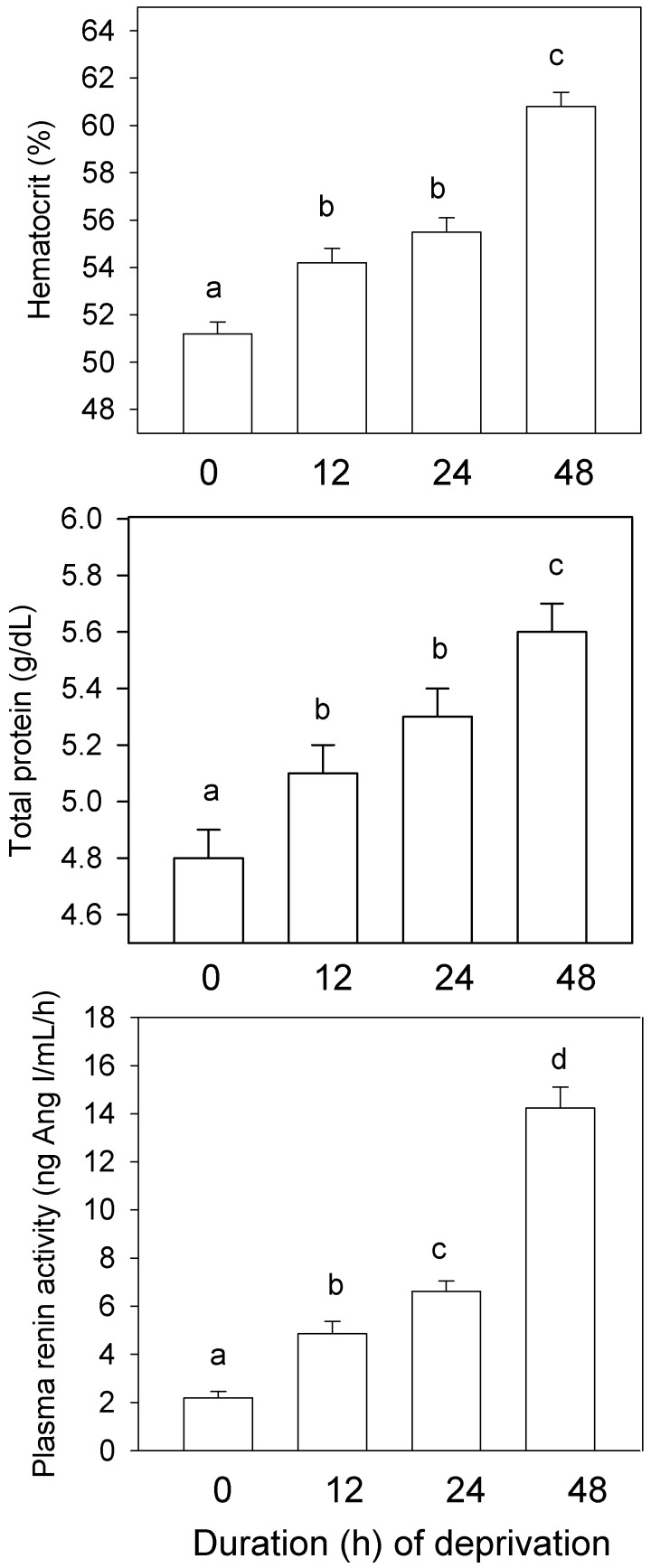
PCV (F3,180 = 46.99, P < 0.001), total plasma protein (F3,174 = 19.98, P < 0.0001), and PRA (F3,167 = 85.33, P < 0.001) increased significantly with duration of deprivation (Figure 4). After 12, 24, and 48 h, the mean elevations relative to control values were 5.9%, 8.4%, and 11.9% (PCV) and 6.2%, 10.4%, and 16.7% (protein); PRA after water deprivation was 2.2-, 3.0-, and 6.5-fold greater than the corresponding control value.
Figure 4.
Hematocrit (top panel), total protein (middle panel), and plasma renin activity (bottom panel) in mice deprived of water for as long as 48 h: experiment 1. Caging types have been combined; data are given as mean ± SE (n = 42 to 45). Within a panel, bars marked with different letters differ significantly (P < 0.05); values that are marked with the same letter are not significantly different.
Other blood parameters (Table 3) showed significant (P < 0.05) variation as a function of deprivation but not caging type. BUN was higher (P < 0.05) than baseline only after 48 h deprivation, but creatinine did not change from the level in ad libitum controls. Total WBC count decreased with duration of deprivation and was significantly (P < 0.05) lower than the control value after 24 and 48 h. Platelet count increased with deprivation and was significantly (P < 0.05) higher than that in controls after 24 h; the count at 48 h was significantly (P < 0.05) higher than those at all other time points. Plasma corticosterone concentration increased with duration of deprivation and was significantly (P < 0.05) elevated from control values after 12 and 24 h and further elevated at 48 h.
Table 3.
Select blood parameters after various durations of water deprivation
| Duration (h) | BUN (mg/dL) | WBC count (x103/μL) | Platelet count (x103/ μL) | Corticosterone (ng/mL plasma) |
| 0 | 26.3 ± 0.5a | 4.5 ± 0.3a | 1426 ± 35a | 145 ± 19a |
| 12 | 24.6 ± 0.5a | 4.0 ± 0.2ab | 1423 ± 53a | 236 ± 26b |
| 24 | 25.8 ± 0.4a | 3.5 ± 0.2bc | 1613 ± 50b | 311 ± 27b |
| 48 | 31.3 ± 0.5b | 3.2 ± 0.2c | 1855 ± 56c | 466 ± 41c |
Data are expressed as mean ± SE for control and water-deprived mice (n = 38 to 51). Within columns, values marked with different letters differ significantly (P < 0.05); values marked with the same letter are not significantly different.
Chronic restriction.
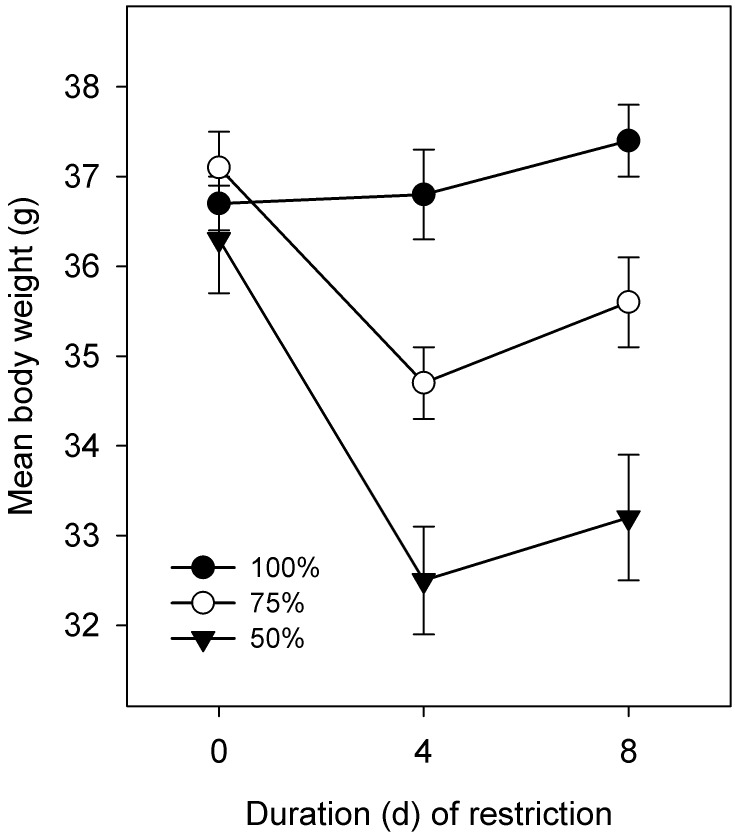
The attitude and appearance scores of mice worsened with chronic water restriction. After 7 d, the ad libitum group received scores of 5.0 on both measures. The appearance scores (mean ± SE) for the 75% and 50% ration groups were 3.9 ± 0.1 and 3.7 ± 0.1, respectively, and attitude scores were 4.1 ± 0.1 and 3.9 ± 0.1, respectively. Most mice scored 4, and none scored less than 3. These values were significantly (P < 0.05) different from those of controls but did not differ between the 75% and 50% ration groups. The mean body weight changes are shown in Figure 5. Restricted groups lost weight in the first 4 d and then resumed weight gain that paralleled weight gain in the controls. The 50% ration group lost more weight than did the 75% group (P < 0.05 pairwise comparisons). The mean weight losses relative to control values after 7 d were approximately 5% and 11% for the 75% and 50% groups. Food intake (4 d averages for days 0 to 4 and 5 to 8) was not significantly affected by water ration, although over the second period (days 5 to 8), the intakes of the 75% and 50% ration groups were 94% and 88% of control values. Group means averaged between 5.1 and 5.8 g of food per mouse daily for both control and experimental groups.
Figure 5.
Body weight (g; mean ± SE, n = 13 or 14) in mice allowed ad libitum (100% ration) water intake or restricted to either 75% or 50% of that ration daily: experiment 2. The body weights of the 50% and 75% groups differ (P < 0.05) from controls and from each other on days 4 and 8 of restriction.
The plasma variables measured in this study are shown in Table 4. None of the direct hydration-related variables (plasma osmolality, PCV, and total protein) showed a significant change from that in the control group or from each other. Although mice on chronic water restriction did not have any significant changes in these dehydration-related variables, the mice on both paradigms displayed apparent thirst, as evidenced by the informal observation that mice on 75% ration consumed their entire allocation of water within 6 h of presentation, whereas the 50% ration mice consumed theirs within 2 h of presentation (data not shown). PRA was increased (P < 0.05) by approximately 4-fold in both restriction groups. Corticosterone was elevated significantly (P < 0.05) only in the 50% ration group.
Table 4.
Blood parameters after 8 d of water restriction
| 100% ration | 75% ration | 50% ration | |
| Plasma osmolality | 327 ± 4 | 339 ± 4 | 338 ± 4 |
| (mOsm/kg) | |||
| Plasma protein (g/dL) | 7.0 ± 0.1 | 7.1 ± 0.1 | 7.3 ± 0.1 |
| Hematocrit (%) | 42.4 ± 0.3 | 42.7 ± 0.6 | 43.9 ± 0.5 |
| Renin activity | 2.2 ± 0.4a | 7.9 ± 1.3b | 8.7 ± 1.3b |
| (ng angiotensin I/mL/h) | |||
| Corticosterone (ng/mL) | 44 ± 9a | 99 ± 28a | 182 ± 27b |
Data are expressed as mean ± SE for control (that is, 100% ration) and water-deprived mice (n = 13-14). Within rows, values marked with different letters differ significantly (P < 0.05); values that are marked with the same letter are not significantly different.
Histologically, renal distal tubular dilatation was a consistent finding in mice from both experiments but was especially prominent in the chronically restricted groups. In addition, there was occasional rounding and exfoliation of the cells in the distal tubules and (rarely) the collecting ducts. No other noteworthy pathologic changes were found in the other organs examined.
Discussion
Acute deprivation of mice from water for more than 24 h has significant physiologic effects, as demonstrated by 18% body weight loss at 48 h. A weight loss of 15% is generally considered the benchmark for a humane endpoint.19,24 This widely accepted standard is exceeded in mice subjected to 48 h of water deprivation. In addition, the appearance and attitude of mice at 48 h, as determined on a 5-point scale, declined beginning at 24 h and further deteriorated after 48 h of water deprivation. These data demonstrate that until 24 h, mice do not display overt physical or behavioral signs of clinical dehydration, but they do increasingly show these signs of dehydration between 24 and 48 h. Acutely deprived mice lost a larger proportion of their body weight per unit time during deprivation than do larger mammals: therefore, the weight loss after 24 h in mice (approximately 12%) was comparable to that observed after 72 h water deprivation in rats weighing 10-fold more.2,10,17,19
Assessing the effect of water deprivation requires an understanding of the physiologic mechanisms that occur with inadequate water intake. During dehydration, osmolality of the extracellular fluid increases as the volume of the circulating fluid decreases; these are measured as plasma osmolality and plasma volume, respectively.16-18,22,24,28 After 12 h of acute water deprivation in our mice, both plasma osmolality and sodium increased significantly as compared with controls. Plasma osmolality did not continue to increase after the initial 12 h, most likely because of the loss of intracellular water. Changes in the intracellular environment can affect the activity of other hormones and enzymes in the body altering the overall physiology of the animal.7 Decreased water intake is associated with fluid loss in both the intracellular and extracellular compartments. In our mice, change in the extracellular compartment was reflected by increased Hct and plasma total protein at all 3 time points. Both Hct and total protein continued to increase in a linear fashion through the 48 h of deprivation. These changes in intra- and extracellular environments demonstrate potential physiologic distress.
Maintenance of the body's fluid volume is also regulated by renin and angiotensin, measured as PRA. As expected, PRA increased in the face of dehydration, with a 3-fold increase after 24 h and 6-fold increase after 48 h. With decreased body fluid, vasoconstriction occurs to maintain blood pressure and cause release of aldosterone. Aldosterone decreases sodium output from the proximal tubules, preserving plasma sodium and driving the maintenance of the body fluid by cellular dehydration.3,20 The significant increase in PRA at 48 h in the current study illustrates the body's increasing inability to maintain fluid volume and demonstrates significant physiologic distress,6,15,21,26 In addition, serum corticosterone was elevated at all time points, indicating a homeostatic challenge.23
Food intake decreased with duration of water deprivation, indicating that the amount of food that a mouse consumes is dependent on the availability of water. This compensatory effect is referred to as dehydration anorexia.5,19 This reduced food intake serves to decrease the amount of solutes present within the body.19
Chronic restriction of water to only 75% or 50% of the normal daily intake for 7 d resulted in apparent physiologic adaptation, as evidenced by a minimal body weight loss of 9% in the more severely (50%) restricted group. In addition, all the standard indices for dehydration approached normal, with no significant differences in any of the indices between the 2 ration groups. Given the maintenance of the plasma volume throughout the restriction period, the observed weight loss can be attributed to dehydration anorexia,1,5,27 The food intake decreased during the first 4 d but then increased and was comparable to that of the ad libitum controls. This pattern is similar to the findings of another study, in which mice restricted to 2 or 4 h water daily acclimated to chronic water restriction and recovered prerestriction body weights within 3 to 4 d.4,25 These results indicate that mice are equipped physiologically to deal with chronic water restriction. Although not explored in the current study, others have found that wild and laboratory rodents demonstrate a decrease in reproductive parameters or indices when chronically water restricted; therefore chronic water restriction may affect studies that involve breeding.11-14,25 The minor renal alterations that we observed microscopically may be reversible with rehydration, although this was not studied.
Serum corticosterone increased significantly only in the 50% ration group. The less-restricted 75% ration group had relatively high interindividual variability, and levels were not statistically different from those of controls. We do not have a good explanation for this high interindividual variability; different subjects may display different complements of physiologic adaptations. In previous studies, water-restricted and control rats performed similarly in open-field studies, as both groups entered a similar number of zones.6 This behavioral test can be a sensitive and reliable indicator of behavioral changes attributed to stress.6 Therefore, rodents may have elevated corticosterone levels but exhibit normal behavior on water-restriction protocols.
The current study used young CD1 mice, which may not be an accurate model for all mice on water deprivation or restriction studies, particularly inbred or genetically modified animals. During the acute deprivation experiment, we assessed an additional group of older (approximately 18 mo) C57Bl/6. These mice were water-deprived for 24 h and exhibited similar changes in attitude and appearance scores and weight loss as did the outbred CD1 mice at the same time points (results not shown). Although our results may not be directly applicable to all strains, our findings represent a basic framework for animal use protocols using water deprivation and restriction. Given our findings, we conclude that acute deprivation of 24 h in mice results in clinical dehydration and that deprivation beyond 24 h is not recommended for mice in light of noteworthy physiologic changes. Mice on chronic restriction regimens show considerable physiologic adaption. However, when water restriction is performed, the animals’ health and behavior should be closely monitored for signs of pain and distress, as recommended in the Guide.9
Acknowledgments
We thank Dr Chris Baylis for use of the flame photometer and micro-osmometer. This research was supported by a grant-in-aid from the American College of Laboratory Animal Medicine Foundation.
References
- 1.Armstrong S, Grahame C, Singer G. 1980. Food and water deprivation: changes in rat feeding, drinking, activity, and body weight. Neurosci Biobehav Rev 4:377–402 [DOI] [PubMed] [Google Scholar]
- 2.Bealer S, Crofton J, Share L.1983. Hypothalmic knife cuts alter fluid regulation, vasopressin secretion, and natriruresis during water deprivation. Neuroendocrinology 36: 364–370.
- 3.Fitzsimons JT. 1998. Angiotensin, thirst, and sodium appetite. Physiol Rev 78:583–686 [DOI] [PubMed] [Google Scholar]
- 4.Haines H, Shield C. 1971. Reduced evaporation in house mice (Mus musculus) acclimated to water restriction. Comp Biochem Physiol A Comp Physiol 39:53–61 [DOI] [PubMed] [Google Scholar]
- 5.Hamilton L, Flaherty C. 1973. Interactive effects of deprivation in the albino rat. Learn Motiv 4:148–162 [Google Scholar]
- 6.Heiderstadt KM, McLaughlin R, Wright D, Walker S, Gomez-Sanchez C. 2000. The effect of chronic food and water restriction on open-field behavior and serum corticosterone levels in rats. Lab Anim 34:20–28 [DOI] [PubMed] [Google Scholar]
- 7.Hohenegger M, Laminer U, Om P, Sadjak A, Gutmann K, Vermes M. 1986. Metabolic effects of water deprivation. J Clin Chem Clin Biochem 24:277–282 [DOI] [PubMed] [Google Scholar]
- 8.Homma S, Takeda S, Kusano E, Matsuo T, Simizu T, Nakamura M, Oohara T, Makino S, Assano Y. 2002. Impaired urinary concentrating ability in genetically polyuric mice. Nephron 92:889–897 [DOI] [PubMed] [Google Scholar]
- 9.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 10.Kleiner SM. 1999. Water: an essential but overlooked nutrient. J Am Diet Assoc 99:200–206 [DOI] [PubMed] [Google Scholar]
- 11.Nelson R. 1988. Restricted water intake influences male reproduction in 2 strains of house mice (Mus musculus). Physiol Behav 43:217–221 [DOI] [PubMed] [Google Scholar]
- 12.Neslon RJ. 1985. Restricted water availability induces reproductive regression in deer mice. Biol Reprod 32:265 [Google Scholar]
- 13.Nelson RJ, Dark J, Zucker I. 1983. Influence of photoperiod, nutrition, and water availability on reproduction of male California voles (Microtus californicus). J Reprod Fertil 69:473–477 [DOI] [PubMed] [Google Scholar]
- 14.Nelson RJ, Desjardins C. 1987. Water availability affects reproduction in deer mice. Biol Reprod 37:257–260 [DOI] [PubMed] [Google Scholar]
- 15.Ottenweller JE, Servatius R, Tapp W, Drastal S, Bergen M, Natelson B. 1992. A chronic stress state in rats: effects of repeated stress on basal corticosterone and behavior. Physiol Behav 51:289–298 [DOI] [PubMed] [Google Scholar]
- 16.Rolls BJ, Rolls E. 1981. The control of drinking. Br Med Bull 37:127–130 [DOI] [PubMed] [Google Scholar]
- 17.Rolls BJ, Rolls E. 1982. Thirst. Cambridge (UK): Cambridge University Press. [Google Scholar]
- 18.Rolls BJ, Wood R, Rolls E. 1980. Thirst: the initiation, maintenance, and termination of drinking. Prog Psychobiol Physiol Psychol 9:263–321 [Google Scholar]
- 19.Rowland NE.2007. Food or fluid restriction in common laboratory animals: balancing welfare considerations with scientific inquiry. Comp Med 57: 149-160.
- 20.Rowland NE, Farnbauch L, Crews E. 2004. Sodium deficiency and salt appetite in ICR: CD1 mice. Physiol Behav 80:629–635 [DOI] [PubMed] [Google Scholar]
- 21.Silverman P. 1978. Animal behavior in the laboratory. New York (NY): Pica Press. [Google Scholar]
- 22.Stricker EM, Verbalis JG. 1988. Hormones and behavior: the biology of thirst and sodium appetite. Am Sci 76:261–267 [Google Scholar]
- 23.Sutanto W, de Kloet E. 1994. The use of various animal models in the study of stress and stress-related phenomena. Lab Anim 28:293–306 [DOI] [PubMed] [Google Scholar]
- 24.Toth L, Gardiner T.2000. Food and water restriction protocols: physiological and behavioral considerations. Contemp Top Lab Anim Sci 39: 9–17.
- 25.Tucci V, Hardy A, Nolan P. 2006. A comparison of physiological and behavioral parameters in C57BL/6J mice undergoing food or water restriction regimes. Behav Brain Res 173:22–29 [DOI] [PubMed] [Google Scholar]
- 26.Walsh RN, Cummins R. 1976. The open-field test: a critical review. Psychol Bull 83:482–504 [PubMed] [Google Scholar]
- 27.Watts AG. 1999. Dehydration-associated anorexia: development and rapid reversal. Physiol Behav 65:871–878 [DOI] [PubMed] [Google Scholar]
- 28.Wolf A. 1958. Thirst, physiology of the urge to drink and problems of water lack. Springfield (IL): Thomas. [Google Scholar]