Abstract
Social housing of nonhuman primates (NHP) in an infectious disease setting presents unique challenges, and individual housing is often scientifically justified. At our institute, we recognized an opportunity to limit individual housing to the minimal period necessary by pair-housing NHP after quarantine and separating them just before they are moved into holding rooms for infectious disease studies. To alleviate concerns that pair-housing followed by separation affects the immune system of NHP and makes them unfit as research candidates, we designed a short-term pair-housing study. After a 3-wk baseline period, juvenile rhesus macaques (age, 3 to 4 y) were paired for 7 wk and then separated for 7 wk. During the study, serum cortisol, lymphocyte subsets, and proinflammatory cytokines were measured. The average values for all parameters were significantly lower after separation than during the baseline period. We conclude that short-term pair housing is a viable option at our institute for social housing of NHP.
Abbreviation: NHP, nonhuman primates; ABSL, Animal Biosafety Level
Social housing of nonhuman primates (NHP) has garnered much needed attention. The eighth edition of the Guide for the Care and Use of Laboratory Animals strengthened its position on social housing needs of all animals stating “20 Social animals should be housed in stable pairs or groups of compatible individuals unless they must be housed alone for experimental reasons or because of social incompatibility. Single housing should be limited to the minimum period necessary; enrichment should be offered to enhance animal wellbeing through facilitation of species-typical behaviors.”20 The Animal Welfare Act states “The environment enhancement plan must include specific provisions to address the social needs of nonhuman primates of species known to exist in social groups in nature.”1 To benefit animals and to maintain compliance with the rules and guidelines that regulate the field of laboratory animal medicine, social housing of NHP should be considered the accepted professional standard, and every NHP should be allowed the opportunity for social housing.
“Social interactions,” which can be achieved through social housing, are “considered to be one of the most important factors influencing the psychological wellbeing of NHP.”19 There are various forms of social housing of NHP. Troops and small groups can be formed, animals can be pair-housed or allowed to comingle during parts of the day, and play cages can be used. In addition, partial or protected contact pairing, accomplished through the use of grooming contact bars and perforated panels, can be considered for social housing in a laboratory setting.2 The formation of stable groups does present some risk of injury until dominant–subordinate order is established. Pair housing is an appealing option to group housing in that pair housing is logistically easier and allows socialization with less likelihood of injury.
The benefits of social housing, including pair housing, are well-documented.12,22-26 A recent review article examined the beneficial effects of pair housing, including the ability to cope effectively, demonstration of species-typical behavior, absence of maladaptive behaviors, balance of temperament, and absence of chronic distress.11 Social deprivation can lead to stress that “may distort processes, both physiological and behavioural” and create animals that are suboptimal research candidates.23
Routine social housing of NHP in infectious disease studies under Animal Biosafety Level (ABSL) 3 or ABSL4 conditions poses several unique challenges. For example, numerous infectious agents are aerosolized for the purpose of infecting animals. Because of the possibility of disease transmission, control or naïve NHP cannot be cohoused with animals that have been infected via aerosolization. Socially housing only infected NHP or only control animals would introduce confounders into a study; all animals must be housed identically. In addition, social housing of NHP on infectious disease studies presents logistical challenges, because many studies use central venous catheters with jackets and tethers. Cages currently in use in our facility do not accommodate multiple NHP on tethers. Tether security is important to the safety of the animal and the quality of the research. In addition, tethered and jacketed NHP may be limited in their ability to express species-specific behaviors that are seen with social housing, such as playing, hugging, and mutual grooming. Personnel safety issues are an ongoing concern, particularly when it is necessary to separate socially housed and infected NHP under ABSL3 or ABSL4 conditions by using heavy cage dividers.
Until recently, NHP at our institute were individually housed for 2 prevailing reasons: 1) the rapid use and reuse of NHP did not allow the time necessary to create pairings or groups or to maintain stable pairings or groups; and 2) infectious disease study design precluded social housing. Although many of the factors that deter the social housing of NHP in an infectious disease study could not be mitigated, we recognized the opportunity to allow social housing of NHP for a limited period of time. NHP that are released from quarantine could be socially housed before they are placed on an infectious disease study. This practice would allow us to provide social housing and would limit single housing to the minimal period necessary (that is, the study period), as directed by the Guide. Short-term social housing may be an ideal option at other research facilities in which long-term social housing is limited for any reason.
Much of the published literature on the positive effects of the social housing of NHP is based on long-term stable groups or pairs. Several previous studies evaluating the physiologic effects of NHP social housing included stable pair or group formations that ranged from 4 mo to 2 y.7,8,12,26 However, little information has been published on the effects of short-term social housing. The few short-term social-housing studies focused on effects on grooming and social behaviors that were followed for 8 wk after long-term or short-term individual housing,27 the effect of pair-housing on operant behavior testing for 8 wk, in which pair-housing negatively affected some test parameters,17 and the beneficial effect of environmental enrichment for animals individually housed short-term for 5 wk.29 No studies have examined pair housing and its effect on NHP psychological wellbeing during and after short-term pairing, and no studies in the literature have investigated the effects of separating paired NHP after short-term social housing. Indeed, a US Department of Agriculture memorandum states that “Once animals have been compatibly paired or grouped, separating them is stressful.”30 Understandably, one of the concerns faced at our institute was separation stress, as would occur on assigning NHP to an infectious disease study involving housing under ABSL3 or ABSL4 conditions, and its real or perceived effect on the animals’ immune systems.
For the purpose of the current study, we evaluated proinflammatory cytokines, lymphocyte subsets, and serum cortisol levels. Diminished immune function can occur in response to stressful social manipulation, and immune function may be enhanced in response to stable social environments.26 Immune parameters measured in previous studies on NHP social housing include lymphocyte subset levels, lymphocyte proliferation in response to gastrointestinal pathogens, natural killer cell activity, and cytokine assays.8,24,26 The lymphocyte subset parameter involves the levels of CD4+ (helper) and CD8+ (killer) T cells. The ratio of CD4+ to CD8+ cells (that is, CD4:CD8 ratio) should be positively correlated with a ‘healthy’ immune system.28 In addition, cytokine assays can be relevant measures of immune system function, because interferon γ and numerous interleukin concentrations show significant differences over time and between housing conditions.26 Cortisol, colloquially known as the ‘stress hormone,’ can be measured from plasma, serum, feces, saliva, and even hair and has been studied in NHP in regard to housing conditions,12 anesthetic protocols,31 environmental conditions,21 and many other variables.
The benefits or possible detriments of short-term social housing followed by separation of NHP are not well understood. We designed the current study to examine short-term pair housing and subsequent separation and its effect on proinflammatory cytokines, lymphocyte subsets, and serum cortisol. For the purpose of this study, short-term pair housing occurred for 7 wk. This duration was chosen as the typical amount of time that NHP are housed before moving onto an infectious disease study under ABSL3 or ABSL4 housing. We implemented a plan of routine pair housing, under which pair housing is attempted for all NHP expected to be housed for at least 60 days after quarantine before experimental use. The goal of this study was to ensure that the implementation of routine pair housing would not adversely affect infectious disease studies at our institute by altering the NHP immune system. Our hypothesis was that baseline levels of immunologic parameters would not be significantly different from those after separation of animals that had been pair-housed briefly. We hoped to determine the appropriate time frame for separating NHP, if necessary, before moving them onto an infectious disease study under ABSL3 or ABSL4 housing such that their immune system and related parameters would return to a baseline level. To our knowledge, the current study is the first social-housing study of juvenile rhesus macaques that includes measures of cytokine function in addition to serum cortisol and lymphocyte subset measurements. In addition, this study is the first to examine short-term housing of juvenile rhesus macaques followed by separation.
Materials and Methods
Research was conducted under an IACUC-approved protocol, in compliance with the Animal Welfare Act, PHS Policy, and other Federal statutes and regulations relating to animals and experiments involving animals. The facility where this research was conducted is AAALAC-accredited and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals.20
Animals.
Juvenile rhesus macaques (Macaca mulatta; 12 male, 12 female; age, 3 to 4 y) from the colony at the United States Army Medical Research Institute of Infectious Diseases (Fort Detrick, MD) were selected for the study. These animals were from various sources and weighed between 3.6 and 4.0 kg. Each macaque was seronegative for macacine herpesvirus 1, simian retrovirus type D, SIV, and simian T-lymphotropic leukemia virus. They were tested twice annually for tuberculosis, and all were negative. Before the study, all of the macaques were housed individually in 4.5-ft2 (0.42-m2) cages with 4 cages per rack (Allentown Caging Equipment, Allentown, NJ), and environmental conditions were maintained as recommended in the Guide (temperature, 16 to 29 °C; humidity, 30% to 70%; and 12:12-h light:dark cycle). Macaques received a standard primate diet (no. 2050, Harlan Teklad, Madison, WI) supplemented with fruit and other food treats. Fresh water, provided ad libitum, was chlorinated at the municipal level and filtered (Edstrom Industries, Waterford, WI). Environmental enrichment (various manipulanda; Bio-Serv, Frenchtown, NJ) was provided in accordance with institutional standard operating procedures, and cages were arranged so that macaques were facing each other across the room. All animals were cared for by the same husbandry staff.
Study design.
We used 24 juvenile rhesus macaques that previously had been housed individually since they arrived at the institute 6 mo prior to the start of the study. From this population, 12 male macaques were combined into 6 pairs, and 12 female macaques were combined into 6 pairs. During the baseline period, one of the female macaques died unexpectedly due to acute heart failure that went undetected during the prestudy physical examination. The animal that was scheduled to be her pair-mate then was removed from the study, and thus only 5 female pairs completed the study, making a total of 22 NHP. The study included a baseline period and a social-housing manipulation period. During the baseline period (weeks 1 through 3), the 24 NHP designated for pairing were housed in one room, animals were individually housed and were considered to be controls. During the social-housing manipulation period, macaques were same-sex paired during weeks 4 through 10 in the same room as during the baseline period. At week 11, each pair was separated and moved to simulate the movement of animals into an ABSL3 or ABSL4 room. They remained separated until the end of the study (week 17). On separation at week 11, one NHP from each pair was moved to a different room and placed in a specific cage position so that the animal did not have direct visual contact, was not directly across from, or was not in the same cage position that it had during the pairing period. Separated macaques that were not moved into a different room were moved to a different cage position in the same room, again to ensure there was no direct visual contact with the same animals as during the paired period. No other animals were introduced into either room throughout the duration of the study. The macaques were maintained in the initial study room for approximately 8 wk before the start of the study. Throughout the entire 17-wk study, blood was collected weekly from each macaque for immunologic profiling, CBC analysis, and serum chemistry evaluation including serum cortisol.
Pairing process.
Six weeks prior to the start of the study, macaques were placed next to randomly selected partners of the same sex. At the start of the study, macaques were left in place for 3 wk for collection of baseline data. The first step of the pairing process was removing the solid middle stainless steel divider, leaving a double mesh divider and allowing partial contact. The NHP were monitored for a maximum of 20 min, until all pairs exhibited neutral or bidirectional affiliative behavior. After this achievement, the double mesh divider was removed, allowing full contact between paired macaques. Veterinary personnel remained in the room for approximately 5 min and then monitored the macaques remotely by using video cameras already installed in the room. After approximately 1 h of remote monitoring, neutral or bidirectional affiliative behavior was noted in all pairs. A pairing assessment form was used daily for 2 d to document successful pairing, which was defined as macaques exhibiting neutral or bidirectional affiliative behavior with minimal aggression and no required veterinary intervention. For 10 of the 11 pairs, there was a brief period of wrestling and minimally aggressive behavior while dominance was established. The last pair took longer to establish dominance, and the wrestling and aggressive behavior lasted for approximately 20 min with some vocalization from the submissive partner. However, no NHP required veterinary intervention, and 100% of attempted pairs were successful.
Blood collection.
On a weekly basis, each NHP was sedated with Telazol (dose, 3 mg/kg; 100 mg/mL, Fort Dodge Animal Health, Fort Dodge, IA) given intramuscularly in the caudal thigh by using a 1 mL tuberculin syringe with a 25-gauge needle. Adequate depth of anesthesia was achieved in 12 to 15 min. An additional half-dose of Telazol was used when an NHP required additional anesthesia. Telazol is a mixture of tiletamine and zolazepam, which is a benzodiazepine. Benzodiazepines can reduce the activity of the hypothalamo–pituitary–adrenal axis by inhibiting the release of corticotropin releasing hormone3 and possibly subsequently lowering measured serum cortisol. However, in the current study, all macaques received approximately the same dose of Telazol and thus any potential reductions in serum cortisol levels would be consistent among all animals. Once each macaque was sedated, approximately 7 mL blood was collected from a femoral vein by using a 20- to 22-gauge Vacuette needle and hub (Greiner Bio-One, Monroe, NC). The blood was collected into a single EDTA-containing and 2 serum-separator blood collection tubes. Whole blood was aliquoted into 2 equal amounts, which were used for the CBC and CD4:CD8 analyses done on the day of collection. The blood samples were collected at the same time in the morning and on the same day each week. In addition, macaques were sedated and blood was collected in the same order within the rooms each week.
Cytokine assays.
Proinflammatory cytokine assays were performed for each week throughout the 17 wk of the study. Assays were completed at the end of the study by using previously frozen serum. Serum cytokine levels were determined by using a sandwich immunoassay method as applied in a commercially available electrochemiluminesence detection kit (Human Proinflammatory 9-Plex Ultrasensitive Kit, Meso-Scale Discovery, Gaithersburg, MD) according to the manufacturer's instructions. The kit assayed granulocyte–macrophage colony-stimulating factor, IFNγ, IL10, IL12p70, IL1β, IL2, IL6, IL8, and TNFα.
Analyses of CD4+ and CD8+ T cells.
Flow cytometry of whole blood was used to determine the absolute numbers of CD4 and CD8 cells and the CD4:CD8 ratio. Staining cocktail was prepared at a 50-μL working volume containing 5 μL CD3 V450 (clone SP34-2, BD Biosciences, San Jose, CA), 5 μL CD8 eFluor 605NC (clone RPA-T8, eBioscience, San Jose, CA), 20 μL CD45 PERCP (clone D058-1283, BD Biosciences), and 20 μL of CD4 APC (clone OKT4, BD Biosciences). Staining cocktail was added to wells of a 1-mL 96-well plate for each sample to be analyzed. EDTA-anticoagulated whole blood (50 μL) from each macaque was added to wells containing staining cocktail, 1 macaque per well. Sample and staining cocktail were mixed briefly by pipette and allowed to incubate for 15 min at room temperature and protected from light. Samples then were fixed and RBC were lysed in a single 15-min, room-temperature step by adding 850 μL BD FACS Lysing solution (BD Biosciences) prepared at a 1× concentration with distilled water. The plate was centrifuged at 450 × g for 10 min to pellet cells, buffer was aspirated from the plate by using a 12-well vacuum manifold (VP Scientific, San Diego, CA), and cells were washed twice in 850 μL BD FACS Stain Buffer containing BSA (BD Biosciences). After washes, cells were resuspended in 300 μL BD FACS Stain Buffer containing BSA and transferred to tubes (FACS Tubes, BD Biosciences) for signal acquisition (LSRFortessa, BD Biosciences).
Serum cortisol.
Blood samples for serum cortisol analyses were collected in serum separator tubes and allowed to clot at room temperature for not more than 90 min before being centrifuged. All samples were assayed on the day of collection. Serum cortisol analyses were run inhouse (Vitros 5600, Ortho Clinical Diagnostics, Raritan, NJ).
Data analysis.
In general, statistical analyses included comparisons of overall weekly trends, average values of male and female macaques, and interactions. Baseline values were compared with predetermined week clusters after separation of paired NHP between weeks 11 and 17 to determine whether and when values returned to baseline.
SPSS version 16.0 (SPSS, Chicago, IL) was used to analyze data. Repeated-measures ANOVA was used to analyze data from weeks 1 through 3 and weeks 4 through 17. ANOVA was used to determine whether differences between sexes were present and whether overall trends or differences in trends were present between sexes throughout the 3 (baseline) or 14 (social-housing manipulation) weeks. To more accurately test for differences during the social-housing manipulation period, the 3 baseline values (weeks 1 through 3) for each NHP for each parameter were averaged and used as a covariate. The purpose of the covariate was to adjust future observations for each NHP. Using the covariate allowed us to account for natural variability and differences in measured parameters before the social-housing manipulation even began so that NHP with naturally high or low values would not skew the results. Within the ANOVA test, interactions between factors were tested to assess whether combinations of pairing or sex groups showed different trends through the weeks. Estimated marginal means, which are the unweighted averages adjusted for other variables in the model, were calculated through the ANOVA model. To determine whether differences were present, an F-statistic and corresponding P value for each factor and interaction were calculated during ANOVA. As a secondary test, to help identify when measured parameters returned to baseline, an independent 1-sample t test was used. Values for predetermined week clusters (weeks 11 and 12, 13 and 14, and 15 through 17) during the separation period were averaged and compared with the 3-wk baseline average. When the difference was positive, the baseline average was greater than the average for the separation week cluster; when the difference was negative, baseline was lower. P values from t tests were used to determine the similarity between the values for baseline and the separation period clusters: the closer a P value to 1.00, the more alike the values.
Only when the blood samples were analyzed for cytokines, a lower limit of detection was generated for each cytokine and used as a calibration point for all assays of that particular cytokine. Reported cytokine values below the lower limit of detection were considered to be inaccurate and unreliable; the square of the lower limit of detection was substituted for the purposes of data analysis. After the replacement of data points that were below the limit of detection, data were checked for normality; data for each cytokine were heavily skewed to the right. To alleviate this problem, the data were log10-transformed. Histograms before and after data transformation showed that the transformed data adequately satisfied the normality assumption. Therefore, the log10-transformed data were used for all analyses.
Results
For all measured parameters (CD4:CD8 ratio, serum cortisol, proinflammatory cytokine concentrations), average values, trends, and interactions were not significantly different between male and female macaques; these results are not addressed further.
Comparison of CD4:CD8 values.
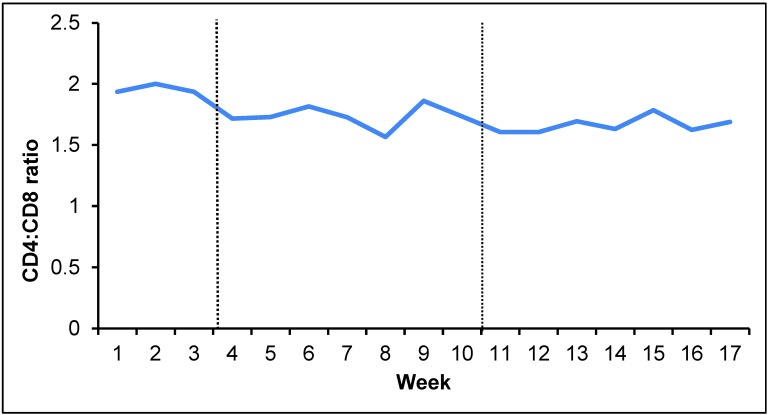
For the lymphocyte subset analysis, we assessed the CD4:CD8 ratio (Figure 1); weekly averages ranged from approximately 1.6 to 1.9 during the 17-wk study. During the 3-wk baseline period, CD4:CD8 averages did not differ significantly between weeks. During the 14-wk social-housing manipulation period, the weekly CD4:CD8 averages differed significantly (P < 0.001) from each other. Baseline CD4:CD8 averages were significantly (P ≤ 0.001) higher than those measured after separation. The CD4:CD8 average for weeks 15 to 17 was most similar to the baseline value (Table 1).
Figure 1.
CD4:CD8 values over 17-wk study period. Baseline period, weeks 1 through 3; pair housing, weeks 4 through 10; postseparation period, weeks 11 through 17.
Table 1.
CD4:CD8 ratio and serum cortisol (mg/dL) after separation of pair-housed juvenile macaques
| Weeks during separation period |
|||
| 11 and 12 | 13 and 14 | 15–17 | |
| CD4:CD8 | |||
| Changea | 0.276 | 0.245 | 0.160 |
| Pb | < 0.001 | < 0.001 | 0.001 |
| Serum cortisol | |||
| Change | 1.52 | 2.10 | 3.71 |
| P | 0.150 | 0.080 | 0.003 |
Relative to baseline value.
Compared with baseline value.
Comparison of serum cortisol values.
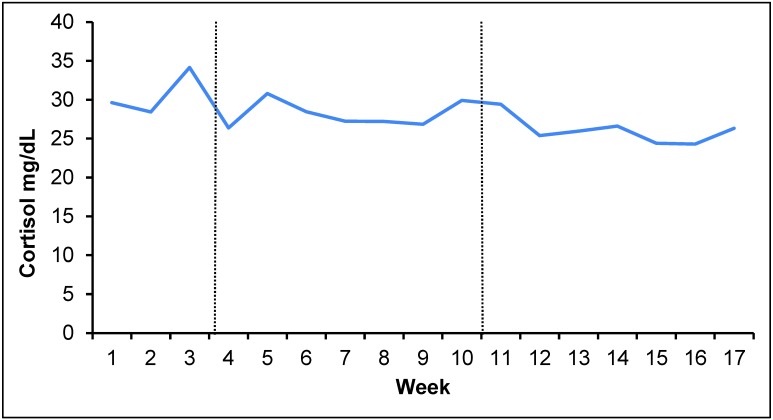
Serum cortisol was measured weekly, and averages ranged from approximately 22.8 to 38.4 mg/dL (Figure 2). During the 3-wk baseline period, average serum cortisol levels differed significantly (P = 0.024) across weeks but did not differ significantly between weeks during the 14-wk social-housing manipulation period. Baseline average serum cortisol was significantly greater than that for separation weeks 15 to 17. Serum cortisol during weeks 11 and 12 was most similar to that for the baseline period (Table 1).
Figure 2.
Serum cortisol values over 17-wk study period. Baseline period, weeks 1 through 3; pair housing, weeks 4 through 10; postseparation period, weeks 11 through 17.
Comparison of proinflammatory cytokines.
During the 3-wk baseline period, average proinflammatory cytokine values showed significant differences between weeks with P values ≤ 0.006 for all cytokines except IL10 (P value = 0.327). During the 14-wk social-housing period, values were also significantly different between weeks for all cytokines (P ≤ 0.001), except for IL8 (P = 0.062). Baseline average values were significantly (P ≤ 0.019 for all comparisons) lower than those measured after separation in weeks 11 and 12 for 7 of the 9 cytokines evaluated. By weeks 13 and 14, baseline values and those measured after separation did not differ for all 9 cytokines. By weeks 15 through 17, baseline values were significantly higher (P ≤ 0.001 for all comparisons) than values measured after separation for 8 of the 9 cytokines evaluated. Average values at weeks 13 through 14 were most comparable to baseline averages for all proinflammatory cytokines except IL1β, which was most similar to baseline at weeks 11 through 12. By week 15 through 17, all cytokine values significantly (P < 0.05) below baseline values (Table 2).
Table 2.
Proinflammatory cytokines (pg/mL) after separation of pair-housed juvenile rhesus macaques
| Weeks during separation period |
||||
| 11 and 12 | 13 and 14 | 15–17 | ||
| GM–CSF | Changea | –0.092 | 0.069 | 0.296 |
| Pb | 0.019 | 0.110 | 0.000 | |
| IL10 | Change | –0.047 | 0.075 | 0.298 |
| P | 0.360 | 0.383 | 0.000 | |
| IL12 p70 | Change | –0.247 | –0.020 | 0.264 |
| P | 0.000 | 0.745 | 0.000 | |
| IL1β | Change | –0.067 | 0.129 | 0.408 |
| P | 0.074 | 0.063 | 0.000 | |
| IL2 | Change | –0.241 | 0.009 | 0.392 |
| P | 0.000 | 0.881 | 0.000 | |
| IL6 | Change | –0.237 | –0.071 | 0.082 |
| P | 0.000 | 0.171 | 0.083 | |
| IL8 | Change | –0.230 | 0.026 | 0.411 |
| P | 0.000 | 0.642 | 0.000 | |
| IFNγ | Change | –0.177 | –0.006 | 0.293 |
| P | 0.000 | 0.891 | 0.000 | |
| TNFα | Change | –0.122 | –0.036 | 0. 231 |
| P | 0.009 | 0.409 | 0.000 | |
GM-CSF, granulocyte–macrophage colony-stimulating factor
Relative to baseline level.
Compared with baseline value.
Discussion
Short-term pair housing of juvenile rhesus macaques appears to provide an opportunity to comply with guidelines for social housing. This novel housing scheme can encourage beneficial species-specific behavior, lead to improvements in wellbeing, and allow NHP to meet their social needs without jeopardizing the health of animals destined for infectious disease studies and without negatively affecting immune parameters.
In the current study, CD4:CD8 baseline averages were higher than averages after separation. This decline in CD4:CD8 after separation was due to an increase in CD8 cells. These results did not support our hypothesis, because we predicted CD4:CD8 values would return to baseline or increase. This finding did not allow us to determine an appropriate time point to separate pair-housed NHP before assigning them to a study as the average CD4:CD8 values did not return to baseline but showed a steady decline. These results are in contrast to a previous study,26 in which CD4:CD8 rose in paired or group-housed NHP at the 4-mo time point, the first measurement after baseline. CD4:CD8 levels have not previously been measured after the separation of NHP that had experienced short-term pair housing, as we did in the current study. Perhaps juvenile macaques need to be pair-housed longer than 7 wk before an increase in CD4:CD8 values occurs.
There are no standard reference ranges for CD4:CD8 ratios in healthy, juvenile rhesus macaques. However, in 4 previous studies, 112 healthy rhesus macaques (age, 2 y and older) had CD4:CD8 values ranging from 0.68 to 1.52.5,21,26,32 These studies5,21,26,32 used flow cytometry to measure lymphocyte subsets, as we did. Over the course of our study, CD4:CD8 ranged from 1.585 to 1.932, thus exceeding all of the values in the 4 cited studies.5,21,26,32 What constitutes a ‘normal’ reference range for CD4:CD8 in humans is a matter of great discussion, given the occurrence of differences among various countries and age groups; the same is likely true for NHP. Given the average values, the decline in CD4:CD8 in our study does not seem to be biologically significant, and NHP that are placed on infectious disease studies after short-term pair housing are likely to mount a normal cell-mediated immune response when challenged.
Baseline serum cortisol values were most similar to those for weeks 11 through 12. Values for weeks 15 through 17 were significantly lower than the baseline average. The lower concentrations after separation indicate that short-term pair housing followed by separation does not adversely elevate serum cortisol levels and supports our hypothesis that values after separation do not indicate a cortisol response consistent with stress. The average values after short-term pair housing and separation were significantly lower than the baseline average, with no measurable difference between serum cortisol levels during the weeks just after pairing and just after separating. On the basis of these results, any real or perceived stress of pairing and separating must be quickly abrogated and does not result in a long-term or chronic increase in serum cortisol levels. As found in other studies9,10,14 any acute increase in serum cortisol levels as a result of social manipulation becomes statistically insignificant as early as 24 h later. Although high serum cortisol levels can affect an animal's ability to fight infection, our current findings did not reveal any clinically important elevations, and the process of short-term pair housing of juvenile rhesus macaques would not likely affect infectious disease studies. Consequently, macaques could be placed on a study as soon as 7 d after separation.
Baseline average values for 7 of the 9 proinflammatory cytokines evaluated were lower than the average values at weeks 11 and 12. However, by weeks 13 and 14, levels of all 9 cytokines were not statistically different from those during the baseline period. By weeks 15 through 17, 8 of the 9 proinflammatory cytokine average values were significantly lower than those during the baseline period. Thus, cytokine values after separation do not indicate the presence of an inflammatory state. Because our macaque pairs were separated at week 10, we infer that 3 to 4 wk is an adequate time frame to ensure that cytokine levels have returned to baseline. Cytokines have been measured (with cortisol) to examine their relationship to social rank.16 In addition, cytokine expression and alterations are measured frequently in infectious disease studies4,6,13,15,18,33 and are used regularly by researchers at our institute. Because there are no published reference ranges for proinflammatory cytokines in NHP, the biologic significance of our findings is unknown. For our purposes, however, investigating proinflammatory cytokine levels in the current study was useful because we can reassure our research scientists that cytokine alterations that may arise during their studies are truly due to the infectious disease process and not related to stress surrounding social housing and subsequent separation.
Throughout the 17-wk study, we collected blood each Thursday for serum cortisol, proinflammatory cytokine, and CD4:CD8 analysis. Macaques were paired on a Thursday after the weekly blood collection and blood was not collected blood again until the next Thursday. The pairs were separated in the same manner. It would have been judicious to collect blood 24 h after pairing and again 24 h after separation to capture any acute changes in the cortisol values that may have been abolished 7 d later.
In the current study, we found that the average values for all parameters (serum cortisol, CD4:CD8 ratio, and proinflammatory cytokines) for juvenile rhesus macaques that were pair-housed short-term were significantly lower after separation than during the baseline period. The data indicate that concerns about stress associated with short-term pairing and subsequent separation process in juvenile rhesus macaques may be unwarranted. Thus, we now have evidence that the process of short term pair-housing followed by separation is not associated with increased proinflammatory cytokine or cortisol levels and the decline seen in CD4:CD8 likely is not biologically significant. The changes we observed in serum cortisol and proinflammatory cytokine values indicate that the process of short-term pair housing of juvenile rhesus macaques would not likely adversely affect infectious disease studies, and the immune systems of juvenile rhesus macaques that are placed on a study 3 to 4 wk after separation would neither be stimulated nor depressed as a result of separation. The improvement in psychological wellbeing that may be afforded with short-term pair housing “would not be outweighed by the potential for a short-term decrement in wellbeing over the introduction (or separation) process”.12
We used juvenile rhesus macaques as our research subjects because they are used extensively at our institute and because they are easy to form into pairs for social housing. Older NHP and other species may exhibit more changes in physiologic parameters when they are short-term pair-housed and then separated. Further studies could include the use of other NHP species or age groups. Another future study could be to determine whether short-term pair housing has any long-term beneficial or detrimental effect on morbidity and mortality rates of NHP after infection.
Acknowledgments
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the US Army or the Department of Defense. We thank Dr Anna Honko and Dr Rebecca Erwin-Cohen for help in the design and conduct of the study. In addition, we thank Stacey Robinson for providing clinical laboratory support, the USAMRIID Veterinary Medicine NHP section led by SGT Steven Mraz for study support, and Dr Christine Ege for editorial review.
References
- 1. Animal Welfare Regulation. 2009. 9 CFR 3.81.
- 2.Baker KC, Crockett C, Lee G, Oettinger B, Schoof V, Thom J. 2012. Pair housing for female longtailed and rhesus macaques in the laboratory: behavior in protected contact versus full contact. J Appl Anim Welf Sci 15:126–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentson KL, Capitanio JP, Mendoza SP. 2003. Cortisol responses to immobilization with Telazol or ketamine in baboons (Papio cynocephalus/anubis) and rhesus macaques (Macaca mulatta). J Med Primatol 32:148–160 [DOI] [PubMed] [Google Scholar]
- 4.Bosio CM, Aman JM, Grogan C, Hogan R, Ruthel G, Negley D, Mohamadzadeh M, Bavari S, Schmaljohn A. 2003. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J Infect Dis 188:1630–1638 [DOI] [PubMed] [Google Scholar]
- 5.Brignolo L, Spinner A, Yee JL, Lerche NW. 2004. Subsets of T cells in healthy rhesus macaques (Macaca mulatta) infected with simian T-lymphotropic virus type I. Comp Med 54:271–274 [PubMed] [Google Scholar]
- 6.Burke RL, West M, Erwin-Cohen R, Selby E, Fisher D, Twenhafel N. 2010. Alterations in cytokines and effects on dexamethasone immunosuppression during subclinical infections of invasive Klebsiella pneumoniae with hypermucoviscosity phenotype in rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) macaques. Comp Med 60:62–70 [PMC free article] [PubMed] [Google Scholar]
- 7. Capitanio JP. 1998. Social experience and immune system measures in laboratory-housed macaques: implications for management and research. ILAR J 39: 12–20. [DOI] [PubMed]
- 8.Capitanio JP, Mendoza SP, Lerche NW. 1998. Individual differences in peripheral blood immunological and hormonal measures in adult rhesus macaques (Macaca mulatta): evidence for temporal and situational consistency. Am J Primatol 44:29–41 [DOI] [PubMed] [Google Scholar]
- 9.Clarke MR, Harrison RM, Didier ES. 1996. Behavioral, immunological, and hormonal responses associated with social change in rhesus monkeys (Macaca mulatta). Am J Primatol 39:223–233 [DOI] [PubMed] [Google Scholar]
- 10.Czoty PW, Gould RW, Nader MA. 2009. Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fascicularis). J Neuroendocrinol 21:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiVincenti L, Jr, Wyatt JD. 2011. Pair housing of macaques in research facilities: a science-based review of benefits and risks. J Am Assoc Lab Anim Sci 50:856–863 [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle LA, Baker KC, Cox LD. 2008. Physiological and behavioral effects of social introduction on adult male rhesus macaques. Am J Primatol 70:542–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giavedoni L, Velasquillo C, Parodi L, Hubbard G, Hodara V. 2000. Cytokine expression, natural killer cell activation, and phenotypic changes in lymphoid cells from rhesus macaques during acute infections with pathogenic simian immunodeficiency virus. J Virol 74:1648–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gust DA, Gordon T, Wilson ME, Ahmed-Ansari A, Brodie AR, McClure HM. 1991. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain Behav Immun 5:296–307 [DOI] [PubMed] [Google Scholar]
- 15.Hensley LE, Young HA, Jahrling PB, Geisbert TW. 2002. Proinflammatory response during Ebola virus infection of primate models: possible involvement of the tumor necrosis factor receptor superfamily. Immunol Lett 80:169–179 [DOI] [PubMed] [Google Scholar]
- 16.Hoffman CL, Higham J, Heistermann M, Coe C, Prendergast B, Maestripieri D. 2011. Immune Function and HPA axis activity in free-ranging rhesus macaques. Physiol Behav 104:507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotchkiss CE, Paule M. 2003. Effect of pair-housing on operant behavior task performance by rhesus monkeys. Contemp Top Lab Anim Sci 42:38–41 [PubMed] [Google Scholar]
- 18.Hutchinson KL, Villinger F, Miranda ME, Ksiazek T, Peters C, Rollin P. 2001. Multiplex analysis of cytokines in the blood of cynomolgus macaques naturally infected with Ebola virus (Reston serotype). J Med Virol 65:561–566 [PubMed] [Google Scholar]
- 19.Institute for Laboratory Animal Research. 1998. The psychological well-being of nonhuman primates: a report of the Committee on Well-Being of Nonhuman Primates. Washington (DC): National Academies Press. [Google Scholar]
- 20.Institute for Laboratory Animal Research. 2011. The guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 21.Lilly AA, Mehlman PT, Higley JD. 1999. Trait-like immunological and hematological measures in female rhesus across varied environmental conditions. Am J Primatol 48:197–223 [DOI] [PubMed] [Google Scholar]
- 22.Reinhardt V. 2008. Taking better care of monkeys and apes: refinement of housing and handling practices for caged nonhuman primates. Washington (DC): Animal Welfare Institute. [Google Scholar]
- 23.Reinhardt V, Reinhardt A. 2008. Environmental enrichment and refinement for nonhuman primates kept in research laboratories: a photographic documentation and literature review. Washington (DC): Animal Welfare Institute. [Google Scholar]
- 24.Schapiro SJ. 2002. Effects of social manipulations and environmental enrichment on behavior and cell-mediated immune responses in rhesus macaques. Pharmacol Biochem Behav 73:271–278 [DOI] [PubMed] [Google Scholar]
- 25.Schapiro SJ, Bloomsmith M. 1994. Behavioral effects of enrichment on pair-housed juvenile rhesus monkeys. Am J Primatol 32:159–170 [DOI] [PubMed] [Google Scholar]
- 26.Schapiro SJ, Neheste PN, Perlman JE, Sastry KJ. 2000. A comparison of cell-mediated immune responses in rhesus macaques housed singly, in pairs, or in groups. Appl Anim Behav Sci 68:67–84 [DOI] [PubMed] [Google Scholar]
- 27.Taylor W, Brown DA, Richardson RL, Laudenslager ML. 1998. The effect of duration of individual housing on social behavior of adult male bonnet macaques (Macaca radiata). Contemp Top Lab Anim Sci 37:47–50 [PubMed] [Google Scholar]
- 28.Tizard IR.2000. Veterinary immunology: an introduction, p 89. Philadelphia (PA): WB Saunders.
- 29.Turner PV, Grantham L. 2002. Short-term effects of an environmental enrichment program for adult cynomolgus monkeys. Contemp Top Lab Anim Sci 41:13–17 [PubMed] [Google Scholar]
- 30.United States Department of Agriculture. 1999. Final report on environmental enhancement to promote the psychological well-being of nonhuman primates. Beltsville (MD): Animal Welfare Information Center. [Google Scholar]
- 31.Winterborn AN, Bates W, Feng C, Wyatt J. 2008. The efficacy of orally dosed ketamine and ketamine–medetomidine compared with intramuscular ketamine in rhesus macaques (Macaca mulatta) and the effects of dosing route on haematological stress markers. J Med Primatol 37:116–127 [DOI] [PubMed] [Google Scholar]
- 32.Woodward RA, Weld KP. 1997. A comparison of ketamine, ketamine–acepromazine, and tiletamine–zolazepam on various hematologic parameters in rhesus monkeys (Macaca mulatta). Contemp Top Lab Anim Sci 36:55–57 [PubMed] [Google Scholar]
- 33.Yang C, Xiao L, Tongren J, Sullivan J, Lal A, Collins W. 1999. Cytokine production in rhesus monkeys infected with Plasmodium coatneyi. Am J Trop Med Hyg 61:226–229 [DOI] [PubMed] [Google Scholar]