Abstract
Dry bedding has been shown to be an effective enrichment strategy for small groups of captive nonhuman primates housed in cages or in small enclosures with concrete flooring. However, dry bedding is used infrequently for large groups because of the perception that its use is time- and resource-intensive. We investigated the cost-effectiveness of this enrichment strategy in large groups (30 to 50 subjects) of rhesus macaques. Macaques were housed under 3 comparison conditions for 4 wk: pine shavings (n = 4), aspen and pine shaving mixture (n = 4), and nonbedded control (n = 4). As measures of resource consumption, husbandry tasks were documented by using time-in-motion methodology, and water usage was determined. In addition, groups underwent behavioral observations to assess the effect of dry bedding. The time required to care for units did not differ between bedded and nonbedded units. However, significantly less water was used for sanitization of bedded compared with nonbedded units. Monkeys housed in bedded units showed more foraging (13.8% ± 1.6% of time in bedded compared with 4.0% ± 0.3% of time in nonbedded units) and less aggression and self-grooming. Dry bedding benefited the macaques, reduced water usage and costs, and did not affect human resources.
Abbreviation: NHP, nonhuman primate; SH, shelter housing
In 1998 the National Research Council codified the requirement for providing for the psychologic wellbeing of nonhuman primates used in biomedical research.18 This necessity was strengthened by the performance standards outlined in the eighth edition of the Guide for the Care and Use of Laboratory Animals.19 One goal of enrichment programs is to improve and refine practices that promote behavioral wellness and increase the opportunities for animals to express natural behaviors.19 The environments of captive primates often do not have the types of challenges that primates would find in the wild. Therefore, providing opportunities for animals to express species-specific behaviors in captivity is an important tool to improve their quality of life.
Foraging is a behavior that is greatly reduced in captivity. In the wild, nonhuman primates (NHP) spend as much as 70% of their day foraging.15,28,29 In contrast, in captivity, meals are provided at specific times and locations, and animals often spend relatively little time foraging. This lack of foraging opportunities has been posited as a potential cause of overgrooming behavior.5 An enrichment strategy that can help to promote foraging is the use of dry bedding as a substrate. This strategy is particularly important for animals living in cages or in enclosures with a concrete floor. Previous studies in many NHP species have demonstrated the positive effects of providing bedding, including decreased aggression and increased foraging,2,7-10,24 decreased overgrooming,4 decreased labor, and decreased water and chemical usage.18 Still, facilities often encounter obstacles when trying to implement dry bedding. For example, despite the evidence that dry bedding is beneficial to captive primates, its use is perceived as time-intensive and costly; therefore dry bedding is used only infrequently.3 Little information is available on the operational costs of a bedded substrate. One of few studies on this topic examined the use of a bedding substrate in indoor–outdoor group-housed bonnet macaques and found the bedding to be a cost-effective enrichment strategy.6 Although information on the benefits of dry bedding for NHP in cages or in small (that is, fewer than 10 animals) groups is available, few (if any) studies examine dry bedding use for large, outdoor-housed groups. This paucity of information occurred because many NHP facilities in the United State and elsewhere house large groups of macaques in outdoor enclosures with grass or gravel substrate. However, several environmental constraints may direct facilities to house on artificial substrates. Solid flooring provides an opportunity for radiant heating in colder climates. Solid flooring also provides a structure for the controlled disposal of waste through drain-to-sewer systems, thereby decreasing run-off and ground water contamination.
Our facility houses approximately 4500 macaques in various types of facilities and social configurations. The 2800 animals of the SPF Indian-origin rhesus macaque breeding colony are maintained in open-top 1-acre corrals and in smaller shelter-housing (SH) units, which are the focus of the current study. Management of breeding colony animals provides opportunity to manipulate the environment to promote enrichment and socialization for the benefit of the NHP health and productivity. The enrichment program for the SH macaques includes social housing; various play structures; seasonal water enrichment (ice blocks, misters, and pools); forage devices that contain fruits, vegetables, and seeds on a rotating schedule; and interaction with caretakers. Prior to the start of the current study, dry bedding was used sparingly as an intervention strategy for groups with social unrest. During times of social stress (that is, group formation, removal or reintroduction of key animals, social instability of unknown etiology), dry bedding was distributed as a distraction to reduce fighting, injuries, and removal of animals. Historic resistance to widespread implementation rested on personnel concerns of increased work load, decreased cleanliness, increased facility-related complications, and a general perception that dry bedding may cause more harm than good (contaminated wounds, eating of wood chips, and so forth). The current study was designed to address these concerns.
Animal facilities frequently are designed for economic and ergonomic simplicity.26 The SH units at our facility have concrete surfaces that are easily cleaned with high-pressure water. The daily wash-down of 32 SH units requires substantial water resources that is inconsistent with our facility's philosophy of environmental sustainability. Animal welfare and reduced water usage were strong motivators at the management level to evaluate dry bedding in the SH area. Other authors6 correctly identified the need to involve all stakeholders in the evaluation of costs and benefits of dry bedding to ensure its adoption. Without acceptance by the various teams encountered at the facility, change would be challenged. As is appropriate in assessments of all novel enrichment strategies, addressing the concerns of the participants was an explicit goal of the current study.6,23
Here we examined the use of 2 kinds of wood shavings (pine and pine–aspen mix) in large (30 to 60 animals) outdoor groups of rhesus macaques over a 1-mo period. We focused our study on behavior and operational aspects of management (water use, time to maintain, and so forth). We hypothesized that the presence of wood shavings would increase normal behaviors, decrease abnormal behaviors, and decrease operational costs, including labor.
Materials and Methods
Humane care guidelines.
The study was conducted in compliance with all federal regulations, including the IACUC of the Oregon National Primate Research Center, and the Guide for the Care and Use of Laboratory Animals.19 The Oregon National Primate Research Center is AAALAC-accredited.
Subjects.
Evaluation of the bedding substrates was conducted in the SH complex of our facility. This complex holds approximately two thirds (approximately 1200 animals) of the facility's SPF Indian-origin rhesus macaque (Macaca mulatta) breeding colony, ranging in age from infants to adults. SPF is defined as negative viral status for SIV, simian T-cell lymphotrophic virus, simian retrovirus, and Macacine herpesvirus 1. The SPF breeding colony is surveilled annually via serologic assay and is maintained in housing areas separate from those for conventional, nonSPF NHP. Procedures and practices are in place to ensure biosecurity of the SPF breeding population, including designated staff, support areas, and personal protection equipment. Macaques evaluated in the current study were housed in 12 social groups each consisting of 40 to 60 animals. Groups were matched as closely as possible for density and demographic distribution (for example, age of the group, male to female ratio). Prior to the start of the study, 2 adult female macaques (4 to 10 y of age) were selected randomly from each of the 12 social groups (n = 24) as subjects for behavioral observations. Female macaques selected were age-matched to the best of our ability. Because social rank can affect behaviors in which we were interested, including grooming and aggression, we also matched female macaques for their dominance status.
Shelter housing units.
Each unit was 1400 ft2 and was divided into 3 sections that were accessed via 2 guillotine doors to allow animal movement between the rooms. Each unit had 2 exterior bays enclosed in welded-wire mesh and a single center bay consisting of 3 concrete masonry walls and a roll-down door. Two units formed a complex that was 20 ft high, with gabled roofs of clear corrugated polycarbonate. Each section contained a play structure or swings (or both) as well as other toys and enrichment devices. The units were outdoors and partially environmentally controlled, with overhead heating and heated floors for the winter months and water misters for summer months. Each section contained a feeding bin and lixits for water. Macaques were fed high-protein lab diet (Purina 5000 diet, Animal Specialties, Woodburn, OR) ad libitum twice daily and received produce or other enrichment (for example, grains) daily. Water was available ad libitum. All units received the same enrichment on any given day.
Bedding.
Prior to the start of the current study, we assessed several types of bedding, including pine shavings, aspen shavings, wood pellets, corncob bedding, and reclaimed pulp waste. According to factors such as appearance, absorbance, cost, and ease of use, we chose pine shavings and a mixture of pine and aspen shavings as the 2 bedding types for further study. Bedding was placed into the SH of 8 of the 12 groups. Half of the bedded groups (n = 4) received pine shavings (GEM Shavings, Auburn, WA), and the other bedded groups (n = 4) received a combination of pine and aspen shavings (NEPCO, Caspian, MI); the remaining 4 groups served as nonbedded controls. Bedding covered the entire floor of the SH units and was used for 4 wk. Bedding was replaced every 2 wk and ‘spot-cleaned’ once weekly. Replacement of bedding consisted of shoveling out the shavings, washing the enclosures with high-pressure water, and restocking the enclosures with clean shavings. Enclosures for the nonbedded (control) groups were washed daily with high-pressure water according to the standard husbandry practices for the facility. The drains in all bedded units were capped to prevent bedding from entering the drainage system.
Behavioral observations.
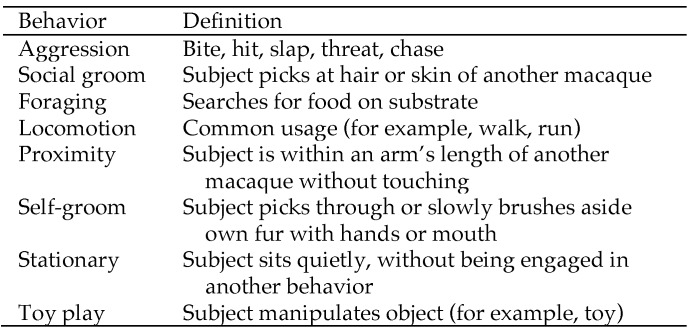
To assess behavior of the macaques, 10-min focal observations of the 24 subjects 2 or 3 times each week. A single trained observer used instantaneous focal sampling techniques,1 in which the behavior of the subject was recorded at 20-s intervals for 10 min. Behaviors scored included foraging, self-grooming, and play (Figure 1 for ethogram). Because aggression has a relatively short duration, all occurrence sampling was used to record this behavior (that is, the observer recorded any instance of aggression that occurred during the 10-min focal observation). Prior to taking observations in any given group, the observer stood in front of the SH unit for 5 min to acclimate the monkeys to his presence. Observations were taken on all 12 SH units for 2 wk before bedding was added (baseline phase) and throughout the 4 wk during which bedding was present (bedded phase). Observations were taken during 2 time periods, morning (0900 to 1200) and afternoon (1300 to 1600) and were balanced so that each macaque was observed the same number of times for each time period. Enrichment was provided at random times, depending on the schedule of the animal care technicians; therefore we were unable to balance observations for the presence or absence of food. Observation periods were scheduled to not coincide with morning or afternoon feeding and took place at least 30 min before or after feeding activities.
Figure 1.
Behavioral ethogram.
Time in motion.
To determine the human resource investment in the various husbandry tasks, animal care staff recorded the amount of time it took to complete each task for all 12 SH units during the 4 wk of the study. Technicians documented the time associated with the following tasks: filling out daily task sheets, morning and evening feeding including enrichment and produce distribution, performing health observations, washing the shelters, checking lixit function, picking through bedding, changing bedding, and cleaning the outside of the enclosure and walkways.
Cost calculations.
The costs associated with water usage and garbage disposal were determined. Water usage was determined by measuring the flow of water (gallons per minute) and the time used to wash the individual shelters. The average water use over 2-wk time periods was compared between shelters with and without bedding. There were additional costs associated with disposing of the used bedding. The cost for additional dumpsters on site was divided by the volume of used bedding per shelter to find the approximate cost of bedding disposal per shelter.
Data analysis.
To determine the time investment associated with cleaning bedded and nonbedded SH units, we summed all variables involved in cleaning (wash, remove and add bedding, pick through bedding, and clean outside of the pen) for each 2-wk period, which corresponded to a single complete cycle of bedding use. This time was averaged for each unit, and the averages compared across the 3 treatments (pine, pine–aspen mix, control). In addition, water use over a 2-wk period was calculated for each unit and then compared across the treatment groups. For behavioral data, we calculated the average time each of the 24 focal subjects spent in various behaviors before (baseline) and after (bedded) the shavings were added to the SH units. Assumptions of normality were tested for all variables. Nonbehavioral data were analyzed by using one-way ANOVA (or Kruskall–Wallis when data did not meet the assumption of normality even after transformation) with treatment (for example, pine, pine–aspen mix, or control) as the grouping variable. Kruskall–Wallis is a nonparametric test that does not make any assumptions about distributions; no power analyses were performed because none of those available are appropriate for this test type.27 Behavioral data were analyzed by using 2-way mixed ANOVA, with before and after bedding as the within-subject variable and treatment as the between-subject variable. An exception was made for foraging data; because so few animals forage when bedding is not present, we categorized the groups as ‘showed foraging behavior’ or ‘did not show foraging behavior’ and looked for differences across treatment by using χ2 analysis. Data are presented as mean ± SEM; α values were set at 0.05. SyStat 11 (Systat Software, San Jose, CA) was used for all analyses.
Results
The addition of dry bedding to SH units had no perceptible negative effects on the macaques, personnel, or facility. Clinical concerns regarding the bedding (increased wound contamination, impaired ability to perform health observations, excessive eating of bedding) did not occur. Environmental temperature regulation was not affected by the bedding, although the application of water (mist, swimming pools, ice blocks) as an enrichment modality was excluded during the study in all groups to avoid excessive soiling. Despite the perception of increased physical effort for the staff, no occupational health concerns (repetitive motion injury, dust irritation) were reported during the study period.
Behavioral observations.
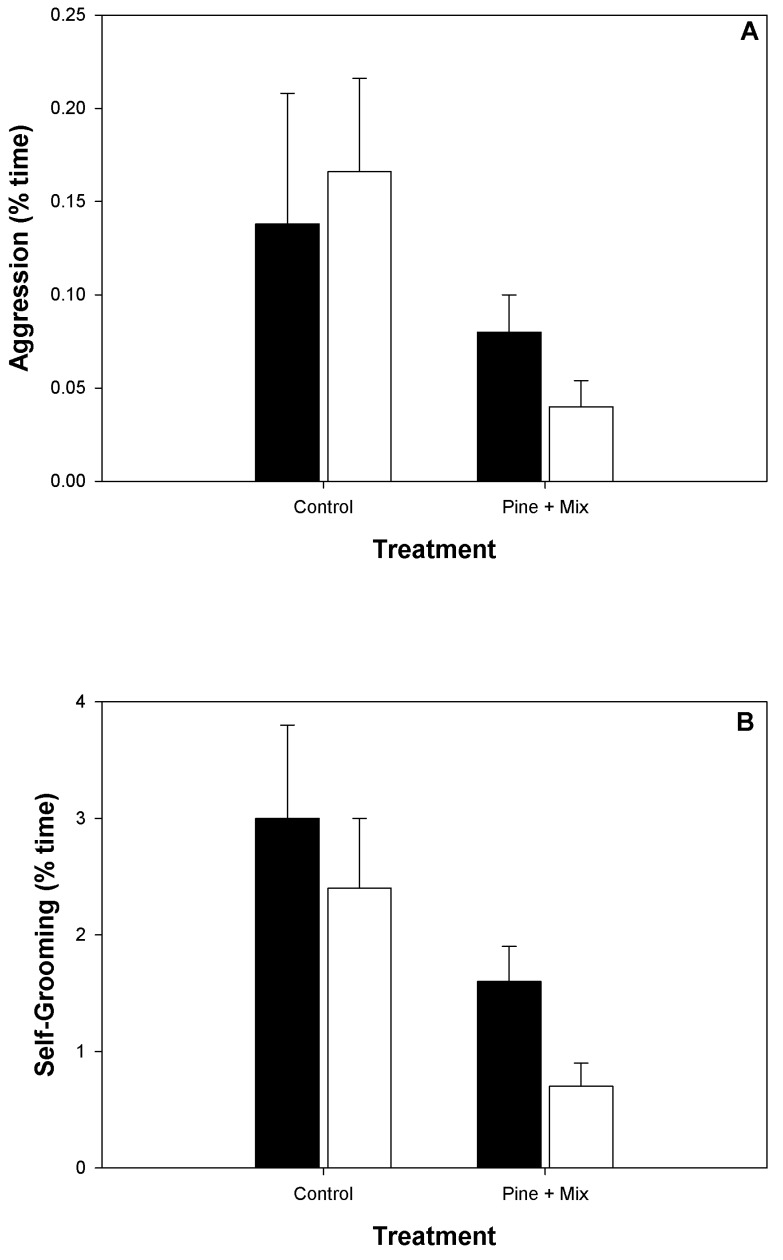
There were no differences in behavior between female macaques in the SH units bedded with pine or the pine–aspen mix (data not shown). Therefore we combined the 2 groups for further analyses. Animals in bedded units showed less aggression and self-grooming compared with those in control units (aggression: F1,22 = 10.10, P = 0.004, Figure 2 A; self-grooming: F1,22 = 9.75, P = 0.005, Figure 2 B). There were no differences in other behaviors including social grooming (F1, 22 = 2.57, P = 0.12) and time spent in social behavior (proximity, touch, and so forth; F1,22 = 0.044, P = 0.52).
Figure 2.
(A) Frequency of aggression (threats, bites, and so forth) for animals in control and bedded units during the baseline (black) and treatment (white) portions of the study. Subjects in bedded units showed less (F1,22 = 10.1, P = 0.004) aggression than did those in control units. (B) Percentage of time macaques in control and bedded units engaged in self-grooming behavior during the baseline (black) and treatment (white) portions of the study. Subjects in the bedded units spent less (F1,22 = 9.7, P = 0.005) time self-grooming than did those in the control units.
Macaques in bedded SH (n = 16) were more likely to forage than were subjects in control units (that is, nonbedded SH, n = 8; χ2 = 4.36, df = 1, P = 0.037). Only 2 of the 8 macaques in the control units showed any foraging behavior (overall average for 8 animals = 4.04% ± 0.31% of time spent in foraging behavior). In contrast, all 16 female macaques in bedded SH spent some time foraging (average time, 13.83% ± 1.60%). Macaques in the bedded groups spent a small amount of time eating the bedding (1.39% ± 0.19%), but this behavior did not result in any clinical problems. Animals that ate shavings tended to do so right after the bedding was placed in the SH units.
Time in motion.
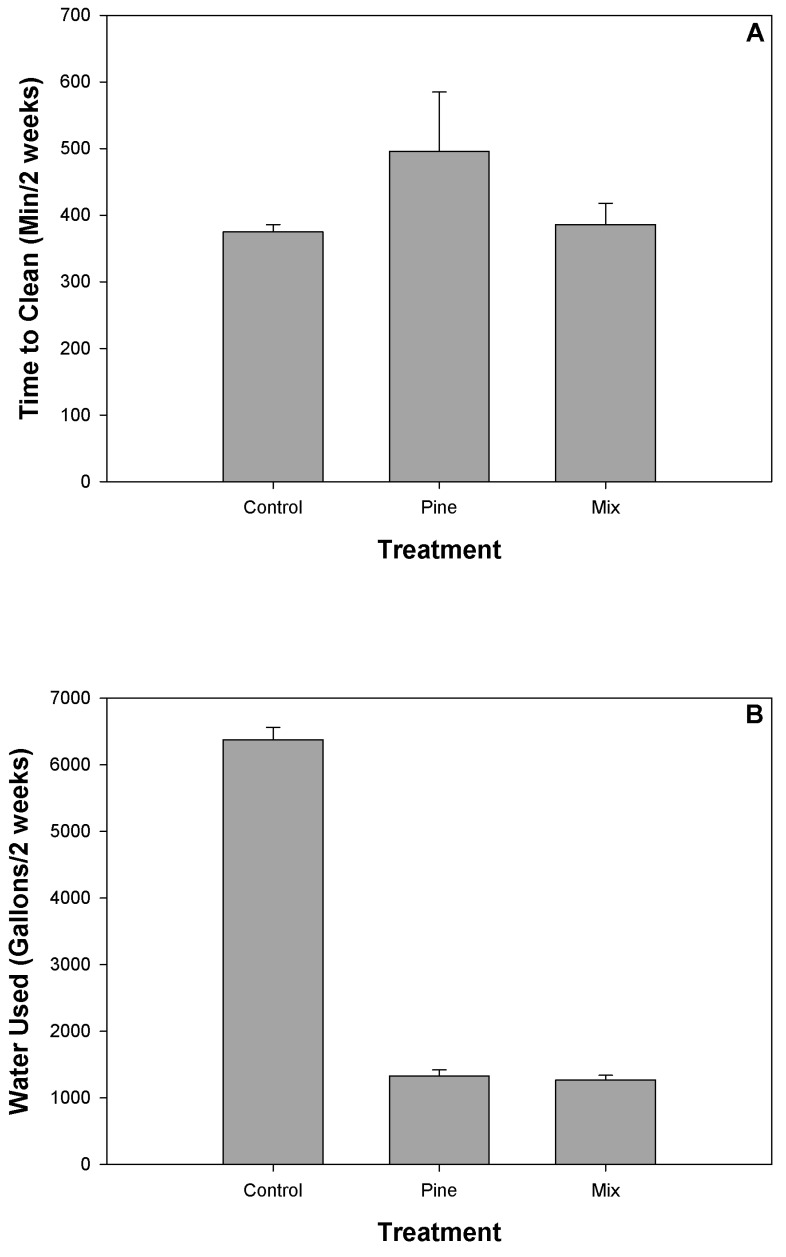
Data for the time-in-motion analysis did not meet the assumptions of normality, even after transformation; we therefore used nonparametric analyses. Husbandry time needed to maintain units did not differ between SH with or without bedding over a 2-wk period (Kruskall–Wallis H= 0.73, P = 0.69, Figure 3 A). Control units required 375 ± 11 min to service, whereas units with mixed shavings took 386 ± 32 min and SH with pine shavings needed 496 ± 89 min.
Figure 3.
(A) Amount of personnel time (min) needed to clean (wash, change bedding, spot-clean bedding, clean outside units) control units and those with either pine or pine–aspen mix shavings over a 2-wk period. There was no significant difference in technician time (H = 0.73, P = 0.69) among bedding types. (B) The amount of water used (gallons per 2-wk period) in sanitization procedures for the nonbedded control units and units bedded with pine or pine–aspen mix shavings. Units with shavings used significantly (Kruskall–Wallis H = 7.1, P = 0.03) less water than did nonbedded control units.
Operational costs.
Units with shavings used significantly (Kruskall–Wallis H = 7.1, P = 0.03) less water over a 2-wk period than did nonbedded control units. Control units used 6375 ± 187 gallons whereas units bedded with mixed shavings used 1269 ± 69 gal and pine shavings used 1328 ± 91 gallons (Figure 3 B). Again, the difference in water usage between mixed and pine shavings was not significant. The cost of water to wash down a single nonbedded shelter every day for 2 wk was $44.75 (852 ft3 of water used)—this figure includes both the cost of the water from the local water district and the sewage fees. The cost of water to wash down after 2 wk of bedding with pine shavings was $9.32 (178 ft3) and $8.17 (156 ft3) with mixed shavings. These data demonstrate an 80% reduction in water use for bedded shelters compared with nonbedded shelters.
There were additional costs associated with the disposal of the used bedding. The cost to dispose of the bedding removed by spot-cleaning after the first week was $6.14 per SH. The cost to dispose of the used bedding every 2 wk was $24.56 per SH. The total cost for water and waste removal for 2 wk of the pine-shavings treatment was $40.02 (11% reduction compared with those for controls). The total cost associated with the use of the mixed-shavings treatment was $38.87 (13% reduction).
Discussion
Our 4-wk study revealed that the use of a bedding substrate both increased normal behavior (foraging) and decreased undesired behaviors (aggression and self-grooming), and is therefore an important tool to improve wellbeing for captive NHP. This outcome supports our first and second hypotheses: that dry bedding increases normal behavior and decreases abnormal or undesired behavior. Not surprisingly, the presence of bedding promoted foraging behavior. All macaques were given the same food and enrichment items, but the animals in the control group tended to consume these foods rather quickly and therefore showed little foraging behavior during the observation periods. In contrast, the bedded groups continued to forage for food long after it was provided. Social aggression in breeding groups incurs considerable costs. Approximately one third of the clinical cases at our facility are due to conspecific trauma. Reducing fighting injuries improves welfare, increases the long-term stability of the breeding group, and thus increases productivity.5,11-14,16-17,20-22,25,30 Other behaviors, such as social grooming and eating, did not differ with the presence of substrate. More work is needed to determine whether bedding would affect behavior over the long-term. For example, will the effect diminish with time of exposure as the monkeys habituate to the stimulus, or will the beneficial effect be sustained? Further, although we noted a decrease in self-grooming, we do not know whether that outcome resulted in a decrease in alopecia; we were unable to assess alopecia due to the short duration of our study. Furthermore, we were unable to determine whether the bedding affected seasonal differences in behavior. We ended our study right before the start of the breeding season, a time known for increased aggression.31 These questions are being addressed in a long-term study.
No clinical concerns (for example, contamination of wounds) were raised in the units that contained bedding. Some monkeys did eat the bedding, but none required clinical intervention as a result. We did not evaluate the incidence of conspecific trauma, but a next logical next step would be to determine whether less aggressive behavior translates into fewer traumatic injuries and less need for clinical and behavioral intervention.
Unlike others,6 we did not see a decrease in personnel time necessary to maintain the SH in this study. This result may reflect our small sample size, which can lead to the lack of sufficient statistical power to reveal differences among groups. The amount of time necessary to clean the bedded SH varied much more widely than did that for the nonbedded groups. The SEM for the control groups was only 11 min, whereas the SEM for the mixed-shavings group was 32 min and for the pine-shaving group was 89 min. This variation is likely due, at least in part, to the fact that although we tried to control for demographic variables such as density and juvenile-to-adult ratios across treatment groups, we were not unable to control all factors completely. Density in particular seemed to have a greater effect on the time needed to clean bedded SH compared with nonbedded SH. The presence of urine made it difficult to shovel out the shavings but had no effect on daily cleaning. The amount of urine in the bedding was presumably related to the number of macaques. One of the pine-bedded groups comprised more than 50 macaques and that unit was the one that took the longest to clean. Unfortunately, our small sample size (4 groups per treatment) did not allow us to examine the effect of treatment on density. More work with larger sample sizes and longer study durations is needed to determine whether the use of shavings differentially affects groups with high or low densities.
In a modification from the current study, personnel currently are using a large vacuum to remove the bedding. It now takes a single caregiver about 1 h to remove and dispose of the bedding, whereas it took 2 or 3 people about 90 min to remove the wood shavings without the vacuum. The finding that dry bedding had a negligible effect on human resources was exceedingly important psychologically for the husbandry staff. The acceptance and adoption of dry bedding hinged on this result in particular, and therefore this finding is crucial to the change initiative.
The use of bedding decreased water use, as found previously.6 In fiscal year 2011, our facility spent approximately $335,000 on water-related costs, including sewage fees, permits, and monthly payments toward service development charges. Switching to consistent and regular use of dry bedding in 12 SH provides direct annual water savings of about $11,000. If dry bedding were implemented in all 32 SH units, savings approach $30,000. This outcome supports our third hypothesis: that dry bedding decreases operational expenses although labor is unchanged. If the use of dry bedding becomes a consistent regular part of our operations, alternate methods of disposal that may be more cost effective and provide additional benefits can be explored. For example, the soiled bedding might be used as an alternative fuel source or for composting.
The current study indicates that for our facility, the benefits of wood-shaving bedding substrate include financial savings and water conservation. In addition, the labor associated with maintaining bedded units is equivalent to that of nonbedded units and, with the addition of the vacuum system, may even be less. Overall, dry bedding proved to be a cost-effective enrichment strategy for group-housed NHP and added value to multiple systems.
Acknowledgments
We thank Rick Doughty and Bill Morris for providing facility-management–related expertise and support. We also thank Kirsten Olsen and Adriane Maier as well as the Division of Animal Resources staff for their help with husbandry and operational aspects of the project. This work was supported by NIH grant 8P51OD011092-53.
References
- 1.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49:227–267 [DOI] [PubMed] [Google Scholar]
- 2.Anderson JR, Chamove A. 1984. Allowing captive primates to forage, p 253–256. Standards in laboratory animal management. Potters Bar (UK): Universities Federation for Animal Welfare. [Google Scholar]
- 3.Baker KC, Weed JL, Crockett CM, Bloomsmith MA. 2007. Survey of environmental enhancement programs for laboratory primates. Am J Primatol 69:377–394 [DOI] [PubMed] [Google Scholar]
- 4.Bayne K, Dexter S, Mainzer H, McCully C, Campbell G, Yamada F. 1992. The use of artificial turf as foraging substrate for individually housed rhesus monkeys (Macaca mulatta). Anim Welf 1:39–53 [Google Scholar]
- 5.Beisner BA, Isbell LA. 2011. Factors affecting aggression among females in captive groups of rhesus macaques (Macaca mulatta). Am J Primatol 73:1152–1159 [DOI] [PubMed] [Google Scholar]
- 6.Bennett AJ, Corcoran CA, Hardy VA, Miller LR, Pierre PJ. 2010. Multidimensional cost–benefit analysis to guide evidence-based environmental enrichment: providing bedding and foraging substrate to pen-housed monkeys. J Am Assoc Lab Anim Sci 49:571–577 [PMC free article] [PubMed] [Google Scholar]
- 7.Blois-Heulin C, Jubin R. 2004. Influence of the presence of seeds and litter on the behaviour of captive red-capped mangabeys Cerocebus torquatus torquatus. Appl Anim Behav Sci 85:349–362 [Google Scholar]
- 8.Boccia M, Hijazi A. 1998. A foraging task reduces agonistic and stereotypic behaviors in pigtail macaques. Lab Primate Newletter 37:1–5 [Google Scholar]
- 9.Brent L. 1992. Woodchip bedding as enrichment for captive chimpanzees in an outdoor enclosure. Anim Welf 1:161–170 [Google Scholar]
- 10.Chamove A, Anderson JR, Morgan-Jones S, Jones S. 1982. Deep woodchip litter: hygeine, feeding, and behavioral enhancement in 8 primate species. Int J Study Anim Probl 3:308–318 [Google Scholar]
- 11.De Waal F. 1989. The myth of a simple relation between space and aggression in captive primates. Zoo Biol 8:S141–S148 [Google Scholar]
- 12.Eaton G, Modahl KB, Johnson DF. 1981. Aggressive behavior in a confined troop of Japanese macaques: effects of density, season, and gender. Aggress Behav 7:145–164 [Google Scholar]
- 13.Erwin J, Sackett G. 1990. Effects of management methods, social organization, and physical space on primate behavior and health. Am J Primatol 20:23–30 [DOI] [PubMed] [Google Scholar]
- 14.Erwin N, Erwin J. 1976. Social density and aggression in captive groups of pigtail monkeys (Macaca nemestrina). Appl Anim Ethol 2:265–269 [Google Scholar]
- 15.Goldstein S, Richard A. 1989. Ecology of rhesus macaques (Macaca mulatta) in northwest Pakistan. Int J Primatol 10:531–567 [Google Scholar]
- 16.Ha JC, Alloway H, Sussman A. 2011. Aggression in pigtailed macaque (Macaca nemestrina) breeding groups affects pregnancy outcome. Am J Primatol 73:1169–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ha JC, Robinette RL, Sackett GP. 1999. Social housing and pregnancy outcome in captive pigtailed macaques. Am J Primatol 47:153–163 [DOI] [PubMed] [Google Scholar]
- 18.Institute for Laboratory Animal Research. 1998. The psychological wellbeing of nonhuman primates: a report of the Committee on Well-Being of Nonhuman Primates. Washington (DC): National Academies Press.
- 19.Institute for Laboratory Animal Research. 2011. The guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press.
- 20.Judge PG, De Waal FBM. 1997. Rhesus monkey behaviour under diverse population densities: coping with long-term crowding. Anim Behav 54:643–662 [DOI] [PubMed] [Google Scholar]
- 21.Kaplan JR, Manning P, Zucker E. 1980. Reduction of mortality due to fighting in a colony of rhesus monkeys (Macaca mulatta). Lab Anim Sci 30:565–570 [PubMed] [Google Scholar]
- 22.Kessler MJ, London WT, Rawlins RG, Gonzalez J, Martinez HS, Sanchez J. 1985. Management of a harem breeding colony of rhesus monkeys to reduce trauma-related morbidity and mortality. J Med Primatol 14:91–98 [PubMed] [Google Scholar]
- 23.Nelson RJ, Mandrell TD. 2005. Enrichment and nonhuman primates: “first, do no harm”. ILAR J 46:171–177 [DOI] [PubMed] [Google Scholar]
- 24.Novak MA, Rulf A, Munroe H, Parks K, Price C, O'Neill P, Suomi S. 1995. Using a standard to evaluate the effects of environmental enrichment. Lab Anim (NY) 24:37–42 [Google Scholar]
- 25.Oates-O'Brien RS, Farver TB, Anderson-Vicino KC, McCowan B, Lerche NW. 2010. Predictors of matrilineal overthrows in large captive breeding groups of rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 49:196–201 [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenbaum MD, Vandewoude S, Johnson T. 2009. Effects of cage-change frequency and bedding volume on mice and their microenvironment. J Am Assoc Lab Anim Sci 48:763–773 [PMC free article] [PubMed] [Google Scholar]
- 27.Siegel S, Castellan NJ. 1988. Nonparametric statistics for the behavioral sciences, 2nd ed. New York (NY): McGraw-Hill.
- 28.Seth P, Seth S. 1986. Ecology and behaviour of rhesus monkeys in India, p 89–103. In: Else JG, Lee P. Primate ecology and conservation. New York (NY): Cambridge University Press. [Google Scholar]
- 29.Teas J, Richie T, Taylor H, Southwick C. 1980. Population patterns and behavioral ecology of rhesus macaques (Macaca mulatta) in Nepal, p 247–262. In: Lindburg D. The macaques studies in ecology, behavior, and evolution. New York (NY): Van Nostrand Reinhold. [Google Scholar]
- 30.Westergaard GC, Izard MK, Drake JH. 2000. Reproductive performance of rhesus macaques (Macaca mulatta) in 2 outdoor housing conditions. Am J Primatol 50:87–93 [DOI] [PubMed] [Google Scholar]
- 31.Wilson AP, Boelkins RC. 1970. Evidence for seasonal variation in aggressive behaviour by Macaca mulatta. Anim Behav 18:719–724 [DOI] [PubMed] [Google Scholar]